Abstract
Objective
Evaluate the safety and tolerability of oral rimegepant when used for acute treatment concomitantly with a monoclonal antibody (mAb) targeting the calcitonin gene‐related peptide (CGRP) ligand or receptor (CGRP mAb) for the preventive treatment of migraine.
Background
The efficacy of CGRP mAbs for the preventive treatment of migraine and the small molecule CGRP receptor antagonist rimegepant for acute treatment has been demonstrated in randomized controlled clinical trials. Over the past few years, the US Food and Drug Administration has approved 4 CGRP mAbs for the preventive treatment of migraine and 2 small molecule CGRP receptor antagonists for the acute treatment of migraine. A previous case report of 2 patients receiving concomitant treatment with rimegepant and erenumab suggested that rimegepant may be safely used as acute treatment in patients who are also receiving a preventive regimen involving CGRP mAbs. We report here 13 additional patients with migraine who simultaneously used rimegepant and either erenumab, fremanezumab, or galcanezumab and assess the rate of on‐treatment adverse events (AEs).
Methods
This was a substudy nested within a multicenter, open‐label, long‐term safety study in adults with 2‐14 monthly migraine attacks of moderate to severe pain intensity. A subgroup experiencing 2‐8 monthly attacks and taking a stable dose of a CGRP mAb also took rimegepant 75 mg as needed up to once daily for acute treatment for 12 weeks.
Results
The 13 patients (11 women [85%]; mean age 49.9 years) enrolled in the substudy were being treated with CGRP mAbs (erenumab [n = 7], fremanezumab [n = 4], or galcanezumab [n = 2]). Mean (SD) time in the rimegepant treatment period was 9.6 (4.6) weeks. Mean (SD) 4‐week rimegepant exposure was 7.8 (5.5) doses; a total of 224 doses were taken. Five (38%) patients reported ≥1 on‐treatment AE. Of these, 2 (15%) patients had mild or moderate nasopharyngitis; no other AEs occurred in ≥2 patients. Three patients had AEs of mild or moderate severity that were considered potentially treatment‐related. No patients had serious AEs, AEs leading to discontinuation, or aminotransferase levels >3× the upper limit of normal.
Conclusion
Rimegepant, when used as an oral acute treatment in patients receiving CGRP mAbs as preventive treatment, was well tolerated; no safety issues were identified. Studies involving larger patient populations are needed to confirm these findings.
Keywords: migraine, prevention, calcitonin gene‐related peptide, rimegepant
Abbreviations
- AE
adverse event
- ALT
alanine transaminase
- AST
aspartate transaminase
- CGRP
calcitonin gene‐related peptide
- CYP
cytochrome P450
- IRB
institutional review board
- mAb
monoclonal antibody
- SD
standard deviation
- ULN
upper limit of normal
Introduction
Pharmacotherapy for migraine can be used acutely, to treat individual attacks in progress, or preventively, to reduce the frequency and severity of attacks. 1 , 2 , 3 Virtually everyone with migraine needs acute treatment, while preventive treatments are often added for people with more frequent and disabling attacks. Because acute treatments are used for breakthrough attacks during preventive treatment, the safety and tolerability issues associated with the coadministration of acute and preventive treatments can influence drug selection, adherence, and the success of therapy.
Calcitonin gene‐related peptide (CGRP) has become an important target for both the acute and preventive treatment of migraine. 4 , 5 , 6 Randomized controlled trials have established the efficacy of CGRP signal‐blocking monoclonal antibodies (CGRP mAbs) for the preventive treatment of migraine 7 , 8 , 9 and small molecule CGRP receptor antagonists (gepants) for acute treatment. 10 , 11 , 12 , 13 , 14 The US Food and Drug Administration has approved 4 CGRP mAbs for the prevention of migraine and 2 gepants for the acute treatment of migraine. 15 A previous case report of 2 patients receiving erenumab suggests that rimegepant (Nurtec ODT, Biohaven Pharmaceutical Holding Company Ltd., New Haven, CT, USA) may be used acutely to relieve attacks without tolerability or safety problems in patients receiving preventive CGRP mAbs. 16
Herein, we expand on the previous case report and present the results of a substudy of 13 patients with migraine who simultaneously used rimegepant and mAbs targeting the CGRP ligand or receptor and assess the rate of on‐treatment adverse events (AEs). The substudy objective was to evaluate the safety and tolerability of oral rimegepant when used for acute treatment concomitantly with CGRP mAbs for migraine prevention in adults.
Methods
Ethics
This study was conducted in accordance with the ethical principles of Good Clinical Practice, per the International Council on Harmonization Harmonized Tripartite Guideline, and all applicable local regulations. The protocol was approved by a central institutional review board (IRB) or an IRB at each study center. Before study initiation, investigators were required to have written and dated approval/favorable opinion from the IRB for the protocol, consent form, patient recruitment materials/process (eg, advertisements), and other written information to be provided to patients, and patients provided written informed consent. The study was prospectively registered at clinicaltrials.gov (Study 201, NCT03266588). The authors take full responsibility for the data, the analyses and interpretation, and the conduct of the research, and they confirm their full access to all the data throughout the course of the study.
Study Conduct
This was a substudy within a multicenter, open‐label, long‐term safety study in adults with migraine. A detailed description of the entire long‐term safety study is available in the study protocol, which is available at clinicaltrials.gov. The analysis presented here is of a cohort of patients added by amendment after CGRP mAbs were approved for use in the United States. Participation in the substudy was limited to 12 weeks to obtain a preliminary assessment of safety and tolerability of concomitant use of rimegepant with CGRP mAbs. The cohort comprised patients with a history of 2 to 8 migraine attacks of moderate to severe pain intensity per month who were receiving a stable dose (≥2 months) of a Food and Drug Administration‐approved CGRP mAb, specifically erenumab (Aimovig, Amgen, Thousand Oaks, CA, USA), fremanezumab (Ajovy, Teva Pharmaceuticals, Parsippany, NJ, USA), or galcanezumab (Emgality, Eli Lilly and Company, Indianapolis, IN, USA).
There were 3 study periods, as detailed below: a screening visit/observation period of 30 days, a long‐term rimegepant treatment period of 12 weeks, and a follow‐up safety visit 14 ± 2 days after rimegepant treatment ended.
In the 30‐day observation period, patients eligible after screening were provided an electronic diary to document attack occurrence, severity, and treatment, plus a paper diary to record their use of nonstudy migraine treatments, throughout the study. After the observation period, patients returned to the study site for a review of both diaries to ensure study compliance. Patients in compliance were enrolled in the long‐term treatment period, given a 30‐day supply of study medication, and instructed to wait until baseline laboratory results confirmed their continued eligibility before administering any medication. During the treatment period, patients could treat attacks of mild, moderate, or severe pain intensity as needed with no more than 1 rimegepant 75 mg tablet per calendar day.
During the treatment period, patients visited the study site approximately every 2 weeks in the first month and then every 4 weeks through Week 12 for the assessment of study medication compliance and monitoring of tolerability and safety (including vital signs, laboratory tests, and electrocardiography). At the end of Week 12, patients returned to the study site for electronic diary review and assessments of medication compliance, tolerability, and safety.
After an additional 14 ± 2 days, patients returned to the study site for a follow‐up safety visit that included the collection of laboratory tests, vital signs, electrocardiography, and AEs, including assessment for AEs consistent with drug dependency or withdrawal effects.
Additional details about the study design are available in the study protocol.
Patients
In the long‐term safety study and the substudy, eligible patients included males or females 18 years and older who had migraine with or without aura per the International Classification of Headache Disorders, 3rd Edition, beta version. 17 Those taking preventive migraine medication were permitted to remain on therapy if on a stable dose for at least 2 months prior to baseline and the dose was not expected to change during the study. Patients in whom triptans were contraindicated could participate if they met all eligibility criteria.
Key exclusion criteria included a history of basilar migraine or hemiplegic migraine; human immunodeficiency virus disease; Gilbert’s Syndrome or active hepatic or biliary disease; or aspartate transaminase (AST), alanine transaminase (ALT), and/or serum bilirubin (total, direct, or indirect) greater than 1× the upper limit of normal (ULN). Full exclusion criteria are provided in the study protocol.
Statistical Analysis
All statistical analyses were performed using SAS Version 9.4.
Analyzed populations included screened patients (signed informed consent and assigned to an enrollment group), enrolled patients (screened and enrolled in long‐term treatment), and treated patients (enrolled and took any amount of rimegepant).
Categorical variables were summarized using frequency and expressed as the number of patients and percentage. Continuous variables were summarized with univariate statistics and expressed as mean, median, standard deviation (SD).
The primary safety and tolerability endpoints were frequency and severity of on‐treatment AEs occurring in at least 5% of treated patients, serious AEs, AEs leading to study drug discontinuation, and clinically significant laboratory test abnormalities. The 5% threshold was applied to the percentage of patients with a preferred term in any enrollment group, regardless of severity. Investigators determined the severity (eg, mild, moderate, severe) of AEs and the relationship of AEs to study drug; their terms were coded and grouped by system organ class using the Medical Dictionary for Regulatory Activities. An AE was considered related to study drug if the relationship was not reported (missing), unlikely related, possibly related, or related. Clinically significant laboratory abnormalities were identified as Grade 3 or 4 according to numeric laboratory test criteria. 18 , 19
Secondary safety endpoints included frequency of on‐treatment elevations of AST or ALT > 3× ULN concurrent with total bilirubin > 2× ULN; frequency and severity of on‐treatment hepatic‐related AEs; and frequency of on‐treatment hepatic‐related AEs leading to study drug discontinuation. Elevations of AST or ALT > 3× ULN concurrent with total bilirubin > 2× ULN were defined as elevations on the same collection date.
The sample size was not formally calculated for this analysis. However, investigators prospectively planned to enroll approximately 20 patients into the substudy to enhance the clinical relevance and generalizability of results.
Results
Patients
The first patient was screened on January 23, 2019, and the last patient completed the study on July 15, 2019. In this substudy, 16 patients on CGRP mAbs were screened, 13 (81%) were enrolled, treated with rimegepant, and evaluated for safety, and 10 (77%) completed the study (Fig. 1). Of the 16 patients screened, 3 withdrew before starting treatment with rimegepant. Of the 3 patients who initiated treatment with rimegepant and then discontinued, 2 were also being treated with fremanezumab 225 mg, and 1 was also being treated with erenumab 140 mg. One patient taking the fremanezumab‐rimegepant combination discontinued for lack of efficacy, and the other 2 patients withdrew from the study without providing reasons.
Fig. 1.
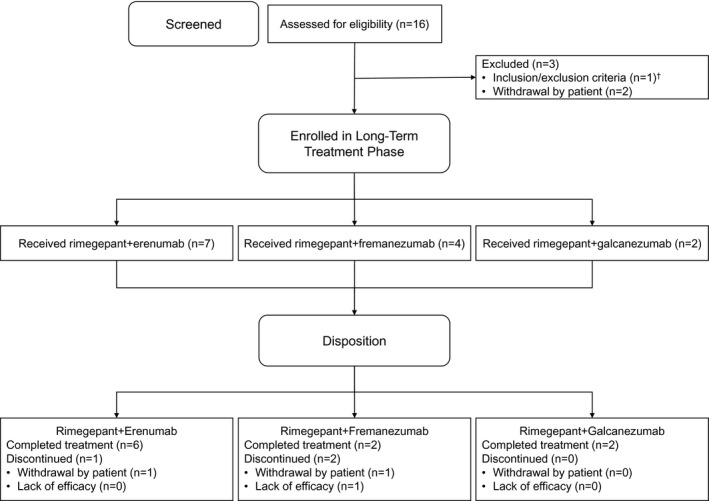
Patient flow. †Noncompliance with or inability to complete the electronic diary during the observation period.
The 13 safety‐evaluable patients participated in the long‐term treatment period for a mean (SD) of 9.6 (4.6) weeks and a median (range) of 12.1 (1.1‐13.1) weeks. The 4‐week average rimegepant exposure (mean [SD] tablets per 4 weeks) was 7.8 (5.5) tablets; the median (range) was 6.5 (3.0‐20.5) tablets. The mean (SD) cumulative rimegepant exposure was 17.2 (13.2) tablets, and the median was 17.0 (interquartile range 13) tablets. Total exposure across the cohort was 224 tablets.
The treated population had a mean (SD) age of 49.9 (13.7) years. Most patients (85% [11/13]) were female, 92% (12/13) were white, and the mean (SD) body mass index was 27.1 (4.2) kg/m2. Demographics and other baseline characteristics are shown in Table 1.
Table 1.
Demographics and Baseline Characteristics
| Rimegepant N = 13 | |
|---|---|
| Age, years, mean (SD) | 49.9 (13.7) |
| Sex, n (%) | |
| Female | 11 (85) |
| Male | 2 (15) |
| Race, n (%) | |
| American Indian or Alaska Native | 0 |
| Asian | 1 (8) |
| Black or African American | 0 |
| White | 12 (92) |
| Multiple | 0 |
| Weight, kg, mean (SD) | 74.3 (13.0) |
| Height, cm, mean (SD) | 165.3 (7.7) |
| Body Mass Index, kg/m2, mean (SD) | 27.1 (4.2) |
Safety and Tolerability
Table 2 details the on‐treatment AEs experienced by the 13 patients. Five (38%) patients reported at least 1 AE, the most common of which was nasopharyngitis (2/13 [15%]). All other AEs – back pain, myalgia, contusion, dizziness, viral gastroenteritis, sinusitis, and first‐degree atrioventricular block – affected single patients (8%) in the subgroup and were generally of mild or moderate severity.
Table 2.
On‐treatment Adverse Events During Concomitant Treatment With Rimegepant Plus a CGRP Monoclonal Antibody
| Patient | Age | Sex | Moderate or Severe Attacks/Month† | CGRP mAb Monthly Dose | Adverse Events | ||
|---|---|---|---|---|---|---|---|
| Event | Severity | Relationship to Study Drug | |||||
| 1 | 29 | Female | 3 | Erenumab 140 mg | — | — | — |
| 2 | 47 | Female | 4 | Fremanezumab 225 mg | Viral gastroenteritis | Moderate | Unlikely |
| Nasopharyngitis | Mild | No | |||||
| 3 | 69 | Male | 5 | Erenumab 140 mg | Back pain | Moderate | No |
| Nasopharyngitis | Moderate | No | |||||
| Myalgia | Moderate | No | |||||
| 4 | 50 | Female | 4 | Galcanezumab 120 mg | — | — | — |
| 5 | 44 | Female | 3 | Galcanezumab 120 mg | — | — | — |
| 6 | 30 | Female | 3 | Erenumab 140 mg | First‐degree atrioventricular block‡ | Mild | Possibly |
| Sinusitis | Severe | No | |||||
| 7 | 51 | Female | 2 | Erenumab 140 mg | Contusion | Mild | No |
| 8 | 49 | Male | 8 | Erenumab 70 mg | — | — | — |
| 9§ | 71 | Female | 8 | Fremanezumab 225 mg | — | — | — |
| 10¶ | 58 | Female | 8 | Fremanezumab 225 mg | — | — | — |
| 11 | 30 | Female | 3 | Fremanezumab 225 mg | — | — | — |
| 12§ | 56 | Female | 5 | Erenumab 140 mg | — | — | — |
| 13 | 58 | Female | 4 | Erenumab 140 mg | Dizziness | Mild | Possibly |
CGRP = calcitonin gene‐related peptide; mAb = monoclonal antibody; — = none reported.
History prior to screening.
Baseline PR interval = 196 ms, Week 12 PR interval = 206 ms (~2 days after the last rimegepant dose), follow‐up electrocardiography PR interval = 200 ms.
Discontinued, patient withdrew.
Discontinued, lack of efficacy.
Events considered by the investigators to be potentially related to treatment were observed in 3 (23%) patients and are summarized below. Viral gastroenteritis occurred in a 47‐year‐old woman receiving concomitant fremanezumab; the event was moderate in severity and considered unlikely to be related to treatment. First‐degree atrioventricular block occurred in a 30‐year‐old woman receiving erenumab and was considered mild and possibly related to treatment. Electrocardiography showed that this patient had a PR interval of 196 ms at baseline, 206 ms at Week 12 (~2 days after the last rimegepant dose), and 200 ms at the follow‐up visit 21 days later. Dizziness in a 58‐year‐old woman was also considered mild and possibly related to treatment. In all 3 patients who experienced AEs considered potentially treatment‐related, the rimegepant dose was not changed, and the events resolved without treatment.
There were no serious AEs or AEs leading to study drug discontinuation.
Of the 12 patients with liver function test data, 1 patient had an AST level that was above normal (45 U/L, 1.1× ULN). No patients had ALT or AST levels > 3× ULN, and no patients had elevations in total bilirubin or any other liver function test parameters.
Discussion
We assessed the tolerability and safety of oral rimegepant for the acute treatment of migraine among 13 patients in a long‐term, open‐label safety study who were concomitantly receiving erenumab, fremanezumab, or galcanezumab for preventive treatment of migraine. The incidence, type, and severity of AEs experienced by this cohort were consistent with findings in patients who did not receive CGRP mAbs, 20 and there were no apparent distinctions based on which mAb was coadministered. In addition, no patients discontinued the study for reasons associated with safety or tolerability, and there was no evidence of hepatotoxicity with any of the rimegepant‐CGRP mAb combinations.
The safety and tolerability profiles of rimegepant 11 , 12 and of the CGRP mAbs 7 , 8 , 9 have been favorable to date. The probability of pharmacokinetic interactions between the 2 classes of antagonists is low. 21 While certain biologics may affect drug metabolism by modifying the expression of cytochrome P450 (CYP) enzymes, 21 , 22 the prescribing information for erenumab, 23 fremanezumab, 24 and galcanezumab 25 states that they are not metabolized by CYP enzymes and that interactions with concomitant medications that are substrates, inducers, or inhibitors of CYP enzymes are unlikely. These characteristics, together with the favorable safety profiles of rimegepant and the CGRP mAbs, support the safety of concomitant use. Nonetheless, caution is warranted during concomitant use given the limited body of evidence to date.
Although not specifically studied, the mechanism(s) that might underlie the therapeutic benefit of oral rimegepant acute treatment combined with injectable CGRP mAb preventive therapy are of interest. The notion that CGRP mAb therapy completely blocks peripheral CGRP signaling, and therefore adding acute rimegepant treatment cannot provide additional biologic effect, is erroneous. Modeling of ligand‐targeting mAbs indicates that during a given month, CGRP plasma levels first drop and then rise, with up to 36% to 55% of CGRP circulating freely. 26 A target engagement model for galcanezumab at the 240 mg loading dose shows initially low circulating free CGRP levels that progressively increase ~10‐fold over 4 weeks: ~3% free on day 1, ~9% free at 1 week, ~18% free at 2 weeks, ~28% free at 3 weeks, and ~36% free at 4 weeks. 26 After each of the 4 subsequent 120 mg monthly injections, circulating free CGRP levels likewise fall then rise, but with higher free levels, where minimum free CGRP is ~22 to ~28%, and maximum free CGRP is ~50 to 55%. With this abundance of free circulating CGRP, it should not be surprising that rimegepant acute treatment could provide additional therapeutic benefit. Since the 3 ligand‐targeting CGRP mAbs (fremanezumab, galcanezumab, eptinezumab) exhibit similar treatment effects on migraine prevention, 27 it is likely that all 3 leave comparable free levels of CGRP circulating during mAb treatment.
Further, the preventive efficacy profile is similar for the receptor‐targeting CGRP mAb erenumab, indicating that CGRP receptor occupancy may also decrease between injections as exposures drop over time, providing an opportunity for added benefit with rimegepant. Support for the potential benefit of a CGRP receptor‐targeting small molecule in combination with a receptor‐targeting mAb comes from a case report showing that open‐label rimegepant consistently and successfully treated breakthrough migraine attacks in 2 patients on preventive erenumab therapy. 16 Taken together, these data suggest that not all CGRP signaling is blocked by preventive treatment with the CGRP mAbs.
While it is unknown what precise mechanism(s) might underlie the added therapeutic benefit of oral rimegepant acute treatment in combination with injectable CGRP mAb preventive treatment, the presence of up to 55% circulating free CGRP levels each month may increase the propensity for breakthrough attacks in people with migraine, despite ongoing CGRP mAb treatment. As presented previously, 16 other factors contributing to the added benefit of rimegepant may include ~280× smaller physical size; higher inherent membrane permeability, greater functional inhibition of CGRP signaling, differential receptor kinetics, and/or the ability to withstand a wave of CGRP release. Additional studies are needed to determine whether these or other differences are the primary factors underlying the therapeutic benefits of combination therapy with rimegepant and CGRP mAbs.
The findings in the present study of 13 safety‐evaluable patients are consistent with those in the case report involving 2 patients who simultaneously received rimegepant for acute treatment and erenumab for preventive treatment. 16 While those patients were being treated with the rimegepant‐CGRP mAb combination, no AEs related to treatment were observed. The efficacy and safety of concomitant use of rimegepant and CGRP mAbs merits additional study.
It remains unknown if there is an optimal period for this approach or a subset of patients for whom this combination therapy might be of particular benefit or risk. Use of rimegepant during periods of low circulating CGRP mAb concentrations and/or higher free CGRP levels, such as after the first week of each dosing interval, might be of particular clinical utility. Additionally, combination therapy of rimegepant with CGRP mAbs might prove to be useful to those with suboptimal response, poor tolerability, or contraindications to triptans, and clinical trials are needed to investigate these possibilities. Although drugs for acute treatment will remain the foundational element in migraine pharmacotherapy, the results of the 13 safety‐evaluable cases in this study, together with the previous 2 cases capturing efficacy and safety, provide additional evidence that combination treatment has the potential to satisfy the unmet acute and preventive therapeutic needs for a large subset of patients with migraine, potentially without an added safety burden.
A strength of this study is the novelty of its approach to the treatment of migraine; patients receiving acute and preventive treatment with different agents administered via different routes focused on the same molecular target have not been previously evaluated. Of note, this cohort included patients treated monthly with fremanezumab, but not when it was administered every 3 months, and did not include any patients who received the recently approved eptinezumab, as it was investigational at the time of this study. While the enrolled patients were broadly representative of the general migraine population, the strength and generalizability of these findings are limited by the relatively small number of patients analyzed, the open‐label design, and the short duration of follow‐up.
Conclusions
Rimegepant was well tolerated, with minimal side effects reported, for the acute treatment of migraine in 13 safety‐evaluable patients receiving concomitant preventive treatment with an injectable CGRP mAb over 12 weeks. These data support and extend the safe use of combination treatment initially reported in 2 earlier cases. Research in a larger patient population is needed to further confirm and extend these results.
Statement of Authorship
Category 1
(a) Conception and Design
Robert Croop, Meghan Lovegren, Alexandra C. Thiry, Vladimir Coric, Richard B. Lipton
(b) Acquisition of Data
Gary Berman, Robert Croop, David Kudrow, Philip Halverson, Meghan Lovegren, Alexandra C. Thiry, Vladimir Coric
(c) Analysis and Interpretation of Data
Gary Berman, Robert Croop, David Kudrow, Philip Halverson, Meghan Lovegren, Alexandra C. Thiry, Charles M. Conway, Vladimir Coric, Richard B. Lipton
Category 2
(a) Drafting the Manuscript
Gary Berman, Robert Croop, David Kudrow, Philip Halverson, Meghan Lovegren, Alexandra C. Thiry, Charles M. Conway, Vladimir Coric, Richard B. Lipton
(b) Revising It for Intellectual Content
Gary Berman, Robert Croop, David Kudrow, Philip Halverson, Meghan Lovegren, Alexandra C. Thiry, Charles M. Conway, Vladimir Coric, Richard B. Lipton
Category 3
(a) Final Approval of the Completed Manuscript
Gary Berman, Robert Croop, David Kudrow, Philip Halverson, Meghan Lovegren, Alexandra C. Thiry, Charles M. Conway, Vladimir Coric, Richard B. Lipton
Acknowledgments
The authors are grateful to Christopher M. Jensen, PharmD (Biohaven Pharmaceuticals, New Haven, CT, USA), and Yushan M. Zhang, PharmD (University of Connecticut School of Pharmacy, Storrs, CT, USA), for review of preliminary data and early versions of the manuscript. Medical writing services were provided by Christopher Caiazza.
Disclosures: Gary Berman, MD, has received fees for the speaker’s bureau from Amgen, Lilly, and Teva. He has consulted for Alder BioPharmaceuticals. He has received research grants from Amgen, Lilly, Teva, Alder, CoLucid, Dr. Reddy’s Laboratories, Allergan, and Biohaven. Robert Croop is employed by and holds stock/stock options in Biohaven Pharmaceuticals. David Kudrow has received fees for the advisory board from Alder, Biohaven, Eli Lilly, Amgen, and Xoc and for speaker’s bureau from Xoc, Teva, Amgen, Novartis, and Eli Lilly. He has also received research support from Amgen, Novartis, Eli Lilly, Teva, Alder, Biohaven, Biogen, and Roche‐Genentech. Philip Halverson has received research support from Biohaven Pharmaceuticals. Meghan Lovegren is employed by and holds stock/stock options in Biohaven Pharmaceuticals. Alexandra Thiry is employed by and holds stock/stock options in Biohaven Pharmaceuticals. Charles M. Conway is employed by and holds stock/stock options in Biohaven Pharmaceuticals. Vladimir Coric is employed by and holds stock/stock options in Biohaven Pharmaceuticals. Richard B. Lipton is the Edwin S. Lowe Professor of Neurology at the Albert Einstein College of Medicine in New York. He receives research support from the NIH. He also receives support from the Migraine Research Foundation and the National Headache Foundation. He serves on the editorial board of Neurology, senior advisor to Headache, and associate editor to Cephalalgia. He has reviewed for the NIA and NINDS, holds stock options in eNeura Therapeutics and Biohaven Holdings; serves as consultant, advisory board member, or has received honoraria from American Academy of Neurology, Abbvie/Allergan, American Headache Society, Amgen, Axsome, Biohaven, BioVision, Dr. Reddy’s (Promius), electroCore, Eli Lilly, eNeura Therapeutics, Equinox, GlaxoSmithKline, Lundbeck (Alder), Merck, Novartis, Pernix, Teva, Trigemina, Vector, Vedanta. He receives royalties from Wolff’s Headache 7th and 8th Edition, Oxford Press University, 2009, Wiley and Informa.
Funding: This study was funded and sponsored by Biohaven Pharmaceuticals, Inc.
Trial Registration: ClinicalTrials.gov identifier: NCT03266588.
References
- 1. Hu XH, Markson LE, Lipton RB, Stewart WF, Berger ML. Burden of migraine in the United States: Disability and economic costs. Arch Intern Med. 1999;159:813‐818. [DOI] [PubMed] [Google Scholar]
- 2. Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343‐349. [DOI] [PubMed] [Google Scholar]
- 3. Silberstein SD, Holland S, Freitag F, Dodick DW, Argoff C, Ashman E. Evidence‐based guideline update: Pharmacologic treatment for episodic migraine prevention in adults: Report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337‐1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goadsby PJ, Edvinsson L, Ekman R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann Neurol. 1988;23:193‐196. [DOI] [PubMed] [Google Scholar]
- 5. Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28:183‐187. [DOI] [PubMed] [Google Scholar]
- 6. Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: Studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993;33:48‐56. [DOI] [PubMed] [Google Scholar]
- 7. Goadsby PJ, Reuter U, Hallstrom Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377:2123‐2132. [DOI] [PubMed] [Google Scholar]
- 8. Stauffer VL, Dodick DW, Zhang Q, Carter JN, Ailani J, Conley RR. Evaluation of galcanezumab for the prevention of episodic migraine: The EVOLVE‐1 randomized clinical trial. JAMA Neurol. 2018;75:1080‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med. 2017;377:2113‐2122. [DOI] [PubMed] [Google Scholar]
- 10. Marcus R, Goadsby PJ, Dodick D, Stock D, Manos G, Fischer TZ. BMS‐927711 for the acute treatment of migraine: A double‐blind, randomized, placebo controlled, dose‐ranging trial. Cephalalgia. 2014;34:114‐125. [DOI] [PubMed] [Google Scholar]
- 11. Lipton RB, Croop R, Stock EG, et al. Rimegepant, an oral calcitonin gene‐related peptide receptor antagonist, for migraine. N Engl J Med. 2019;381:142‐149. [DOI] [PubMed] [Google Scholar]
- 12. Croop R, Goadsby PJ, Stock DA, et al. Efficacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: A randomised, phase 3, double‐blind, placebo‐controlled trial. Lancet. 2019;394:737‐745. [DOI] [PubMed] [Google Scholar]
- 13. Dodick DW, Lipton RB, Ailani J, et al. Ubrogepant for the treatment of migraine. N Engl J Med. 2019;381:2230‐2241. [DOI] [PubMed] [Google Scholar]
- 14. Lipton RB, Dodick DW, Ailani J, et al. Effect of ubrogepant vs placebo on pain and the most bothersome associated symptom in the acute treatment of migraine: The ACHIEVE II randomized clinical trial. JAMA. 2019;322:1887‐1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. US Food and Drug Administration . FDA Approves New Treatment for Adults With Migraine. Available at: https://www.fda.gov/news‐events/press‐announcements/fda‐approves‐new‐treatment‐adults‐migraine. Accessed July 14, 2020. [Google Scholar]
- 16. Mullin K, Kudrow D, Croop R, et al. Potential for treatment benefit of small molecule CGRP receptor antagonist plus monoclonal antibody in migraine therapy. Neurology. 2020;94:e2121‐e2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Headache Classification Committee of the International Headache Society (IHS) . The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33:629‐808. [DOI] [PubMed] [Google Scholar]
- 18. National Cancer Institute . Common Technical Criteria for Adverse Events (CTCAE) Version 5.0 (2017). Available at: https://evs.nci.nih.gov/ftp1/CTCAE/About.html. Accessed July 14, 2020. [Google Scholar]
- 19. Division of AIDS . Table for Grading the Severity of Adult and Pediatric Adverse Events (Corrected Version 2.1, July 2017). Available at: https://rsc.niaid.nih.gov/sites/default/files/daidsgradingcorrectedv21.pdf. Accessed July 14, 2020. [Google Scholar]
- 20. Nurtec ODT (rimegepant) Orally Disintegrating Tablets Prescribing Information. Available at: https://www.nurtec.com/pi. Accessed July 14, 2020. [Google Scholar]
- 21. Ferri N, Bellosta S, Baldessin L, Boccia D, Racagni G, Corsini A. Pharmacokinetics interactions of monoclonal antibodies. Pharmacol Res. 2016;111:592‐599. [DOI] [PubMed] [Google Scholar]
- 22. Mallick P, Taneja G, Moorthy B, Ghose R. Regulation of drug‐metabolizing enzymes in infectious and inflammatory disease: Implications for biologics‐small molecule drug interactions. Expert Opin Drug Metab Toxicol. 2017;13:605‐616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aimovig (erenumab‐aooe) Injection Prescribing Information. Available at: https://www.pi.amgen.com/united_states/Aimovig/Aimovig_pi_hcp_english.pdf. Accessed July 14, 2020. [Google Scholar]
- 24. Ajovy (fremanezumab‐vfrm) Injection Prescribing Information. Available at: https://www.ajovy.com/globalassets/ajovy/ajovy‐pi.pdf. Accessed July 14, 2020. [Google Scholar]
- 25. Emgality (galcanezumab‐gnlm) Injection Prescribing Information. Available at: http://uspl.lilly.com/emgality/emgality.html#pi. Accessed July 14, 2020. [Google Scholar]
- 26. Kielbasa W, Helton DL. A new era for migraine: Pharmacokinetic and pharmacodynamic insights into monoclonal antibodies with a focus on galcanezumab, an anti‐CGRP antibody. Cephalalgia. 2019;39:1284‐1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deng H, Li GG, Nie H, et al. Efficacy and safety of calcitonin‐gene‐related peptide binding monoclonal antibodies for the preventive treatment of episodic migraine – An updated systematic review and meta‐analysis. BMC Neurol. 2020;20:57. [DOI] [PMC free article] [PubMed] [Google Scholar]


