Summary
Background
Guselkumab is an interleukin‐23 inhibitor indicated for the treatment of moderate‐to‐severe plaque psoriasis in adults. Guselkumab has demonstrated additional benefit in patients with early inadequate response to ustekinumab. Long‐term efficacy comparisons of guselkumab and ustekinumab are currently lacking among ustekinumab‐naive patients.
Objectives
To assess the relative efficacy of guselkumab and ustekinumab for maintenance therapy of moderate‐to‐severe plaque psoriasis, using individual patient data (IPD) from randomized controlled trials.
Methods
IPD for guselkumab from the VOYAGE 1 and 2 trials were pooled and compared with IPD for ustekinumab from the NAVIGATE trial. Multivariable logistic regression analyses compared guselkumab 100 mg and ustekinumab 45 mg or 90 mg for the achievement and maintenance of Psoriasis Area and Severity Index (PASI) 90, 75 and 100 responses up to 40 weeks. The regression models accounted for a range of clinically relevant covariates (e.g. age, sex, psoriasis duration). Relative efficacy was expressed using odds ratios (ORs) and predicted probability of treatment response associated with each intervention.
Results
Patients receiving guselkumab had significantly higher probabilities of achieving a PASI 90 response than patients receiving ustekinumab, at both week 16 [70·4% vs. 46·0%, OR 2·79, 95% confidence interval (CI) 2·22–3·45] and week 40 (74·2% vs. 54·5%, OR 2·40, 95% CI 1·89–3·13]. Guselkumab was also associated with a significantly increased likelihood of achieving both PASI 75 and PASI 100 responses at weeks 16 and 40, compared with ustekinumab.
Conclusions
Adjusted analyses leveraging IPD demonstrate that guselkumab has a significantly higher probability of achieving and maintaining PASI treatment responses through week 40 than ustekinumab does.
Linked Comment: Yiu. Br J Dermatol 2020; 183:202–203.
Short abstract
What's already known about this topic?
The NAVIGATE trial demonstrated improved treatment responses in patients with moderate‐to‐severe plaque psoriasis who switched to guselkumab after inadequate responses to ustekinumab induction therapy.
What does this study add?
To date, no head‐to‐head randomized controlled trials have compared ustekinumab and guselkumab in a combination of biologic‐naive and biologic‐experienced patients.
The present study leveraged individual patient data from randomized controlled trials to compare guselkumab and ustekinumab maintenance therapy indirectly in a combination of biologic‐naive and biologic‐experienced patients.
Linked Comment: Yiu. Br J Dermatol 2020; 183:202–203.
Plain language summary available online
Psoriasis is a painful, disfiguring and chronic dermatological condition characterized by autoimmune‐mediated inflammation of the skin.1, 2 The most common form of the condition is psoriasis vulgaris, or plaque‐type psoriasis, which affects approximately 80–90% of patients.3 Plaque‐type psoriasis is characterized by raised red lesions on the skin that often cause considerable pain and itchiness. These signs and symptoms can severely reduce health‐related quality of life.4, 5 Treatment of moderate‐to‐severe plaque psoriasis typically involves systemic therapy or phototherapy. Several biologic therapies have been shown to have efficacy in this patient population.
Interleukin (IL)‐23 exerts an early effect in the pathogenesis of psoriasis via upstream regulation of the two key immune axes in psoriasis: the IL‐23/T helper cell 17 (Th17) axis, which is responsible for the inflammatory cascade, and the regulatory T cell (Treg)/Th17 axis, which contributes to the chronicity of the disease. From one side, IL‐23 activates Th17 cells to produce effector cytokines (i.e. IL‐17, tumour necrosis factor‐α and IL‐22) that drive the inflammatory response and, on the other side, IL‐23 maintains the Th17 secretory phenotype leading to the chronic inflammation seen in psoriasis.6, 7 Inhibiting IL‐23 is an effective strategy that downregulates the inflammatory cascade (IL‐23/Th17 axis) and its perpetuation (Treg/Th17 axis). Ustekinumab, an anti‐IL‐12/IL‐23 monoclonal antibody that binds to the p40 subunit shared by IL‐12 and IL‐23, is indicated for the treatment of moderate‐to‐severe plaque psoriasis and is consistently associated with the highest rates of time on treatment in real‐world settings.8, 9, 10, 11 Guselkumab is the first approved monoclonal antibody that inhibits IL‐23 by targeting its p19 subunit with high specificity and affinity.6 The more selective blockade of IL‐23 via the p19 subunit, as opposed to the dual blockade of IL‐12/IL‐23 via the p40 subunit, may provide additional clinical benefit.7
The NAVIGATE study, a randomized controlled trial (RCT), provides data regarding the relative efficacy of guselkumab compared with ustekinumab in patients who were inadequate responders to ustekinumab [i.e. an Investigator's Global Assessment (IGA) score ≥ 2 after 16 weeks of treatment].12 The VOYAGE 1 and VOYAGE 2 trials included patients with or without prior biologic therapy (i.e. 21·6% and 20·4% of patients had prior biologic treatment in VOYAGE 1 and 2, respectively).13, 14 However, there are no head‐to‐head RCTs comparing guselkumab and ustekinumab in a broader patient population of patients not treated previously with ustekinumab. The objective of the current study was to compare the efficacy of guselkumab vs. ustekinumab in a mix of ustekinumab‐naive and ustekinumab‐experienced patients with moderate‐to‐severe plaque psoriasis, using individual patient data (IPD) from pivotal guselkumab RCTs (i.e. VOYAGE 1 and VOYAGE 2) and ustekinumab RCTs (i.e. NAVIGATE, PHOENIX 1 and PHOENIX 2).
Patients and methods
Overview of study populations
Overviews of the NAVIGATE, VOYAGE and PHOENIX RCTs are presented in the Appendix (see Supporting Information).12, 13, 14, 15, 16 The ACCEPT trial of ustekinumab was not included because it did not have sufficient data beyond the induction period.17 In the current analysis, IPD from VOYAGE 1 and VOYAGE 2 were combined to form a pooled dataset for patients treated with guselkumab.13, 14 IPD from NAVIGATE and PHOENIX 1 were included for patients treated with ustekinumab.12, 15, 16 Primary analyses only included ustekinumab IPD from NAVIGATE,12 while sensitivity analyses included IPD for patients treated with ustekinumab in the PHOENIX trials according to approved labelled dosing (i.e. 45 mg for patients weighing ≤ 100 kg, and 90 mg for patients > 100 kg).15, 16 In addition, NAVIGATE, VOYAGE 1 and VOYAGE 2 were all conducted in a similar timeframe (i.e. initiated in 2014),12, 13, 14 making them more suitable for comparison, whereas the PHOENIX trials were conducted earlier (i.e. initiated in 2005).15, 16
Adjusting for treatment switching in the NAVIGATE and VOYAGE 2 trials
Primary analyses were performed using IPD from NAVIGATE for the ustekinumab cohort (n = 853) and from the VOYAGE trials for the guselkumab cohort (n = 825).12, 13, 14 The ustekinumab cohort included both responders (i.e. patients with an IGA score of 0 or 1 at week 16) who received open‐label ustekinumab for 40 weeks, and inadequate responders (i.e. IGA ≥ 2 at week 16) who were randomized to continued ustekinumab at week 16 (i.e. instead of switching to guselkumab). To adjust for treatment switching at week 16, inadequate responders who continued ustekinumab were reweighted to represent the outcome for patients who switched to guselkumab, through a commonly accepted method known as inverse probability of censoring weighted (IPCW) analysis.18 In using this approach, we attempted to simulate a trial design where patients receiving ustekinumab in NAVIGATE all continued receiving ustekinumab (i.e. did not switch).
Despite lacking long‐term response rates for ustekinumab in patients who switched to guselkumab, we were able to estimate missing information by leveraging information from rerandomized patients who continued on ustekinumab. The alternative approach of excluding nonresponder patients who switched to guselkumab from our analysis would cause selection bias by including only responders, and overestimate response rates for ustekinumab (i.e. a half‐cohort of nonresponders would have been left out). IPCW is expected to be unbiased because of randomization, as nonresponding patients who continued using ustekinumab are treated as being similar to or exchangeable with patients who switched to guselkumab. Due to the randomized nature of treatment switching at week 16, no adjustment for confounders was applied. In VOYAGE 2, patients who achieved ≥ 90% improvement in Psoriasis Area and Severity Index (PASI 90 response) at week 28 were rerandomized to either continued guselkumab or placebo.14 This rerandomization was accounted for via an approach that was similar to the adjustment for crossover described for NAVIGATE (Fig. 1).12
Figure 1.
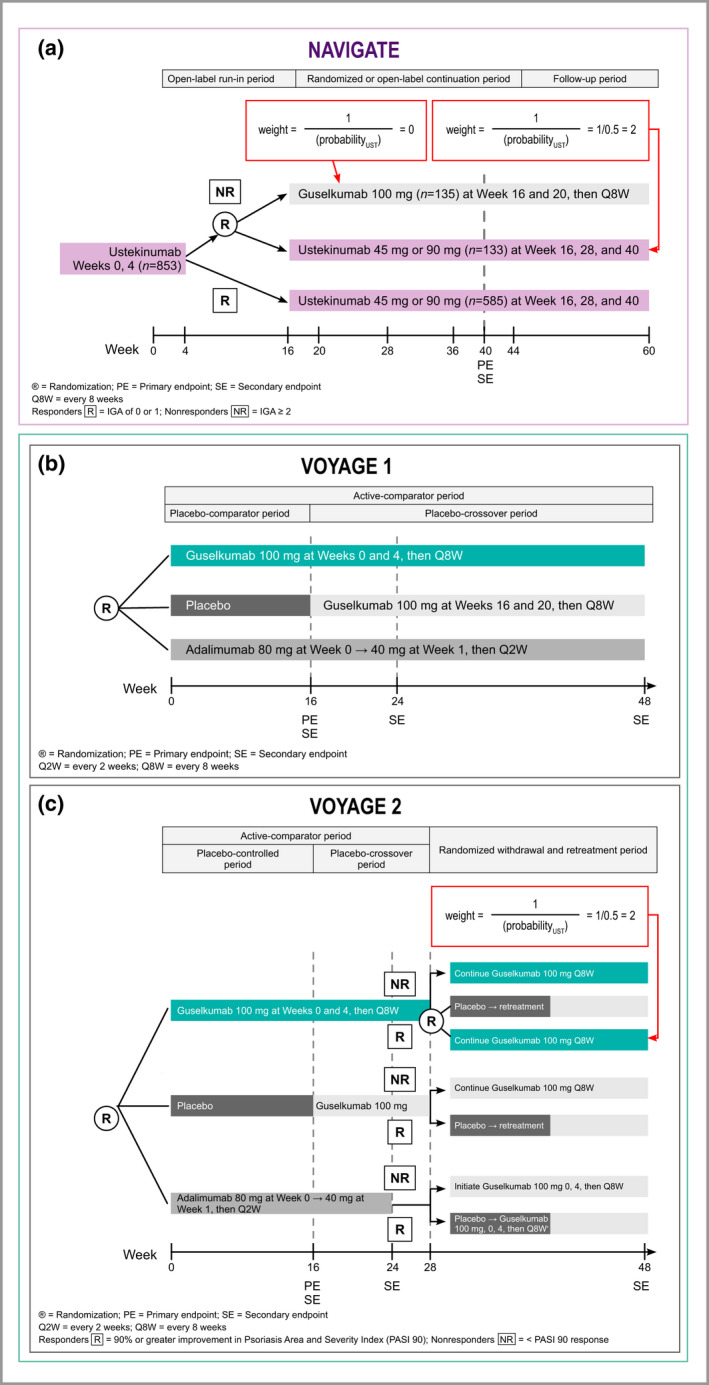
Overview of study designs and approach to adjustments for crossovers in the VOYAGE 2 and NAVIGATE studies. (a) NAVIGATE. As nonresponding patients were randomly assigned to continue ustekinumab vs. switching to guselkumab, patients in both arms can be considered exchangeable, and outcomes for the nonresponding ustekinumab patients who continued on ustekinumab after randomization can be considered to constitute a valid counterfactual for the patients randomized to guselkumab. To estimate unbiased outcomes for the entire cohort and eliminate the impact of treatment switching at week 16, nonresponders who continued with ustekinumab were upweighted to represent the outcome for the patients switched to guselkumab as if they had continued ustekinumab. (b) VOYAGE 1. No adjustments for crossovers needed. (c) A similar approach as described for NAVIGATE was applied to VOYAGE 2 to deal with rerandomization of responding patients in the guselkumab arm at week 28 data. IGA, Investigator's Global Assessment; R, randomized; UST, ustekinumab; W, week.
Approach for multivariable regression analysis
Weighting was added to selected intervention arms as needed to account for the rerandomization of patient subgroups in NAVIGATE and VOYAGE 2.12, 14 PASI 90, PASI 75 and PASI 100 response rates were analysed through 48 weeks of follow‐up, using weighted multivariable logistic regression methods to derive adjusted treatment comparisons between guselkumab and ustekinumab (Fig. 2). Covariates adjusted for in the analyses were prespecified and were selected based on clinical relevance and discussions with clinical experts. Prognostic factors included sex (male vs. female), baseline age (< 45 years, 45–64 years, ≥ 65 years), baseline bodyweight (< 70 kg, 70–79 kg, 80–89 kg, 90–99 kg, 100–109 kg, ≥ 110 kg), psoriasis duration (< 15 years vs. ≥ 15 years), baseline PASI score (< 16, 16–18, 18–19, 20–21, ≥ 22), baseline IGA score (< 4, 4), presence of psoriatic arthritis (yes vs. no), history of phototherapy (ever used vs. never used) and history of prior therapies (systemic and biologic, biologic, systemic, naive).
Figure 2.
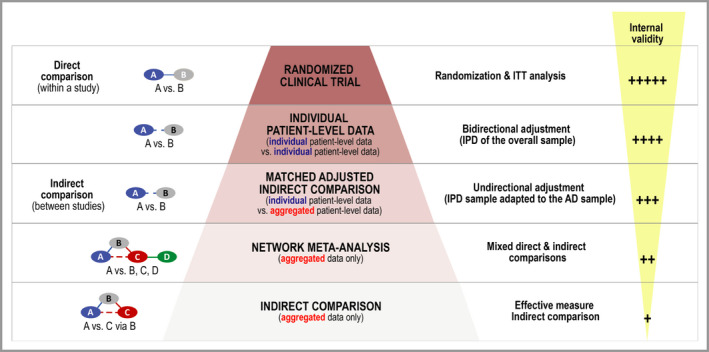
Hierarchy of evidence for comparing interventions; adapted from Coyle et al.26 AD, aggregated data; IPD, individual patient data; ITC, indirect treatment comparison; ITT, intention to treat; MAIC, matching‐adjusted indirect comparison; NMA, network meta‐analysis; RCT, randomized controlled trial.
Findings from all analyses were expressed in terms of both odds ratios (ORs) and risk differences (RDs) with corresponding 95% confidence intervals (CIs) to provide a more robust analysis. Results were considered statistically significant if the 95% CI for ORs excluded the null value of 1 (and for RDs if the 95% CI excluded 0%). Inadequate responder imputation was used for missing data in the analyses. Multivariable logistic models were also used to estimate the predicted probability of treatment response for each therapy up to week 40 of follow‐up. All analyses were performed using SAS software (version 9·4; SAS Institute Inc., Cary, NC, U.S.A.).
Sensitivity analysis using patients from PHOENIX 1 and PHOENIX 2
Sensitivity analyses were performed using a second cohort of patients who used ustekinumab, from the PHOENIX 1 and PHOENIX 2 trials.15, 16 Only patients treated as per approved labelled dosing (i.e. ustekinumab 45 mg for patients weighing ≤ 100 kg, or ustekinumab 90 mg for patients weighing > 100 kg) were included. These analyses were performed to validate findings from the primary analyses.
Results
Overview of patient populations
Table 1 and Figure S1 (see Supporting Information) provide an overview of patient demographics by intervention group and trial for NAVIGATE, VOYAGE 1 and VOYAGE 2.12, 13, 14 Statistically significant differences were observed between the studies, with respect to sex (64·4–71·4% male, with higher values in the VOYAGE studies); disease duration (46·8–55·3% with duration ≥ 15 years, with higher values in the VOYAGE studies); prior therapies (28·5–42·1% of patients were treatment naive, with higher values in the NAVIGATE study); and history of phototherapy (51·2–58·4% ever used, with higher values in the VOYAGE studies). The other patient characteristics that were assessed were comparable. Characteristics of patients from the PHOENIX trials of ustekinumab are provided in the Appendix (see Supporting Information).15, 16
Table 1.
Baseline patient characteristics by trial
| Characteristic | VOYAGE 1 & 2, n (%)a | NAVIGATE, n (%)b | P‐value |
|---|---|---|---|
| Sex | |||
| Male | 589 (71·4) | 549 (64·4) | 0·002 |
| Female | 236 (28·6) | 304 (35·6) | |
| Baseline age (years) | |||
| ˂ 45 | 432 (52·4) | 475 (55·7) | 0·23 |
| 45–64 | 352 (42·7) | 330 (38·7) | |
| ≥ 65 | 41 (5·0) | 48 (5·6) | |
| Baseline weight (kg) | |||
| < 70 | 125 (15·2) | 173 (20·3) | 0·06 |
| 70–79 | 146 (17·7) | 148 (17·4) | |
| 80–89 | 182 (22·1) | 153 (17·9) | |
| 90–99 | 165 (20·0) | 152 (17·8) | |
| 100–109 | 92 (11·2) | 112 (13·1) | |
| ≥ 110 | 115 (13·9) | 115 (13·5) | |
| Psoriasis disease duration (years) | |||
| < 15 | 369 (44·7) | 454 (53·2) | < 0·001 |
| ≥ 15 | 456 (55·3) | 399 (46·8) | |
| Baseline PASI score | |||
| < 16 | 240 (29·1) | 264 (31·0) | 0·68 |
| 16–17 | 134 (16·2) | 134 (15·7) | |
| 18–19 | 93 (11·3) | 107 (12·6) | |
| 20–21 | 57 (6·9) | 61 (7·2) | |
| ≥ 22 | 301 (36·5) | 286 (33·5) | |
| IGA score | |||
| < 4 | 633 (76·7) | 679 (79·5) | 0·16 |
| 4 | 192 (23·3) | 174 (20·5) | |
| BSA (%) | |||
| < 20 | 309 (37·5) | 345 (40·4) | 0·21 |
| ≥ 20 | 516 (62·5) | 508 (59·6) | |
| Psoriatic arthritis | |||
| Yes | 153 (18·5) | 119 (14·0) | 0·01 |
| No | 672 (81·5) | 734 (86·0) | |
| Previous therapy | |||
| Naive | 235 (28·5) | 359 (42·1) | < 0·001 |
| Systemic use only | 418 (50·7) | 367 (43·0) | |
| Biologic use only | 49 (5·9) | 45 (5·3) | |
| Systemic and biologic use | 123 (14·9) | 82 (9·6) | |
| Phototherapy | |||
| Never used | 343 (41·6) | 416 (48·8) | 0·003 |
| Ever used | 482 (58·4) | 437 (51·2) | |
PASI, Psoriasis Area and Severity Index; IGA, Investigator's Global Assessment; BSA, body surface area; aguselkumab (N = 825 patients); bustekinumab (N = 853 patients).
The Psoriasis Area and Severity Index 90 response at 40 weeks
Figure 3 presents a forest plot summarizing the effects of the different covariates included in the multivariable model of the PASI 90 response at week 40. PASI 90 response rates were generally consistent across covariates, although weight 90–99 kg and prior phototherapy were both associated with a significantly lower likelihood of a PASI 90 response at week 40 (P < 0·05 vs. weight < 70 kg and no phototherapy, respectively). Figure 4 presents a longitudinal plot of the predicted PASI 90 response rates based on the multivariable regression model from the pooled VOYAGE and NAVIGATE trials.
Figure 3.
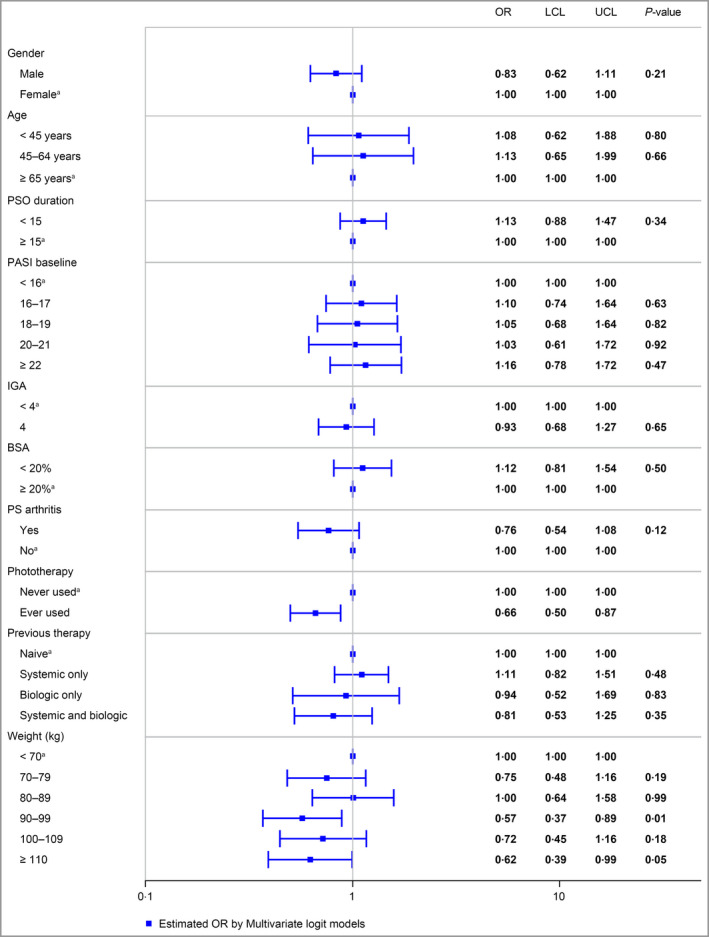
Estimated covariate effects from logistic regression, Psoriasis Area and Severity Index 90 response at week 40. Treatment and covariate effects were estimated using multivariable logistic regression and are expressed as odds ratios with corresponding 95% confidence intervals. BSA, body surface area; IGA, Investigator's Global Assessment; LCL, lower 95% confidence limit; OR, odds ratio; PASI, Psoriasis Area and Severity Index; PS, psoriatic; PSO, psoriasis; UCL, upper 95% confidence limit; areference value.
Figure 4.
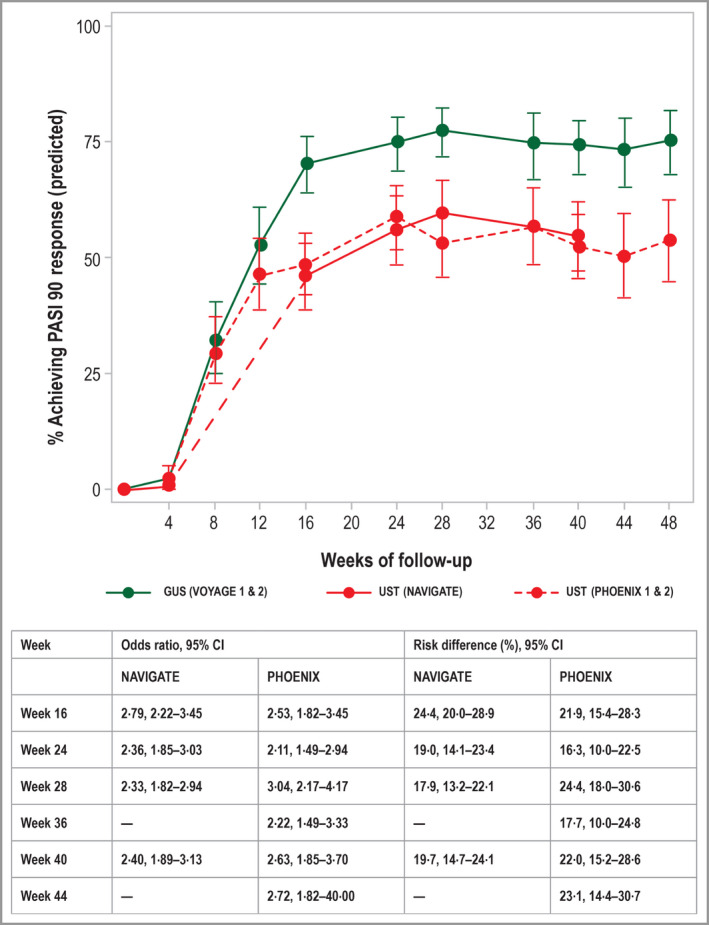
Predicted rates of a Psoriasis Area and Severity Index 90 treatment response for guselkumab and ustekinumab, based on data from the VOYAGE, NAVIGATE and PHOENIX trials, with 95% confidence intervals. Predicted rates were derived from the multivariable logistic regression model, as described in the study methods. CI, confidence interval; GUS, guselkumab; PASI, Psoriasis Area and Severity Index; UST, ustekinumab.
The predicted response rates account for differences between study populations and were found to align well with the observed rates from the individual studies (Appendix; see Supporting Information). Based on the regression analysis, significantly more patients treated with guselkumab achieved a PASI 90 response at week 40, compared with patients treated with ustekinumab. The predicted probability of achieving a PASI 90 response at week 40 was 74·2% for patients treated with guselkumab, compared with 54·4% for patients treated with ustekinumab (adjusted OR 2·40, 95% CI 1·89–3·13; Fig. 4). The corresponding RD at week 40 was 19·7% (95% CI 14·7–24·1%). This difference was statistically significant in favour of guselkumab for weeks 16–48.
Psoriasis Area and Severity Index 75 and 100 responses
Longitudinal plots of the observed and predicted response rates for PASI 75 and PASI 100 are presented in the Appendix (see Supporting Information); a summary of covariate effects is provided in the Appendix (see Supporting Information). Based on the multivariable regression analysis, the adjusted OR for the PASI 75 response at week 40 was 2·14 (95% CI 1·54–2·94) in favour of guselkumab (Fig. S3; see Supporting Information). The predicted probability of a PASI 75 response at week 40 was 87·8% for guselkumab and 77·1% for ustekinumab, with a corresponding RD of 10·7% (95% CI 6·2–15·0%).
Similarly, the adjusted OR for a PASI 100 response at week 40 was 2·53 (95% CI 2·00–3·23) in favour of guselkumab (Fig. 5). The predicted probability of a PASI 100 response at week 40 was 49·7% for guselkumab and 28·1% for ustekinumab, with a corresponding RD of 21·6% (95% CI 16·7–26·4%). The relative treatment effect (expressed as the OR) was greater for higher PASI thresholds. Similar differences in PASI 75 and PASI 100 response rates between cohorts were observed for the entire maintenance treatment phase between weeks 16 and 40.
Figure 5.
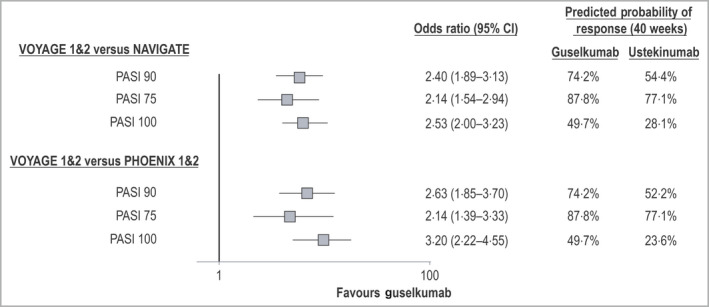
Adjusted comparisons for guselkumab vs. ustekinumab: achievement of Psoriasis Area and Severity Index (PASI) 90, 75 and 100 responses at week 40. Odds ratios from multivariable logistic regression analyses comparing guselkumab and ustekinumab for PASI response outcomes at 40 weeks of follow‐up are shown. In both primary and secondary analyses, guselkumab was associated with a statistically significantly increased likelihood of treatment response. Predicted probabilities of response associated with each intervention are also provided for each outcome measure. CI, confidence interval.
Sensitivity analyses
Multivariable logistic regression analyses for all PASI response end points were repeated using ustekinumab IPD from the PHOENIX trials; predicted and observed PASI response rates are presented in Figure 4 and the Appendix (see Supporting Information), respectively. Predicted treatment effects were similar to those obtained in the primary analyses, with statistically significant differences in favour of guselkumab for the PASI 90 response (OR 2·63, 95% CI 1·85–3·70), the PASI 75 response (OR 2·14, 95% CI 1·39–3·33) and the PASI 100 response (OR 3·20, 95% CI 2·22–4·55; Fig. 5). The corresponding predicted probabilities of PASI responses were 74·2% vs. 52·2% (PASI 90), 87·8% vs. 77·1% (PASI 75) and 49·7% vs. 23·6% (PASI 100; Fig. 5).
Discussion
To date, no head‐to‐head RCTs have compared ustekinumab and guselkumab in a combination of biologic‐naive and biologic‐experienced patients. The present study leveraged IPD from the NAVIGATE and VOYAGE trials to compare guselkumab and ustekinumab indirectly in a combination of biologic‐naive and biologic‐experienced patients.12, 13, 14 IPD analysis is the gold‐standard methodology for comparing treatments in the absence of head‐to‐head RCTs.19, 20 The current study found that guselkumab was associated with significantly greater achievement of PASI 90, PASI 75 and PASI 100 responses than ustekinumab throughout 40 weeks of treatment, using IPD from the NAVIGATE, VOYAGE 1 and VOYAGE 2 trials.12, 13, 14
There was consistency in long‐term results among analyses using different data sources. The primary analyses included ustekinumab IPD from NAVIGATE,12 while sensitivity analyses included IPD for patients treated with ustekinumab in the PHOENIX trials, according to approved labelled dosing (i.e. 45 mg for patients weighing ≤ 100 kg, and 90 mg for patients weighing > 100 kg).15, 16 The primary analyses focused on NAVIGATE,12 to reduce potential bias, as the timeframe for that study was similar to that for VOYAGE 1 and VOYAGE 2 (i.e. it was initiated in 2014).13, 14 It is reassuring that sensitivity analyses using IPD from the PHOENIX trials, which were initiated in 2005,15, 16 were consistent with the primary analyses of PASI response rates.
This is the first indirect treatment comparison of its kind to compare guselkumab and ustekinumab for induction and maintenance treatment in moderate‐to‐severe psoriasis in a combination of biologic‐naive and biologic‐experienced patients. The findings leveraged IPD from clinical trials of two treatments and adjusted for cross‐trial differences by using multivariable regression. As we used a more methodologically rigorous form of indirect treatment comparison than naive indirect treatment comparisons,21 our study demonstrates the comparative effectiveness of guselkumab and ustekinumab in the absence of a head‐to‐head clinical trial.
Clinical and real‐world studies have shown that ustekinumab is associated with sustained treatment response.22, 23, 24, 25 However, the higher rates of PASI 90 response observed with guselkumab compared with ustekinumab up to week 40 in the present analysis suggest that guselkumab may be associated with rates of sustained treatment response that are similar to or higher than those for ustekinumab. Future studies are needed to assess the long‐term comparative efficacy of guselkumab relative to ustekinumab and other treatments beyond 40 weeks.
Multivariable regression analysis using IPD allowed adjustment for the observed differences between patients from the NAVIGATE trial and those from the VOYAGE trials.12, 13, 14 Given that only small differences were seen among patient characteristics before multivariable adjustments, and only a limited set of covariates was predictive of PASI response, the alignment of the results from both naive and adjusted comparisons estimates lends credibility to the findings of our analyses. Nonetheless, the following limitations of this study must be acknowledged.
Firstly, we could only adjust for differences between the NAVIGATE and the VOYAGE trials with respect to known characteristics;12, 13, 14 differences in unknown characteristics could potentially affect PASI responses, and are not adjusted for in the current study. Secondly, each of the RCTs included in the IPD analyses was conducted in different settings to address different questions, and this may have introduced confounding factors and potential biases. We attempted to address this by adjusting for a wide range of clinically relevant patient characteristics; the fact that the results remained consistent across a number of analyses suggests that our findings are robust. Additionally, even though we used two different studies to provide patient data for ustekinumab, with the two studies having been conducted at different times and using different study designs and patient populations, our findings were consistent, a result that further strengthens their credibility. Finally, comparing blinded arms for guselkumab with partly unblinded arms for ustekinumab may have introduced information bias. However, the potential impact that such a bias might have had on the results is unclear; in addition, the fact that results from the sensitivity analysis based on the PHOENIX trial confirmed long‐term findings suggests that any impact was minimal.
In summary, adjusted analyses leveraging IPD demonstrate that guselkumab has a significantly higher probability of achieving and maintaining PASI treatment responses through week 40 than ustekinumab does.
Supporting information
Fig S1. Bar charts of patient demographics from the VOYAGE and NAVIGATE studies.
Fig S2. Observed (‘naive comparison’) and predicted (‘adjusted comparison’) rates of Psoriasis Area and Severity 90 treatment response, guselkumab (VOYAGE 1, 2) and ustekinumab (NAVIGATE, PHOENIX 1, 2).
Fig S3. Observed (‘naive comparison’) and predicted (‘adjusted comparison’) rates of Psoriasis Area and Severity 75 treatment response, guselkumab (VOYAGE 1, 2) and ustekinumab (NAVIGATE, PHOENIX 1, 2).
Fig S4. Observed (‘naive comparison’) and predicted (‘adjusted comparison’) rates of Psoriasis Area and Severity 100 treatment response, guselkumab (VOYAGE 1, 2) and ustekinumab (NAVIGATE, PHOENIX 1, 2).
Table S1 Patient characteristics across randomized controlled trials of guselkumab and ustekinumab, including PHOENIX trials.
Table S2 Estimated covariate effects from multivariable logistic regression analyses of Psoriasis Area and Severity 75 and 100 responses at 40 weeks.
Appendix Overviews of studies informing the adjusted treatment comparisons.
Powerpoint S1 Journal Club Slide Set.
Video S1 Author video.
Funding sources This study was funded by Janssen Inc.
Conflicts of interest J.D., P.T., A.S. and S.M. are employees of Janssen Inc. C.C. is an employee and shareholder of Cornerstone Research Group Inc. L.P. has received consultancy and speaker's honoraria from AbbVie, Almirall, Amgen, Baxalta, Biogen, Boehringer Ingelheim, Celgene, Genro, Janssen, LEO Pharma, Eli Lilly, Merck‐Serono, MSD Mylan, Novartis, Pfizer, Regeneron, Roche, Sandoz, Samsung Bioepis, Sanofi and UCB and participated in clinical trials sponsored by AbbVie, Almirall, Amgen, Boehringer Ingelheim, Janssen, LEO Pharma, Eli Lilly, Novartis, Pfizer, Regeneron, Roche, Sanofi and UCB. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Plain language summary available online
References
- 1. Queiro R, Tejon P, Alonso S et al Age at disease onset: a key factor for understanding psoriatic disease. Rheumatology (Oxford) 2014; 53:1178–85. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . Global Report on Psoriasis. World Health Organization, 2016. Available at: https://apps.who.int/iris/bitstream/handle/10665/204417/9789241565189_eng.pdf.psoriasis;jsessionid=54912784D28C9F36ECCD45471AC5775B?sequence=1 (last accessed 8 November 2019). [Google Scholar]
- 3. Di Meglio P, Villanova F, Nestle FO. Psoriasis. Cold Spring Harb Perspect Med 2014; 4:a015354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee YW, Park EJ, Kwon IH et al Impact of psoriasis on quality of life: relationship between clinical response to therapy and change in health‐related quality of life. Ann Dermatol 2010; 22:389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lebwohl MG, Bachelez H, Barker J et al Patient perspectives in the management of psoriasis: results from the population‐based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol 2014; 70:871–81. [DOI] [PubMed] [Google Scholar]
- 6. Puig L. The role of IL 23 in the treatment of psoriasis. Expert Rev Clin Immunol 2017; 13:525–34. [DOI] [PubMed] [Google Scholar]
- 7. Girolomoni G, Strohal R, Puig L et al The role of IL‐23 and the IL‐23/TH 17 immune axis in the pathogenesis and treatment of psoriasis. J Eur Acad Dermatol Venereol 2017; 31:1616–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davila‐Seijo P, Dauden E, Carretero G et al Survival of classic and biological systemic drugs in psoriasis: results of the BIOBADADERM registry and critical analysis. J Eur Acad Dermatol Venereol 2016; 30:1942–50. [DOI] [PubMed] [Google Scholar]
- 9. Menter A, Papp KA, Gooderham M et al Drug survival of biologic therapy in a large, disease‐based registry of patients with psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Eur Acad Dermatol Venereol 2016; 30:1148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Warren RB, Smith CH, Yiu ZZN et al Differential drug survival of biologic therapies for the treatment of psoriasis: a prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J Invest Dermatol 2015; 135:2632–40. [DOI] [PubMed] [Google Scholar]
- 11. Egeberg A, Ottosen MB, Gniadecki R et al Safety, efficacy and drug survival of biologics and biosimilars for moderate‐to‐severe plaque psoriasis. Br J Dermatol 2018; 178:509–19. [DOI] [PubMed] [Google Scholar]
- 12. Langley RG, Tsai TF, Flavin S et al Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double‐blind, phase III NAVIGATE trial. Br J Dermatol 2018; 178:114–23. [DOI] [PubMed] [Google Scholar]
- 13. Blauvelt A, Papp KA, Griffiths CE et al Efficacy and safety of guselkumab, an anti‐interleukin‐23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double‐blinded, placebo‐ and active comparator‐controlled VOYAGE 1 trial. J Am Acad Dermatol 2017; 76:405–17. [DOI] [PubMed] [Google Scholar]
- 14. Reich K, Armstrong AW, Foley P et al Efficacy and safety of guselkumab, an anti‐interleukin‐23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double‐blind, placebo‐ and active comparator‐controlled VOYAGE 2 trial. J Am Acad Dermatol 2017; 76:418–31. [DOI] [PubMed] [Google Scholar]
- 15. Leonardi CL, Kimball AB, Papp KA et al Efficacy and safety of ustekinumab, a human interleukin‐12/23 monoclonal antibody, in patients with psoriasis: 76‐week results from a randomised, double‐blind, placebo‐controlled trial (PHOENIX 1). Lancet 2008; 371:1665–74. [DOI] [PubMed] [Google Scholar]
- 16. Papp KA, Langley RG, Lebwohl M et al Efficacy and safety of ustekinumab, a human interleukin‐12/23 monoclonal antibody, in patients with psoriasis: 52‐week results from a randomised, double‐blind, placebo‐controlled trial (PHOENIX 2). Lancet 2008; 371:1675–84. [DOI] [PubMed] [Google Scholar]
- 17. Griffiths CE, Strober BE, van de Kerkhof P et al Comparison of ustekinumab and etanercept for moderate‐to‐severe psoriasis. N Engl J Med 2010; 362:118–28. [DOI] [PubMed] [Google Scholar]
- 18. Latimer NR, Henshall C, Siebert U et al Treatment switching: statistical and decision‐making challenges and approaches. Int J Technol Assess Health Care 2016; 32:160–6. [DOI] [PubMed] [Google Scholar]
- 19. Phillippo D, Ades T, Dias S et al Methods for population‐adjusted indirect comparisons in submissions to NICE. Available at: http://nicedsu.org.uk/wp-content/uploads/2018/08/Population-adjustment-TSD-FINAL-ref-rerun.pdf (last accessed 8 November 2019).
- 20. Schneeweiss S. Developments in post‐marketing comparative effectiveness research. Clin Pharmacol Ther 2007; 82:143–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dias S, Sutton AJ, Welton NJ et al Heterogeneity: subgroups, meta‐regression, bias and bias‐adjustment. Available at: http://nicedsu.org.uk/wp-content/uploads/2016/03/TSD3-Heterogeneity.final-report.08.05.12.pdf (last accessed 8 November 2019). [PubMed]
- 22. Kimball AB, Gordon KB, Fakharzadeh S et al Long‐term efficacy of ustekinumab in patients with moderate‐to‐severe psoriasis: results from the PHOENIX 1 trial through up to 3 years. Br J Dermatol 2012; 166:861–72. [DOI] [PubMed] [Google Scholar]
- 23. Langley RG, Lebwohl M, Krueger GG et al Long‐term efficacy and safety of ustekinumab, with and without dosing adjustment, in patients with moderate‐to‐severe psoriasis: results from the PHOENIX 2 study through 5 years of follow‐up. Br J Dermatol 2015; 172:1371–83. [DOI] [PubMed] [Google Scholar]
- 24. Talamonti M, Galluzzo M, Bianchi L et al What happened after the clinical trials: long‐term safety and efficacy of ustekinumab in daily clinical practice. Dermatology 2014; 229:324–32. [DOI] [PubMed] [Google Scholar]
- 25. Kimball AB, Papp KA, Wasfi Y et al Long‐term efficacy of ustekinumab in patients with moderate‐to‐severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol 2013; 27:1535–45. [DOI] [PubMed] [Google Scholar]
- 26. Coyle PK, Shang S, Xiao Z et al Matching‐adjusted comparisons demonstrate better clinical outcomes with SC peginterferon beta‐1a every two weeks than with SC interferon beta‐1a three times per week. Mult Scler Relat Disord 2018; 22:134–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Bar charts of patient demographics from the VOYAGE and NAVIGATE studies.
Fig S2. Observed (‘naive comparison’) and predicted (‘adjusted comparison’) rates of Psoriasis Area and Severity 90 treatment response, guselkumab (VOYAGE 1, 2) and ustekinumab (NAVIGATE, PHOENIX 1, 2).
Fig S3. Observed (‘naive comparison’) and predicted (‘adjusted comparison’) rates of Psoriasis Area and Severity 75 treatment response, guselkumab (VOYAGE 1, 2) and ustekinumab (NAVIGATE, PHOENIX 1, 2).
Fig S4. Observed (‘naive comparison’) and predicted (‘adjusted comparison’) rates of Psoriasis Area and Severity 100 treatment response, guselkumab (VOYAGE 1, 2) and ustekinumab (NAVIGATE, PHOENIX 1, 2).
Table S1 Patient characteristics across randomized controlled trials of guselkumab and ustekinumab, including PHOENIX trials.
Table S2 Estimated covariate effects from multivariable logistic regression analyses of Psoriasis Area and Severity 75 and 100 responses at 40 weeks.
Appendix Overviews of studies informing the adjusted treatment comparisons.
Powerpoint S1 Journal Club Slide Set.
Video S1 Author video.


