Abstract
Background
Transanal total mesorectal excision (TaTME) has been proposed as an approach in patients with mid and low rectal cancer. The TaTME procedure has been introduced in the Netherlands in a structured training pathway, including proctoring. This study evaluated the local recurrence rate during the implementation phase of TaTME.
Methods
Oncological outcomes of the first ten TaTME procedures in each of 12 participating centres were collected as part of an external audit of procedure implementation. Data collected from a cohort of patients treated over a prolonged period in four centres were also collected to analyse learning curve effects. The primary outcome was the presence of locoregional recurrence.
Results
The implementation cohort of 120 patients had a median follow up of 21·9 months. Short‐term outcomes included a positive circumferential resection margin rate of 5·0 per cent and anastomotic leakage rate of 17 per cent. The overall local recurrence rate in the implementation cohort was 10·0 per cent (12 of 120), with a mean(s.d.) interval to recurrence of 15·2(7·0) months. Multifocal local recurrence was present in eight of 12 patients. In the prolonged cohort (266 patients), the overall recurrence rate was 5·6 per cent (4·0 per cent after excluding the first 10 procedures at each centre).
Conclusion
TaTME was associated with a multifocal local recurrence rate that may be related to suboptimal execution rather than the technique itself. Prolonged proctoring, optimization of the technique to avoid spillage, and quality control is recommended.
During the learning curve, the transanal total mesorectal excision procedure is associated with a high multifocal local recurrence rate, which appears to be related to suboptimal execution rather than the technique, and necessitates prolonged proctoring, optimization of the technique avoiding spillage, participation in controlled clinical trials with surgical quality control, and centralization until oncological safety is demonstrated.

Defines a learning curve
Antecedentes
La escisión total del mesorrecto por vía transanal (Transanal Total Mesorectal Excision , TaTME) se ha propuesto como abordaje quirúrgico en pacientes con cáncer de recto medio e inferior. La técnica TaTME se ha introducido en los Países Bajos mediante un proceso de formación estructurado que incluye la supervisión. Este estudio evaluó el porcentaje de recidiva local durante la fase de implementación de TaTME.
Métodos
Se recogieron los resultados oncológicos de los primeros 10 procedimientos realizados mediante TaTME en cada uno de los 12 centros participantes como parte de una auditoría externa de implementación del procedimiento. Se reunió una cohorte más amplia de pacientes procedentes de 4 centros para analizar los efectos de la curva de aprendizaje. El criterio de valoración principal fue la presencia de recidiva locorregional.
Resultados
La cohorte de implementación de 120 pacientes tuvo una mediana de seguimiento de 21,9 meses. Los resultados a corto plazo incluyeron una tasa del margen de resección circunferencial positivo del 5% y una tasa de fuga anastomótica del 17,4%. La tasa global de recidiva local en la cohorte de implementación fue del 10% (12/120) con un intervalo medio de recidiva de 15,2 (DE 7) meses. El patrón de recidiva local fue multifocal en 8 de 12 casos (67%). En la cohorte ampliada (n = 266), la tasa global de recidiva fue del 5,6% (4,0%, excluyendo a los primeros 10 pacientes).
Conclusión
TaTME se asoció con un porcentaje de recidiva local multifocal que puede relacionarse con una ejecución subóptima, más que con la técnica en sí. Se recomienda una supervisión prolongada, la optimización de la técnica para evitar la diseminación tumoral, así como un control de calidad.
Introduction
The transanal total mesorectal excision (TaTME) technique has been introduced for patients with low rectal cancer, with the aim of improving clinical outcomes, such as a greater degree of radical resection, lower rates of anastomotic leakage, more sphincter‐saving procedures, better functional results and, most importantly, similar or lower local recurrence rates1, 2. Direct visualization facilitates purse‐string suture placement. The technique has been met with tremendous enthusiasm in the colorectal surgical community, and more than 300 centres worldwide have implemented the technique3. In expert centres, TaTME is associated with promising pathological and clinical outcomes4, 5, 6, 7, 8. The first long‐term outcome data from two expert centres showed a favourable low recurrence rate of 2 per cent after 3 years9.
Despite these positive results, it is also acknowledged that TaTME is a difficult technique and has a long learning curve with associated morbidity10, 11. The international TaTME registry3 and a systematic review4 have shown that widespread adoption results in less favourable clinical outcomes than reported in the initial cohorts treated in expert centres. The TaTME registry3, representing more than 300 centres voluntarily entering data, recorded an anastomotic failure rate of 15·6 per cent among 1594 patients, which is higher than rates from expert centres. In addition, a population‐based study12 documented an overall morbidity rate of 42·3 per cent, anastomotic leakage in 16·0 per cent and a circumferential resection margin (CRM)‐positive rate of 4·4 per cent. These latter studies show that the promise of TaTME has not yet been met on a large scale.
The long‐term oncological safety of TaTME remains to be proven. Although the first report with long‐term outcome data showed a low level of local recurrence, the question remains whether such results can be achieved with more widespread adoption of TaTME9. As TaTME is substantially different from abdominal techniques in terms of open access to the tumour, purse‐string closure and a subsequent endoluminal approach to the mesorectal dissection, it is especially important to assess long‐term outcomes properly. RCTs such as COLOR III13 and GRECCAR 1114 are investigating long‐term outcomes of TaTME, and are currently including patients. Recently, concern has been raised by the first report15 of national Norwegian data which showed an increase in the incidence of local recurrence with an extensive or multifocal pattern following TaTME, leading to a national halt to TaTME16.
In the Netherlands, a structured training pathway, including proctoring sessions by dedicated trainers, has been set up to ensure safe implementation of TaTME and minimization of learning curve effects17. A collective review of the short‐term outcomes of the first ten patients in 12 proctored centres revealed a major morbidity rate of 19·2 per cent and involved CRMs in 5·0 per cent of patients17. The aim of the present study was to evaluate the oncological outcomes of the initial patients who underwent TaTME within the structured training pathway. In addition, a cohort treated over a prolonged period after the implementation of TaTME in four high‐volume centres was evaluated to analyse learning curve effects in terms of local recurrence rates.
Methods
Structured training pathway
The structured training pathway was set up in the Netherlands in 2014 as a programme for postgraduate colorectal surgeons in centres with an annual volume of total mesorectal excision (TME) surgery for rectal cancer of 20 procedures or more and with known proficiency in laparoscopic TME. The clinical data from patients in the structured training pathway were collected prospectively, as described previously17. The first five procedures were discussed with and assisted by an experienced proctor, after which the following procedures were performed independently. The first ten patients in each of the centres that completed the structured training pathway were included to evaluate clinical outcomes during the implementation of TaTME17. In addition, a larger cohort of patients from four centres that continued TaTME after training, with a procedure volume greater than 45, was collected to assess learning curve effects. Long‐term clinical data were obtained as part of an external audit to assure high quality and completeness of the data set. The anonymized operative notes and full imaging reports of locoregional recurrences were obtained and audited by senior TaTME surgeons. All patients consented to a TaTME procedure as required under the Dutch national patient–physician relation regulations. The Medical Ethics Review Board of Amsterdam UMC, Location VUmc, approved the study and waived the need for additional informed consent for the present study.
Outcomes
The primary outcome of this study was the incidence of local recurrence confirmed by either imaging (MRI, CT or PET–CT) and/or pathology (biopsy, salvage surgery). A local recurrence was defined as a mass in the pelvis with a biopsy positive for adenocarcinoma, or growth on sequential imaging in the absence of histopathological confirmation. A multifocal local recurrence was defined by the presence of two or more separate foci of recurrence in the pelvic area, as seen on MRI or PET–CT. Secondary outcomes included location of local recurrence and distant metastasis, treatment of recurrence and distant metastasis, and overall mortality. All potential risk factors were evaluated for an association with recurrence. Pelvic sepsis was defined by the occurrence of early anastomotic leakage, early pelvic abscess or late complications (leakage, abscess or presacral sinus occurring more than 30 days after operation)18. Complications were graded according to the Clavien–Dindo classification19. Rectal perforation, purse‐string failure and an insufficient anastomosis requiring reinforcement or refashioning were deemed to increase the risk of spillage of tumour cells into the pelvis. A positive CRM was defined by the presence of tumour cells 1 mm or less from the circumferential plane.
Statistical analysis
Categorical data are shown as number with percentage, whereas continuous outcomes are recorded as mean(s.d.) or median (range). Dichotomous and categorical values were analysed using Pearson's χ2 test or Fisher's exact test. Comparison of continuous data was done using the independent Student's t test, or Mann–Whitney U test if the data were not distributed normally.
Univariable logistic regression analysis was performed to identify potential risk factors for local recurrence. Multivariable analysis was not possible because the event rate did not exceed the threshold for entry of multiple univariable significant predictors into a multivariable model. Case–control analysis between the present TaTME group and the laparoscopic TME group from the original COLOR II study was performed by matching sex, age, tumour height, neoadjuvant chemoradiotherapy, type of procedure (low anterior resection or abdominoperineal resection) and pathological risk factors, R1 and CRM and pT4 category20, 21. Patients with a final pT4 category or positive margins were excluded to enable evaluation of the technique as a potential individual risk factor for recurrence. For all tests, two‐sided P ≤ 0·050 was considered statistically significant. Statistical analyses were done using SPSS® version 24 for Windows® and Mac® (IBM, Armonk, New York, USA).
Results
Baseline characteristics and clinical outcomes
A cohort of 120 patients, comprising the first ten patients in each of 12 centres who underwent TaTME between March 2015 and October 2018, was included. Median follow‐up was 21·9 (range 2·0–46·7) months. The median interval between the first and tenth procedures in each hospital was 12·5 (range 3·5–35·5) months. Baseline characteristics have been published previously and are shown in Table 1, 17.
Table 1.
Patient characteristics
| No. of patients*(n = 120) | |
|---|---|
| Age (years) † | 65·4(9·6) |
| Sex ratio (M : F) | 91 : 29 |
| BMI (kg/m 2 ) † | 26·9(4·1) |
| ASA fitness grade | |
| I | 26 (21·7) |
| II | 77 (64·2) |
| III | 17 (14·2) |
| Tumour height from anal verge (cm) † | 6·9(3·1) |
| Clinical tumour category | |
| (y)cT1 | 7 (5·8) |
| (y)cT2 | 24 (20·0) |
| (y)cT3 | 89 (74·2) |
| Clinical node category | |
| cN0 | 52 (43·3) |
| cN1 | 44 (36·7) |
| cN2 | 24 (20·0) |
| Persistent MRF+ after RT ‡ | 6 (5·0) |
| Preoperative therapy | |
| None | 43 (35·8) |
| RT | 41 (34·2) |
| CRT | 36 (30·0) |
| Transanal total mesorectal excision | |
| Low anterior resection | 110 (91·7) |
| Intersphincteric resection | 10 (8·3) |
With percentages in parentheses unless indicated otherwise;
values are mean(s.d.).
All patients with a persistent theatened mesorectal fascia (MRF+) initially had cT3 tumours (3 anterior, 2 lateral, 1 unknown). RT, radiotherapy; CRT, chemoradiotherapy.
Short‐term outcomes are summarized in Table 2. The overall 30‐day morbidity rate was 45·0 per cent, including an anastomotic leakage rate of 17 per cent and pelvic sepsis in 17·5 per cent. The involved CRM rate was 5·0 per cent; no patient had an involved distal resection margin. The quality of the specimen was rated as complete in 89·2 per cent of procedures and nearly complete in 10·8 per cent; none of the specimens were considered incomplete.
Table 2.
Short‐term clinicopathological outcomes
| No. of patients(n = 120) | |
|---|---|
| Intraoperative events | |
| Purse‐string failure | 1 (0·8) |
| Perforation | 1 (0·8) |
| Reinforcement | 3 (2·5) |
| 30‐day mortality | 0 (0) |
| 30‐day overall morbidity | 54 (45·0) |
| Major morbidity (Clavien–Dindo grade ≥ III) | 23 (19·2) |
| 30‐day anastomotic leakage | 17 of 98 (17) |
| Pelvic sepsis (early leak, abscess and late sinus) * | 21 (17·5) |
| Pathological tumour category | |
| (y)pT0 | 11 (9·2) |
| (y)pT1 | 16 (13·3) |
| (y)pT2 | 34 (28·3) |
| (y)pT3 | 59 (49·2) |
| (y)pT4 | 0 (0) |
| Quality of specimen (Quirke) * | |
| Complete | 107 (89·2) |
| Nearly complete | 13 (10·8) |
| Incomplete | 0 (0) |
| CRM involvement ≤ 1 mm | 6 (5·0) |
| DRM involvement < 5 mm | 0 (0) |
Values in parentheses are percentages.
All patients (anastomosis and colostomy). CRM, circumferential resection margin; DRM, distal resection margin.
Long‐term outcomes
Long‐term outcomes are shown in Table 3. Twelve of 120 patients (10·0 per cent) developed local recurrence, which was multifocal in eight patients. The median interval to local recurrence was 15·9 months, ranging from 6·0 to 26·4 months (Table 4). The recurrences were located presacrally (2), anterior (1), at the rectal stump (1) or in multiple regions in the pelvis (8) (Fig. 1). Nine of the 12 patients with local recurrence presented with or developed distant metastasis, whereas only 14 of 108 patients without local recurrence had distant metastases diagnosed (P < 0·001).
Table 3.
Long‐term outcomes
| No. of patients*(n = 120) | |
|---|---|
| Follow‐up (months) | |
| Mean(s.d.) | 23·4(9·5) |
| Median (range) | 21·9 (2·0–46·7) |
| Local recurrence (total) | 12 (10·0) |
| Multifocal local recurrence | 8 of 12 (67) |
| Interval to local recurrence (months) † | 15·2(7·0) |
| Overall distribution of disease (recurrence and metastasis) | |
| Isolated local | 3 (12) |
| Local + liver | 4 (15) |
| Local + lung | 2 (8) |
| Local + liver + lung | 2 (8) |
| Local + lung + peritoneal + brain | 1 (4) |
| Liver + lung | 4 (15) |
| Isolated liver | 5 (19) |
| Isolated lung | 5 (19) |
| Disease‐free surival | 94 (78·3) |
| Overall survival | 115 (95·8) |
With percentages in parentheses unless indicated otherwise;
values are mean(s.d.).
Table 4.
Location and treatment of local recurrences
| No. of patients*(n = 12) | |
|---|---|
| Interval to local recurrence (months) | |
| Mean(s.d.) | 15·2(7·0) |
| Median (range) | 15·9 (6·0–26·4) |
| Location | |
| Presacral | 2 |
| Anterior | 1 |
| Rectal stump | 1 |
| Multiple sites | 8 |
| Focality (no. of sites) | |
| 1 | 4 |
| 2 | 4 |
| 3 | 4 |
| Treatment | |
| Exenteration† | 4 |
| CRS + HIPEC | 1 |
| Abdominoperineal resection + IORT | 1 |
| Palliative chemotherapy | 5 |
| Further CRT; multivisceral resection planned | 1 |
Unless indicated otherwise.
Also intraoperative radiotherapy (IORT) in one patient. CRS + HIPEC, cytoreductive surgery and hyperthermic intraperitoneal chemotherapy; CRT, chemoradiotherapy.
Figure 1.
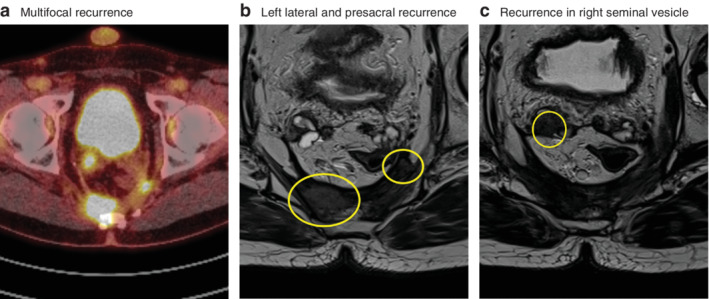
Images from a patient with multifocal recurrence after transanal total mesorectal excision a PET images showing multifocal recurrence. b,c T2‐weighted axial MRI images showing left lateral and presacral local recurrence (b) and recurrence in right seminal vesicle (c).
The local recurrences were distributed over the 12 participating sites as follows: three in one centre, two in three centres, one in three centres and none in five centres. There was no relationship between the time to include ten procedures and the incidence of local recurrence.
Details of the 12 patients who developed local recurrence are shown in Table 5. Two patients initially presented with a synchronous liver metastasis which was treated by a liver‐first approach. One of these developed lung metastasis simultaneous with the local recurrence. Pathological examination showed two poorly differentiated tumours, and three patients had an involved margin, one due to perineural growth that intersected the circumferential plane.
Table 5.
Details of patients with local recurrence
| Patient no. | Baseline data (sex, age, tumour height, cTNM stage) | Neoadjuvant treatment, MRF status* | Surgery | Anastomotic leakage | Pathological stage | Differentiation | CRM (mm) | Follow‐up details | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M, 68 years, 2 cm from AV, cT2 N0 M0 | No, MRF– | LAR + diversion | No | pT3 N0 | W/M | 10 | 18 months LR (multifocal) + M (hepatic) | Metastasectomy, APR + IORT | M+ (pulmonary), palliative chemotherapy. Alive at 35 months |
| 2 | F, 50 years, 8 cm from AV, cT3 N2 M1 (hepatic) | CRT, MRF– | Liver‐first laparoscopic segmentectomy VI + VII. LAR + diversion | Yes | pT3 N0 | W/M | 5 | 19 months LR (unifocal) | Exenteration | Further recurrence after 3 months. Alive at 28 months |
| 3 | F, 54 years, 3 cm from AV, cT3 N0 M0 | No, MRF– | LAR + diversion | No | pT3 N0 | W/M | 3 | 26 months LR (unifocal) + M (hepatic) | Metastasectomy, exenteration | Disease‐free. Alive at 39 months |
| 4 | M, 65 years, 4 cm from AV, cT3 N1 M0 | CRT, MRF– | LAR, no stoma | No | pT2 N1 | Poor | 3 | 12 months LR (multifocal) | Exenteration (R1) | M+ (hepatectomy) after 5 months, palliative chemotherapy. Died 36 months after TME |
| 5 | M, 55 years, 8 cm from AV, cT3 N0 M0 | 5 × 5, MRF– | LAR, no stoma | Yes | pT3 N1 | W/M | 4 | 7 months LR (multifocal) + M (hepatic) | Palliative | Alive at 23 months |
| 6 | M, 40 years, 8·3 cm from AV, cT3 N2 M0 | CRT, MRF– | LAR + diversion | No | pT3 N2 | W/M | 7 | 14 months LR (multifocal) + M (pulmonary) | Further CRT, systemic chemotherapy | Progression, palliative. Alive at 25 months |
| 7 | M, 85 years, 2 cm from AV, cT3 N2 M0 | CRT, MRF– | LAR + colostomy | No | pT3 N0 | Poor | 0 | 10 months LR (unifocal) | Palliative | Died 15 months after TME |
| 8 | M, 51 years, 5 cm from AV, cT3 N2 M1 (hepatic) | CRT, MRF– | Liver‐first laparoscopic segmentectomy IVb. LAR + diversion | No | pT3 N1 | W/M | < 1 | 8 months LR (multifocal) + M (pulmonary) | Pulmonary RT. Response to induction chemotherapy. Recurrent M+ (pulmonary) | Palliative chemotherapy. Alive at 34 months |
| 9 | F, 54 years, 3 cm from AV, cT3 N1 M0 | CRT, MRF– | LAR + diversion | No | pT3 N1 | W/M | > 10 | 25 months LR (multifocal) | Induction chemotherapy + further CRT. CRS + HIPEC (R0) | Alive 36 at months |
| 10 | M, 60 years, 7 cm from AV, cT3 N1 M0 | 5 × 5, MRF– | LAR + diversion; air leak reinforced by sutures | Yes | pT3 N0 | W/M | > 10 | 20 months LR (multifocal) | Induction chemotherapy + further CRT. Exenteration (R0) | Alive at 22 months |
| 11 | F, 75 years, 5 cm from AV, cT3 N1 M0 | 5 × 5, MRF– | LAR, no stoma; air leak reinforced by sutures | Yes | pT3 N1 | W/M | 0† | 19 months LR (multifocal) + M (pulmonary). Also previous 10 months M (hepatic) | Work‐up to plan treatment for LR + M (pulmonary) | Alive at 22 months |
| 12 | M, 73, 10 cm AV, cT3 N1 M0 | 5 × 5, MRF– | LAR + diversion | No | pT3 N1 | W/M | 7 | 6 months LR (unifocal) + M (pulmonary, peritoneal, brain) | Palliative | Alive at 18·5 months |
After neoadjuvant treatment if applicable.
Perineural growth. MRF, mesorectal fascia; CRM, circumferential resection margin; AV, anal verge; MRF–, MRF not threatened; LAR, low anterior resection; W/M, well to moderate; LR, local recurrence; M, distant metastasis; APR, abdominoperineal resection; IORT, intraoperative radiotherapy; CRT, chemoradiotherapy; TME, total mesorectal excision; 5 × 5, short‐course radiotherapy (RT) 5 × 5 Gy; CRS + HIPEC, cytoreductive surgery and hyperthermic intraperitoneal chemotherapy.
Treatment of recurrences
Of the 12 patients with local recurrence, five with unresectable and/or systemic disease received palliative treatment. Six patients had local exenterative surgery with curative intent. Four patients underwent exenteration (1 combined with intraoperative radiotherapy (IORT)), one had abdominoperineal excision with IORT and one had cytoreductive surgery with hyperthermic intraperitoneal chemotherapy as salvage surgery. At the time of writing, the final patient was receiving further chemoradiotherapy before salvage surgery.
Risk factors for recurrence
Risk factors for recurrence were identified by univariable logistic regression analysis. Prognostic factors associated with local recurrence (12 patients) were: positive CRM (odds ratio (OR) 11·67; P = 0·006), intraoperative complication (OR 7·00; P = 0·005), (y)pT3 category (OR 6·02; P = 0·025) and pelvic sepsis (OR 4·12; P = 0·029) (Table S1 , supporting information). Risk factors associated with multifocal recurrence (8 patients) were: intraoperative complication (OR 12·11; P = 0·013), positive CRM (OR 9·00; P = 0·022), pathological N‐positive status (OR 6·88; P = 0·022), (y)pT3 category (OR 3·34; P = 0·150) and pelvic sepsis (OR 5·59; P = 0·023) (Table S2 , supporting information).
Proctoring effect
There were four patients with local recurrence among the first five proctored TaTME procedures per centre (4 of 60 overall) and eight occurred in the second five proctored TaTME procedures (8 of 60) (P = 0·362). Clinicopathological outcomes for the first and second five procedures per centre were an intraoperative complication rate of 3 versus 5 per cent respectively, an anastomotic leakage rate of 19 versus 16 per cent, and involved CRM rate of 2 versus 8 per cent.
Comparative case‐matched analysis of transanal versus laparoscopic total mesorectal excision
To focus on the procedure itself rather than pathological risk factors for local recurrence, case‐matched pairing of patients with good‐quality specimens and no CRM involvement yielded two groups of 109 patients with similar baseline characteristics, abdominoperineal resection rate and incidence of anastomotic leakage (Table S3 , supporting information). The pathological outcomes were comparable in terms of stage, and no patient in either matched group had a non‐radical resection or incomplete specimen. The overall local recurrence rate was higher for TaTME than laparoscopic TME: 8·3 per cent (nine patients) and 1·8 per cent (2) respectively.
Long‐term outcomes of four hospitals with experience of more than 45 procedures
A prolonged cohort from four hospitals with experience of more than 45 procedures included a total of 266 patients who underwent TaTME for primary rectal cancer. Median follow‐up was 23·8 (range 1·0–62·4) months. The crude local recurrence rate was 15·0 per cent after the first ten procedures in each centre, 4·2 per cent after procedures 11–40, and 3·8 per cent for procedure 41 onwards (Table 6). Overall, 15 patients (5·6 per cent) in this cohort of 266 patients who underwent TaTME developed local recurrence.
Table 6.
Local recurrence according to number of transanal total mesorectal excision procedures at each centre in prolonged cohort
| Local recurrence rate | ||||
|---|---|---|---|---|
| Procedures 1–10 | Procedures 11–40 | Procedures ≥ 41 | Total | |
| Centre A | 2 of 10 | 2 of 30 | 0 of 31 | 4 of 71 (6) |
| Centre B | 1 of 10 | 2 of 30 | 3 of 28 | 6 of 68 (9) |
| Centre C | 2 of 10 | 0 of 30 | 1 of 7 | 3 of 47 (6) |
| Centre D | 1 of 10 | 1 of 30 | 0 of 40 | 2 of 80 (3) |
| Overall | 6 of 40 (15) | 5 of 120 (4·2) | 4 of 106 (3·8) | 15 of 266 (5·6) |
Values in parentheses are percentages.
Discussion
In this study, the local recurrence rate during the learning curve was 10·0 per cent, despite the low positive CRM rate and the presence of a structured training pathway, including on‐site proctoring. The multifocal pattern of recurrence seemed to be substantially different from that after abdominal TME (open, laparoscopic or robotic) and confirmed the pattern encountered in Norway15, which calls for further evaluation of the safety of TaTME. TaTME has been shown to be a difficult technique with a relatively long learning curve and associated morbidity10. Therefore, it was expected that some learning curve‐related problems would be encountered in the present cohort, despite the presence of a structured training pathway aimed at minimizing harm during implementation. The effect of the learning curve is demonstrated by the relatively high rate of anastomotic leakage and relatively high rate of local recurrences in the longer term. The present cohort size in each centre was inadequate for cumulative sum analysis with the endpoint local recurrence, but an increased recurrence rate among the first ten patients was clearly shown. This could reflect difficulties with poor execution of the technique causing unwanted tumour spillage. These data also demonstrate that the structured training as set out in this programme was not capable of diminishing all adverse outcomes, and should therefore be made more extensive for centres implementing this technique in the future. Proctoring of more than ten procedures should be advised until proficiency is met according to independent competency assessment using video analysis22.
Execution of the procedure rather than the technique itself may explain the observed recurrences. This is supported by the results of univariable analysis, which identified intraoperative events as the biggest risk factor. Two expert centres reported a 3‐year local recurrence rate of 2·0 per cent9. In the present study, long‐term outcomes from four centres with experience of more than 45 TaTME procedures after training indicated that the first ten procedures (early experience) are more at risk of local recurrence than the following 30. The 4·0 per cent local recurrence rate achieved after exclusion of the first ten procedures at each centre is more in line with the results reported by Hol and colleagues9 for the two expert centres starting this technique in the Netherlands. Longer follow‐up is needed to confirm the present recurrence rates, which should be interpreted with caution owing to inclusion of more challenging cases23.
The learning curve for implementation of new surgical techniques and its influence on long‐term oncological outcome is an important issue. Data are scarce, but a study of laparoscopic TME surgery demonstrated a significantly higher recurrence rate among the first 100 procedures compared with the following 200 (10·5 versus 4·9 per cent respectively)24. Robotic‐assisted TME surgery is being implemented worldwide, but data on the learning curve have focused on duration of operation, involved CRM rates and/or complications, and not on long‐term recurrence rates. A series by Polat and co‐workers25, reporting the first 77 procedures, documented a recurrence rate of 9·5 per cent despite a relatively low positive margin rate. This relatively high local recurrence rate was probably related to suboptimal technical execution within the learning curve.
The full report of the National Norwegian audit16 of 157 TaTME procedures revealed 12 local recurrences (7·6 per cent) after a median follow‐up of 19 months, with an estimated local recurrence rate of 11·6 per cent at 2·4 years according to Kaplan–Meier analysis. Wasmuth and colleagues16 stated that TaTME was responsible for the increased local recurrence rate, and that poor outcome could not be attributed to the learning curve effect because several of these recurrences occurred late in the series. However, four high‐volume centres performed 152 procedures over 4 years, which breaks down to an average annual volume of 9·5 procedures. This raises the question of whether the learning curve had been completed owing to the low exposure. A high rate of positive margins despite low tumour stage, the high rate of permanent stomas and perioperative morbidity may be indicative of suboptimal TaTME procedures. An unsupervised learning curve without proctoring, as shown by experienced single‐port surgeons, takes over 40 procedures10, 11.
The crucial difference in the TaTME technique is the endoluminal approach and potential direct contact with the tumour, whereas in the other abdominal techniques distal closure is assured by stapling below the tumour26. Poor tumour handling and inadequate closure of the lumen by failing purse strings could lead to tumour cells spilling into the pelvic dissection area during the procedure causing (multifocal) recurrences. This could be a similar mechanism to that described in early reports of laparoscopy demonstrating port‐site metastasis27. Careful evaluation led to the acknowledgement of tumour cell aerosolization combined with a chimney effect at the trocar sites. After implementation of sufficient training and clinical trials, it has now been proven that laparoscopy is safe when executed proficiently.
The multifocal local recurrence shown in this series and reported by Larsen and co‐workers15 seems to be a new pattern. In the Dutch TME trial28, the multifocality of recurrences was not evident on review of the imaging of patients with local recurrence. Other data regarding the incidence of multifocal local recurrences are scarce; large trials have not reported multifocality as a separate entity. In the present study, seven of 12 patients with local recurrence developed distant metastasis, similar to rates found in the Dutch TME29 and COLOR II21 trials, in which 50–60 per cent of patients with local recurrence also had distant metastasis. The question remains whether recurrence is related to the biology of the cancer rather than the surgical technique driving distant haematogenous spread of the disease30.
The explanation for both the high rate of multifocal recurrences and the local recurrence rate of 10·0 per cent, despite a relatively low CRM positivity rate of 5·0 per cent in this implementation cohort, could be multifactorial. Theoretically, unsuccessful execution of a TaTME procedure might result in inadequate purse‐string closure of the lumen. During the subsequent pelvic dissection, spilled tumour cells might be scattered as a result of the continuous high‐flow insufflation used in the dissection area in TaTME, leading to multifocal local recurrence. A high rate of positive bacterial cultures during TaTME, as reported by Velthuis and colleagues31, might provide support for this hypothesis. The authors have preliminary data showing that cancer cells can be cultured from rectal wash‐out (J. Tuynman; unpublished observation). Although the exact aetiology remains to be proven, all COLOR III sites have been instructed to secure the purse‐string closure with a second over‐running suture after the rectotomy with a secondary wash‐out32. Intraoperative perforation of the rectal tube in conventional TME might be regarded as a similar mechanism whereby tumour cells can seed in the pelvic cavity. In the present risk analysis, occurrence of intraoperative complications was the strongest predictor of multifocal local recurrence and second strongest for overall local recurrence. A previous study by Eriksen and colleagues33 showed a tremendous negative impact of perforation on 5‐year local recurrence, with the incidence rising from 9·9 per cent to 28·8 per cent in the presence of perforation (P < 0·001). The relatively high rate of pelvic sepsis (17·5 per cent) in the present learning curve cohort might also have contributed to the increased recurrence rate. A consistent hypothesis is that pelvic sepsis leads to an increased inflammatory reaction, and increased levels of growth factors associated with stimulation of adhesion and seeding of tumour cells34, 35, 36.
A potential weakness of this cohort study is the possible inclusion of some patients with advanced‐stage disease in the learning curve cohort. Overall, selection bias could be present within these data, but all patients who underwent TaTME for primary rectal cancer were included consecutively and the data were audited externally by an independent clinical researcher. Furthermore, case‐matched analysis of TaTME and laparoscopic TME procedures, excluding CRM‐positive and T4 tumours, demonstrated that TaTME during the learning curve was the only risk factor for local recurrence and not the pathology, showing that case selection was not an issue in the present cohort. Video analysis with surgical quality assessment could have revealed potential risk features for local recurrence. Quality assessment of every procedure is the central ingredient in the current COLOR III trial22, in which all data including MRI and the entire video of each procedure are captured centrally.
As stated in the IDEAL framework, a new innovation or technique should be evaluated stepwise, and not be implemented broadly before standardized indications and procedures have been developed. In this way, adverse effects and consistent outcomes can be established during the learning curve, which new centres can set as a benchmark37. The surgical community should focus on demonstrating oncological safety rather than surrogate endpoints for new innovative surgical techniques for patients with cancer. High‐quality data accrual in a clinical (randomized) trial is key, including establishing a safety commission and frequent external data monitoring38. The international TaTME guidance also states that TaTME should be implemented only in centres with a high volume of TME practice and with adequate training, including individual proctoring2.
Contributors
Other proctors in the training pathway: H. B. A. C. Stockmann, R. C. L. M. Vuylsteke (Spaarne Hospital, Hoofddorp); P. G. Doornebosch (IJsselland Hospital, Cappelle aan den IJssel).
Supporting information
Table S1 Univariate analysis of risk factors for local recurrences
Table S2 Univariate analysis of risk factors for Multifocal Local recurrence
Table S3 Case matched analysis TaTME versus LapTME local recurrences
Acknowledgements
The authors acknowledge the COLOR II study group for providing data on the laparoscopic TME cohort. Support for the on‐site proctoring was provided by: Applied Medical, Conmed, Ethicon J&J, Medtronic and Olympus.
Disclosure: The authors declare no other conflict of interest.
References
- 1. Sylla P, Rattner DW, Delgado S, Lacy AM. NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg Endosc 2010; 24: 1205–1210. [DOI] [PubMed] [Google Scholar]
- 2. Adamina M, Buchs NC, Penna M, Hompes R; St. Gallen Colorectal Consensus Expert Group. St. Gallen consensus on safe implementation of transanal total mesorectal excision. Surg Endosc 2018; 32: 1091–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Penna M, Hompes R, Arnold S, Wynn G, Austin R, Warusavitarne J et al Incidence and risk factors for anastomotic failure in 1594 patients treated by transanal total mesorectal excision: results from the International TaTME Registry. Ann Surg 2019; 269: 700–711. [DOI] [PubMed] [Google Scholar]
- 4. Deijen CL, Tsai A, Koedam TW, Veltcamp Helbach M, Sietses C, Lacy AM et al Clinical outcomes and case volume effect of transanal total mesorectal excision for rectal cancer: a systematic review. Tech Coloproctol 2016; 20: 811–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Oostendorp SE, Koedam TWA, Sietses C, Bonjer HJ, Tuynman JB. Transanal total mesorectal excision compared to laparoscopic TME for mid and low rectal cancer – current evidence. Ann Laparosc Endosc Surg 2018; 3: 41. [Google Scholar]
- 6. Lacy AM, Tasende MM, Delgado S, Fernandez‐Hevia M, Jimenez M, De Lacy B et al Transanal total mesorectal excision for rectal cancer: outcomes after 140 patients. J Am Coll Surg 2015; 221: 415–423. [DOI] [PubMed] [Google Scholar]
- 7. Buchs NC, Wynn G, Austin R, Penna M, Findlay JM, Bloemendaal AL et al A two‐centre experience of transanal total mesorectal excision. Colorectal Dis 2016; 18: 1154–1161. [DOI] [PubMed] [Google Scholar]
- 8. Veltcamp Helbach M, Deijen CL, Velthuis S, Bonjer HJ, Tuynman JB, Sietses C. Transanal total mesorectal excision for rectal carcinoma: short‐term outcomes and experience after 80 cases. Surg Endosc 2016; 30: 464–470. [DOI] [PubMed] [Google Scholar]
- 9. Hol JC, van Oostendorp SE, Tuynman JB, Sietses C. Long‐term oncological results after transanal total mesorectal excision for rectal carcinoma. Tech Coloproctol 2019; 23: 903–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koedam TWA, Veltcamp Helbach M, van de Ven PM, Kruyt PM, van Heek NT, Bonjer HJ et al Transanal total mesorectal excision for rectal cancer: evaluation of the learning curve. Tech Coloproctol 2018; 22: 279–287. [DOI] [PubMed] [Google Scholar]
- 11. Lee L, Kelly J, Nassif GJ, deBeche‐Adams TC, Albert MR, Monson JRT. Defining the learning curve for transanal total mesorectal excision for rectal adenocarcinoma. Surg Endosc 2020; 34: 1534–1542. [DOI] [PubMed] [Google Scholar]
- 12. Detering R, Roodbeen SX, van Oostendorp SE, Dekker JT, Sietses C, Bemelman WA et al Three‐year nationwide experience with transanal total mesorectal excision for rectal cancer in the Netherlands: a propensity score‐matched comparison with conventional laparoscopic total mesorectal excision. J Am Coll Surg 2019; 228: 235.e1–244.e1. [DOI] [PubMed] [Google Scholar]
- 13. Deijen CL, Velthuis S, Tsai A, Mavroveli S, Lange‐de Klerk ES, Sietses C et al COLOR III: a multicentre randomised clinical trial comparing transanal TME versus laparoscopic TME for mid and low rectal cancer. Surg Endosc 2016; 30: 3210–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lelong B, de Chaisemartin C, Meillat H, Cournier S, Boher JM, Genre D et al A multicentre randomised controlled trial to evaluate the efficacy, morbidity and functional outcome of endoscopic transanal proctectomy versus laparoscopic proctectomy for low‐lying rectal cancer (ETAP‐GRECCAR 11 TRIAL): rationale and design. BMC Cancer 2017; 17: 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Larsen SG, Pfeffer F, Korner H; Norwegian Colorectal Cancer Group . Norwegian moratorium on transanal total mesorectal excision. Br J Surg 2019; 106: 1120–1121. [DOI] [PubMed] [Google Scholar]
- 16. Wasmuth HH, Faerden AE, Myklebust TA, Pfeffer F, Norderval S, Riis R et al; Norwegian TaTME Collaborative Group, on behalf of the Norwegian Colorectal Cancer Group . Transanal total mesorectal excision for rectal cancer has been suspended in Norway. Br J Surg 2020; 107: 121–130. [DOI] [PubMed] [Google Scholar]
- 17. Veltcamp Helbach M, van Oostendorp SE, Koedam TWA, Knol JJ, Stockmann H, Oosterling SJ et al Structured training pathway and proctoring; multicenter results of the implementation of transanal total mesorectal excision (TaTME) in the Netherlands. Surg Endosc 2020; 34: 192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Borstlap WAA, Westerduin E, Aukema TS, Bemelman WA, Tanis PJ; Dutch Snapshot Research Group . Anastomotic leakage and chronic presacral sinus formation after low anterior resection: results from a large cross‐sectional study. Ann Surg 2017; 266: 870–877. [DOI] [PubMed] [Google Scholar]
- 19. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van der Pas MH, Haglind E, Cuesta MA, Furst A, Lacy AM, Hop WC et al Laparoscopic versus open surgery for rectal cancer (COLOR II): short‐term outcomes of a randomised, phase 3 trial. Lancet Oncol 2013; 14: 210–218. [DOI] [PubMed] [Google Scholar]
- 21. Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MH, Lange‐de Klerk ES et al A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 2015; 372: 1324–1332. [DOI] [PubMed] [Google Scholar]
- 22. Tsai AY, Mavroveli S, Miskovic D, van Oostendorp S, Adamina M, Hompes R et al Surgical quality assurance in COLOR III: standardization and competency assessment in a randomized controlled trial. Ann Surg 2019; 270: 768–774. [DOI] [PubMed] [Google Scholar]
- 23. D'Andrea AP, McLemore EC, Bonaccorso A, Cuevas JM, Basam M, Tsay AT et al Transanal total mesorectal excision (taTME) for rectal cancer: beyond the learning curve. Surg Endosc 2019; doi: 10.1007/s00464-019-07172-4 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 24. Kim CH, Kim HJ, Huh JW, Kim YJ, Kim HR. Learning curve of laparoscopic low anterior resection in terms of local recurrence. J Surg Oncol 2014; 110: 989–996. [DOI] [PubMed] [Google Scholar]
- 25. Polat F, Willems LH, Dogan K, Rosman C. The oncological and surgical safety of robot‐assisted surgery in colorectal cancer: outcomes of a longitudinal prospective cohort study. Surg Endosc 2019; 33: 3644–3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rondelli F, Trastulli S, Cirocchi R, Avenia N, Mariani E, Sciannameo F et al Rectal washout and local recurrence in rectal resection for cancer: a meta‐analysis. Colorectal Dis 2012; 14: 1313–1321. [DOI] [PubMed] [Google Scholar]
- 27. Berends FJ, Kazemier G, Bonjer HJ, Lange JF. Subcutaneous metastases after laparoscopic colectomy. Lancet 1994; 344: 58. [DOI] [PubMed] [Google Scholar]
- 28. Kusters M, Marijnen CA, van de Velde CJ, Rutten HJ, Lahaye MJ, Kim JH et al Patterns of local recurrence in rectal cancer; a study of the Dutch TME trial. Eur J Surg Oncol 2010; 36: 470–476. [DOI] [PubMed] [Google Scholar]
- 29. Peeters KC, Marijnen CA, Nagtegaal ID, Kranenbarg EK, Putter H, Wiggers T et al The TME trial after a median follow‐up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg 2007; 246: 693–701. [DOI] [PubMed] [Google Scholar]
- 30. Tohme S, Simmons RL, Tsung A. Surgery for cancer: a trigger for metastases. Cancer Res 2017; 77: 1548–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Velthuis S, Veltcamp Helbach M, Tuynman JB, Le TN, Bonjer HJ, Sietses C. Intra‐abdominal bacterial contamination in TAMIS total mesorectal excision for rectal carcinoma: a prospective study. Surg Endosc 2015; 29: 3319–3323. [DOI] [PubMed] [Google Scholar]
- 32. Koch MJ, Tanis PJ, Bemelman WA, Tuynman JB, Hompes R. Belgers H.J. Purse‐string reinforcement in transanal total mesorectal excision: a further essential step to increase oncological safety – a video vignette. Colorectal Dis 2020; 22: 219–220. [DOI] [PubMed] [Google Scholar]
- 33. Eriksen MT, Wibe A, Syse A, Haffner J, Wiig JN; Norwegian Rectal Cancer Group; Norwegian Gastrointestinal Cancer Group . Inadvertent perforation during rectal cancer resection in Norway. Br J Surg 2004; 91: 210–216. [DOI] [PubMed] [Google Scholar]
- 34. Weese JL, Ottery FD, Emoto SE. Do operations facilitate tumor growth? An experimental model in rats. Surgery 1986; 100: 273–277. [PubMed] [Google Scholar]
- 35. Oosterling SJ, van der Bij GJ, Bögels M, ten Raa S, Post JA, Meijer GA et al Anti‐beta1 integrin antibody reduces surgery‐induced adhesion of colon carcinoma cells to traumatized peritoneal surfaces. Ann Surg 2008; 247: 85–94. [DOI] [PubMed] [Google Scholar]
- 36. Ramphal W, Boeding JRE, Gobardhan PD, Rutten HJT, de Winter LJMB, Crolla RMPH et al Oncologic outcome and recurrence rate following anastomotic leakage after curative resection for colorectal cancer. Surg Oncol 2018; 27: 730–736. [DOI] [PubMed] [Google Scholar]
- 37. McCulloch P, Feinberg J, Philippou Y, Kolias A, Kehoe S, Lancaster G et al Progress in clinical research in surgery and IDEAL. Lancet 2018; 392: 88–94. [DOI] [PubMed] [Google Scholar]
- 38. Abis GSA, Stockmann H, Bonjer HJ, van Veenendaal N, van Doorn‐Schepens MLM, Budding AE et al; SELECT study group. Randomized clinical trial of selective decontamination of the digestive tract in elective colorectal cancer surgery (SELECT trial). Br J Surg 2019; 106: 355–363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Univariate analysis of risk factors for local recurrences
Table S2 Univariate analysis of risk factors for Multifocal Local recurrence
Table S3 Case matched analysis TaTME versus LapTME local recurrences


