In this issue, Müller‐Thomas et al. 1 explored the predictive value of indoleamine 2,3 dioxygenase (IDO) expression in 96 patients with high‐risk myelodysplastic syndromes (MDS) and secondary AML (sAML) treated with azacitidine.
MDS are clonal haematopoietic stem cell (HSC) disorders characterised by progressive bone marrow (BM) failure resulting in cytopenias, with approximately one‐third of patients progressing to sAML, 2 a process during which the increased ratio of apoptosis to proliferation is inversed and replaced by a differentiation block. The pathophysiology of early‐stage MDS is a much‐debated topic, and it remains unclear whether (i) the MDS clone emerged first (e.g. via mutations), 2 followed by CD8+ cytotoxic T cell (CTL) attack against aberrantly expressed tumour‐associated antigens (‘T against the clone scenario’); (ii) the immune defect came first, with expansion of autoreactive or cross‐reactive polyclonal CTLs targeting normal HSCs (‘autoimmune attack’); or (iii) microenvironmental defects came first, with (ii) and (iii) resulting in selection pressure for MDS clones. 3
What is clear, however, is that both the microenvironment and the immune system are severely disturbed in MDS and AML (hereafter referred to as MDS/AML), and that disease progression is paralleled by a progression from immunosurveillance to immunoselection and ultimately immuno‐subversion, resulting in tumour immune escape. MDS/AML are characterised by a plethora of numerical and functional changes in virtually all cellular components of the immune system and microenvironment. Much of the immune suppression and evasion results from intense crosstalk between the MDS/AML clone with mesenchymal stem and progenitor cells (MSPCs). Under inflammatory licensing conditions, which prevail in the BM of MDS/AML patients, tumour‐educated Type‐2 MSPCs exert their strongly immunosuppressive function, that is, via secretion of high levels of IDO. IDO is expressed by different cells throughout the body, including macrophages and dendritic cells, but is also found in tumour cells and the tumour microenvironment. 4 IDO has been linked to the development of several cancer types, including MDS/AML, via suppression of the immune system, propagation of cancer cell growth, migration and invasion. 5 , 6 , 7 , 8 , 9 IDO is an endocellular monomeric enzyme that degrades the essential amino acid L‐tryptophan to L‐kynurenine. Tryptophan starvation results in T‐cell cycle arrest, and kynurenine and its metabolites are also directly toxic for many T‐ and natural killer cells (NKCs). IDO also induces a plethora of regulatory cells and the switch from M0‐ to M2‐macrophages, which, together with the induction of T‐cell anergy, results in a tolerogenic microenvironment, strong immunosuppression, tumour immune escape and disease progression (Fig 1). Furthermore, the enzyme inhibits the production of erythropoietin and may also be mutagenic, thus contributing to genetic instability. 10 , 11
Fig 1.
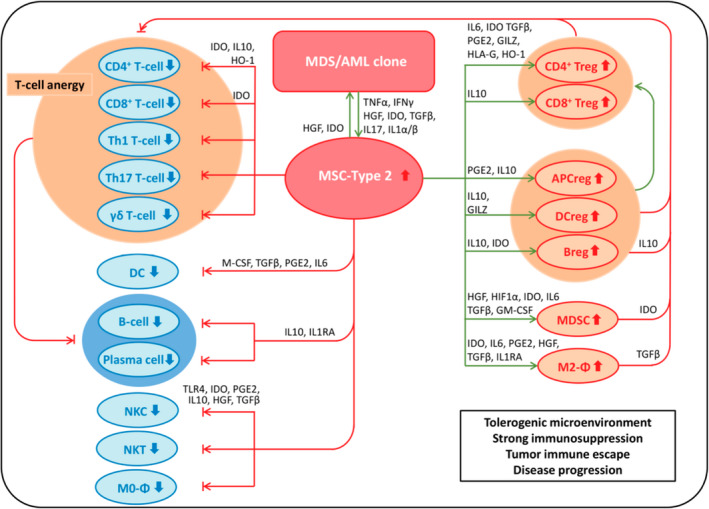
Mechanisms of immune evasion in MDS/AML. Reprinted with permission from Pleyer et al.10 Early stage MDS/AML are ssociated with a stage of inflammation. The inflammatory BM microenvironment is believed to recruit proinflammatory Type‐1 MSCs and license them to adopt a Type‐2 immunosuppressing and tumour‐promoting phenotype. Together with the leukemic clone, tumour‐educated Type‐2 MSCs recruit additional immunosuppresive cells, and suppress those cells capable of targeting the leukemic clone, resulting in a strongly immunosuppressive environment, enabling tuour immune escape and disease progression.
Thus far, only a few groups have studied IDO in the context of MDS/AML. Constitutive overexpression of the strongly‐immunosuppressive enzyme has been detected in primary human AML blasts 11 and patient sera 12 . It has been correlated with increased levels of circulating T regulatory cells (T‐regs) at initial diagnosis 13 and linked with decreased relapse‐free and overall survival. 14 In MDS, elevated IDO metabolites were detected in patient sera and correlated with the degree of cytopenia. 15 Primary MSPCs from MDS patients have been shown to secrete IDO. 16 The fact that IDO has emerged as a key target in cancer immunotherapy, and the paucity of data regarding this critical switch towards immune suppression and evasion in MDS/AML, highlight the relevance and need of the current report by Müller‐Thomas et al. 1 Immunohistochemistry staining for IDO in BM sections revealed that 37% of their cohort showed moderate to high expression of IDO, with cytoplasmic positivity being observed mainly in CD11c+ myelomonocytic cells and in a few mature CD68+ macrophages, while BM blasts remained negative. In line with the reported immunosuppressive function of IDO, the group observed a significantly lower CD8/CD3 ratio (P < 0·0001) and a trend for lower FOXP3 expression (P = 0·060), which serves as a lineage specification factor for T regulatory cells, in the BM of patients with high IDO expression.
Müller‐Thomas et al. 1 are the first to analyse IDO in a patient cohort uniformly treated with the hypomethylating agent (HMA) azacitidine. According to current NCCN guidelines and numerous expert opinions, 18 , 19 , 20 HMA are the recommended front line treatment of choice in patients with MDS and AML who are unfit for intensive chemotherapy and/or allogeneic stem cell transplantation. Of note, azacitidine is approved for both MDS and AML in the EU and the US, whereas decitabine is approved for AML (but not MDS) in the EU and for MDS (but not AML) in the US. So far, azacitidine is the only treatment modality shown to prolong survival in MDS in a phase III randomised trial, 20 whereas both azacitidine and decitabine (albeit in a post hoc sensitivity analysis) demonstrated prolonged overall survival (OS) in patients with AML. 22 , 23 However, approximately half of patients do not respond to HMA, and all of the responders eventually experience progressive disease and die. Therefore, several groups have attempted to predict which patients will benefit from HMA treatment. 24 , 25 , 26 , 27
In their report, Müller‐Thomas et al. 1 explored the predictive value of IDO expression in 95 MDS/AML patients treated with azacitidine. Median OS was 12·6 and 7·5 months, and the overall response rate (ORR) was 42% and 37% for MDS and AML patients, respectively. Notably, both are lower than those reported by others, including ourselves. In an analysis of 339 MDS/AML patients treated with azacitidine within the Austrian Registry of Hypomethylating Agents, median OS was 23·7, 18·9, 13·5 and 13·1 months for patients classified as MDS‐RAEB‐I, MDS‐RAEB‐II, low blast count sAML and AML with >30% BM blasts, respectively. ORR ranged from 49·0 to 55·9%. 9 These discrepancies might be due to the high percentage of patients with poor and very poor‐risk karyotype in Müller‐Thomas’s cohort (54%). Importantly, the group demonstrated that high expression of IDO in the BM predicts azacitidine treatment failure (83 vs. 48%, P < 0·001) and significantly shorter OS (10·8 vs. 21·4 months, P = 0·034) in IDO positive versus negative patients respectively, despite the fact that the IDO positive group had significantly fewer patients with poor and very poor IPSS‐R risk categories than the IDO negative group (40 vs. 62%, P = 0·014). IDO expression remained prognostically significant for OS in multivariate analysis.
Azacitidine is known to increase CD8+ CTLs 27 and has also been reported to induce T regulatory cells. 28 Müller‐Thomas et al. 1 demonstrated that IDO positivity significantly correlated with a lack of increase of CD8+ CTLs (P < 0·001) in a small subgroup of patients (n = 15) with a follow‐up BM sample. These results may be seminal if reproduced in a larger set of samples, as they show that IDO (i) is expressed in the BM of MDS/AML patients, (ii) induces an immunosuppressive microenvironment, thereby relevantly contributing to azacitidine treatment failure and poor survival associated therewith, and (iii) may thus represent an interesting drug target in MDS/AML, especially for combination therapies.
So how can the expression of an enzyme in mainly non‐blast cells be related to the efficacy of an HMA on a mechanistic basis? It has been shown that HMAs exert pleiotropic effects on a plethora of cells relevant to MDS/AML development and progression. 29 Besides hypomethylation of silenced tumour‐suppressor genes and direct cytotoxicity, HMAs modulate numbers and functions of various immune cells (i.e., T‐cell subsets, NKCs, MSCs and myeloid‐derived suppressor cells (MDSCs)) to reactivate dormant anti‐tumour immune responses. 31 , 32 , 33 One could speculate that high IDO expression in the BM of MDS/AML patients might be a surrogate marker for advanced disease in terms of advanced tumour immune evasion and strong immunosuppression, without necessarily being strictly associated with BM blast count or other laboratory parameters currently included in prognostic scoring systems. In MDS/AML patients with high IDO levels and a severely dysfunctioning immune system, monotherapy with azacitidine might not suffice to reverse these changes. As such, the present study forms a basis for further exploring therapeutic inhibition of IDO in MDS/AML. Several IDO inhibitors exist, including indoximod, epacadostat and BMS‐986205. 33 A recent phase II trial with epacadostat monotherapy in MDS demonstrated safety, but did not reveal relevant activity, with disease stabilisation in 80% of the patients being the best reported outcome. 34 From the mechanisms reported above, this does not seem too surprising, as one might expect IDO inhibition to be more effective as an add‐on, rather than as a stand‐alone therapy. IDO inhibitors are currently being tested in solid tumours, including combination strategies with checkpoint inhibitors (NCT04106414, NCT04047706, NCT03915405, NCT03695250, NCT03414229, NCT03347123, NCT03291054, NCT03085914, NCT02073123), but selected trials are also starting to look at this potentially promising target in AML (NCT02835729). As immune therapies (most excitingly the field of chimeric antigen receptor T‐cell therapies) develop forwards in the field of MDS and AML, combinatorial targeting of IDO will be an interesting avenue to explore, with the aim to further increase therapeutic efficacy of existing treatments. Clinical trials with these combination strategies are therefore eagerly awaited.
Author Contribution
M.L. and L.P. contributed equally.
Conflict of Interest
L.M.: Honoraria from Bristol‐Myers‐Squibb, Pfizer, Takeda; travel support from Novartis, Roche. R.G.: Honoraria from Bristol‐Myers‐Squibb, Cephalon, Amgen, Eisai, Mundipharma, Merck, Janssen‐Cilag, Genentech, Novartis, AstraZeneca, Boehringer Ingelheim, Pfizer, Roche, and Sanofi Aventis; research funding from Cephalon, Celgene, Amgen, Mundipharma, Genentech, Pfizer, GSK, and Ratiopharm; consulting work for Bristol‐Myers‐Squibb, Cephalon, and Celgene. L.P.: Honoraria from Abbvie, Agios, Bristol‐Myers‐Squibb, Celgene, Inflection Point Biomedical Advisors, Novartis; travel support from Celgene, Gilead, and Novartis.
References
- 1. Müller‐Thomas C, Heider M, Piontek G, Schlensog M, Bassermann F, Kirchner T, et al. Prognostic value of indoleamine 2,3 dioxygenase in patients with higher‐risk myelodysplastic syndromes treated with azacytidine. Br J Haematol. 2020(3);190:361–70. [DOI] [PubMed] [Google Scholar]
- 2. Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405. [DOI] [PubMed] [Google Scholar]
- 3. Leisch M, Jansko B, Zaborsky N, Greil R, Pleyer L. Next generation sequencing in AML—on the way to becoming a new standard for treatment initiation and/or modulation? Cancers. 2019;11:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pleyer L, Neureiter D, Faber V, Greil R. Myelodysplastic syndromes (MDS) In: Chronic myeloid neoplasias and clonal overlap syndromes. Vienna: Springer Vienna; 2010. p. 153–222. [Google Scholar]
- 5. Ye Z, Yue L, Shi J, Shao M, Wu T. Role of IDO and TDO in cancers and related diseases and the therapeutic implications. J Cancer. 2019;10:2771–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prendergast GC, Malachowski WJ, Mondal A, Scherle P, Muller AJ. Indoleamine 2,3‐dioxygenase and its therapeutic inhibition in cancer. Int Rev Cell Mol Biol. 2018;336:175–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang R, Gao N, Chang Q, Meng X, Wang W. The role of IDO, IL‐10, and TGF‐β in the HCV‐associated chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. J Med Virol. 2019;91:265–71. [DOI] [PubMed] [Google Scholar]
- 8. Moffett JR, Namboodiri MA. Tryptophan and the immune response. Immunol Cell Biol. 2003;81:247–65. [DOI] [PubMed] [Google Scholar]
- 9. Thackray SJ, Mowat CG, Chapman SK. Exploring the mechanism of tryptophan 2,3‐dioxygenase. Biochem Soc Trans. 2008;36:1120–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pleyer L, Burgstaller S, Stauder R, Girschikofsky M, Sill H, Schlick K, et al. Azacitidine front‐line in 339 patients with myelodysplastic syndromes and acute myeloid leukaemia: comparison of French‐American‐British and World Health Organization classifications. J Hematol Oncol. 2016;9:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pleyer L, Valent P, Greil R. Mesenchymal stem and progenitor cells in normal and dysplastic hematopoiesis—masters of survival and clonality? Int J Mol Sci. 2016;17:1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Curti A, Aluigi M, Pandolfi S, Ferri E, Isidori A, Salvestrini V, et al. Acute myeloid leukemia cells constitutively express the immunoregulatory enzyme indoleamine 2,3‐dioxygenase. Leukemia. 2007;21:353–5. [DOI] [PubMed] [Google Scholar]
- 13. Corm S, Berthon C, Imbenotte M, Biggio V, Lhermitte M, Dupont C, et al. Indoleamine 2,3‐dioxygenase activity of acute myeloid leukemia cells can be measured from patients’ sera by HPLC and is inducible by IFN‐gamma. Leuk Res. 2009;33:490–4. [DOI] [PubMed] [Google Scholar]
- 14. Curti A, Pandolfi S, Valzasina B, Aluigi M, Isidori A, Ferri E, et al. Modulation of tryptophan catabolism by human leukemic cells results in the conversion of CD25‐ into CD25+ T regulatory cells. Blood. 2007;109:2871–7. [DOI] [PubMed] [Google Scholar]
- 15. Chamuleau MED, van de Loosdrecht AA, Hess CJ, Janssen JJWM, Zevenbergen A, Delwel R, et al. High INDO (indoleamine 2,3‐dioxygenase) mRNA level in blasts of acute myeloid leukemic patients predicts poor clinical outcome. Haematologica. 2008;93:1894–8. [DOI] [PubMed] [Google Scholar]
- 16. Berthon C, Fontenay M, Corm S, Briche I, Allorge D, Hennart B, et al. Metabolites of tryptophan catabolism are elevated in sera of patients with myelodysplastic syndromes and inhibit hematopoietic progenitor amplification. Leuk Res. 2013;37:573–9. [DOI] [PubMed] [Google Scholar]
- 17. Ling W, Zhang J, Yuan Z, Ren G, Zhang L, Chen X, et al. Mesenchymal stem cells use IDO to regulate immunity in tumor microenvironment. Cancer Res. 2014;74:1576–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gangat N, Patnaik MM, Tefferi A. Myelodysplastic syndromes: Contemporary review and how we treat. Am J Hematol. 2016;91:76–89. [DOI] [PubMed] [Google Scholar]
- 19. Schuh AC, Döhner H, Pleyer L, Seymour JF, Fenaux P, Dombret H. Azacitidine in adult patients with acute myeloid leukemia. Crit Rev Oncol Hematol. 2017;116:159–77. [DOI] [PubMed] [Google Scholar]
- 20. Santini V, Ossenkoppele GJ. Hypomethylating agents in the treatment of acute myeloid leukemia: a guide to optimal use. Crit Rev Oncol Hematol. 2019;140:1–7. [DOI] [PubMed] [Google Scholar]
- 21. Fenaux P, Mufti GJ, Hellstrom‐Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher‐risk myelodysplastic syndromes: a randomised, open‐label, phase III study. Lancet Oncol. 2009;10:223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dombret H, Seymour JF, Butrym A, Wierzbowska A, Selleslag D, Jang JH, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with %3e30% blasts. Blood. 2015;126:291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thomas XG, Arthur C, Delaunay J, Jones M, Berrak E, Kantarjian HM. A post hoc sensitivity analysis of survival probabilities in a multinational phase III trial of decitabine in older patients with newly diagnosed acute myeloid leukemia. Clin Lymphoma Myeloma Leuk. 2014;14:68–72. [DOI] [PubMed] [Google Scholar]
- 24. Bally C, Adès L, Renneville A, Sebert M, Eclache V, Preudhomme C, et al. Prognostic value of TP53 gene mutations in myelodysplastic syndromes and acute myeloid leukemia treated with azacitidine. Leuk Res. 2014;38:751–5. [DOI] [PubMed] [Google Scholar]
- 25. Falantes JF, Trujillo P, Piruat JI, Calderón C, Márquez‐Malaver FJ, Martín‐Antonio B, et al. Overexpression of GYS1, MIF, and MYC is associated with adverse outcome and poor response to azacitidine in myelodysplastic syndromes and acute myeloid leukemia. Clin Lymphoma Myeloma Leuk. 2015;15:236–44. [DOI] [PubMed] [Google Scholar]
- 26. Itzykson R, Kosmider O, Cluzeau T, Mansat‐De Mas V, Dreyfus F, Beyne‐Rauzy O, et al. Impact of TET2 mutations on response rate to azacitidine in myelodysplastic syndromes and low blast count acute myeloid leukemias. Leukemia. 2011;25:1147–52. [DOI] [PubMed] [Google Scholar]
- 27. Itzykson R, Thépot S, Quesnel B, Dreyfus F, Beyne‐Rauzy O, Turlure P, et al. Prognostic factors for response and overall survival in 282 patients with higher‐risk myelodysplastic syndromes treated with azacitidine. Blood. 2011;117:403–11. [DOI] [PubMed] [Google Scholar]
- 28. Li K, Hu C, Mei C, Ren Z, Vera JC, Zhuang Z, et al. Sequential combination of decitabine and idarubicin synergistically enhances anti‐leukemia effect followed by demethylating Wnt pathway inhibitor promoters and downregulating Wnt pathway nuclear target. J Transl Med. 2014;12:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Landman S, Cruijsen M, Urbano PCM, Huls G, van Erp PEJ, van Rijssen E, et al. DNA methyltransferase inhibition promotes Th1 polarization in human CD4+CD25high FOXP3+ regulatory T cells but does not affect their suppressive capacity. J Immunol Res. 2018;2018:4973964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pleyer L, Greil R. Digging deep into "dirty" drugs ‐ modulation of the methylation machinery. Drug Metab Rev. 2015;47:252–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Greil R, Hutterer E, Hartmann TN, Pleyer L. Reactivation of dormant anti‐tumor immunity ‐ a clinical perspective of therapeutic immune checkpoint modulation. Cell Commun Signal. 2017;15:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lindblad KE, Goswami M, Hourigan CS, Oetjen KA. Immunological effects of hypomethylating agents. Exp Rev Hematol. 2017;10:745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pleyer L, Greil R. Digging deep into “dirty” drugs – modulation of the methylation machinery. Drug Metab Rev. 2015;47:252–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Prendergast GC, Malachowski WP, DuHadaway JB, Muller AJ. Discovery of IDO1 inhibitors: from bench to bedside. Cancer Res. 2017;77:6795–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Komrokji RS, Wei S, Mailloux AW, Zhang L, Padron E, Sallman D, et al. A phase II study to determine the safety and efficacy of the oral inhibitor of indoleamine 2,3‐dioxygenase (IDO) enzyme INCB024360 in patients with myelodysplastic syndromes. Clin Lymphoma Myeloma Leuk. 2019;19:157–61. [DOI] [PubMed] [Google Scholar]


