Abstract
To improve efficiency of somatic cell nuclear transfer (SCNT), it is necessary to modify differentiated donor cells to become more amendable for reprogramming by the oocyte cytoplasm. A key feature that distinguishes somatic/differentiated cells from embryonic/undifferentiated cells is cellular metabolism, with somatic cells using oxidative phosphorylation (OXPHOS) while embryonic cells utilize glycolysis. Inducing metabolic reprogramming in donor cells could improve SCNT efficiency by priming cells to become more embryonic in nature before SCNT hypoxia inducible factor 1‐α (HIF1‐α), a transcription factor that allows for cell survival in low oxygen, promotes a metabolic switch from OXPHOS to glycolysis. We hypothesized that chemically stabilizing HIF1‐α in donor cells by use of the hypoxia mimetic, cobalt chloride (CoCl2), would promote this metabolic switch in donor cells and subsequently improve the development of SCNT embryos. Donor cell treatment with 100 µM CoCl2 for 24 hr preceding SCNT upregulated messenfer RNA abundance of glycolytic enzymes, improved SCNT development to the blastocyst stage and quality, and affected gene expression in the blastocysts. After transferring blastocysts created from CoCl2‐treated donor cells to surrogates, healthy cloned piglets were produced. Therefore, shifting metabolism toward glycolysis in donor cells by CoCl2 treatment is a simple, economical way of improving the in vitro efficiency of SCNT and is capable of producing live animals.
Keywords: cellular reprogramming, hypoxia inducible factor, metabolism, porcine, somatic cell nuclear transfer
Hypoxic culture of donor cells used for porcine somatic cell nuclear transfer (SCNT) has previously been shown to induce cellular metabolic reprogramming and improve development of cloned embryos. In this study, treating donor cells with a hypoxia mimetic, cobalt chloride, further improved SCNT efficiency as compared with hypoxic culture. Therefore, cobalt chloride treatment can be used as a simple and economical alternative to hypoxic culture for porcine SCNT donor cells.
Abbreviations
- BCL2
B‐cell leukemia/lymphoma 2
- BNIP3
BCL2/adenovirus E1B 19 kDa protein‐interacting protein 3
- CoCl2
cobalt chloride
- COCs
cumulus‐oocyte complexes
- HIF1‐α
hypoxia inducible factor 1‐α
- HIFs
hypoxia inducible factors
- LDHA
lactate dehydrogenase A
- OXPHOS
oxidative phosphorylation
- PDK1
pyruvate dehydrogenase kinase 1
- PGAM1
phosphoglycerate mutase 1
- PKM2
pyruvate kinase muscle isozyme M2
- PVA
polyvinyl alcohol
- SCNT
somatic cell nuclear transfer
- TL‐Hepes
Tyrode's lactate 4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid
- TUNEL
terminal deoxynucleotidyl transferase (TdT) dUTP nick‐end labeling
1. INTRODUCTION
Since the birth of the first animal cloned with a somatic cell in 1996, somatic cell nuclear transfer (SCNT) has developed into a useful research tool (Wilmut, Schnieke, McWhir, Kind, & Campbell, 1997). Today SCNT is used for biomedical models, including xenotransplantation, as well as agricultural models that have led to the discovery of novel treatments for human diseases, animals that are disease resistant, and have put animal‐to‐human organ transplant within reach (Whitworth & Prather, 2017; Prather, Lorson, Ross, Whyte, & Walters, 2013). Even with the current success of SCNT‐created animals, the overall efficiency of SCNT remains low (<5%) with few live births resulting from the SCNT process (Whitworth & Prather, 2010). Due to the lack of authentic embryonic stem cells and induced pluripotent stem cell lines capable of producing live pigs, porcine SCNT is limited to the use of somatic cell types. Since somatic cells have already undergone some degree of differentiation, a possible explanation for poor SCNT efficiency is the inability to successfully remodel somatic nuclei through the SCNT process. A key feature that distinguishes embryonic/undifferentiated cells from somatic/differentiated cells is the metabolism that is used. Differentiated cells utilize mitochondrial oxidative phosphorylation (OXPHOS), while undifferentiated cells use glycolysis. There is mounting evidence to suggest that metabolic reprogramming, or the switch from OXPHOS to glycolysis, is necessary to revert cells back to an undifferentiated state and maintain stemness (Prigione et al., 2014).
HIFs are a class of master transcription factors responsible for the cellular survival response to hypoxic conditions. HIF stabilization promotes the transcription of target genes related to glycolysis, angiogenesis, cell survival and proliferation, cell migration, apoptosis, and erythropoiesis (Hu, Wang, Chodosh, Keith, & Simon, 2003). Hypoxic stress is alleviated by these downstream targets by modifying the need for oxygen for cellular mechanisms, such as energy production, or allowing for greater oxygen delivery. For example, downstream targets related to glucose metabolism, such as the glucose transporters SLC2A1 and SLC2A3, allow for energy production through glycolysis as opposed to mitochondrial OXPHOS, which can only occur in the presence of oxygen (Semenza, 2000).
Previous studies have shown that donor cell culture in hypoxia (1.25% O2) results in an upregulation of genes related to glycolysis in donor cells, as well as increased blastocyst production and in utero survivability following SCNT (Mordhorst et al., 2018, 2019). However, hypoxic cell culture can be costly and often requires specialized mixed gas tanks to achieve low oxygen tensions. There is also no reliable way to monitor the oxygen tension that the donor cells are being exposed to when cultured in hypoxia, as it requires culture in chambers that must remain sealed. In addition, HIF 1‐α, the modulator of the hypoxic response in cells, has a high turnover rate with degradation occurring in 5–8 min once cells are exposed to atmospheric oxygen levels. During the SCNT process, the time between cell collection and cell‐oocyte fusion/activation is typically greater than 1 hr. Therefore, the influence of HIF 1‐α in these cells may be greatly diminished by the conclusion of the SCNT process.
Due to the possible instability of hypoxia inducible factor 1‐α (HIF1‐α) in hypoxia cultured cells, we proposed a chemical hypoxia mimetic that allows a sustained effect of HIF1‐α outside of physiological hypoxia. In normoxia, HIF1‐α is hydroxylated by prolyl hydroxylases that require oxygen and iron for their enzymatic activity. This hydroxylation serves as a docking site for Von Hippel Lindeau protein that marks HIF1‐α for degradation by the 26S proteasome. In hypoxic conditions, the oxygen required for the prolyl hydroxylases is not available; and therefore, the cascade of events leading to HIF1‐α degradation cannot be initiated. This allows HIF1‐α protein to accumulate in the cytoplasm and subsequently translocate to the nucleus to dimerize with HIF1‐β and direct transcription of downstream targets (Semenza, 2000). Cobalt chloride (CoCl2) is a known hypoxia mimetic that inhibits the activity of prolyl hydroxylases by replacing the required iron domain of the prolyl hydroxylases with cobalt (Hirsila et al., 2005). This chemical simulation allows stabilization of the volatile HIF1‐α, even in the presence of atmospheric oxygen. Once stabilized, HIF1‐α can activate its downstream targets including genes that induce the reprogramming of metabolic processes to favor glycolytic metabolism over OXPHOS.
Therefore, the objective of this study was to determine if treatment of somatic donor cells with the hypoxia mimetic, CoCl2, can induce metabolic reprogramming in the donor cells and promote better nuclear reprogramming before SCNT to improve development of SCNT embryos.
2. RESULTS
2.1. Impact of CoCl2 on cell viability
Cell number and viability was determined by Trypan blue exclusion after culture in 50, 100, or 150 µM of CoCl2 for 24, 48, or 72 hr (Figure 1). Live cell number was not different between any CoCl2 concentrations after 24 hr of culture. After 48 hr of culture, live cell number was significantly lower in the 150 µM treatment group as opposed to the 50 and 100 µM, or untreated cell groups. After 72 hr of culture, live cell number was negatively impacted in the 100 and 150 µM treatment groups as compared with the 50 µM and untreated groups.
Figure 1.
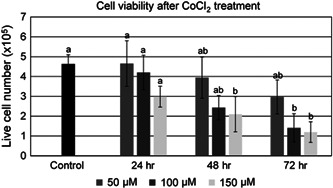
Cell viability after treatment with 0, 50, 100, or 150 µM of CoCl2 for 24, 48, or 72 hr. Data represented as means ± SEM. Statistical differences represented by different lowercase letters (a, b). SEM, standard error of mean
Long‐term effects of CoCl2 treatment were determined by analysis of cell viability after a 3‐day recovery period following CoCl2 exposure (Figure 2). Only the 24‐hr 50 µM treatment of CoCl2 was capable of recovering cell viability to numbers comparable to the untreated control. The 50 µM treatment of CoCl2 did become detrimental to cell viability following 48 and 72 hr of exposure. The 100 µM CoCl2 treatment was comparable to the 50 µM treatment at all time points. The 150 µM treatment was significantly lower than the 50 µM treatment after 48 and 72 hr of CoCl2 exposure. Based on the results of these two studies, a treatment of 24‐hr exposure to 100 µM CoCl2 was chosen for the remainder of the study.
Figure 2.
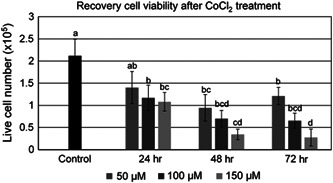
Cell viability following a 72 hr recovery period after treatment with 0, 50, 100, or 150 µM of CoCl2 for 24, 48, or 72 hr. Data represented as means ± SEM. Statistical differences represented by different lowercase letters (a, b, c, d). SEM, standard error of mean
2.2. Gene expression in donor cells following CoCl2 exposure
Real‐time quantitative polymerase chain reaction (PCR) was used to analyze differences in message abundance between CoCl2 treated donor cells, hypoxia treated donor cells, and untreated control cells (Table 1) for HIF1‐α and non HIF1‐α gene targets (Liu, Shen, Zhoa, & Chen, 2012). Glucose transporters, SLC2A1 and SLC2A3, as well as glycolytic enzymes HK1, HK2, GPI, ALDOC, GAPDH, PGK1, PGAM1, ENO1, PKM2, PDK1, and LDHA were upregulated in the CoCl2 group compared with the control. The same transcripts, with the exception of SLC2A1, ALDOC, GAPDH, and PGAM1 were also upregulated in the hypoxia group compared with the control. Transcript abundance of the mitophagy‐associated gene BNIP3, GPI, and PDK1 were differentially expressed between all treatment groups with the lowest expression present in the control cells and the highest expression in the CoCl2 cells. Non HIF1‐α targets, TALDO1, EPAS1, YWHAG, LDHB, and BCL2 were not differentially expressed between the groups.
Table 1.
Normalized abundance ± SEM of gene products related to glycolysis and mitophagy. Treatments include a control (cultured at 5% O2 for 3 days), CoCl2 treatment (100 µM CoCl2 for 24 hr), and a hypoxic treatment (cultured at 1% O2 for 3 days)
| Gene name | Control | CoCl2 | Hypoxia |
|---|---|---|---|
| SLC2A1 * | 1.88 ± 0.38a | 3.25 ± 0.32b | 2.29 ± 0.12a,b |
| SLC2A3 * | 1.61 ± 0.26b | 3.25 ± 0.39b | 3.75 ± 0.34b |
| HK1 * | 2.02 ± 0.17a | 3.30 ± 0.25b | 3.02 ± 0.18b |
| HK2 * | 10.59 ± 1.92a | 22.36 ± 1.68b | 19.24 ± 1.03b |
| GAPDH * | 3.27 ± 0.34a | 6.38 ± 0.49b | 4.33 ± 0.44b |
| PGK1 * | 1.03 ± 0.10a | 2.06 ± 0.09b | 1.77 ± 0.10b |
| ENO1 * | 5.88 ± 0.44a | 10.28 ± 0.68b | 9.15 ± 0.97b |
| PKM2 * | 3.70 ± 0.25a | 6.30 ± 0.59b | 5.59 ± 0.48b |
| PDK1 * | 3.82 ± 0.48a | 7.10 ± 0.05b | 5.66 ± 0.51c |
| LDHA * | 2.16 ± 0.22a | 3.45 ± 0.28b | 3.57 ± 0.26b |
| LDHB | 0.11 ± 0.01 | 0.11 ± 0.001 | 0.12 ± 0.02 |
| BNIP3 * | 2.02 ± 0.40a | 5.54 ± 0.32b | 3.69 ± 0.31c |
| TALDO1 | 0.81 ± 0.10 | 1.03 ± 0.09 | 0.85 ± 0.09 |
| EPAS1 | 0.32 ± 0.07 | 0.55 ± 0.17 | 0.24 ± 0.04 |
| YWHAG | 0.39 ± 0.04 | 0.44 ± 0.04 | 0.39 ± 0.02 |
| BCL2 | 0.55 ± 0.03 | 0.65 ± 0.07 | 0.53 ± 0.05 |
Note: a,b,cRepresent differences between treatments with p < .05 considered significant.
Abbreviation: SEM, standard error of mean.
Indicates genes that are HIF targets.
2.3. SCNT embryo development and quality
The use of CoCl2‐treated donor cells for SCNT resulted in an increased rate of development to the blastocyst stage compared with untreated control donor cells (50.3 ± 2.6% vs. 32.6 ± 1.9%; p = .0002; Figure 3), as well as an increase in the total number of nuclei within the blastocyst‐stage embryos (52.0 ± 3.3 vs. 39.0 ± 3.0; p = .014; Figure 4). Evaluation of DNA damage by the terminal deoxynucleotidyl transferase dUTP nick‐end labeling (TUNEL) assay revealed no difference in the number of apoptotic nuclei between the groups (p = .64; Table 2).
Figure 3.
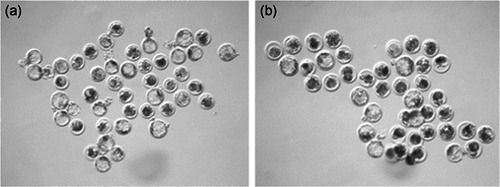
Representative images of blastocyst stage embryos created from (a) CoCl2 treated donor cells and (b) control donor cells
Figure 4.
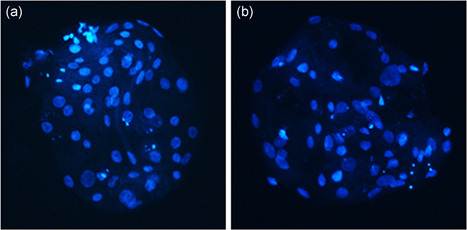
Representative images of Hoechst stained blastocyst stage embryos created from (a) CoCl2 treated donor cells and (b) control donor cells
Table 2.
Blastocyst‐stage embryo development and quality parameters on Day 6 between embryos created from CoCl2 treated donor cells and control donor cells
| Quality parameter | Control | CoCl2 |
|---|---|---|
| Blastocyst rate (%) ± SEM | 32.55 ± 1.87a | 50.29 ± 2.57b |
| Total cell number ± SEM | 38.99 ± 3.03a | 51.96 ± 3.34b |
| % TUNEL positive ± SEM | 7.04 ± 0.78 | 6.51 ± 0.72 |
Note: a,bRepresent differences between treatments with p < .05 considered significant.
Abbreviations: SEM, standard error of mean; TUNEL, terminal deoxynucleotidyl transferase dUTP nick‐end labeling.
2.4. Gene expression in SCNT blastocyst stage embryos produced by CoCl2 donor cells
Genes that were evaluated in donor cells were also analyzed in blastocyst‐stage embryos created with CoCl2 treated donor cells and blastocyst‐stage embryos created from untreated control cells (Table 3). Of the genes evaluated, SLC2A1, PGAM1, and LDHA were upregulated in Day 6 blastocyst‐stage embryos created from CoCl2 treated donor cells compared with control donor cells (p < .05).
Table 3.
Normalized abundance ± SEM of gene products related to glycolysis and mitophagy. Treatments include Day 6 blastocyst stage embryos created from control donor cells and CoCl2 treated donor cells (100 µM CoCl2 for 24 hr)
| Gene name | Control | CoCl2 | p Value |
|---|---|---|---|
| SLC2A1 | 5.86 ± 0.66a | 8.06 ± 0.44b | .0497 |
| SLC2A3 | 2.05 ± 0.47 | 2.74 ± 0.16 | .2370 |
| HK1 | 0.12 ± 0.01 | 0.16 ± 0.03 | .0978 |
| HK2 | 24.30 ± 3.32 | 30.87 ± 2.68 | .0989 |
| GPI | 0.66 ± 0.10 | 0.88 ± 0.04 | .0917 |
| ALDOC | 0.37 ± 0.12 | 0.50 ± 0.03 | .3755 |
| GAPDH | 2.45 ± 0.52 | 2.38 ± 0.50 | .4626 |
| PGK1 | 0.18 ± 0.02 | 0.25 ± 0.04 | .0955 |
| PGAM1 | 3.08 ± 0.10a | 3.88 ± 0.26b | .0446 |
| ENO1 | 1.31 ± 0.12 | 1.55 ± 0.09 | .0916 |
| PKM2 | 0.47 ± 0.11 | 0.66 ± 0.11 | .1518 |
| PDK1 | 2.26 ± 0.58 | 2.48 ± 0.56 | .3997 |
| LDHA | 0.08 ± 0.01a | 0.15 ± 0.02b | .0315 |
| BNIP3 | 4.99 ± 0.66 | 6.76 ± 0.68 | .1348 |
| TALDO1 | 3.90 ± 0.45 | 4.58 ± 0.74 | .2414 |
| YWHAG | 0.09 ± 0.01 | 0.13 ± 0.03 | .1302 |
| BCL2 | 4.49 ± 0.57 | 4.94 ± 0.69 | .6432 |
| POU5F1 | 476.97 ± 136.52 | 614.25 ± 35.49 | .1928 |
| VEGFA | 2.99 ± 0.23 | 3.73 ± 0.53 | .2678 |
Note: a,bRepresent differences between treatments with p < .05 considered significant.
Abbreviations: SEM, standard error of mean.
2.5. Cloned piglet production with CoCl2 treated donor cells
Following surgical embryo transfer to two recipient surrogates, both surrogates were confirmed pregnant by ultrasound at 25 and 38 days of gestation. At 52 days of gestation, one of the two surrogates had exhibited estrus and was no longer pregnant. At 120 days of gestation, the remaining pregnant surrogate farrowed naturally and delivered five piglets. Three of the five piglets were stillborn, and the surviving two piglets were healthy with no signs of abnormalities (Figure 5). No obvious defects were detected in the stillborn piglets; however, a necropsy was not performed. Birthweights ranged from 0.800 to 1.155 kg, with an average birthweight of 0.955 kg. Weaning weights recorded at 3 weeks were 4.720 and 4.120 kg, for an average weight of 4.420 kg (Table 4).
Figure 5.

Images of cloned piglets produced from SCNT embryos created from CoCl2 treated donor cells. SCNT, somatic cell nuclear transfer
Table 4.
Birthweights and status of piglets born from SCNT embryos created from CoCl2 treated donor cells
| Piglet # | Birth weight, kg | Weaning weight, kg |
|---|---|---|
| 1 | 0.845 | 4.120 |
| 2 | 1.155 | 4.720 |
| 3 (stillborn) | 0.980 | – |
| 4 (stillborn) | 0.800 | – |
| 5 (stillborn) | 0.995 | – |
| Avg | 0.955 | 4.420 |
Abbreviation: SCNT, somatic cell nuclear transfer.
3. DISCUSSION
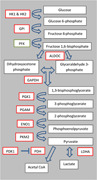
The purpose of this study was to understand the effect of CoCl2 treatment on metabolism in SCNT donor cells and the resultant effect on SCNT efficiency in vitro with these donor cells. Analysis of HIF1‐α targets related to glycolysis and cell survival in donor cells cultured in either 5% O2 (control), 1% O2 (hypoxia), or 5% O2 with CoCl2 treatment was analyzed to understand the effect that HIF1‐α stabilization through physiological or chemical means had on gene expression (Table 5). Hypoxic culture of fibroblasts and fibroblasts cultured with CoCl2 resulted in an increase in messenger RNA (mRNA) abundance of glucose transporters SLC2A1 and SLC2A3, as well as glycolysis‐related enzymes HK1 and HK2, GPI, ALDOC, GAPDH, PGK1, PGAM, ENO1, PKM2, PDK1, and LDHA, all of which are HIF downstream targets. (Figure 6).
Table 5.
RT‐PCR primers
| Gene | Forward primer 5′ → 3′ | Reverse primer 5′ → 3′ | Accession # |
|---|---|---|---|
| YWHAG | TCCATCACTGAGGAAAACTGCTAA | TTTTTCCAACTCCGTGTTTCTCTA | XM_005661962.3 |
| PKM2 | ATGCAGTCTTGGATGGAGCTGACT | ATTGCAAATGGTAGATGGCGGCCT | AJ557236.1 |
| SLC2A1 | TCCACACCCACTTTGTCACACTGA | AGCCTCAACTCCCACATCACTGAA | XM_021096908.1 |
| SLC2A3 | CCCTCAGCTGCATTCTATTT | GTCTCAGGGACTTTGAAGAAG | XM_021092392.1 |
| PGK1 | CGCTTTCTGCATCTCCACTTGGCA | GCTGTGCAATGGTTCAAGGGTTCCT | NM_001099932.2 |
| PDK1 | ACCAGGACAGCCAATACAAGTGGT | ACGTGGACTTGAATAGGCGGGTAA | NM_001159608.1 |
| TALDO1 | TGAAGCGGCAGAGGATGGAGAGC | TCGTCGATGGCGTTGAAGTCGC | NM_001244935.1 |
| EPAS1 | AAGCAAAGACATGTCCACCGAGCG | GTGGCTGACTTGAGGTTGACGGTG | NM_001097420.1 |
| HK1 | TCTTGATCGACTTCACCAAGAGGG | TCGCTCTCGATCTGCGAGAGATACTT | NM_001243184.1 |
| HK2 | GAATTTGATGCGGCCGTGGATGAA | CCAGGTACATGCCGCTGATCATTT | NM_001122987.1 |
| ENO1 | TCGGAGTTCTACAGGTCGGGCAAG | TGGTCCGGTGAGATGTACCTGCTG | XM_021095280.1 |
| PGAM1 | CAGTGCTGGATGCCATTGACCAAA | GCTTGGCAGCAGTTTCTGCCTTAT | XM_003483535.4 |
| LDHA | TTCAGCCCGGTTCCGTTACCTAAT | TTCTTCAGGGAGACACCAGCAACA | NM_001172363.2 |
| LDHB | TAAGCATGGGCTTTGACTCTGGGA | ACTCCCGGCTTCTAGGTTGTAGTA | NM_001113287.1 |
| VEGFA | CAAACCTCACCAAGGCCAGCACAT | CGAGCAAGGCCCACAGGGATTTTC | NM_214084.1 |
| GPI | CCAGGAGACCATCACAAATG | TAGACAGGGCGACAAAGT | NM_214330.1 |
| ALDOC | TCTTCCATGAGACCCTCTAC | TACACCCTTGTCCACCTT | NM_001243928.1 |
| BNIP3 | GGATTACATGGAGAGGAGGA | GTGCTTGAAGAGGAGGAAC | XM_003359404.4 |
| BCL2 | ACTGAATGCCCTCCGGTACC | ATCCCCATGGCTGCAGTGAA | XM_003130557.2 |
| ACTB | TCTGGCACCACACCTTCT | TGATCTGGGTCATCTTCTCAC | DQ178122.1 |
| POU5F1 | TTTGGGAAGGTGTTCAGCCAAACG | TCGGTTCTCGATACTTGTCCGCTT | NM_001113060.1 |
Abbreviation: RT‐PCR, real‐time polymerase chain reaction.
Figure 6.
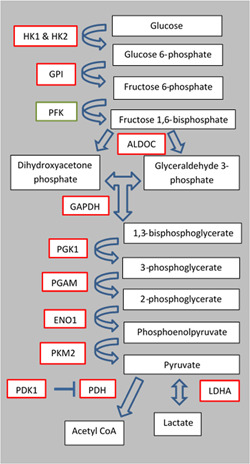
Schematic of glycolysis. Red rectangles represent enzyme transcripts that were differentially expressed between CoCl2 treated fibroblasts and control fibroblasts. Green rectangles represent enzyme transcripts that were not evaluated. ALDOC, aldolase C; CoA, coenzyme A; ENO1, enolase 1; HK1, hexokinase 1; GAPDH, glyceraldehyde 3‐phosphate dehydrogenase; GPI, glucose‐6‐phosphate isomerase; LDHA, lactate dehydrogenase A; PDH, pyruvate dehydrogenase; PDK1, pyruvate dehydrogenase kinase 1; PFK, phosphofructokinase; PGAM, phosphoglycerate mutase 1; PGK1, phosphoglycerate kinase 1; PKM2, pyruvate kinase muscle isozyme M2
Although the majority of these enzymes are basic glycolytic enzymes that could indicate that an increase in glycolytic activity is occurring, enzymes, such as pyruvate kinase muscle isozyme M2 (PKM2), pyruvate dehydrogenase kinase 1 (PDK1), and lactate dehydrogenase A (LDHA) have unique roles that are specific to less differentiated cells, such as cancer cells, that are being pushed away from oxidative metabolism. Pyruvate kinase muscle isozyme M2 is one of the four isoforms of pyruvate kinase, produced by alternative splicing, and is specifically associated with proliferating cells and cancer cells (as reviewed by Dong et al., 2016). In the analysis of mRNA abundance of glycolytic enzymes associated with the Warburg effect, it was determined that blastocyst stage‐embryos exclusively expressed the fetal PKM2 as opposed to the adult PKM1 (Redel et al., 2011).
In an aerobic system, once pyruvate has been produced through glycolysis, it is subsequently converted to acetyl coenzyme A (CoA) through the mitochondrial enzyme pyruvate dehydrogenase. However, in glycolytic systems, the production of the enzyme PDK1 results in phosphorylation of pyruvate dehydrogenase which inactivates the complex and directs pyruvate away from the TCA cycle, inhibiting its oxidation. PDK1 has been demonstrated by microarray and chromatin immunoprecipitation to be a direct target of HIF1‐α, and is an important player in the switch from aerobic to anaerobic metabolism through its ability to block acetyl CoA production so that pyruvate can be converted to lactate (Kim, Tchernyshyov, Semenza, & Dang, 2006).
Since PDK1 increases availability of pyruvate in the cell, it is then able to be converted to lactate by LDHA. The conversion of pyruvate to lactate is crucial for anaerobic glycolysis. In human pancreatic cancer cells, LDHA is upregulated by hypoxia and is directly activated by HIF1‐α. Induced expression of LDHA promotes the proliferation and migration of pancreatic cancer cells, and knocked down expression inhibits cell growth and migration (Cui et al., 2017). This indicates that LDHA and its effect in hypoxic conditions is crucial for cancer cell survival.
Although the majority of gene expression changes found in this study relate to the SCNT donor cells, there were also several genes upregulated in CoCl2 treated donor cell SCNT blastocyst stage embryos (Table 3). Glucose transporter SLC2A1, and glycolytic enzymes PGAM1 and LDHA were found to be upregulated in embryos created from CoCl2 treated donor cells as compared with those created from control donor cells. Although glucose is not a component of the embryo culture media used in this study, increased glucose uptake has been shown to be associated with improved embryo viability in bovine (Renard, Philippon, & Menezo, 1980), mouse (Gardner & Leese, 1987) and human (Gardner, Wale, Collins, & Lane, 2011) systems. Phosphoglycerate mutase 1 (PGAM1) enzymatic activity has been proposed as a potential alternative glycolytic pathway in rapidly proliferating cells that do not have increased pyruvate kinase activity. Phosphorylation of PGAM1 by the phosphate donor phosphoenolpyruvate, which is typically associated with PKM2 activity, promotes increased pyruvate production and allows for a higher glycolytic flux (Vander Heiden et al., 2010). LDHA promotes lactate production, and aligning with the Warburg effect, lactate production in the presence of oxygen is associated with rapidly proliferating cells. During blastocyst formation, there is a transition from the lactate dehydrogenase B isoform to the LDHA isoform which is associated with lactate production as opposed to pyruvate production (as reviewed by Krisher & Prather, 2012). Therefore, the upregulation of LDHA at the blastocyst stage in the embryos created from CoCl2 treated donor cells as compared with control SCNT embryos could indicate that a more natural gene expression profile in the blastocysts is promoted by metabolic reprogramming of CoCl2 treated donor cells before SCNT.
CoCl2 treatment of donor cells resulted in greater (~18% increase) blastocyst stage embryo development and improved embryo quality (13 more cells per blastocyst) as compared with control embryos (Table 2). However, previous studies have shown that analysis of blastocyst stage embryo qualities alone is not indicative of the in utero survival and live birth potential of embryos (Redel et al., 2016). To demonstrate that CoCl2 treatment of donor cells could result in the live birth of piglets following SCNT, embryo transfer was conducted. An untreated control donor cell comparison was not conducted in this study due to the number of animals that would need to be utilized, and surgeries that would have to be performed. The purpose of the embryo transfer was to ensure that there were no lethal effects of the donor cell treatment that would prevent the in utero survival of these embryos. Of the two surrogates used for embryo transfer, one was able to maintain pregnancy to term. This surrogate delivered five piglets unassisted. Of the five piglets that were delivered, three were stillborn. From outward visual inspection and birth weights, the three piglets did not have any obvious abnormalities that would have resulted in their death and had healthy birthweights for an Ossabaw breed (Table 4). Due to the lack of outward abnormalities in these stillborn piglets, along with the birth of two live piglets that proved there was no lethal effect of the CoCl2 treatment, postmortem necropsies were not conducted. The two surviving piglets had healthy birth weights and weaning weights and have had no issues since their birth. Therefore, the birth of healthy clones from this experiment indicates that CoCl2 treatment of donor cells results in SCNT embryos that are capable of producing piglets and can be used as a viable option for future cloning studies.
Our findings indicate that the use of CoCl2 as a novel treatment for SCNT donor cells induces the same glycolytic response as culture in 1% oxygen (hypoxia) for 3 days. The use of the hypoxia mimetic allows the cells to be maintained in any oxygen tension, without the need for specialized gas tanks or chambers and eliminates the need for long term culture of donor cells in hypoxic conditions to establish the same effect. The upregulation of genes that are known to be downstream targets of HIF1‐α in the CoCl2 treated and hypoxia treated donor cells, along with the lack of differential expression of non‐HIF1‐α targets suggests that the transcription factor may be activated through these treatments. Therefore, promoting metabolic reprogramming in donor cells through CoCl2 treatment improves the efficiency of the SCNT process through alterations in gene expression in donor cells and resultant SCNT blastocysts, improvement in the quality and development rate of SCNT embryos, and production of healthy, cloned piglets.
4. MATERIALS AND METHODS
4.1. Ethics statement
Collection of ovaries from prepubertal gilts and use of live animals were in accordance with approved protocol and standard operating procedures by the Animal Care and Use Committee of the University of Missouri.
4.2. Determining optimal CoCl2 concentration
Dorsal tissue of gestational Day 35 wild‐type fetuses was removed and dissociated. Cells were cryopreserved in 500 µl aliquots and stored in liquid nitrogen until needed. Cells were thawed and cultured in Dulbecco's modified Eagle's medium (1 g/L glucose with phenol red) supplemented with 15% fetal bovine serum (Corning, Corning, NY) for 4 days in T25 flasks (Corning). For determining the working CoCl2 concentration, cobalt chloride hexahydrate (C8661; Thermo Fisher Scientific, Waltham, Massachusetts) was mixed fresh daily for each use. To achieve a 10 mM concentration of CoCl2, 11.89 mg of CoCl2 was dissolved into 5 ml MilliQ H2O. The solution was then added at a 1:100 µl ratio to culture media to achieve a final concentration of 100 µM. To evaluate the effect of increased CoCl2 concentrations on cell viability, cells were plated at equal density of 7.5 × 104 cells/flask and the CoCl2 solution was added at 50, 100, and 150 µM. All concentrations were applied to cells for 24, 48, or 72 hr. Control cells were left untreated. After the 72 hr, CoCl2 treated and control cells were trypsinized and Trypan blue exclusion was used to determine live and total cell number. To evaluate the recovery ability of cells after CoCl2 exposure, the beforementioned conditions were applied to cells plated at equal densities, followed by aspiration of media containing CoCl2 and replacement with fresh media. The cells were grown for 3 days subsequent to CoCl2 removal and then trypsinized and subjected to Trypan blue exclusion to determine live and total cell number.
For SCNT, fibroblast cells were thawed 4 days before SCNT, counted by Trypan blue exclusion, plated at a density of 7.5 × 104 cells/T25 flask and cultured in a humidified incubator with an atmosphere of 5% CO2, 5% O2, and 90% N2 at 37.5°C. On Day 3, 24 hr before SCNT, CoCl2 was added at a 100 µM concentration. The control cells were left untreated.
4.3. Oocyte collection and somatic cell nuclear transfer
Ovaries from a local abattoir (Smithfield, Milan, MO) were harvested and 18‐gauge needles attached to disposable 10 ml syringes were used to aspirate follicles that were 3–6 mm in size and showed normal morphology. Cumulus‐oocyte complexs (COCs) in follicular fluid were washed three times in Tyrode's lactate 4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid (TL‐Hepes) before being placed in 100 mm polystyrene petri dishes. COC's displaying uniform cytoplasm and at least three layers of cumulus cells were selected and placed in maturation medium (TCM‐199 medium supplemented with 0.1% polyvinyl alcohol [PVA], 3.05 mM d‐glucose, 0.91 mM sodium pyruvate, 10 μg/ml of gentamicin, 0.57 mM cysteine, 10 ng/ml of EGF, 0.5 μg/ml of FSH, 0.5 μg/ml of LH, 40 ng/ml FGF2, 20 ng/ml LIF, and 20 ng/ml IGF1; Yuan et al., 2017) for 42–44 hr in a humidified incubator with an atmosphere of 5% CO2 in air at 37.5°C. Cumulus cells were stripped from oocytes by gentle vortex for 3 min in 0.1% (wt/vol) hyaluronidase in TL‐HEPES‐buffered saline with 0.1% PVA. Metaphase II oocytes were selected based on the presence of an extruded first polar body in the perivitelline space.
Metaphase II oocytes were placed on the stage of an inverted microscope equipped with micromanipulators in drops containing manipulation medium (Lai & Prather, 2004) supplemented with 7.0 µg/ml cytochalasin B. A hand‐tooled glass pipette was used to remove the polar body, and approximately 10% of the adjacent cytoplasm (presumably containing the metaphase plate). Following enucleation, a fibroblast cell was injected into the perivitelline space and pressed against the cytoplasm. Donor cells were then trypsinized and harvested for SCNT, with CoCl2 treated cells resuspended in manipulation medium containing 7.0 µg/ml cytochalasin B and 100 µM CoCl2. While injecting CoCl2 treated cells, 100 µM CoCl2 was present in the micromanipulation drops to sustain the treatment effect and prohibit HIF1‐α degradation. Oocyte‐donor cell couplets were then fused in fusion medium (0.3 M mannitol, 0.1 mM CaCl2, 0.1 mM MgCl2, 0.5 mM HEPES buffer, pH 7.2) by two direct current pulses (1‐s interval) at 1.2 kV/cm for 30 µs by using a BTX Electro Cell Manipulator (Harvard Apparatus, Holliston, MA). At least 1 hour after fusion, reconstructed embryos were fully activated for 30 min with 200 µM N,N,N′,N′‐tetrakis (2‐pyridylmethyl) ethane‐1,2‐diamine (Lee et al., 2015) in TL‐HEPES. Embryos were then incubated in MU‐2 media with 0.5 µM of histone deacetylase inhibitor Scriptaid, for 14–16 hr in a 5% carbon dioxide (atmospheric oxygen) incubator (Whitworth, Zhao, Spate, Li, & Prather, 2011; Zhao et al., 2009). The following morning, embryos were removed from Scriptaid treatment, washed, and placed in fresh MU‐2 media and cultured in an incubator with a humidified atmosphere of 5% CO2, 5% O2, and 90% N2 at 37.5°C until Day 6 post‐activation.
4.4. Blastocyst quality evaluation
Day 6 blastocyst‐stage embryos collected in pools of 15–25 per treatment were fixed in 4% paraformaldehyde in TL‐HEPES for 20 min, followed by permeabilization with 0.1% Triton X‐100 for 30 min. To assess DNA damage, blastocyst stage embryos were incubated with TUNEL stain for 30 min, and then Hoechst nuclear stain (10 µg/ml) for 5 min. Blastocyst‐stage embryos were visualized at 20x magnification on a microscope equipped with epi‐fluorescence, and total cells and TUNEL positive cells were quantified. The ratio of TUNEL positive cells to total cells was calculated to determine a percentage of DNA damaged cells per blastocyst‐stage embryo.
4.5. RNA extraction and complementary DNA synthesis
To evaluate gene expression in donor cells, cells were subjected to either CoCl2 treatment, hypoxic treatment, or left untreated. For all treatments, cells were plated at equal densities in T25 plates. For CoCl2 treatment, cells were maintained at 5% CO2, 5% O2, and 90% N2 at 37.5°C and 100 µM CoCl2 was added on the third day of culture, 24 hr before cell collection. For the hypoxic treatment, cells were placed in an incubator maintained at 5% CO2, 5% O2, and 90% N2 at 37.5°C for at least 4 hours before being transferred to a hypoxic chamber (Billups‐Rothenburg, San Diego, CA) supplemented with a 100 mm petri dish of milliQ H2O. The chamber was sealed and gassed for 2 min with 1% O2 using a mixed gas LiquidGas tank (1% O2, 5% CO2). The chamber was then placed back into the incubator at 5% CO2, 5% O2, and 90% N2 at 37.5°C and were left to grow for 3 days following hypoxic exposure.
Day 6 blastocyst‐stage embryos created with either CoCl2 treated donor cells or control donor cells were collected in pools of 35–50 and washed in diethyl pyrocarbonate‐treated phosphate‐buffered saline before being snap‐frozen in liquid nitrogen for storage at −80°C. Fibroblast cells cultured in CoCl2 for 24 hr, untreated, or cultured in 1% hypoxia for 3 days were trypsinized, pelleted, and snap‐frozen in liquid nitrogen for storage at −80°C. Three biological replicates were collected for each treatment. For blastocyst‐stage embryos, total RNA was extracted by using an RNeasy Micro Kit (Qiagen, Germantown, MD) and eluted in 12 μl of nuclease‐free water. All 12 μL of eluted RNA was used for complementary DNA (cDNA) synthesis by the SuperScript VILO cDNA Synthesis Kit (11754050; Thermo Fisher Scientific). For fibroblast cells, total RNA was extracted by using an RNeasy Mini Kit (Qiagen, Germantown, MD) and eluted in 30 µl of nuclease‐free water. RNA content was determined by using a Nanodrop 1000 Spectrophotometer (Thermo Fisher Scientific), and an appropriate amount of eluted RNA was added accordingly for cDNA synthesis by the SuperScript VILO cDNA Synthesis Kit (11754050; Thermo Fisher Scientific).
4.6. Relative quantitative PCR
Relative quantitative PCR was performed with each sample from cDNA synthesis. Message evaluated included HIF1‐α targets associated with glycolysis, autophagy, and pluripotency in fibroblast cells and blastocyst stage embryos (Table 5). Samples from each biological replicate were diluted to 5 ng/µL, and quantitative PCR was run in triplicate to determine differential expression of the selected transcripts with the conditions: 95°C for 3 min, and 40 cycles of 95°C for 10 s, 55°C for 10 s, and 72°C for 30 s. A dissociation curve was generated after amplification to ensure that a single product was amplified. Abundance of each mRNA transcript was calculated relative to a housekeeping gene, β actin, and a pig genome reference sample. The comparative quantification cycle method was used to determine relative mRNA expression for each treatment.
4.7. Surgical embryo transfer
For the embryo transfer experiment, donor cells used for SCNT were a wild‐type Ossabaw cell line (RRID NSRRC:0008) that had been proven clonable (Mordhorst et al., 2019). Following SCNT, Day 6 blastocyst‐stage embryos created from CoCl2‐treated donor cells were transferred into recipient surrogates. Briefly, two gilts 4 days post‐observed estrus were aseptically prepared for surgery, and the infundibulum was exposed by entry though the lower abdominal wall. A Tomcat catheter containing 42 blastocyst‐stage embryos was inserted into one ampullary‐isthmic junction of each surrogate where the blastocysts were deposited. Pregnancy was determined by ultrasound on Day 25 and monitored by biweekly ultrasounds thereafter. After farrowing, birth weights, weaning weights, and phenotypes were recorded.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ACKNOWLEDGMENTS
The authors would like to thank Jason Dowell for his assistance with the embryo transfer surgeries, as well as the pig care workers that assisted with surrogate and piglet care. The authors would like to acknowledge funding from Food for the 21st Century and the National Institutes of Health via R01 HD080636 and NHLBI and NIAID via U42 OD011140.
Cecil RF, Chen PR, Benne JA, et al. Chemical simulation of hypoxia in donor cells improves development of somatic cell nuclear transfer‐derived embryos and increases abundance of transcripts related to glycolysis. Mol Reprod Dev. 2020;87:763–772. 10.1002/mrd.23392
REFERENCES
- Cui, X. , Han, Z. , He, S. , Chen, T. , Shao, C. , Chen, D. , … Xiao, J. (2017). HIF1/2a mediates hypoxia‐induced LDHA expression in human pancreatic cancer cells. Ongotarget, 8(15), 24840–24852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, G. , Mao, Q. , Xia, W. , Xu, Y. , Wang, J. , Xu, L. , & Jiang, F. (2016). PKM2 and cancer: The function of PKM2 beyond glycolysis. Oncology Letters, 11(3), 1980–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner, D. K. , & Leese, H. J. (1987). Assessment of embryo viability before transfer by the noninvasive measurement of glucose uptake. Journal of Experimental Zoology, 242, 103–105. [DOI] [PubMed] [Google Scholar]
- Gardner, D. K. , Wale, P. L. , Collins, R. , & Lane, M. (2011). Glucose consumption of single post‐compaction human embryos is predictive of embryo sex and live birth outcome. Human Reproduction, 26(8), 1981–1986. [DOI] [PubMed] [Google Scholar]
- Hirsila, M. , Koivunen, P. , Leon, X. , Seeley, T. , Kivirikko, K. I. , & Myllyharju, J. (2005). Effect of desferrioxamine and metals on the hydroxylases in the oxygen sensing pathway. The FASEB Journal, 19(10), 10.1096/fj.04-3399fje [DOI] [PubMed] [Google Scholar]
- Hu, C. , Wang, L. , Chodosh, L. A. , Keith, B. , & Simon, M. C. (2003). Differential roles of hypoxia‐inducible factor 1a (HIF‐1a) and HIF‐2a in hypoxia gene regulation. Molecular and Cellular Biology, 23(24), 9361–9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. W. , Tchernyshyov, I. , Semenza, G. L. , & Dang, C. V. (2006). HIF‐1‐mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metabolism, 3(3), 177–185. [DOI] [PubMed] [Google Scholar]
- Krisher, R. , & Prather, R. S. (2012). A role for the Warburg Effect in preimplantation embryo development: Metabolic modification to support rapid cell proliferation. Molecular Reproduction and Development, 79(5), 311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, L. , & Prather, R. S. (2004). A method for producing cloned pigs by using somatic cells as donors. Germ Cell Protocols, 245, 149–163. [DOI] [PubMed] [Google Scholar]
- Lee, K. , Davis, A. , Zhang, L. , Ryu, J. , Spate, L. D. , Park, K. W. , … Prather, R. S. (2015). Pig oocyte activation using a Zn2+ chelator, TPEN. Theriogenology, 84(6), 1024–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, W. , Shen, S. , Zhoa, X. , & Chen, G. (2012). Targeted genes and interacting proteins of hypoxia inducible factor‐1. International Journal of Biochemistry and Molecular Biology, 3(2), 165–178. [PMC free article] [PubMed] [Google Scholar]
- Mordhorst, B. R. , Benne, J. A. , Cecil, R. F. , Whitworth, K. M. , Samuel, M. S. , Spate, L. D. , … Prather, R. S. (2019). Improvement of in vitro and early in utero porcine clone development after somatic donor cells are cultured under hypoxia. Molecular Reproduction and Development, 86(5), 558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordhorst, B. R. , Murphy, S. L. , Schauflinger, M. , Salazar, S. R. , Ji, T. , Behura, S. K. , … Prather, R. S. (2018). Porcine fetal‐derived fibroblasts alter gene expression and mitochondria to compensate for hypoxic stress during culture. Cellular Reprogramming, 20(4), 225–235. 10.1089/cell.2018.0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather, R. S. , Lorson, M. , Ross, J. W. , Whyte, J. J. , & Walters, E. (2013). Genetically engineered pig models for human diseases. Annual Review of Animal Biosciences, 1, 203–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigione, A. , Rohwer, N. , Hoffmann, S. , Mlody, B. , Drews, K. , Bukowiecki, R. , … Adjaye, J. (2014). HIF1α modulates cell fate reprogramming through early glycolytic shift and upregulation of PDK1‐3 and PKM2. Stem Cells, 32(2), 364–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redel, B. K. , Brown, A. N. , Spate, L. S. , Whitworth, K. M. , Green, J. A. , & Prather, R. S. (2011). Glycolysis in preimplantation development is partially controlled by the Warburg effect. Molecular Reproduction and Development, 79(4), 262–271. [DOI] [PubMed] [Google Scholar]
- Redel, B. K. , Spate, L. D. , Lee, K. , Mao, J. , Whitworth, K. M. , & Prather, R. S. (2016). Glycine supplementation in vitro enhances porcine preimplantation embryo cell number and decreases apoptosis but does not lead to live births. Molecular Reproduction and Development, 83(3), 246–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard, J. P. , Philippon, A. , & Menezo, Y. (1980). In‐vitro uptake of glucose by bovine blastocysts. Journal of Reproduction and Fertility, 58, 161–164. [DOI] [PubMed] [Google Scholar]
- Semenza, G. L. (2000). Expression of hypoxia‐inducible factor 1: Mechanisms and consequences. Biochemical Pharmacology, 59, 47–53. [DOI] [PubMed] [Google Scholar]
- Vander Heiden, M. G. , Locasale, J. W. , Swanson, K. D. , Sharfi, H. , Heffron, G. J. , Amador‐Noguez, D. , … Cantley, L. C. (2010). Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science, 329(5998), 1492–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth, K. , & Prather, R. S. (2010). Somatic cell nuclear transfer efficiency: How can it be improved through nuclear remodeling and reprogramming? Molecular Reproduction and Development, 77(12), 1001–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth, K. , & Prather, R. S. (2017). Gene editing as applied to prevention of reproductive porcine reproductive and respiratory syndrome. Molecular Reproduction and Development, 84(9), 926–933. [DOI] [PubMed] [Google Scholar]
- Whitworth, K. M. , Zhao, J. , Spate, L. D. , Li, R. , & Prather, R. S. (2011). Scriptaid corrects gene expression of a few aberrantly reprogrammed transcripts in nuclear transfer pig blastocyst stage embryos. Cellular Reprogramming, 13(3), 191–204. [DOI] [PubMed] [Google Scholar]
- Wilmut, I. , Schnieke, A. E. , McWhir, J. , Kind, A. J. , & Campbell, K. H. S. (1997). Viable offspring derived from fetal and adult mammalian cells. Nature, 385, 810–813. [DOI] [PubMed] [Google Scholar]
- Yuan, Y. , Spate, L. D. , Redel, B. K. , Tian, Y. , Zhou, J. , Prather, R. S. , & Roberts, R. M. (2017). Quadrupling efficiency in production of genetically modified pigs through improved oocyte maturation. Proceedings of the National Academy of Sciences, 114(29), E5796–E5804. 10.1073/pnas.1703998114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, J. , Ross, J. W. , Hao, Y. , Spate, L. D. , Walters, E. M. , Samuel, M. S. , … Prather, R. S. (2009). Significant improvement in cloning efficiency of an inbred miniature pig by histone deacetylase inhibitor treatment after somatic cell nuclear transfer. Biology of Reproduction, 81(3), 525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]


