Abstract
Background
Benign prostatic hyperplasia (BPH) etiology remains poorly understood, but chronic low‐grade inflammation plays a role. Pulsed electromagnetic field therapy (PEMF) (1‐50 Hz) is effective in reducing tissue inflammation.
Objectives
We designed a pilot study to evaluate the effects of PEMF on prostate volume (PV) in BPH.
Materials and Methods
This is a prospective interventional trial on 27 naive patients with BPH and lower urinary tract symptoms (LUTS). At baseline (V0), all patients had blood tests, transrectal ultrasound, and questionnaires (IPSS, IIEF‐15) and received a perineal PEMF device (Magcell®Microcirc, Physiomed Elektromedizin). PEMF was delivered on perineal area 5 minutes twice daily for 28 days, then (V1) all baseline evaluations were repeated. Afterward, nine patients continued therapy for 3 more months (PT group) and 15 discontinued (FU group). A 4‐month evaluation (V2) was performed in both groups.
Results
A reduction was observed both at V1 and at V2 in PV: PVV0 44.5 mL (38.0;61.6) vs PVV1 42.1 mL (33.7;61.5, P = .039) vs PVV2 41.7mL (32.7;62.8, P = .045). IPSS was reduced both at V1 and at V2: IPSSV0 11 (5.7;23.2) vs IPSSV1 10 (6;16, P = .045) vs IPSSV2 9 (6;14, P = .015). Baseline IPSS was related to IPSS reduction both at V1 (rs = 0.313;P = .003) and at V2 (rs = 0.664;P < .001). PV reduction in patients without metabolic syndrome (ΔPVV1nMetS −4.7 mL, 95%CI −7.3;‐2.0) was greater than in affected patients (ΔPVV1MetS 1.7 mL, 95%CI −2.69;6.1)(P = .017, Relative RiskMetS = 6). No changes were found in gonadal hormones or sexual function.
Discussion
PEMF was able to reduce PV after 28 days of therapy. Symptoms improved in a short time, with high compliance and no effects on hormonal and sexual function or any side effects. Patients with moderate‐severe LUTS and without MetS seem to benefit more from this treatment.
Conclusion
PEMF reduces PV and improves LUTS in a relative short time, in BPH patients. These benefits seem greater in those patients with moderate‐severe LUTS but without MetS.
Keywords: BPH, LUTS, PEMF, prostate volume, IPSS, inflammation
1. INTRODUCTION
Benign prostatic hyperplasia (BPH) is a prostate volume (PV) enlargement due to a non‐malignant cellular proliferation of the parenchyma and stroma of the gland, mainly in the transition area. BPH is a common age‐related pathology, often causing lower urinary tract symptoms (LUTS) due to compression of the urethra by the enlarged prostate, which reduces the quality of life of affected patients.1, 2
The underlying etiology is not completely understood yet. Risk factors include age, diabetes, cardiovascular disease, hypertension, and metabolic syndrome (MetS).3 Due to the wide expression of androgen receptors (AR), hormonal stimulation of prostate growth may play a role4: This is mainly due to dihydrotestosterone (DHT), an active metabolite with a higher affinity for the AR compared with testosterone. However, the most supported etiological hypothesis for BPH identifies inflammatory damage5, 6 as the trigger for subsequent fibrosis and tissue hypoxia resulting in structural changes in the prostate.7, 8 To confirm this, some histological studies have shown intraprostatic inflammatory infiltration in 43%‐98% of BPH tissues.9, 10 During inflammation, in fact, mitogen substances (cytokines, growth factors) are released, causing abnormal proliferation of prostatic cells and stroma11, 12 (Figure 1). The net result is the triggering of a vicious cycle of inflammation‐fibrosis‐hypoxia‐inflammation which in turn causes glandular remodeling, alteration of prostatic architecture, and adenoma's growth. This etiopathogenetic hypothesis represents the rationale of our study.
Figure 1.
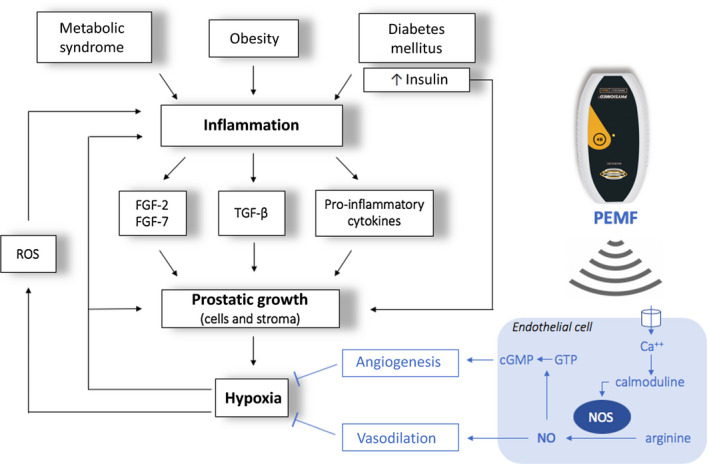
Inflammatory hypothesis underlying BPH pathogenesis and biophysical mechanism of PEMF efficacy: during inflammation mitogen substances such as cytokines (IL‐2, IL‐4, IL‐8, IL‐15, IL‐17, IFN‐γ) and growth factors (VEGF, TGF‐β, FGF‐2, FGF‐7) are released, resulting in an abnormal proliferation of prostatic cells and stroma. FGFs primarily stimulate fibroblasts to produce fibromuscular tissue and also stimulate angiogenesis, proliferation, and differentiation of stromal and epithelial prostatic cells. The TGF‐β stimulates the differentiation of smooth muscle cells and the development of abundant extracellular matrix. The overproduction of stroma strongly increases oxygen consumption, and it is therefore responsible for hypoxia in the transition zone of the prostate. Hypoxia itself activates several signaling pathways that regulate angiogenesis and tissue proliferation. Furthermore, local hypoxia promotes the release of reactive oxygen species (ROS) that, in turn, stimulate the release of growth factors (IL‐8, FGF‐7, TGF‐β, FGF‐2) and consequent glandular hyperplasia. The biophysical mechanism of PEMF efficacy is likely to involve an electrochemical model of the cell membrane: PEMF seems to increase intracellular calcium (Ca2+) binding to calmoduline. This bond activates the calmoduline pathway which catalyzed endothelial nitric oxide synthase isoform (eNOS), an enzyme responsible for the synthesis of nitric oxide (NO) and citrulline from L‐arginine and O2. NO activates an anti‐inflammatory response by recalling lymphocytes from the blood, and it also causes vasodilation with a consequent increase in local blood flow and reduction of hypoxia. Furthermore, NO regulates cGMP signaling cascades that promote angiogenesis and tissue remodeling. The overall effect is a reduction in tissue hypoxia and therefore a reduction in prostatic growth.6, 12, 16, 17
Pulsed electromagnetic field therapy (PEMF) consists of low‐frequency pulsed energy waves (1‐50 Hz)13 that have been employed for many therapeutic purposes mainly because of its anti‐inflammatory effect.14 Moreover, many studies have shown that it is a safe procedure, without side effects.15
The biophysical mechanism of PEMF efficacy is likely to involve an electrochemical model of the cell membrane16 with intracellular pathways that promote angiogenesis, vasodilatation, and tissue remodeling. The overall effect is reduction in tissue hypoxia17 (Figure 1).
Traditional BPH treatment, together with lifestyle changes,18 includes medical and surgical therapy.19, 20 However, they are both expensive21 and can have side effects22 (anejaculation, erectile dysfunction, surgery risks). These factors have led to a growing interest in alternative, non‐invasive procedures for BPH treatment. To date, two studies have used PEMF in BPH treatment, with different in‐office devices, study designs, and outcomes.23, 24
The aim of our study was to evaluate the efficacy of magnetotherapy on BPH using a patient‐applied handheld PEMF device: the main outcome measure was PV reduction after 28 consecutive days of PEMF therapy. Secondary outcomes were changed in PV after 4 months and changed in LUTS during treatment.
2. MATERIALS AND METHODS
2.1. Study population
This is a longitudinal, prospective, interventional pilot study performed in Policlinico Umberto I, Rome, Italy.
We selected 27 male Caucasian patients with diagnosis of BPH and/or referring LUTS among those who underwent an andrological examination from April to December 2018 in our Unit. All patients signed a written informed consent before enrollment.
Exclusion criteria were as follows: any medical treatment for LUTS, androgens, gonadotropins, or cortisone therapy; previous prostatic surgery; PSA values > 10 ng/mL,25 urogenital malformations, genetic syndromes, ongoing tumors, and autoimmune diseases; pacemakers and automatic implantable cardioverter defibrillators.26
2.2. Sample size
Sample size was calculated with the optimal two‐stage design27: the null hypothesis that P ≤ .35 versus the alternative that P ≥ .60 has an expected sample size of 16.04 and a probability of early termination of 0.609. If the therapy is not effective, there is a 0.046 probability of concluding that it is (the target for this value was 0.05). If the therapy is effective, there is a 0.195 probability of concluding that it is not (the target for this value was 0.20). After testing the therapy on nine patients in the first stage, the trial was supposed to be terminated if three or fewer respond. If the trial goes on to the second stage, a total of 27 patients should be studied. If the total number responding is less than or equal to 13, the therapy is rejected.
The first stage was completed in August 2018:six of the first nine patients reported a variable degree of response in terms of PV. Therefore, the second stage started in September 2018. Enrollment was completed in December 2018, and the study ended in April 2019.
2.3. Study design
The study was structured into three visits: (a) a screening visit for evaluation of inclusion and exclusion criteria, presentation of the protocol, and signature for informed consent; (b) a baseline visit (V0) with complete medical history, full physical exam, clinical questionnaires administration, blood tests, transrectal ultrasound (TRUS), handover to patient of PEMF device, and use instructions; (c) a visit after 28 days of PEMF therapy (V1) with same procedures of V0.
The primary outcome measure was the PV change at V1.
After the V1, three patients withdrew from the study for personal reasons, 9 patients were randomized to continue the PEMF up to 3 months (PT group), and 15 stopped the treatment (FU group). In order to evaluate possible time‐dependent effects, a further visit (V2) was then performed for both groups three months after V1, with same procedures.
2.4. Procedures
The device (Magcell® Microcirc, Physiomed Elektromedizin AG, Scnaittach, Germany, Figure 2), with a frequency of 4‐12 Hz and an intensity of 1000 Gauss, was provided to patients at V0. Precise use instructions were given to patients: the effective area was to be placed onto the perineal region without pressure. The device was to be kept in place for 5 minutes, twice daily (morning and evening) for 28 consecutive days. Patients were asked to complete a diary of performed administration of the PEMF. A reminder for each administration was completed by an automatic message sent to each patient's cell phone.
Figure 2.
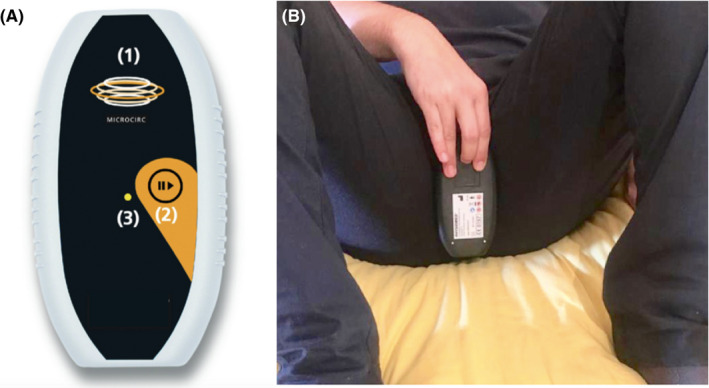
Magcell® Microcirc, Physiomed Elektromedizin AG, Scnaittach, Germany set on frequency of 4–12 Hz and on an intensity of 1000 Gauss. A, (1) effective area; (2) start button; (3) status LED. B, Correct position with the marked active surface placed on the perineal region
Medical history and physical examination (general physical examination, digital rectal exploration, anthropometric measures, blood pressure, and heart rate) were taken at V0.
Self‐administered questionnaires were provided to patients at each visit: (a) the International Prostate Symptom Score (IPSS),28 consisting of seven questions with scores from 0 to 35 (indicating mild, moderate, or severe symptoms with scores ranging, respectively, from 0 to 7, 8 to 19, or 20 to 35), (b) the International Index of Erectile Function‐15 (IIEF‐15) for sexual function (with scores ≤ 25 indicating the presence of erectile dysfunction). Regarding IPSS, question number 8 was also considered separately as an indicator of quality of life (IPSS‐QoL).29
Blood samples for full blood count, kidney function, inflammatory markers, lipid and glucose metabolism, and sexual hormones (gonadotropins, total testosterone, estradiol) were performed at each visit at 8.00 AM, in fasting state. PSA was measured at V0 and V2, but not at V1 for the short time frame occurring from the baseline procedures (DRE and TRUS) which could have been responsible for a high risk of false positives.30, 31
TRUS was performed by two expert operators (GF, VO) using a Philips IU22 units (Philips, Bothell, WA, USA) through a pre‐set transrectal 9.5 Mz end‐fire probe with patient in left and prone decubitus position. The same patient was examined by the same operator at each visit. PV was calculated using the ellipsoid formula.32
2.5. Statistical analysis
Outcome measurements were assessed for normality using the Shapiro‐Wilk test, and non‐parametric tests were used when violations of parametric test assumptions were evident. Values are then expressed as median and interquartile range (IQR). A Wilcoxon signed‐rank test was performed to compare the effects of treatment at different timepoint evaluations (V0 vs V1 and V2). The Mann‐Whitney U test was used to determine whether there were differences between the change over time (delta, Δ) in the two treatment groups. An ANCOVA model was used to determine the effects of the treatment on changes in PV and IPSS among the different timepoints (V0‐V1‐V2), after controlling for baseline values of any dependent variable. A Spearman's rank order correlation was run for baseline univariate correlations.
A first stratification of the cohort was performed based on the severity of LUTS defined as absence or mild symptoms (IPSS < 8, Group 1) or moderate‐severe symptoms (IPSS ≥ 8, Group 2). A second stratification was carried out based on the presence or absence of MetS.
A one‐way ANOVA was conducted to determine whether there were differences in the ΔIPSS between Group 1 and Group 2 and differences in PV between patients with or without MetS. A two‐way ANOVA was conducted to examine the mixed effects of treatment duration (PT/FU) and severity of LUTS (Group 1/Group 2) on IPSS changes.
A relative risk was finally calculated considering the presence or absence of MetS and the treatment response, where responders were defined as patients having a reduction in PV higher than the median of the respective visit (ΔPVV1 = PV1‐PV0, ΔPVV2 = PV2‐PV0). A P‐value < 0.05 was considered statistically significant. Statistical analyses were performed with SPSS Statistics version 25.0 (IBM SPSS Statistics Inc, Chicago, IL, USA).
The protocol has been conducted in accordance with the Declaration of Helsinki and was approved by the internal Ethics Committee of Policlinico Umberto I in Rome (approval number 4906, 31st January 2018).
3. RESULTS
3.1. Study population
A total of 30 patients with diagnosis of BPH and/or complaining of LUTS underwent the screening visit from April to December 2018. Three patients were excluded due to suspicious prostatic lesions (n = 2) and intravesical polyp (n = 1) at V0. Histology confirmed diagnosis of prostatic adenocarcinomas and bladder urothelial carcinoma.
Therefore, 27 patients were enrolled. Median age was 67 years (59;70). Full blood count, kidney function, and lipid and glucose metabolism were within normal limits. PSA median value was 1.9 ng/dL (0.7;3.6), PV was 44.5 mL (38.0;61.6), and IPSS was 11 (6;23) (Table 1).
Table 1.
Characteristic of study population. Comparison between patients (n = 27) before (V0) and after 28 days of therapy (V1). Values are expressed in median (IQR). Wilcoxon test P‐value reported (*P < .05). IPSS‐QoL corresponds to IPSS question number 8. IIEF‐15 domains
| V0 (n = 27) | V1 (n = 27) | P | |
|---|---|---|---|
| Age (years) | 67 (59;70) | – | – |
| BMI (kg/m2) | 25.4 (24.7;28.9) | – | – |
| Ultrasound | |||
| PV (mL) | 44.5 (38.0;61.6) | 42.1 (33.8;61.5) | 0.039* |
| Adenome volume (mL) | 16.7 (12.0;27.3) | 14.3 (10.1;24.0) | 0.089 |
| LUTS questionnaire | |||
| IPSS | 11.0 (5.8;23.2) | 10.0 (6.0;16.0) | 0.045* |
| IPSS‐QoL | 3.0 (1.0;3.2) | 2.0 (1.0;3.) | 0.018* |
| Sexual function questionnaire (IIEF‐15) | |||
| EF | 27.0 (15.0;28.0) | 27.0 (12.0;28.0) | 0.417 |
| IS | 9.0 (7.0;12.0) | 10.0 (5.0;12.0) | 0.554 |
| SD | 7.0 (6.0;9.0) | 8.0 (6.0;8.0) | 0.551 |
| OF | 10.0 (6.0;10.0) | 10.0 (9.0;10.0) | 0.152 |
| OS | 8.0 (4.0;8.0) | 8.0 (6.0;8.0) | 0.542 |
| Hormones | |||
| FSH (mUI/mL) | 7.1 (4.2;12.2) | 6.5 (4.4;11.0) | 0.583 |
| LH (mUI/mL) | 3.4 (2.3;5.9) | 3.0 (2.1;7.1) | 0.075 |
| Testosterone (nmol/L) | 16.0 (13.0;20.3) | 15.6 (12.6;21.1) | 0.738 |
| Estradiol (pg/mL) | 23.8 (18.9;34.1) | 21.5 (17.6;25.7) | 0.073 |
| Lipid and glucose metabolism | |||
| Glycemia (mg/dL) | 97.0 (90.0;108.0) | 102.0 (94.0;113.0) | 0.989 |
| HbA1c (%) | 5.5 (5.2;6.1) | 5.7 (5.2;6.0) | 0.092 |
| Total cholesterol (mg/dL) | 183.0 (149.0;196.0) | 179.0 (155.2;208.0) | 0.109 |
| HDL (mg/dL) | 48.0 (42.0;64.0) | 49.0 (41.9;63.7) | 0.909 |
| LDL (mg/dL) | 98.0 (78.0;112.0) | 99.0 (82.7;124.2) | 0.106 |
| Triglycerides (mg/dL) | 110.0 (81.0;135.0) | 108.0 (72.0;165.5) | 0.611 |
| Kidney function | |||
| Creatinine (mg/dL) | 1.0 (0.8;1.2) | 0.9 (0.9;1.1) | 0.274 |
| Urea (mg/dL) | 36.0 (32;41.4) | 38.9 (31.9;46.7) | 0.679 |
| Inflammation markers | |||
| WBCs (×109/L) | 6.7 (5.2;8.2) | 6.3 (5.4;8.1) | 0.755 |
| Neutrophils (×109/L) | 3.7 (2.9;4.7) | 3.8 (2.9;4.5) | 0.719 |
| Lymphocytes (×109/L) | 1.9 (1.4;2.3) | 1.9 (1.4;2.2) | 0.943 |
| ESR (mm/h) | 9.0 (3.5;15.0) | 6.0 (4.0;10.0) | 0.088 |
| CRP (μg/L) | 1600 (600;2500) | 1400 (500;2025) | 0.078 |
| Fibrinogen (g/L) | 3.1 (2.6;3.3) | 2.9 (2.6;3.3) | 0.548 |
| PSA (ng/mL) | 1.9 (0.7;3.6) | – | – |
Abbreviations: EF, erectile function; IS, intercourse satisfaction; OF, orgasmic function; OS, overall satisfaction; SD, sexual desire.
Excellent compliance was observed: all patients used the device properly and attended V1. No patient showed signs of discomfort, local, or systemic adverse effects through the trial.
3.2. Primary outcome measure
A significant reduction in PV was observed from V0 to V1: PVV0 44.5 mL (38.0;61.6) vs PVV1 42.1 mL (33.7;61.5), median difference (ΔPVV1) −1.0 mL (−6.0;0.9), P = .039 (Table 1).
3.3. Secondary outcome measures
Similarly, IPSS was significantly reduced at V1: IPSSV0 11 (5.7;23.2) vs IPSSV1 10 (6;16), P = .045. IPSS‐QoL also significantly improved at V1: IPSS‐QoLV0 3 (1;3.25) vs IPSSV1 1 (1;3), P = .018 (Table 1).
A reduction in total PV was also observed in V2 compared to V0: PVV0 44.5 mL (38.0;61.6) vs PVV2 41.7 mL (32.7;62.8), median difference (ΔPVV2) −0.4 mL (−3.4;3.4), P = .045. A parallel reduction of symptoms was also observed: IPSSV0 11 (6;23) vs IPSSV2 9 (6;14), P = .015; IPSS‐QoLV0 3 (1;3.25) vs IPSSV2 1 (1;2.75), P = .018 (Table 2).
Table 2.
Characteristic of study population. Comparison of patient measurements at baseline (V0, n = 27) and after 4 months (V2, n = 24). Values are expressed in median (IQR). Wilcoxon test P‐value reported (*P < .05). IPSS‐QoL corresponds to IPSS question number 8. IIEF‐15 domains
| V0 (n = 27) | V2 (n = 24) | P | |
|---|---|---|---|
| Ultrasound | |||
| PV (mL) | 44.5 (38.0;61.6) | 41.7 (32.7;62.8) | 0.045* |
| Adenome volume (mL) | 16.7 (12.0;27.3) | 13.3 (10.6;24.5) | 0.224 |
| LUTS questionnaire | |||
| IPSS | 11.0 (5.7;3.2) | 9.0 (6.0;14.0) | 0.015* |
| IPSS‐QoL | 3.0 (1.0;3.25) | 1.0 (1.0;2.75) | 0.018* |
| Sexual function questionnaire (IIEF‐15) | |||
| EF | 27.0 (15.0;28.0) | 26.0 (17.7;29.0) | 0.694 |
| IS | 9.0 (7.0;12.0) | 10.0 (9.0;12.0) | 0.561 |
| SD | 7.0 (6.0;9.0) | 8.0 (7.0;8.0) | 0.235 |
| OF | 10.0 (6.0;10.0) | 10.0 (7.2;10.0) | 0.362 |
| OS | 8.0 (4.0;8.0) | 8.0 (6.0;10.0) | 0.179 |
| Hormones | |||
| FSH (mUI/mL) | 7.1 (4.2;12.2) | 6.9 (4.72;11.75) | 0.148 |
| LH (mUI/mL) | 3.4 (2.3;5.9) | 4.2 (2.9;6.1) | 0.498 |
| Testosterone (nmol/L) | 16.0 (13.0;20.3) | 15.2 (13.3;18.7) | 0.205 |
| Estradiol (pg/mL) | 25.0 (20.0;35.0) | 20.0 (16.7;22.5) | 0.172 |
| Lipid and glucose metabolism | |||
| Glycemia (mg/dL) | 97.0 (90.0;108.0) | 95.4 (90;106) | 0.126 |
| HbA1c (%) | 5.5 (5.2;6.1) | 5.5 (5.3;5.9) | 0.189 |
| Total cholesterol (mg/dL) | 183.0 (149.0;196.0) | 180.4 (159.7;209.6) | 0.137 |
| HDL (mg/dL) | 48.0 (42.0;64.0) | 50.3 (43.8;59.0) | 0.568 |
| LDL (mg/dL) | 98.0 (78.0;112.0) | 100.5 (85.4;129.9) | 0.137 |
| Triglycerides (mg/dL) | 110.0 (81.0;135.0) | 92.08 (71.7;156.0) | 0.909 |
| Kidney function | |||
| Creatinine (mg/dL) | 1.0 (0.8;1.2) | 1.0 (0.9;1.2) | 0.123 |
| Urea (mg/dL) | 36.0 (32;41.4) | 36.6 (30.3;42.6) | 0.068 |
| Inflammation markers | |||
| WBCs (×109/L) | 6.7 (5.2;8.2) | 7.0 (5.4;7.9) | 0.784 |
| Neutrophils (×109/L) | 3.7 (2.9;4.7) | 3.8 (3;4.9) | 0.403 |
| Lymphocytes (×109/L) | 1.9 (1.4;2.2) | 1.8 (1.1;2.3) | 0.553 |
| ESR (mm/h) | 9.0 (3.5;15) | 5.0 (3;9.7) | 0.132 |
| CRP (μg/L) | 1600 (600;2500) | 1300 (600;1875) | 0.721 |
| Fibrinogen (g/L) | 3.1 (2.6;3.3) | 3.0 (2.5;3.5) | 0.247 |
| PSA (ng/mL) | 1.9 (0.7;3.6) | 2.3 (0.9;4.7) | 0.366 |
Abbreviations: EF, erectile function; IS, intercourse satisfaction; OF, orgasmic function; OS, overall satisfaction; SD, sexual desire.
Interestingly, when comparing FU group and PT group at V2 no differences were found between the groups in terms of PV, IPSS, IPSS‐QoL, or other outcome measures (Table 3).
Table 3.
Characteristic of study population at V2 (n = 24). Comparison between patients who suspended therapy after 1 month (FU group, n = 15) and patients who continued therapy for other 3 months (PT group). Values are expressed in median (IQR). Mann‐Whitney test P‐value reported (*P < .05). IPSS‐QoL corresponds to IPSS question number 8. IIEF‐15 domains
| FU group (n = 15) | PT group (n = 9) | P | |
|---|---|---|---|
| Ultrasound | |||
| PV (mL) | 41.3 (31.6;62.8) | 42.0 (34.3;70.1) | 0.640 |
| Adenome volume (mL) | 11.6 (8.9;23.6) | 13.3 (12.5;38.0) | 0.108 |
| LUTS questionnaire | |||
| IPSS | 9.0 (6.0;14.0) | 8.0 (6.0;14.5) | 0.770 |
| IPSS‐QoL | 2.0 (1.0;3.0) | 1.0 (1.0;2.0) | 0.446 |
| Sexual function questionnaire (IIEF‐15) | |||
| EF | 28.0 (23.0;30.0) | 23.0 (15.0;27.0) | 0.073 |
| IS | 10.0 (9.0;12.0) | 10.0 (4.5;12.5) | 0.815 |
| SD | 8.0 (7.0;9.0) | 7.0 (6.0;8.0) | 0.123 |
| OF | 9.0 (6.0;10.0) | 10.0 (9.0;11.0) | 0.084 |
| OS | 8.0 (4.0;10.0) | 8.0 (6.0;9.0) | 0.861 |
| Hormones | |||
| FSH (mUI/mL) | 8.7 (5.2;14.0) | 5.0 (4.4;9.0) | 0.174 |
| LH (mUI/mL) | 5.3 (3.5;7.2) | 3.3 (2.7;5.0) | 0.104 |
| Testosterone (nmol/L) | 16.1 (13.4;21.7) | 14.2 (10.9;16.4) | 0.121 |
| Estradiol (pg/mL) | 20.6 (16.6;23.9) | 20.5 (16.4;28.6) | 0.097 |
| Lipid and glucose metabolism | |||
| Glycemia (mg/dL) | 102.6 (90.0;113.4) | 95.4 (90.4;103.5) | 0.392 |
| HbA1c (%) | 5.6 (5.3;6.3) | 5.5 (5.3;5.7) | 0.558 |
| Total cholesterol (mg/dL) | 174.4 (158.5; 224.3) | 182.5 (146.4;204.2) | 0.682 |
| HDL (mg/dL) | 49.1 (43.3;57.2) | 52.2 (44.3; 63.8) | 0.411 |
| LDL (mg/dL) | 110.5 (85.1;132.6) | 96.7 (78.3;127.2) | 0.599 |
| Triglycerides (mg/dL) | 95.6 (83.2; 157.6) | 81.4 (66.4;152.7) | 0.318 |
| Kidney function | |||
| Creatinine (mg/dL) | 0.9 (0.9;1.2) | 1.0 (0.9;1.2) | 0.861 |
| Urea (mg/dL) | 33.0 (30.0;46.8) | 39.0 (30.6;41.7) | 0.815 |
| Inflammation markers | |||
| WBCs (×109/L) | 7.0 (5.4;8.5) | 7.0 (4.6;7.7) | 0.548 |
| Neutrophils (×109/L) | 4.0 (3.0;4.9) | 3.7 (2.9;5.0) | 0.925 |
| Lymphocytes (×109/L) | 1.8 (1.5;2.3) | 1.8 (1.2;2.6) | 0.875 |
| ESR (mm/h) | 7.0 (4.0‐10.0) | 3.0 (2.5;7.5) | 0.155 |
| CRP (μg/L) | 1500 (600‐2300) | 800 (600‐1700) | 0.446 |
| Fibrinogen (g/L) | 3.1 (2.9‐3.6) | 2.6 (2.5;3.3) | 0.155 |
| PSA (ng/mL) | 2.1 (0.9;3.2) | 4.9 (0.9;7.2) | 0.165 |
Abbreviations: EF, erectile function; IS, intercourse satisfaction; OF, orgasmic function; OS, overall satisfaction; SD, sexual desire.
When compared to the baseline assessments, no changes were found in PSA values at V2 and in all the other variables (adenoma volume, inflammation markers, glucometabolic test, kidney function, hormonal profile, or sexual function index) both at V1 (Table 1) and at V2 (Table 2).
An ANCOVA test was performed in order to evaluate whether the treatment duration (FU vs PT) could have different impact on PV or IPSS variations (ΔPV, ΔIPSS): no differences were found both in PV (P = .339) and IPSS (P = .295) (Table 4).
Table 4.
ANCOVA models for comparisons of group with different time of therapies (FU group = 1 month vs PT group = 4 months) as fixed factor and basal PV and basal IPSS as covariates, respectively. Values represent the estimated marginal medians (lower‐upper limit of 95% CI)
| FU group (n = 9) | PT group (n = 15) | P | |
|---|---|---|---|
| ΔPVV2‐V0 (mL) | 0.9 (−2.8;5.0) | −2.4 (−6.8;1.7) | 0.339 |
| ΔIPSSV2‐V0 | −1 (−7.2;2.5) | −3 (−11; −1.5) | 0.295 |
In order to identify any correlation between ΔPV and ΔIPSS both at V1 and at V2, a univariate analysis was performed: no correlations were found for ΔPV, whereas a moderate and strong correlation was found between baseline IPSS and ΔIPSSV1 (r s = 0.540; P = .004) or ΔIPSSV2 (r s = 0.800; P < .001), respectively.
Stratification by severity of symptoms resulted in 10 patients in Group 1 (IPSS < 8) and 17 patients in Group 2 (IPSS ≥ 8). Consistent with previous results, patients with higher scores (and therefore worse symptoms) had a higher reduction of IPSS both at V1 (ΔIPSSGroup1 1.3, 95% CI −1.9;4.5 vs ΔIPSSGroup2 −4.1, 95% CI −6.5; −1.8; P = .009) and at V2 (ΔIPSSGroup1 2.0, 95% CI −2.9;6.9 vs ΔIPSSGroup2 −6.7, 95% CI −9.9; −3.5; P = .006). No differences in ΔIPSS were found when comparing the two treatment timings (FU vs PT) between Group 1 and Group 2 (P = .886).
To evaluate possible effects of MetS on treatment success, the same analysis was performed on affected (MetS, n = 7) vs non‐affected (nMetS, n = 19) patients. A reduction was found in PVV1 only for nMetS patients (ΔPVV1MetS 1.7 mL, 95% CI −2.69;6.1 vs ΔPVV1nMetS −4.7 mL, 95% CI −7.3;‐2.0; P = .017) (Figure 3), giving MetS patients a relative risk of non‐response to therapy of 6.0 (95% CI 0.8;43.1, P = .07) (Table 5).
Figure 3.
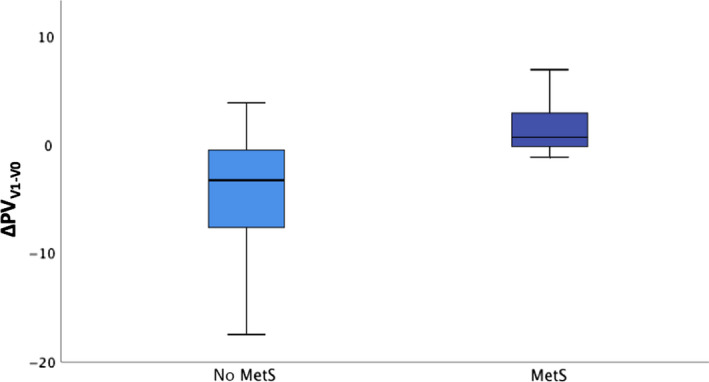
PV reduction (Δ) at V1 in patients without (no MetS) and with metabolic syndrome (MetS) (P = .017). Colored boxes indicate interquartile range (IQR), and center vertical lines indicate median
Table 5.
Relation between MetS and PV response to therapy. Values in the table represent the number of patients and percentages in parentheses. Responders were defined as patients having a reduction in PV higher than the median delta at the respective visit (ΔPVV1 = PV1‐PV0, ΔPVV2 = PV2‐PV0). Relative risk 6.0 (95% CI 0.8;43.1) is higher in MetS patients
| MetS (n = 7) | nMetS (n = 19) | |
|---|---|---|
| PV responders | 1 (14.3) | 12 (63.2) |
| PV non‐responders | 6 (85.7) | 7 (36.8) |
Relative risk 6.0 (95% CI 0.8;43.1, P = .07).
No correlations with response to treatment were found regarding age, smoking habit, obesity, diabetes, or hypertension.
4. DISCUSSION
Our study confirms that a handheld PEMF device is able to reduce PV and IPSS in patients affected by BPH. The effects were already significant after one month of therapy and were sustained even after discontinuation, particularly in patients with moderate‐severe disease and without metabolic derangement.
According to EAU guidelines,1 the current standard therapy for moderate‐to‐severe LUTS/BPH is represented by α‐blockers (AB) and 5α‐reductase inhibitors (5ARI), as monotherapy or in combination. Two large randomized trials33, 34 and a recent meta‐analysis35 demonstrated that, when compared to placebo, the use of these drugs, alone and even more in combination, is able to reduce clinical BPH progression. The exponential efficacy of combined treatment depends on the different mechanism of action of these drugs. ABs improve LUTS providing prostate and bladder neck muscles relaxation, resulting in increased urine flow. 5ARIs, instead, reduce prostate (but not stromal) volume through prostate epithelium cell apoptosis by the inhibition of peripheral testosterone conversion in DHT.
However, despite their proved clinical efficacy, ABs and 5ARIs do not target one of the main triggers for BPH: the prostatic inflammatory infiltrate and consequent fibrosis.5 This has been recently shown to be an independent risk factor for BPH progression, even in patients under combined therapy.36
In this regard, PEMFs therapy could play an important role adding an anti‐inflammatory effect on top of the mentioned pharmacological outcomes. In particular, a pre‐clinical study demonstrated the effectiveness of PEMF therapy in reducing PV in dogs affected by BPH.37 To the best of our knowledge, only two human studies have used PEMF in the treatment of BPH.23, 24 So far, different devices have been used for PEMFs therapy, tailoring treatment duration according to tissue‐specific conductivity and field strengths produced by the device used. In this context, our device was selected taking into account its specific technical features.38
Giannakopoulos et al24 evaluated PEMFs against α‐blockers (AB), demonstrating a reduction of IPSS together with PV in patients treated with electromagnetic waves. However, one of the limitations of this study was the difference in basal PV among the treatment groups: the PEMF group's PV was lower than the minimum threshold (40 mL) needed to justify a first‐line medical treatment prescription, according to EAU Guidelines.1 In our cohort, the baseline median PV was 44.5 mL. Elgohary and Tantawy23 also evaluated PEMF treatment, alone or in combination with pelvic floor exercises, compared to placebo. PEMF effects resulted in a reduction of IPSS and post‐urination residue together with increased urinary flow. No evaluation of PV was performed in this study.
Confirming these results, our analysis demonstrated a median PV reduction of 5.4% after one month of PEMF treatment, accompanied by IPSS and QoL improvement both at V1 and at V2.
We need to acknowledge that V2 data include both patients who continued therapy (PT group) and those who stopped after one month (FU group). However, no differences were found between the two groups in terms of PV and IPSS reduction. We therefore could speculate that those PEMFs effects, achieved shortly after one month, are independent from treatment duration, being maintained also over time. This finding can be affected by the small sample size and should be confirmed in larger cohorts.
PSA values did not change throughout the study. However, the values showed a tendency toward increase in PT group, even if not statistically significant. If in the one hand this could simply be due to the small sample size, on the other hand this finding could be judged as an increase secondary to tissue remodeling during PEMFs’ therapy. Larger cohort and longer follow‐up evaluation are needed to confirm these data.
Notably, IPSS improvement is not associated with adenoma volume reduction, which is likely to be responsible for BPH symptoms. However, as previously mentioned, there is recent evidence supporting the finding that symptoms improvement is strongly related to the reduction of chronic low‐grade inflammation in glandular parenchyma besides adenoma volume itself.5, 6, 8, 39, 40, 41 This is confirmed also by Serenoa repens efficacy studies42 where the direct anti‐inflammatory effect represented a further potential advantage to improve storage and voiding LUTS, regardless of PV reduction.
Bearing all these evidences in mind, it is necessary to identify those patients who are more inclined to benefit from this treatment, in order to plan a tailored therapy. Confirming the hypothesis that more severe symptoms would be more prone to improve after PEMFs therapy, our results showed that patients with moderate‐severe grade LUTS are more likely to respond, as the greater improvement measured in our cohort was in patients with IPSS ≥ 8 compared to those with mild symptoms at baseline.
In addition, the metabolic profile should also be evaluated in the treatment choice. In fact, MetS was a negative prognostic factor regarding the response to treatment in our patients: being affected by MetS gave 6‐times greater risk of not responding to therapy. In line with this result, a greater reduction in PV was measured in nMetS patients. This result has already been reported in literature in the evaluation of BPH response to traditional medical treatment.43 A possible explanation involves MetS as a chronic systemic inflammatory state, which represents continuous stimulation of glandular proliferation, and therefore reduces the efficacy of a localized and temporary anti‐inflammatory treatment. Therefore, in these patients, a preliminary treatment aimed to improve metabolic control could ensure higher therapeutic efficacy.
Electromagnetic waves have been widely demonstrated to be safe and side effect‐free. No local or systemic adverse effects were reported through the trial, and both sexual function and gonadal hormonal profile remained unchanged throughout the study. In this context, AB and 5ARI have been reported to be safe and effective but not free from side effects (such as dizziness, orthostatic hypotension, increased fall risk, erectile dysfunction, ejaculation disorders, reduction of sexual desire) that may reduce quality of life and, consequently, patient adherence to therapies. Furthermore, 5ARI has been very recently associated with a modest increase in development of type 2 diabetes,44 worsening the metabolic condition and therefore, probably, prostatic inflammation.
Our study also showed a good compliance without patients' discomfort. The device used was small, portable, and easy to apply at home by the patient himself. In the previously mentioned studies,23, 24 both of the devices required hospital admission and administration by healthcare professionals with longer daily treatment duration (30 minutes in‐office application 5 days/week).
In summary, if confirmed in larger trials, PEMF may represent a safe and relatively inexpensive add‐on procedure to medical treatment, which can be very useful mainly in elderly men with multimorbidity and consequent polypharmacy.45 However, the improvement we obtained using PEMF was still relatively small when compared to medical treatment or surgery. In this context, further trials aiming to compare the long‐term effect of PEMF vs medical therapy in larger cohorts are warranted to better understand the utility of PEMF in clinical management of BPH.
Our study did have limitations: this was a pilot study on a very small sample size and without a control group. This may limit the interpretation of results. Randomized controlled studies with a larger cohort are certainly needed to confirm our results. Finally, it is critical to confirm PEMF action on the prostate, identifying molecular pathways and specific prostatic inflammation markers involved in the damage that can be modulated with PEMF therapy.
5. CONCLUSIONS
The present trial represented the first attempt to use a portable 4‐12Hz PEMF device for BPH therapy. PEMF was able to reduce PV after 28 consecutive days of therapy.
Our study reported that PEMF provided a highly compliant, safe, side effect‐free therapy which resulted in the reduction of PV and improvement of symptoms in a short time with no side effects in hormonal and sexual function. Patients with moderate‐to‐severe LUTS and without MetS appear to be the most likely to benefit from this treatment.
Although results should be confirmed, PEMF could represent an effective, short‐term, non‐pharmacological add‐on therapy for BPH and LUTS in order to improve therapeutic outcomes. Larger randomized clinical trials are needed to confirm these findings and to identify more accurate predictive factors of treatment response.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest regarding the publication of this article.
AUTHORS' CONTRIBUTIONS
MT and MGT involved in conception, design, and coordination of the study, acquisition, analysis and interpretation of data, draft of the article and critical revision for important intellectual content. PM, LL, and FS involved in acquisition of data, patient's enrollment and follow‐up. CP involved in acquisition of data, analysis, interpretation, and critical revision of data. GF and VO involved in acquisition of data and US performance. FN involved in patient's enrollment, critical revision of the article for important intellectual content, and final approval of the version to be published. DG involved in conception and design, acquisition of data, and interpretation and critical revision of data, and final approval of the version to be published. AL involved in critical revision of the article for important intellectual content and final approval of the version to be published. AMI involved in conception and design, acquisition, analysis, and interpretation of data, draft of the article, critical revision for important intellectual content, and final approval of the version to be published. RP involved in acquisition, analysis and interpretation of data draft of the article and critical revision for important intellectual content, and final approval of the version to be published.
ACKNOWLEDGMENTS
The authors are deeply grateful to Parsemus Foundation for financial assistance, and interest in this study. Particularly, the authors want to sincerely acknowledge Linda Brent for study continuous support, and language revision. The authors are also grateful to Physiomed Elektromedizin AG, Germany for supplying Magcell® Microcirc and for technical support. Finally, authors wish to express their sincere thanks to all patients participating in the study.
Tenuta M, Tarsitano MG, Mazzotta P, et al. Therapeutic use of pulsed electromagnetic field therapy reduces prostate volume and lower urinary tract symptoms in benign prostatic hyperplasia. Andrology. 2020;8:1076–1085. 10.1111/andr.12775
Marta Tenuta and Maria Grazia Tarsitano equally contributed to this study.
Funding information
This study was funded by Amico Andrologo Onlus, Italy through a contribution by the Parsemus Foundation, San Francisco, California, USA; "Sapienza" University of Rome, Italy. Open‐access publication of this manuscript was funded by the Parsemus Foundation.
REFERENCES
- 1. Gratzke C, Bachmann A, Descazeaud A, et al. EAU Guidelines on the assessment of non‐neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol. 2015;67:1099‐1109. [DOI] [PubMed] [Google Scholar]
- 2. Gravas S. Prostate volume as a risk factor for lower urinary tract symptoms: the quest continues. Eur Urol. 2016;69:892‐893. [DOI] [PubMed] [Google Scholar]
- 3. Ngai HY, Yuen KS, Ng CM, Cheng CH, Chu SP. Metabolic syndrome and benign prostatic hyperplasia: an update. Asian J Urol. 2017;4:164‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gianfrilli D, Pierotti S, Pofi R, Leonardo C, Ciccariello M, Barbagallo F. Sex steroid metabolism in benign and malignant intact prostate biopsies: individual profiling of prostate intracrinology. Biomed Res Int. 2014;2014:464869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ficarra V, Rossanese M, Zazzara M, et al. The role of inflammation in lower urinary tract symptoms (LUTS) due to benign prostatic hyperplasia (BPH) and its potential impact on medical therapy. Curr Urol Rep. 2014;15:463. [DOI] [PubMed] [Google Scholar]
- 6. Gandaglia G, Briganti A, Gontero P, et al. The role of chronic prostatic inflammation in the pathogenesis and progression of benign prostatic hyperplasia (BPH). BJU Int. 2013;112:432‐441. [DOI] [PubMed] [Google Scholar]
- 7. Gacci M, Sebastianelli A, Salvi M, et al. Benign prostatic enlargement can be influenced by metabolic profile: results of a multicenter prospective study. BMC Urol. 2017;17:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mishra VC, Allen DJ, Nicolaou C, et al. Does intraprostatic inflammation have a role in the pathogenesis and progression of benign prostatic hyperplasia? BJU Int. 2007;100:327‐331. [DOI] [PubMed] [Google Scholar]
- 9. Kohnen PW, Drach GW. Patterns of inflammation in prostatic hyperplasia: a histologic and bacteriologic study. J Urol. 1979;121:755‐760. [DOI] [PubMed] [Google Scholar]
- 10. Di Silverio F, Gentile V, De Matteis A, et al. Distribution of inflammation, pre‐malignant lesions, incidental carcinoma in histologically confirmed benign prostatic hyperplasia: a retrospective analysis. Eur Urol. 2003;43:164‐175. [DOI] [PubMed] [Google Scholar]
- 11. Berger AP, Kofler K, Bektic J, et al. Increased growth factor production in a human prostatic stromal cell culture model caused by hypoxia. Prostate. 2003;57:57‐65. [DOI] [PubMed] [Google Scholar]
- 12. Ropiquet F, Giri D, Lamb DJ, Ittmann M. FGF7 and FGF2 are increased in benign prostatic hyperplasia and are associated with increased proliferation. J Urol. 1999;162:595‐599. [PubMed] [Google Scholar]
- 13. Frey AH. Differential biologic effects of pulsed and continuous electromagnetic fields and mechanisms of effect. Ann N Y Acad Sci. 1974;238:273‐279. [DOI] [PubMed] [Google Scholar]
- 14. Markov MS. Expanding use of pulsed electromagnetic field therapies. Electromagn Biol Med. 2007;26:257‐274. [DOI] [PubMed] [Google Scholar]
- 15. Hug K, Roosli M. Therapeutic effects of whole‐body devices applying pulsed electromagnetic fields (PEMF): a systematic literature review. Bioelectromagnetics. 2012;33:95‐105. [DOI] [PubMed] [Google Scholar]
- 16. Pilla AA, Muehsam DJ, Markov MS, Sisken BF. EMF signals and ion/ligand binding kinetics: prediction of bioeffective waveform parameters. Bioelectrochem Bioenerg. 1999;48:27‐34. [DOI] [PubMed] [Google Scholar]
- 17. Strauch B, Herman C, Dabb R, Ignarro LJ, Pilla AA. Evidence‐based use of pulsed electromagnetic field therapy in clinical plastic surgery. Aesthet Surg J. 2009;29:135‐143. [DOI] [PubMed] [Google Scholar]
- 18. Yap TL, Brown C, Cromwell DA, van der Meulen J, Emberton M. The impact of self‐management of lower urinary tract symptoms on frequency‐volume chart measures. BJU Int. 2009;104:1104‐1108. [DOI] [PubMed] [Google Scholar]
- 19. Roehrborn CG, Siami P, Barkin J, et al. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4‐year results from the CombAT study. Eur Urol. 2010;57:123‐131. [DOI] [PubMed] [Google Scholar]
- 20. Sterling J, Farber N, Gupta NK. Comparing outcomes of medical management and minimally invasive surgical techniques for lower urinary tract symptoms due to BPH. Curr Urol Rep. 2019;20:29. [DOI] [PubMed] [Google Scholar]
- 21. DeWitt‐Foy ME, Gill BC, Ulchaker JC. Cost comparison of benign prostatic hyperplasia treatment options. Curr Urol Rep. 2019;20:45. [DOI] [PubMed] [Google Scholar]
- 22. Borchert A, Leavitt DA. A review of male sexual health and dysfunction following surgical treatment for benign prostatic hyperplasia and lower urinary tract symptoms. Curr Urol Rep. 2018;19:66. [DOI] [PubMed] [Google Scholar]
- 23. Elgohary HM, Tantawy SA. Pulsed electromagnetic field with or without exercise therapy in the treatment of benign prostatic hyperplasia. J Phys Ther Sci. 2017;29:1305‐1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Giannakopoulos XK, Giotis C, Karkabounas S, et al. Effects of pulsed electromagnetic fields on benign prostate hyperplasia. Int Urol Nephrol. 2011;43:955‐960. [DOI] [PubMed] [Google Scholar]
- 25. Mottet N, Bellmunt J, Bolla M, et al. EAU‐ESTRO‐SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71:618‐629. [DOI] [PubMed] [Google Scholar]
- 26. Gwechenberger M, Rauscha F, Stix G, Schmid G, Strametz‐Juranek J. Interference of programmed electromagnetic stimulation with pacemakers and automatic implantable cardioverter defibrillators. Bioelectromagnetics. 2006;27:365‐377. [DOI] [PubMed] [Google Scholar]
- 27. Simon R. Optimal two‐stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1‐10. [DOI] [PubMed] [Google Scholar]
- 28. Barry MJ, Fowler FJ, O’Leary MP, et al. The American urological association symptom index for benign prostatic hyperplasia. J Urol. 1992;148(5 Part 1):1549‐1557; discussion 1564. [DOI] [PubMed] [Google Scholar]
- 29. Johnson TV, Abbasi A, Ehrlich SS, et al. IPSS quality of life question: a possible indicator of depression among patients with lower urinary tract symptoms. Can J Urol. 2012;19:6100‐6104. [PubMed] [Google Scholar]
- 30. Lechevallier E, Eghazarian C, Ortega JC, Roux F, Coulange C. Effect of digital rectal examination on serum complexed and free prostate‐specific antigen and percentage of free prostate‐specific antigen. Urology. 1999;54:857‐861. [DOI] [PubMed] [Google Scholar]
- 31. Rodriguez‐Rubio FI, Robles JE, Gonzalez A, et al. Effect of digital rectal examination and flexible cystoscopy on free and total prostate‐specific antigen, and the percentage of free prostate‐specific antigen. Differences between two PSA assays. Eur Urol. 1998;33:255‐260. [DOI] [PubMed] [Google Scholar]
- 32. Lee JS, Chung BH. Transrectal ultrasound versus magnetic resonance imaging in the estimation of prostate volume as compared with radical prostatectomy specimens. Urol Int. 2007;78:323‐327. [DOI] [PubMed] [Google Scholar]
- 33. McConnell JD, Roehrborn CG, Bautista OM, et al. The long‐term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2387‐2398. [DOI] [PubMed] [Google Scholar]
- 34. Roehrborn CG, Siami P, Barkin J, et al. The effects of dutasteride, tamsulosin and combination therapy on lower urinary tract symptoms in men with benign prostatic hyperplasia and prostatic enlargement: 2‐year results from the CombAT study. J Urol. 2008;179(2):616‐621; discussion 621. [DOI] [PubMed] [Google Scholar]
- 35. Zhou Z, Cui Y, Wu J, Ding R, Cai T, Gao Z. Meta‐analysis of the efficacy and safety of combination of tamsulosin plus dutasteride compared with tamsulosin monotherapy in treating benign prostatic hyperplasia. BMC Urol. 2019;19:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Macoska JA, Uchtmann KS, Leverson GE, McVary KT, Ricke WA. Prostate transition zone fibrosis is associated with clinical progression in the MTOPS study. J Urol. 2019;202:1240‐1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Leoci R, Aiudi G, Silvestre F, Lissner E, Lacalandra GM. Effect of pulsed electromagnetic field therapy on prostate volume and vascularity in the treatment of benign prostatic hyperplasia: a pilot study in a canine model. Prostate. 2014;74:1132‐1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Funk RH, Knels L, Augstein A, Marquetant R, Dertinger HF. Potent stimulation of blood flow in fingers of volunteers after local short‐term treatment with low‐frequency magnetic fields from a novel device. Evid Based Complement Alternat Med. 2014;2014:543564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chughtai B, Lee R, Te A, Kaplan S. Role of inflammation in benign prostatic hyperplasia. Rev Urol. 2011;13:147‐150. [PMC free article] [PubMed] [Google Scholar]
- 40. Inamura S, Ito H, Shinagawa T, et al. Prostatic stromal inflammation is associated with bladder outlet obstruction in patients with benign prostatic hyperplasia. Prostate. 2018;78:743‐752. [DOI] [PubMed] [Google Scholar]
- 41. Kahokehr A, Vather R, Nixon A, Hill AG. Non‐steroidal anti‐inflammatory drugs for lower urinary tract symptoms in benign prostatic hyperplasia: systematic review and meta‐analysis of randomized controlled trials. BJU Int. 2013;111:304‐311. [DOI] [PubMed] [Google Scholar]
- 42. Novara G, Giannarini G, Alcaraz A, et al. Efficacy and safety of hexanic lipidosterolic extract of serenoa repens (permixon) in the treatment of lower urinary tract symptoms due to benign prostatic hyperplasia: systematic review and meta‐analysis of randomized controlled trials. Eur Urol Focus. 2016;2:553‐561. [DOI] [PubMed] [Google Scholar]
- 43. Cyrus A, Kabir A, Goodarzi D, et al. Impact of metabolic syndrome on response to medical treatment of benign prostatic hyperplasia. Korean J Urol. 2014;55:814‐820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wei L, Lai EC, Kao‐Yang YH, Walker BR, MacDonald TM, Andrew R. Incidence of type 2 diabetes mellitus in men receiving steroid 5alpha‐reductase inhibitors: population based cohort study. BMJ. 2019;365:l1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Oelke M, Becher K, Castro‐Diaz D, et al. Appropriateness of oral drugs for long‐term treatment of lower urinary tract symptoms in older persons: results of a systematic literature review and international consensus validation process (LUTS‐FORTA 2014). Age Ageing. 2015;44:745‐755. [DOI] [PMC free article] [PubMed] [Google Scholar]


