Abstract
The incidence of migraine is higher among women than men and peaks during the reproductive years, when contraceptive medication use is common. Atogepant, a potent, selective antagonist of the calcitonin gene‒related peptide receptor—in development for migraine prevention—is thus likely to be used by women taking oral contraceptives. This phase 1, open‐label, single‐center, 2‐period, fixed‐sequence study examined the effect of multiple‐dose atogepant 60 mg once daily on the single‐dose pharmacokinetics of a combination oral contraceptive, ethinyl estradiol 0.03 mg and levonorgestrel 0.15 mg (EE/LNG), in healthy postmenopausal or oophorectomized women. For participants in period 1, a single dose of EE/LNG was followed by a 7‐day washout. In period 2, atogepant was given once daily on days 1‐17; an oral dose of EE/LNG was coadministered with atogepant on day 14. Plasma pharmacokinetic parameters for EE and LNG were assessed following administration with and without atogepant. Twenty‐six participants aged 45‐64 years enrolled; 22 completed the study in accordance with the protocol. The area under the concentration‐time curve extrapolated to infinity (AUC0‐∞) of LNG was increased by 19% when administered with atogepant. Coadministration of atogepant and a single dose of EE/LNG did not substantially alter the pharmacokinetics of EE; the ∼19% increase in plasma AUC0‐∞ of LNG is not anticipated to be clinically significant. Overall, atogepant alone and in combination with EE/LNG was generally well tolerated, with no new safety signals identified.
Keywords: migraine, prevention, women, calcitonin gene–related peptide, antagonist
Women are approximately 3 times as likely as men to have migraines and have a higher rate of global years lived with disability. 1 , 2 , 3 , 4 The incidence of migraine in women peaks during the reproductive years (18.2 cases per 1000 human years in women between ages 20 and 24), when oral contraceptives (OCs) are commonly used. 5 , 6 Management includes the acute treatment of migraine attacks as well as the use of preventive medications for frequent or severe migraine attacks; these approaches are not mutually exclusive. The American Academy of Neurology guidelines recommend using preventive treatment to reduce attack frequency, severity, and duration, to improve responsiveness to treatment for attacks, and to improve function and reduce disability. 7
Inhibition of calcitonin gene–related peptide (CGRP), a potent vasodilatory protein strongly implicated in the pathophysiology of migraine, has emerged as a targeted approach for migraine treatment. 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 Three monoclonal antibodies that target CGRP or the CGRP receptor are approved for preventive treatment of migraine in US adults. 11 , 16 , 17 , 18 In addition, several small‐molecule CGRP receptor antagonists, known as gepants, are in development for acute and preventive treatment of migraine. 8 , 13 Ubrogepant (Ubrelvy™) and rimegepant (Nurtec™) were recently approved for the treatment of acute migraine.
Atogepant is a novel, orally administered, small‐molecule CGRP receptor antagonist under investigation for migraine prevention. The efficacy and safety of atogepant in migraine prevention were demonstrated in a phase 2/3 clinical trial in which treatment with atogepant, compared with placebo, significantly decreased monthly migraine days over 12 weeks. 19 Atogepant is rapidly absorbed after oral administration, with a median time to maximum plasma drug concentration (tmax) of 1.8 hours and a terminal elimination half‐life (t½) of approximately 10 hours for the 60‐mg dose. Steady‐state concentrations are typically achieved by day 3 of daily dosing. Furthermore, atogepant did not exhibit potent reversible inhibition of cytochrome P450 (CYP)1A2 or CYP3A4 in in vitro studies but did display weak inhibition of CYP2B6, CYP2C8, CYP2C9, CYP2D6, and CYP2C19. Inhibition of CYP3A4 or UDP‐glucuronosyltransferase (UGT)1A1 was not time dependent, and atogepant was shown to induce CYP3A4 in human hepatocytes in a concentration‐dependent manner. Taken together with the anticipated therapeutic unbound maximum plasma drug concentration (Cmax) values (< 0.1 μmol/L) and relative induction score modeling, atogepant is not expected to cause clinically significant interactions with the pharmacokinetics (PK) of compounds whose clearance mechanism is predominantly dependent on CYP3A4. Additionally, atogepant did not induce CYP1A2 or CYP2B6 in human hepatocyte incubations and did not inhibit P‐glycoprotein in vitro.
Combination OCs typically include 2 components, an estrogen and a progestin. Ethinyl estradiol (EE), the estrogen component, is metabolized by hydroxylation via the isoenzymes CYP3A4 and CYP2C9 and by conjugation via sulfation and glucuronidation via UGT1A1. 20 , 21 Levonorgestrel (LNG), the progestin component, is predominately metabolized via CYP3A4. 20 , 21 Atogepant is not anticipated to alter the PK of OCs, as atogepant is not a potent reversible or time‐dependent inhibitor of CYP3A4 or UGT1A1 and is not expected to be an inducer of drug metabolism at clinically relevant concentrations. However, because the mixed elimination pathways of OCs complicate the prospective prediction of interaction, and the particular prevalence of migraine in women of childbearing age, it is important to determine clinically whether any meaningful drug‐drug interactions occur when atogepant and OCs are used together. The primary objective of this study was to evaluate the effect of multiple‐dose administration of atogepant 60 mg on the PK profiles of the components of a combination OC containing EE and LNG. The secondary objectives were to assess the safety and tolerability of coadministering atogepant and EE/LNG in healthy female participants.
Methods
Institutional review board approval of the study protocol (MK‐8031‐P005) was obtained from Chesapeake Research Review, Inc (Columbia, Maryland). The study was conducted in conformance with the ethical principles of the Declaration of Helsinki, the International Council for Harmonisation's Guideline for Good Clinical Practice, and applicable national and/or local statutes and regulations. All participants provided written informed consent.
Study Design
This phase 1, open‐label, 2‐period, fixed‐sequence, single‐site study investigated the effect of multiple doses of atogepant on the single‐dose PK of a combination of EE and LNG (Nordette‐28; Teva Women's Health, Inc, Sellersville, Pennsylvania) in healthy female participants. On day 1 of period 1, participants received a single oral dose (1 tablet) of EE 0.03 mg/LNG 0.15 mg (Figure 1). Period 1 was followed by a 7‐day washout period, which was immediately followed by period 2. On days 1 to 17 of period 2, participants received atogepant 60 mg once daily (6 × 10‐mg tablet); on day 14, atogepant was coadministered with a single oral dose (1 tablet) of EE 0.03 mg/LNG 0.15 mg. Safety was monitored throughout the study, with follow‐up visits scheduled for 14, 28, and 56 days after the final dose of atogepant in period 2. This extended follow‐up period was included due to incidence of liver injury occurring up to 2 months after dosing with a different CGRP antagonist.
Figure 1.
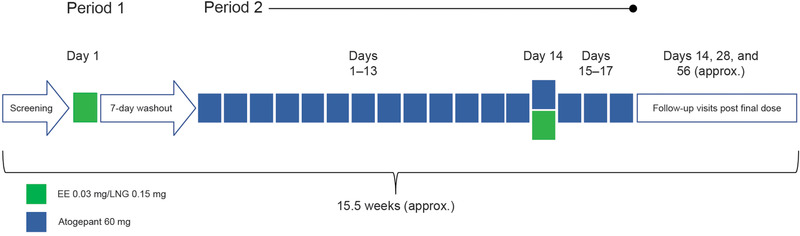
Study design. EE indicates ethinyl estradiol; LNG, levonorgestrel.
Study Population
The study enrolled healthy postmenopausal or bilaterally oophorectomized (≥6 months before start of study) women (estradiol level <35 pg/mL and follicle‐stimulating hormone serum levels in postmenopausal range at screening) in order to minimize any hormonal fluctuations that might influence the interpretation of the PK of the oral contraceptive. Additional inclusion criteria required no use of nicotine‐containing products for at least 3 months and a body mass index of 18 to 30 kg/m2 at screening. Study exclusion criteria were estimated creatinine clearance of ≤80 mL/min; use of any compounds known to be significant inhibitors of CYP enzymes or significant inhibitors or substrates of P‐glycoprotein and/or organic anion‐transporting polypeptide within 14 days of dosing, or 5 times the half‐life of the study drug; use of any compounds known to be inducers of CYP enzymes or P‐glycoprotein within 28 days of dosing, or 5 times the half‐life of the study drug; use of sex hormone–binding globulin agents within 4 weeks or hormone replacement therapy within 6 months; injections of medroxyprogesterone acetate or placement or removal of etonogestrel implants within 1 year; or excessive use of alcohol or caffeine.
Procedures
EE/LNG was administered in the clinical research unit on period 1 (day 1) and period 2 (day 14). Atogepant 60 mg was administered in the clinical research unit on days 1, 2, 7, 10, 11, 14, 15, 16, and 17; participants self‐administered remaining doses at home on days 3, 4, 5, 6, 8, 9, 12, and 13. All doses of atogepant and EE/LNG were to be taken with 240 mL of water under fasting conditions, with additional water restricted 1 hour before and 1 hour after study drug administration.
Blood Sample Collection
Blood samples for determining plasma concentrations of EE and LNG were collected into BD Vacutainer tubes (Becton Dickinson, Franklin Lakes, New Jersey) containing K2EDTA before dosing and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 24, 48, 72, and 96 hours after dosing on day 1 of period 1 and day 14 of period 2. Blood samples for assessing plasma concentrations of atogepant were collected into K2EDTA Vacutainer tubes on day 10 of period 2 before dosing and at 0.33, 0.67, 1, 1.5, 2, 3, 4, 6, 8, 12, 16, and 24 hours after dosing. Whole‐blood samples were centrifuged, plasma was transferred into a cryotube, and samples were immediately stored at –20°C.
Plasma samples for EE/LNG and atogepant were transferred to Pharmanet Canada, Inc (Quebec City, Canada) and Merck & Co, Inc (West Point, Pennsylvania), respectively.
Bioanalytical Methods
Ethinyl Estradiol
EE plasma concentrations were determined by Pharmanet Canada, Inc (Quebec City, Canada) using their fully validated, proprietary liquid chromatography/tandem mass spectrometry assays. Briefly, EE and its internal standard 17α‐EE‐2,4,16,16‐d4 were extracted from a 0.600‐mL aliquot of human K2EDTA plasma using a liquid‐liquid extraction procedure followed by derivatization. Extracted samples were injected into a liquid chromatograph equipped with a Zorbax SB‐C18, 50 × 4.6 mm, 3.5‐μm column (Agilent Technologies, Santa Clara, California) with Milli‐Q type water/methanol with glacial acetic acid for mobile phase A and methanol for mobile phase B. Tandem mass spectrometry was used for detection. The lower limit of quantification (LLOQ) for EE was 1.00 pg/mL, and the linear calibration range was 1.00 to 200 pg/mL. The interassay accuracy bias of the EE assay was −9.4% to −5.4% (precision CV: 1.0% to 6.9%). The intra‐assay accuracy bias was −6.8% to −4.7% (precision CV: 0.5% to 3.8%).
Levonorgestrel
LNG plasma concentrations were determined by Pharmanet Canada, Inc (Quebec City, Canada) using their fully validated, proprietary liquid chromatography/tandem mass spectrometry assays. Briefly, LNG and its internal standard levonorgestrel‐d6 were extracted from a 0.500‐mL aliquot of human K2EDTA plasma using a liquid‐liquid extraction procedure. The extracted samples were injected into a liquid chromatograph equipped with a Zorbax SB‐C18, 50 × 4.6 mm, 3.5‐μm column (Agilent Technologies, Santa Clara, California) with mixtures of Milli‐Q type water and methanol acetic acid in different proportions for mobile phases A and B. Tandem mass spectrometry was used for detection. The LLOQ for LNG was 25.00 pg/mL, and the linear calibration range was 25.00 to 5000.00 pg/mL. The interassay accuracy bias of the LNG assay was −0.5% to −0.01% (precision CV: 2.7% to 4.0%). The intra‐assay accuracy bias was −4.6% to 6.2% (precision CV: 1.4% to 8.3%).
Atogepant
After automated liquid‐liquid extraction of atogepant from the human plasma samples, the analyte and the internal standard ([2H3]‐atogepant) were chromatographed using reversed‐phase chromatography on an XBridge Shield RP18 column (2.1 × 50 mm, 3.5 μm) (Waters Corp, Milford, Massachusetts) and detected with tandem mass spectrometry using a turbo ion spray interface in the positive ionization mode. Two multiple reaction–monitoring transitions were monitored: m/z 604→264 (atogepant) and m/z 608→268 (internal standard). The LLOQ for this method is 1 ng/mL (1.60 nmol/L) when 100 μL of plasma is processed, and the linear calibration range is 1 to 1000 ng/mL. The interassay accuracy bias was −2.1% to 4.0% (precision CV: 0.7% to 3.9%). The intra‐assay accuracy bias was −2.9% to 8.3% (precision CV: 0.4% to 3.7%).
PK Assessments
The area under the plasma drug concentration‐time curve from time 0 to infinity (AUC0‐∞), Cmax, tmax, and t½ of EE and LNG were calculated after administration of EE/LNG alone and after coadministration of multiple‐dose atogepant with single‐dose EE/LNG. AUC from time 0 to 24 hours (AUC0‐24), Cmax, and tmax for atogepant were calculated before dosing EE/LNG and after administration of atogepant alone on day 10 of period 2 (after administration of atogepant 60 mg once daily for 10 days). All standard noncompartmental PK parameters were calculated using WinNonlin Professional Version 5.2 (Certara, LP, St. Louis, Missouri).
Safety and Tolerability
Safety and tolerability were monitored by physical examination, vital signs (heart rate, blood pressure, respiratory rate, temperature), 12‐lead ECGs, and laboratory safety tests (hematology, chemistry, urinalysis) at predefined times. Throughout the study, participants were monitored for adverse events (AEs). AEs were coded according to Version 15.1 of the Medical Dictionary for Regulatory Activities.
Statistical Planning and Analyses
All statistical analyses were performed with SAS/STAT software version 9.1 (SAS Institute Inc, Cary, North Carolina).
Power calculations for the determination of sample size assumed the following within‐subject SDs (variability estimates from previous studies): 0.115 (ln‐[ng.h/mL]) and 0.172 (ln‐[ng/mL]) for EE ln‐AUC0‐∞ and ln‐Cmax, respectively; 0.162 (ln‐[ng.h/mL]) and 0.182 (ln‐[ng/mL]) for LNG ln‐AUC0‐∞ and ln‐Cmax, respectively. Assuming 22 completed participants with available PK data, nonnegative correlation among the 4 test statistics (EE AUC0‐∞, EE Cmax, LNG AUC0‐∞, and LNG Cmax), and true geometric mean ratios (GMRs) of 1.00 for all 4 parameters, there was at least an ∼96% probability that all 4 90%CIs would fall within the 0.80 to 1.25 target interval simultaneously (α = 0.05).
Pharmacokinetics
AUC0‐∞ was calculated using the linear trapezoidal method for ascending concentrations and the log‐linear trapezoidal method for descending concentrations. The apparent terminal t½ was calculated as the quotient of the natural log of 2 (ln [2]) and λ, where λ is the apparent terminal rate constant estimated as the slope of the regression of the terminal log‐linear portion of the plasma concentration‐time profile. AUC0‐∞ and Cmax of EE and LNG values were natural log transformed before analysis and separately evaluated with a linear mixed‐effects model, with a fixed‐effect term for treatment. An unstructured covariance matrix allowed for unequal treatment variances and was used to model the correlation of the 2 treatment measurements within each participant. The Kenward‐Roger method was used to calculate the denominator degrees of freedom for the fixed effects. A 2‐sided 90%CI for the true mean GMR (EE/LNG + atogepant versus EE/LNG alone) was calculated for each parameter. The 90%CI was then exponentiated to obtain the 90%CI for the true GMR (EE/LNG + atogepant versus EE/LNG alone) for each parameter. Other PK parameters (tmax and apparent terminal elimination t½) were summarized with descriptive statistics.
Results
Participant Disposition and Demographics
The study enrolled 26 healthy postmenopausal or oophorectomized women (safety population); 22 participants completed the study in accordance with the protocol. Three participants discontinued the study because of personal reasons or family emergency, although 1 of the 3 received all doses and was included in the Cmax and tmax analyses. The investigator removed a fourth participant from the study because of moderate AEs unrelated to the study drugs (further described in the Safety section). Table 1 lists the participants’ demographics and baseline characteristics.
Table 1.
Participant Demographics and Baseline Characteristics
| Demographic/Characteristic | Total N = 26 a |
|---|---|
| Age, y | |
| Mean (SD) | 56 (5.1) |
| Range, min‐max | 45‐64 |
| Race, n (%) | |
| White | 23 (88.5) |
| Black or African American | 1 (3.8) |
| American Indian/Alaska Native | 1 (3.8) |
| Native Hawaiian/Pacific Islander | 1 (3.8) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 10 (38.5) |
| Not Hispanic or Latino | 16 (61.5) |
| BMI, kg/m2 | |
| Mean (SD) | 26.4 (2.5) |
| Median (min‐max) | 26.4 (19.1‐30.1) |
| Creatinine clearance, b L/h | |
| Mean (SD) | 101.7 (14.7) |
| Median (min‐max) | 99.0 (81.0‐136.0) |
BMI indicates body mass index; max, maximum; min, minimum.
Of these 26 enrolled participants, 4 discontinued. One withdrew for personal reasons on day –1 of period 2; 1 withdrew for a family emergency on day 8 of period 2; 1 withdrew for a family emergency on day 14 of period 2; and 1 was removed from the study by the investigator on day 9 of period 2 for adverse events considered unrelated to the study drugs.
Creatinine clearance was calculated with the Cockcroft‐Gault equation as follows: Creatinine Clearance = (140 – Age [y]) × (Body Weight [kg])/72 × (Serum Creatinine [mg/dL]), where body weight is the weight collected at screening, serum creatinine is the average of the values collected at screening and check‐in, and the result is multiplied by 0.85, as all participants were female.
PK of EE and LNG
Table 2 summarizes the PK parameters for EE and LNG after administration of a single dose of EE/LNG alone or coadministered with multiple‐dose atogepant. For EE, the GMRs (90%CI) for AUC0‐∞ and Cmax comparing EE/LNG coadministered with 60 mg atogepant over EE/LNG alone were 1.00 (0.96‐1.05) and 0.90 (0.84‐0.96), respectively (Table 2 and Figure 2). For LNG, the GMRs (90%CI) for AUC0‐∞ and Cmax were 1.19 (1.13‐1.26) and 1.09 (1.03‐1.17), respectively (Table 2 and Figure 2). The 90%CI of the GMRs fell within the predefined bioequivalence bounds (0.8‐1.25) for all parameters except LNG AUC0‐∞, which was slightly increased after coadministration with atogepant. Mean plasma concentration‐time profiles for EE and LNG were similar with or without coadministration of atogepant (Figure 3A and Figure 3B).
Table 2.
Plasma Pharmacokinetic Parameters of Ethinyl Estradiol and Levonorgestrel
| GM (95%CI) a | ||||||
|---|---|---|---|---|---|---|
| Analyte | Pharmacokinetic Parameter | N | EE/LNG + Atogepant | N | EE/LNG Alone | GMR (90%CI): EE/LNG + Atogepant to EE/LNG Alone |
| EE | AUC0‐∞, a pg·h/mL | 22 |
848 (765, 941) |
26 |
846 (766, 935) |
1.00 (0.96, 1.05) |
| Cmax, a pg/mL | 23 |
68.3 (61.6, 75.9) |
26 |
75.9 (69.5, 83.0) |
0.90 (0.84, 0.96) |
|
| tmax, b h | 23 |
1.5 (1.00, 2.0) |
26 |
1.0 (0.56, 2.0) |
… | |
| Apparent terminal t½, c h | 22 |
21.49 (23.31) |
26 |
19.1 (20.2) |
… | |
| LNG | AUC0‐∞, a ng·h/mL | 22 |
47.9 (41.8, 54.9) |
26 |
40.1 (35.0, 45.9) |
1.19 (1.13, 1.26) |
| Cmax, a ng/mL | 23 |
3.22 (2.82, 3.68) |
26 |
2.95 (2.58, 3.37) |
1.09 (1.03, 1.17) |
|
| tmax, b h | 23 |
1.5 (0.50, 4.0) |
26 |
1.04 (1.0, 3.0) |
… | |
| Apparent terminal t½, c h | 22 |
38.3 (29.8) |
26 |
39.5 (30.6) |
… | |
AUC0‐∞ indicates area under plasma drug concentration‐time curve from time 0 to infinity; Cmax, maximum plasma drug concentration; EE, ethinyl estradiol; GM, geometric mean; GMR, geometric mean ratio; LNG, levonorgestrel; max, maximum; min, minimum; tmax, time when Cmax is reached; t½, terminal elimination half‐life.
Back‐transformed least‐squares mean and CI from mixed‐effects model performed on natural log‐transformed values.
Median (minimum, maximum) provided for tmax.
Geometric mean and geometric coefficient of variation provided for apparent terminal t½.
Figure 2.
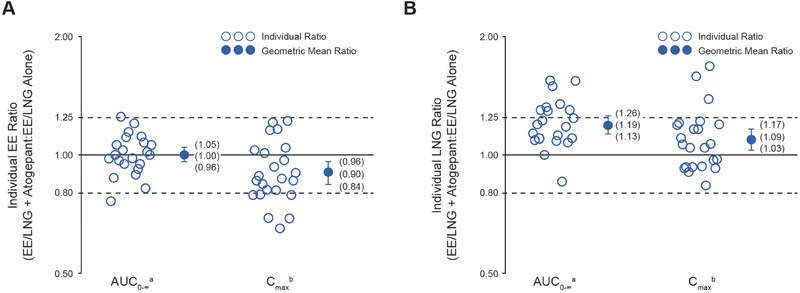
Individual AUC0‐∞ and Cmax ratios, geometric mean ratios, and corresponding 90%CIs for (A) ethinyl estradiol (EE) and (B) levonorgestrel (LNG) after a single dose of EE 0.03 mg/LNG 0.15 mg administered alone and with multiple‐dose atogepant 60 mg daily. AUC0‐∞ indicates area under plasma drug concentration‐time curve from time 0 to infinity; Cmax, maximum plasma drug concentration; EE, ethinyl estradiol; LNG, levonorgestrel. an = 22. bn = 23.
Figure 3.
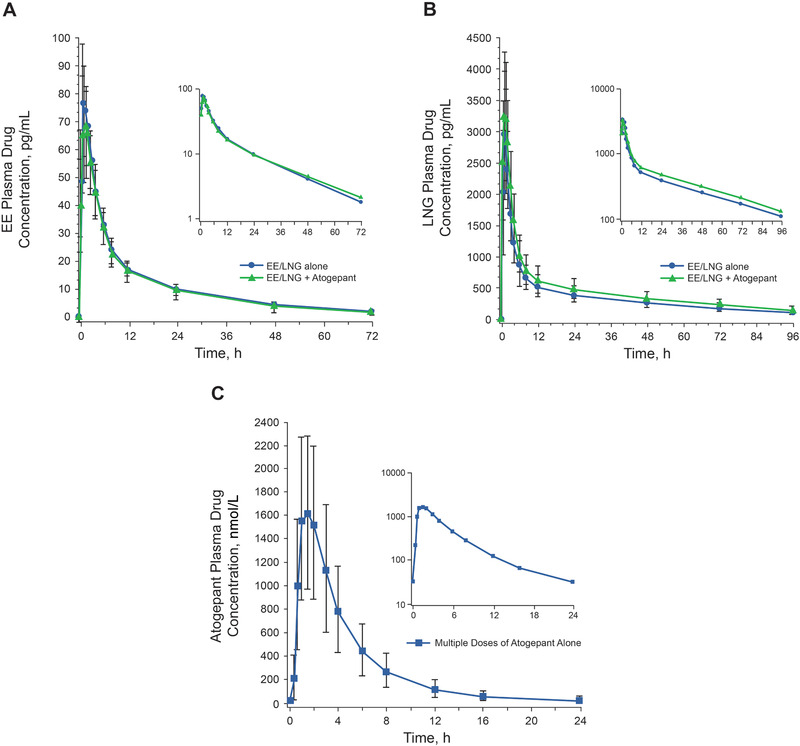
Mean (± SD) plasma drug concentration‐time profiles for (A) ethinyl estradiol (EE) and (B) levonorgestrel (LNG) after a single dose of EE 0.03 mg/LNG 0.15 mg administered alone or with multiple‐dose atogepant 60 mg. C, Multiple‐dose administration of atogepant alone on day 10 of period 2. EE indicates ethinyl estradiol; LNG, levonorgestrel.
PK of Atogepant
Table 3 descriptively summarizes the PK values of atogepant administered alone on day 10 of period 2. Figure 3C shows the mean plasma concentration‐time profile for atogepant after multiple‐dose administration on day 10 of period 2.
Table 3.
Plasma Pharmacokinetic Parameters of Atogepant (Day 10 of Period 2) a
| Pharmacokinetic Parameter | Atogepant 60 mg Alone b |
|---|---|
| AUC0‐24h, c μmol·h/L, mean (SD) | 7.92 (3.19) |
| Cmax, nmol/L, mean (SD) | 1930 (632) |
| tmax, h, median (min, max) | 1.5 (0.68, 2.02) |
AUC0‐24h, area under plasma drug concentration‐time curve from time 0 to 24 hours; Cmax, maximum plasma drug concentration; max, maximum; min, minimum; tlast, time of last measurement; tmax, time when Cmax is reached.
Three participants had missing values because of early discontinuation from study.
Multiple oral doses of atogepant 60 mg (6 × 10‐mg tablets) once daily for 17 consecutive days, starting on day 1 of period 2.
AUC0‐last was used as a close approximation to AUC0‐24 (tlast range, 23.92 to 23.98 hours).
Safety
There were no deaths, serious AEs, or clinically significant laboratory results during the study. Eighteen participants reported a total of 60 AEs, 3 of which occurred before dosing. Eleven participants experienced AEs for both EE/LNG and atogepant alone, and 8 experienced AEs with the combination treatment. Of these AEs, 14 were considered by the investigator to be related to atogepant and 4 to EE/LNG. The most common drug‐related AEs overall were headache, somnolence, diarrhea, and constipation (Table 4). All AEs were mild or moderate in intensity and resolved by the end of the study. There were no consistent treatment‐related changes in laboratory, vital signs, or ECG safety parameters. The moderate AEs that led to the removal of 1 participant from the study (day 9 of period 2) were pneumonia and a ligament sprain, both considered by the investigator to be unrelated to the study drugs.
Table 4.
Number (%) of Adverse Events Reported by More Than 1 Participant a
| AEs, n (%) | EE/LNG Alone(n = 26) | Atogepant Alone(n = 26) | EE/LNG + Atogepant(n = 23) | Total(N = 26) |
|---|---|---|---|---|
| Headache | 4 (15.4) | 1 (3.8) | 2 (8.7) | 6 (23.1) |
| Somnolence | 1 (3.8) | 1 (3.8) | 2 (8.7) | 3 (11.5) |
| Constipation | 1 (3.8) | 3 (11.5) | 0 | 4 (14.5) |
| Diarrhea | 0 | 3 (11.5) | 0 | 3 (11.5) |
| Nausea | 1 (3.8) | 0 | 1 (4.3) | 2 (7.7) |
| Injury, poisoning, procedural complications | 1 (3.8) | 1 (3.8) | 0 | 2 (7.7) |
| Back pain | 0 | 2 (7.7) | 1 (4.3) | 3 (11.5) |
| Upper respiratory tract infection | 1 (3.8) | 0 | 1 (4.3) | 2 (7.7) |
AE indicates adverse event; EE, ethinyl estradiol; LNG, levonorgestrel.
Multiple instances of an AE in the same participant in a dosing category were counted as 1 instance. Participants with only 1 AE in >1 dosing category were counted once in the overall tally for that AE.
Discussion
This study demonstrated that multiple‐dose atogepant does not have a clinically meaningful effect on the exposure of a commonly used OC (EE 0.03 mg/LNG 0.15 mg). Although the AUC0‐∞ of LNG was increased by 19% after coadministration with atogepant compared with EE/LNG alone, a change of this magnitude is not expected to affect the contraceptive efficacy of EE/LNG or to have a clinically meaningful effect on safety. 20 , 22 Because atogepant is a substrate but not an inhibitor of P‐gp, this increase in exposure of LNG may be mediated through substrate binding competition for CYP3A4, as both atogepant and LNG are substrates of CYP3A4 and atogepant is present at much higher concentrations than LNG. Furthermore, the upper bound of the CI for the comparison of LNG with and without atogepant was 1.26, which is only slightly higher than what the Food and Drug Administration considers bioequivalent (1.25). 23 The clinical significance of PK interactions depends on the therapeutic window of interacting drugs. A mean increase of LNG on the order of 20% and an upper bound ranging from 1.4 to 1.8 was reported for fluconazole with no contraindication in the label, further supporting the lack of clinical relevance of the small LNG increase observed here.
The primary mechanism for combination OCs, including EE/LNG, is inhibition of ovulation by suppression of gonadotropins. 21 LNG, the progestin component, is considered primarily responsible for contraceptive activity, whereas the estrogen component (EE) is thought to prevent breakthrough bleeding and other side effects. 24 Given that atogepant resulted in a small increase in LNG AUC0‐∞, no impact on contraceptive efficacy is expected. In addition, large interindividual variation and intraindividual variation in mean serum concentrations and other PK parameters of LNG after administration of the same dose of LNG have been observed in other studies. 25 , 26
EE is eliminated through a combination of metabolism via CYP3A, CYP2C, and CYP2E, sulfation, methylation, and glucuronidation. 21 , 27 , 28 LNG is rapidly absorbed and does not undergo first‐pass metabolism. 25 , 26 The primary metabolic pathways for LNG are oxidation, reduction, and direct sulfation. 21 , 26 , 29 Atogepant does not induce or inhibit CYP2D6, CYP3A4, or P‐glycoprotein at clinically relevant concentrations, and therefore, drug interactions through CYP450s or UGT1A1 or P‐glycoprotein inhibition are unlikely.
Atogepant exposure (AUC0‐24h) following 10 days of daily dosing of atogepant 60 mg in the current study was within range of AUC0‐24h values reported in a previous study in fasted male participants after 10 days of daily dosing of atogepant 50 mg to 100 mg (data on file, Allergan plc). Therapeutic efficacy of atogepant versus placebo for migraine prevention was demonstrated at daily doses of 10 mg, 30 mg, and 60 mg over 12 weeks of dosing. 19
Coadministration of multiple oral doses of atogepant 60 mg and a single dose of EE/LNG was safe and generally well tolerated. There were no deaths, SAEs, or clinically significant laboratory results, and there were no pregnancies reported during the study. Atogepant was generally well tolerated, and the observed AE profile in this study was generally consistent with that reported in the larger phase 2/3 clinical trial. 19
Strengths and Limitations
The study enrolled postmenopausal and oophorectomized women because this population does not have cyclic fluctuations in sex hormones, allowing for comparison of the PK of 2 doses of OCs (with and without atogepant) when background levels of sex hormones were the same under both conditions. Studies of drug interactions with OCs often enroll postmenopausal and oophorectomized women, 30 , 31 as this is considered an optimal population for assessing drug interactions with OCs. The PK of OCs is not expected to differ in postmenopausal women and women of childbearing age.
The 2‐period fixed‐sequence design of this study ensured that the potential interaction of multiple‐dose administration of atogepant with an OC was assessed after steady‐state atogepant exposure had been achieved, ensuring that any potential for CYP3A4 induction was evaluable. Dosing of atogepant continued throughout the collection of EE and LNG PK data in order to fully maintain any potential enzyme inhibition/induction, if present. However, the study design has limited capacity to detect the effect of an OC on the PK of atogepant, as only single doses of OC were evaluated. Other limitations of this study include its relatively small sample size, but the design and sampling allowed for within‐participant comparison of the full PK properties of the OC used alone and coadministered with atogepant. In addition, not all pill intakes were observed, which could have affected the PK results seen with EE/LNG.
Conclusions
Atogepant, an oral CGRP receptor antagonist under investigation for migraine prevention, did not have a clinically relevant effect on the PK of the commonly used OC, EE 0.03 mg/LNG 0.15 mg. Coadministration of multiple daily doses of atogepant 60 mg with a single dose of EE 0.03 mg/LNG 0.15 mg was safe and generally well tolerated in healthy women.
Statement Regarding Accessibility of Data
The authors cannot share the data reported in this manuscript.
Funding
This study was funded by Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, New Jersey.
Author Contributions
J.X., C.M., and M.H.V. were responsible for the study design and writing the paper. J.X., C.M., W.A., and M.D. analyzed the data and wrote the paper. D.A. was responsible for patient recruitment, enrolling patients, collecting and assembling data, and writing the paper. All authors interpreted data, reviewed, revised, and approved the article.
Conflicts of Interest
Wendy Ankrom, PhD, Jialin Xu, PhD, Marie‐Helene Vallee, MSc, and Marissa F. Dockendorf, PhD, are employees of Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, New Jersey and own stock in Merck & Co, Inc.
Danielle Armas, MD, is an employee of Celerion Inc, Phoenix, Arizona.
Ramesh Boinpally, PhD, is an employee and shareholder of Allergan plc, Madison, New Jersey.
K. Chris Min, MD, PhD, was an employee of Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, New Jersey and may own stock in Merck & Co, Inc.
Acknowledgments
This study was sponsored by Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, New Jersey. Medical writing and editorial assistance were provided to the authors by Peloton Advantage, an OPEN Health company, Parsippany, New Jersey, and was funded by Allergan plc, Dublin, Ireland. The authors thank study participants as well as the site/clinical research unit personnel, including clinical and data management staff and the biostatisticians. The results of this study were previously presented, in part, at the American Academy of Neurology 2019 annual meeting, May 4‐10, 2019, Philadelphia, Pennsylvania.
The following authors are Fellows of the American College of Clinical Pharmacology: None
All authors met the ICMJE and GPP3 authorship criteria. Neither honoraria nor other forms of payments were made for authorship.
References
- 1. Buse DC, Loder EW, Gorman JA, et al. Sex differences in the prevalence, symptoms, and associated features of migraine, probable migraine and other severe headache: results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2013;53(8):1278‐1299. [DOI] [PubMed] [Google Scholar]
- 2. Stewart WF, Lipton RB, Celentano DD, Reed ML. Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors. JAMA. 1992;267(1):64‐69. [PubMed] [Google Scholar]
- 3. Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41(7):646‐657. [DOI] [PubMed] [Google Scholar]
- 4. Steiner TJ, Stovner LJ, Vos T, Jensen R, Katsarava Z. Migraine is first cause of disability in under 50s: will health politicians now take notice? J Headache Pain. 2018;19(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Merki‐Feld GS, Imthurn B, Seifert B, Merki LL, Agosti R, Gantenbein AR. Desogestrel‐only contraception may reduce headache frequency and improve quality of life in women suffering from migraine. Eur J Contracept Reprod Health Care. 2013;18(5):394‐400. [DOI] [PubMed] [Google Scholar]
- 6. Vetvik KG, MacGregor EA. Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. Lancet Neurol. 2017;16(1):76‐87. [DOI] [PubMed] [Google Scholar]
- 7. Silberstein SD. Practice parameter: evidence‐based guidelines for migraine headache (an evidence‐based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55(6):754‐762. [DOI] [PubMed] [Google Scholar]
- 8. Holland PR, Goadsby PJ. Targeted CGRP small molecule antagonists for acute migraine therapy. Neurotherapeutics. 2018;15(2):304‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deen M, Correnti E, Kamm K, et al. Blocking CGRP in migraine patients—a review of pros and cons. J Headache Pain. 2017;18(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iyengar S, Ossipov MH, Johnson KW. The role of calcitonin gene‐related peptide in peripheral and central pain mechanisms including migraine. Pain. 2017;158(4):543‐559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Edvinsson L, Haanes KA, Warfvinge K, Krause DN. CGRP as the target of new migraine therapies—successful translation from bench to clinic. Nat Rev Neurol. 2018;14(6):338‐350. [DOI] [PubMed] [Google Scholar]
- 12. Edvinsson L. The journey to establish CGRP as a migraine target: a retrospective view. Headache. 2015;55(9):1249‐1255. [DOI] [PubMed] [Google Scholar]
- 13. Bigal ME, Walter S, Rapoport AM. Calcitonin gene‐related peptide (CGRP) and migraine current understanding and state of development. Headache. 2013;53(8):1230‐1244. [DOI] [PubMed] [Google Scholar]
- 14. Tepper SJ. History and review of anti‐calcitonin gene‐related peptide (CGRP) therapies: from translational research to treatment. Headache. 2018;58(suppl 3):238‐275. [DOI] [PubMed] [Google Scholar]
- 15. Edvinsson L. The trigeminovascular pathway: role of CGRP and CGRP receptors in migraine. Headache. 2017;57(suppl 2):47‐55. [DOI] [PubMed] [Google Scholar]
- 16. Emgality [package insert]. Indianapolis, IN: Eli Lilly and Company; 2018. [Google Scholar]
- 17. Aimovig [package insert]. Thousand Oaks, CA, and East Hanover, NJ: Amgen Inc, and Novartis Pharmaceuticals Corporation; 2018. [Google Scholar]
- 18. Ajovy [package insert]. North Wales, PA: Teva Pharmaceuticals USA, Inc; 2018. [Google Scholar]
- 19. Goadsby PJ, Dodick DW, Trugman JM, et al. Orally administered atogepant was efficacious, safe, and tolerable for the prevention of migraine: results from a phase 2b/3 study (S17.001). Neurology 2019;92(15 suppl):S17.001. [Google Scholar]
- 20. Lee CR. Drug interactions and hormonal contraception. Trends Urol Gynaecol Sexual Health. 2009;14:23‐26. [Google Scholar]
- 21. Nordette‐28 [package insert]. Sellersville, PA: Teva Women's Health; 2012.
- 22. Jusko WJ. Clarification of contraceptive drug pharmacokinetics in obesity. Contraception. 2017;95(1):10‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. US FDA . Clinical Drug Interaction Studies—Study Design, Data Analysis, and Clinical Implications Guidance for Industry. October 24, 2017. https://www.fda.gov/media/82734/download. Accessed March 30, 2020.
- 24. De Leo V, Musacchio MC, Cappelli V, Piomboni P, Morgante G. Hormonal contraceptives: pharmacology tailored to women's health. Hum Reprod Update. 2016;22(5):634‐646. [DOI] [PubMed] [Google Scholar]
- 25. Basaraba CN, Westhoff CL, Pike MC, Nandakumar R, Cremers S. Estimating systemic exposure to levonorgestrel from an oral contraceptive. Contraception. 2017;95(4):398‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fotherby K. Levonorgestrel. Clinical pharmacokinetics. Clin Pharmacokinet. 1995;28(3):203‐215. [DOI] [PubMed] [Google Scholar]
- 27. Ball SE, Forrester LM, Wolf CR, Back DJ. Differences in the cytochrome P‐450 isoenzymes involved in the 2‐hydroxylation of oestradiol and 17 alpha‐ethinyloestradiol. Relative activities of rat and human liver enzymes. Biochem J. 1990;267(1):221‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guengerich FP. Oxidation of 17 alpha‐ethynylestradiol by human liver cytochrome P‐450. Mol Pharmacol. 1988;33(5):500‐508. [PubMed] [Google Scholar]
- 29. Cherala G, Pearson J, Maslen C, Edelman A. An ethinyl estradiol‐levonorgestrel containing oral contraceptive does not alter cytochrome P4502C9 in vivo activity. Drug Metab Dispos. 2014;42(3):323‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Frey R, Unger S, van der Mey D, et al. Pharmacokinetic interaction study between riociguat and the combined oral contraceptives levonorgestrel and ethinylestradiol in healthy postmenopausal women. Pulmonary Circ. 2016;6(suppl 1):S97‐S102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kapitza C, Nosek L, Jensen L, Hartvig H, Jensen CB, Flint A. Semaglutide, a once‐weekly human GLP‐1 analog, does not reduce the bioavailability of the combined oral contraceptive, ethinylestradiol/levonorgestrel. J Clin Pharmacol. 2015;55(5):497‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]


