Abstract
Aim
To assess the clinical performance and patient acceptance of HemaSpot™ blood collection devices as an alternative blood collection method.
Methods
Adult men and women with any type of diabetes, routinely carrying out self‐monitoring of blood glucose were recruited (n = 128). Participants provided a venous blood sample and prepared two HemaSpot dried blood spots, one at clinics and one at home. HbA1c analysis was by Tosoh G8 high‐performance liquid chromatography. Participants also completed a questionnaire.
Results
Strong linear relationships been HbA1c levels in dried blood spots and venous blood were observed and a linear model was fitted to the data. Time between dried blood spot preparation and testing did not impact the model. Participants were accepting of the approach: 69.2% would use this system if available and 60.7% would be more likely to use this system than going to their general practitioner.
Conclusions
The combination of a robust desiccating dried blood spot device, home sample preparation and return by post produces HbA1c data that support the use of a time‐independent linear calibration of dried blood spot to venous blood HbA1c. A robust remote sample collection service would be valuable to people living with diabetes in urban areas who are working or house‐bound as well as those living in remote or rural locations.
What's new?
HbA1c determination from dried blood spots has been reported but results have been affected by stability issues, requiring methodologies which have included extended drying periods, and storage at low temperatures or for a limited period of time.
HbA1c levels determined from HemaSpot blood collection devices show a strong correlation with venous HbA1c results, with the potential for calibration against the venous method used.
Patient acceptance of the blood collection method was high, with 61.7% of participants indicating that they would be more likely to have their testing carried out if this method of blood collection was available.
By providing patients with an opportunity to increase compliance with regular HbA1c testing, the use of venous calibrated HbA1c determination from HemaSpot blood collection devices provides the potential for improved glycaemic control.
What's new?
HbA1c determination from dried blood spots has been reported but results have been affected by stability issues, requiring methodologies which have included extended drying periods, and storage at low temperatures or for a limited period of time.
HbA1c levels determined from HemaSpot blood collection devices show a strong correlation with venous HbA1c results, with the potential for calibration against the venous method used.
Patient acceptance of the blood collection method was high, with 61.7% of participants indicating that they would be more likely to have their testing carried out if this method of blood collection was available.
By providing patients with an opportunity to increase compliance with regular HbA1c testing, the use of venous calibrated HbA1c determination from HemaSpot blood collection devices provides the potential for improved glycaemic control.
Introduction
The benefits of good blood glucose control in preventing long‐term complications of diabetes are well documented 1. Complications, arising from poor blood glucose control over extended periods, place an economic burden on health services and significantly reduce health‐related quality of life in people with diabetes 2, 3.
Ongoing blood glucose control is assessed by regular measurement of HbA1c, with several laboratory methods available for use, with fresh blood obtained using either venepuncture or fingerprick with collection in capillary tubes. Point‐of‐care instruments are now available for measuring HbA1c levels in fresh capillary blood 4.
In the Scottish Highlands (NHS Highland Health Board area), HbA1c determinations are performed centrally in Inverness using ion‐exchange high‐performance liquid chromatography (HPLC) analysis (Tosoh G8 HPLC analyser, Tosoh Bioscience, Tokyo, Japan) on venous blood samples collected locally at general practitioner practices. If there is no recent HbA1c result, DCA Vantage point‐of‐care instruments (Siemens Healthcare GmbH, Erlangen, Germany) are used at hospital appointments.
Current sampling methods are acceptable in terms of HbA1c determination; however, the use of venepuncture with centralized testing is not providing users in the NHS Highland area with an acceptable approach, as evidenced by the frequency with which HbA1c results are not available at clinical appointments.
Individualized HbA1c targets should be agreed and regularly reviewed at diabetes appointments with clinicians and lifestyle and/or medication changes discussed with the aim of optimizing HbA1c levels. The value of diabetes appointments where HbA1c levels are not available is greatly diminished. For hospital‐based appointments, point‐of‐care instruments are available for the immediate determination of HbA1c levels. However, the vast majority of people with diabetes (those with uncomplicated Type 2 diabetes) are managed in the community by general practitioners, where point‐of‐care instruments are not generally available. In the NHS Highland Health Board area, the cost of HbA1c analysis is borne by secondary rather than primary care, so the costs and maintenance implications of general practitioner‐based point‐of‐care instruments are not straightforward.
Method and convenience of place of collection and timeliness of results are potential factors that impact the availability of HbA1c results at appointments and consequently impact the opportunity for discussion of HbA1c levels and targets.
In line with recognition by the UK and Scottish governments that ‘patients need to be empowered to manage their care’ 5, 6, the long‐term aim of our approach is for people with diabetes to be able to take responsibility for sending off their own blood samples and for results to be sent directly to them so that they can attend review appointments having had the opportunity to reflect upon their latest HbA1c result and what it might mean for them in terms of their individual HbA1c target. To address this aim we have identified a robust blood sampling device (HemaSpot, Spot on Sciences, Austin, TX, USA) for preparing dried blood spots (DBS), which has the potential to fulfil the requirements of our overall approach.
HemaSpot blood collection devices comprise a robust plastic wallet enclosing an eight‐bladed filter paper surrounded by desiccant (Fig. 1). A protective cover allows blood application through a central hole. The device is designed to absorb two hanging drops of blood, equivalent to about 65–105 μl of blood.
Figure 1.
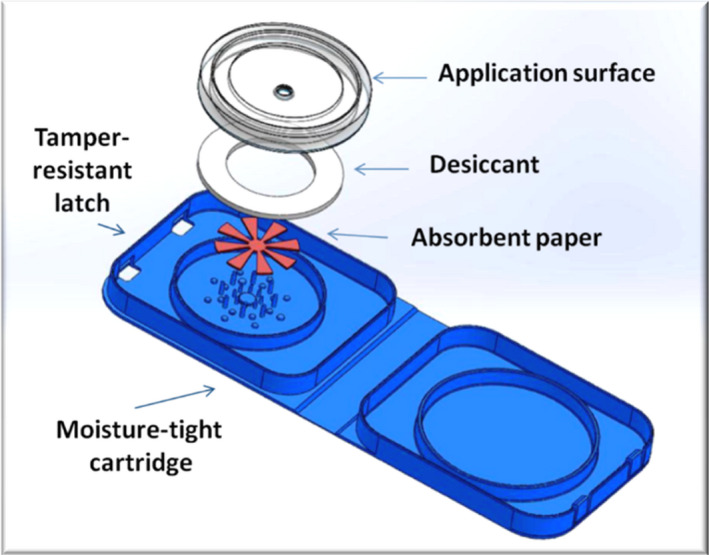
The HemaSpot blood collection device showing the blades of the fan‐shaped filter paper (in red). Once blood has been applied through the hole in the application surface the device is folded over and snapped shut. Image courtesy of Spot on Sciences.
The use of filter papers for collection of DBS is accepted as an alternative method of blood collection for a variety of applications 7 as they are simple to prepare, have low collection and transportation costs, and are safer and more acceptable to study participants 8.
A systematic review identified 17 studies using DBS for HbA1c 9, and two other related studies have recently been published 10, 11. Variation and bias increase with increasing time between sample preparation and testing have been observed, meaning that even when HbA1c can be calibrated against standard venous HbA1c, calibration needs to consider the time between sample preparation and testing.
A laboratory‐based study previously carried out in our laboratory compared HbA1c analysis of laboratory‐prepared HemaSpot DBS (n = 40) with HbA1c values obtained from fresh capillary blood. A strong correlation between HbA1c from DBS and fresh capillary blood was observed, suggesting that HemaSpot devices have the potential to be used as an alternative to current blood collection methods if they are well calibrated using a linear model and DBS are tested within 3 days of preparation (see Table S1).
The purpose of the current study was to assess the clinical performance and user acceptability of DBS prepared using HemaSpot devices by people with diabetes at home, as an alternative blood sample collection method for HbA1c determination.
Methods
Recruitment
Participants (n = 128) were recruited when they attended their routine diabetes clinic appointments in Inverness. It was anticipated that this number of participants would provide as a minimum the recommended 100 returned home ‐ prepared DBS samples for the purposes of comparison 12.
Adult men and women, aged 18–75 years, with any form of diabetes and regularly carrying out self‐monitoring of blood glucose, were included in the study. Pregnant women and patients receiving renal replacement therapy were excluded.
All participants gave written informed consent. NHS research ethics approval was obtained (16/NW/0214).
While attending diabetes clinics, venous blood samples were taken for routine HbA1c analysis. Under the guidance of a research nurse, participants prepared DBS from fingerprick blood using HemaSpot blood collection devices by applying blood from a hanging drop of blood until the filter paper in the device was visibly filled. HemaSpot devices were closed immediately after blood application and stored at 4°C until analysis.
Participants were given a home pack that included a DBS preparation kit, questionnaire and information about HbA1c. Freepost envelopes were provided for the return of home‐prepared DBS and questionnaires. Participants were asked to post their DBS on the day of its preparation.
Questionnaire
A five‐point Likert scale 17‐item questionnaire assessed each participant's experience of preparing DBS, their thoughts on a remote HbA1c service and their views regarding the information provided about HbA1c.
HbA1c analysis
HbA1c analyses were performed using HPLC (Tosoh HPLC 723‐G8 analyser, Tosoh Bioscience) using a cation exchange TSKgel Variant HSi column. The column was calibrated to the International Federation of Clinical Chemistry recommendation using Tosoh calibrators. Lyphocheck Diabetes bi‐level controls (Bio‐Rad Laboratories, CA, USA) were used daily, with coefficient of variation of 2% for the low control [mean of 33 mmol/mol (5.2%)] and 0.9% for the high control [mean of 78 mmol/mol (9.3%)].
Blood from DBS samples was eluted by placing one HemaSpot filter blade in 1 ml hemolysis/wash solution for 2 hours at ambient temperature. For analysis, 4 μl of eluate were aspirated by the analyser before injection onto the column.
DBS were stored at 4°C in the laboratory. Processing and analysis was performed within 4 days of preparation.
Chromatograms were assessed visually using the G8 operator's manual specifications for results acceptability, with guidance from the software flags.
Data analysis
Data analysis was carried out using IBM SPSS versions 19/20 for Windows (IBM Corporation, NY, USA), R and Excel software (Microsoft Corporation, WA, USA). Correlation between sample collection methods was investigated using Pearson coefficient and regression analysis. Agreement and bias between sample collection methods was investigated using Bland‐Altman plots. Statistical significance was determined at the 5% level.
Covariates that were considered to be potential confounders were tested separately to one another within the general linear model and as a full interaction with the main predictor variable (home result) to assess whether a confounder was affecting the relationship (i.e. the calibration) between the home result and the venous reading. Nested models (with and without the additional covariate) were tested for statistical significance using an F‐test (using the function ‘anova ()’ in R).
Results
Participant characteristics
Participant characteristics are presented by type and duration of diabetes (Table 1). More women (61.3%) than men with Type 1 diabetes and more men (69.0%) than women with Type 2 diabetes participated; however, the overall numbers of men (n = 53) and women (n = 51) participants were similar.
Table 1.
Description of study population for whom both venous and home dried blood spots HbA1c levels were available
| Type of diabetes | n | Age (years) | Duration of diabetes (years) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | Minimum | Maximum | Mean | Minimum | Maximum | |||
| Type 1 | Women | 38 | 48.8 | 19 | 71 | 26.8 | 3 | 60 |
| Men | 24 | 44.8 | 19 | 71 | 19.3 | 0.1 | 44 | |
| Total | 62 | 47.3 | 19 | 71 | 23.9 | 0.1 | 60 | |
| Type 2 | Women | 13 | 55.6 | 31 | 69 | 12.0 | 2 | 20 |
| Men | 29 | 64.0 | 39 | 84 | 14.0 | 0.3 | 44 | |
| Total | 42 | 61.4 | 31 | 84 | 13.4 | 0.3 | 44 | |
| Total population | 104 | 53.0 | 19 | 84 | 19.6 | 0.1 | 60 | |
Blood samples available
HbA1c results were available for 127 venous blood samples, 125 clinic and 104 home‐prepared DBS. Minimum and maximum times between home DBS preparation and testing were 1 and 4 days, with 38.5, 43.3, 16.3 and 1.9% of samples tested on day 1, 2, 3 and 4, respectively.
HbA1c analysis
HbA1c from both home‐ and clinic‐prepared DBS exhibited strong correlations with venous HbA1c (R2 values close to 0.98) (Fig. 2). There was a significant difference between the clinic and home DBS relationships with venous blood. We have excluded the time between sample preparation and HbA1c analysis as a potential source of the difference. Early transfer of clinic‐prepared DBS to storage at 4°C differs from home DBS treatment immediately after preparation; however, we have not been able to confirm or exclude this as a reason for the difference observed.
Figure 2.

Home and clinic prepared dried blood spots (DBS) HbA1c plotted against venous HbA1c. Home DBS: solid symbols, dotted line. Clinic DBS: open symbols, dashed line.
The mean bias of home DBS compared with venous blood across the measurement range was +4.27 mmol/mol (+0.39%). A plot of the absolute difference against the mean of venous and home DBS HbA1c indicates that the absolute difference increases with the mean of the pair (Fig. 3), suggesting that while home DBS HbA1c results may not be used directly as equivalent to venous results, they may be successfully calibrated against one another. A general linear model was used to establish a calibrating relationship between home DBS results and venous results. Diagnostic plots supported the assumption of normality among residuals and did not suggest heterogeneity of variance, supporting the use of this model.
Figure 3.
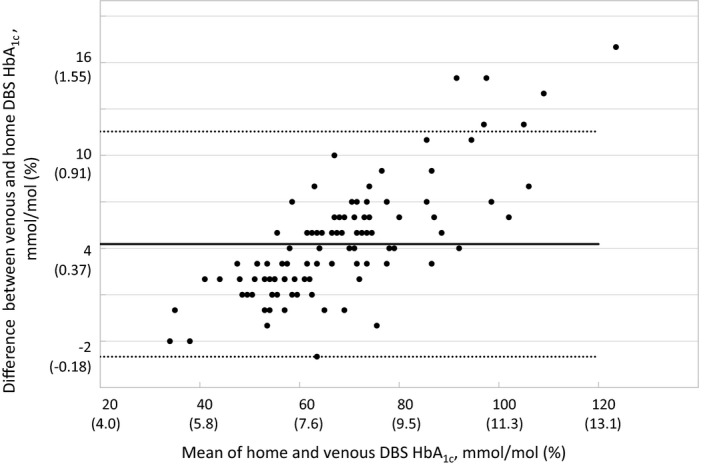
Bland‐Altman plot, the difference between venous and home dried blood spots (DBS) HbA1c showing evidence of a straight line relationship between the absolute difference and the mean values for the pairs of scores. Solid line: mean of difference; dotted lines, upper and lower 95% confidence intervals.
The model fitted is:
The model allows prediction of venous blood HbA1c levels from DBS HbA1c results. Concordance values were 100, 92.5 and 96.6% between venous and predicted values from home DBS for HbA1c values of <48 mmol/mol (<6.5%), 48–64 mmol/mol (6.5–8%) and >64 mmol/mol (>8%), respectively.
The model holds only for the range of HbA1c values in the study sample [35–115 mmol/mol (5.4–12.7%)] and when HbA1c analysis from HemaSpot is performed using a Tosoh G8 Analyser.
A number of covariates were individually tested to assess how they affected the calibration using a set of nested linear models with each of the variables added separately to the main model (Table 2). Of the covariates tested, only a laboratory factor covariate (with laboratory staff carrying out the test as a proxy) statistically significantly affected the results.
Table 2.
The effect of the addition of potential covariates on top of the base model of the venous reading (dependent variable) regressed against the home result (the independent variable). Each additional covariate was added separately and as a full interaction with the home result. Each nested pair of models (without covariate and with) were compared using an analysis of variance. For each nested pairwise comparison of models the dataset used was the full dataset, less any observations that had missing data for either the home result or the added covariate. For further details see Table S2
| Additional covariate | P‐value for full interaction cf. base model |
|---|---|
| Own lancet (Yes = 27, No = 74) | 0.40 |
| Type of diabetes (Type 1 = 64, Type 2 = 42) | 0.47 |
| Sex (F = 52, M = 53) | 0.11 |
| Time between sampling and testing (N = 105) [days (n): 1 (39), 2 (47), 3 (17) and 4(2)] | 0.30 |
| Laboratory factor covariate (A = 15, B = 38, C = 1, D = 2, E = 17, F = 13, G = 3, H = 16) | 0.016 |
Questionnaire
One hundred and ten (86%) questionnaires were returned. Of these, 101 had a corresponding home DBS sample. The majority (99.1%) of respondents found the instructions easy to use and 83.5% found it easy to get their DBS sample in the post on time. Obtaining enough blood, blood application to the device, and deciding when there was enough blood on the device were less easy (Table 3).
Table 3.
Analysis of questionnaire responses to questions relating to the experience of using and potential use of the HemaSpot, and the information provided about HbA1c. The total number of responses to each question (n) is shown in the left hand column
| I found… | Easy or very easy to use (%) | Neither easy nor difficult (%) | Difficult or very difficult (%) |
|---|---|---|---|
| …following the instructions (n = 108) | 99.1 | 0 | 0.9 |
| …using the lancet provided (n = 94) | 89.4 | 8.5 | 2.1 |
| …getting my sample in the post on time (n = 109) | 83.5 | 7.3 | 9.2 |
| …getting enough blood (n = 109) | 50.5 | 21.1 | 28.4 |
| …applying the blood to the device (n = 108) | 44.5 | 22.2 | 33.3 |
| …deciding when I had applied enough blood (n = 109) | 56.9 | 20.2 | 22.9 |
| Agree or strongly agree (%) | Neither agree nor disagree (%) | Disagree or strongly disagree (%) | |
|---|---|---|---|
| Would use if available (n = 107) | 69.2 | 11.2 | 19.6 |
| More likely to use than making an appointment with practice nurse (n = 107) | 61.7 | 15.0 | 23.3 |
| Prefer to use this system at home compared with having blood taken at general practitioner's (n = 107) | 60.7 | 17.8 | 21.5 |
| More likely to have HbA1c test done (n = 107) | 59.8 | 15.0 | 25.2 |
| Help feel more in control of my diabetes (n = 106) | 50.0 | 22.6 | 27.4 |
| Prefer to have pack sent to them by post (n = 105) | 66.7 | 15.2 | 18.1 |
| Happy to collect pack from general practitioner (n = 104) | 51.0 | 19.2 | 29.8 |
| Found information sheet about HbA1c interesting (n = 108) | 88.0 | 10.2 | 1.9 |
| Found information sheet about HbA1c useful (n = 108) | 81.5 | 16.7 | 1.8 |
| HbA1c information sheet detail about right (n = 109) | 83.5 | 14.7 | 1.8 |
| Would like HbA1c information sheet to be more detailed (n = 107) | 28.0 | 32.7 | 39.3 |
When asked if they would use the system if it was available, 69.2% of respondents reported they would, while 61.7% agreed that they would be more likely to use a DBS system than making an appointment with their practice nurse. A similar percentage (60.7%) responded that they would have a preference for using this system at home compared to having blood taken at their general practitioner's surgery.
Sixty per cent of respondents agreed that they would be more likely to have their HbA1c test performed if the system was available, while 50.0% of respondents felt that using a system like this would help them to feel more in control of their diabetes management. The majority (88.0%) of respondents found the information provided about HbA1c interesting and 81.5% found the information useful.
Discussion
The principal finding from the study is that HemaSpot DBS prepared by study participants at home and analysed using the Tosoh G8 system produce clinically acceptable HbA1c results when the DBS method is calibrated to Tosoh G8‐determined venous HbA1c results using a general linear model. Time between sample preparation and testing did not significantly affect the calibration up to 4 days. For this analysis, the unbalanced nature of the number of observations in each ‘bin’ (i.e. the number of days between sampling time and testing) is not ideal, but it does not violate any of the assumptions in a general linear model such as this, it merely reduces the statistical power of the test.
A statistically significant effect on calibration was observed for the laboratory factor covariate tested (Table 2), where laboratory personnel were used as a proxy. For example, as a consequence of logistics, there may have been a relationship between personnel and the day, or time of day, on which a sample was tested. It is also possible that a type I error has occurred (given that five statistical hypotheses were tested, there is an estimated 23% chance of a type I error). In favour of the hypothesis that a type I error may have occurred is the observation that for each of the particular proxies the additive and interaction terms affecting the calibration relationship all had confidence intervals encompassing zero, i.e. no individual proxy appears to be statistically or clinically significantly from the rest. For full details, see Table S2.
Several HbA1c DBS studies have been published 10, 11, 13, 14, 15 with analysis by turbidimetric inhibition immunoassay 13, 16, 17, 18, HPLC analysis 10, 11, 15, 19, 20 and affinity chromatography 21, 22. A systematic review and meta‐analysis by Affan et al. 9 reported that, although results from venous and DBS were different, there was close agreement (meta‐analysis regression equation: [HbA1c]DBS = 0.9553 [HbA1c]venous + 0.2566 (%)) between venous and DBS samples, except when analysis was by affinity chromatography 9. Slope and intercept ranges in the meta‐analysis were 0.88–1.25 and 0.002–1.8%, respectively. Our model slope and intercept fit within these ranges; however, the model is only valid when used with the measurement method used in our study.
In vitro glycation of haemoglobin and degradation of HbA1c during storage have been suggested as possible reasons for differences observed between DBS and fresh blood samples 13, 23. Our results show increasing bias as HbA1c increases, which supports the in vitro glycation theory due to increased blood glucose levels (and hence in vitro glycation) associated with higher HbA1c levels.
Precision of the DBS sample elution and analysis method was assessed by repeat analysis of high and low quality control samples that had been applied to filter papers and eluted using the DBS sample elution and analysis protocol described. Coefficient of variation were comparable to coefficient of variation reported for fresh blood analysis, suggesting that minimal error is introduced due to DBS sample elution and analysis protocols.
Both venous blood collected in EDTA tubes 11, 14, 16, 18, 19, 24 and fingerprick capillary blood 10, 13, 15, 17, 20, 21, 22 have been used to prepare DBS on filter paper, with drying times ranging from 20 minutes to overnight reported 22, before placing the DBS in a storage bag or envelope. The HemaSpot device has no requirement for a pre‐drying step.
With the exception of two previous studies 10, 13, DBS have been prepared by healthcare professionals or researchers. For routine, remote monitoring of HbA1c, a DBS approach needs to be evaluated in the hands of the end user. Our findings show acceptable clinical results when DBS were prepared by users.
The stability of HbA1c on DBS has been a concern, with an indication that a time‐dependent calibration for estimating venous HbA1c levels from DBS is required 15, 16. Our findings suggest that with the HemaSpot device a single calibration could be used for samples tested up to 4 days after preparation.
In agreement with our findings, high patient satisfaction with a potential DBS HbA1c monitoring system was reported in The Netherlands 13.
Although participants in our study reported that the instructions were easy to follow, several participants experienced difficulties with blood application and in deciding when there was sufficient blood on the device; their comments included ‘I don't think I got enough blood into the device’ and ‘[it] does take a bit of work to “fill” the device to the edges’. However, concern about sufficient filling was not reflected in the results, where a strong correlation between venous and DBS HbA1c results was observed. The difficulties reported could be overcome by the inclusion of photographs of sufficiently and insufficiently filled devices with the instructions to give patients confidence that they are providing an adequate amount of blood for analysis. Additional tips on blood application could also be incorporated.
A number of participants felt that they would still prefer to have an appointment at their general practitioner's practice, because they had other tests carried out at the same time and valued the time spent with their healthcare professional. One participant wrote: ‘When visiting the nurse for an HbA1c I also get my blood pressure, weight, and feet checked and the opportunity for questions and instructions/guidance from the nurse. I would miss this testing at home.’ The introduction of a remote monitoring service for HbA1c needs to consider not only what might be gained by this approach, but also what patients might lose through a lack of contact with healthcare professionals.
The provision of information about HbA1c was welcomed by participants. The opportunity to provide simple clear information about HbA1c and its importance in the management of diabetes should be considered in any remote HbA1c monitoring approach.
The combination of a robust desiccating DBS device, home sample preparation and return by post, to our knowledge has not been reported for HbA1c, and provides the opportunity to introduce a remote sample collection service that would be equally valuable for people living with diabetes in urban areas who are working or house‐bound, as well as for those living in remote or rural locations.
The observation that the time between sampling and testing did not significantly affect the linear model indicates that use of the HemaSpot device might enable a single model to be adopted which is independent of the time between preparation of the DBS and the day of testing.
Further studies are planned to assess the performance, acceptability and service delivery options for HemaSpot DBS prepared by people with uncomplicated Type 2 diabetes whose diabetes management is supported solely by general practitioner practices.
The health economics of introducing a remote HbA1c monitoring service using HemaSpot devices needs to be assessed, taking into consideration both the immediate impacts on costs and the longer term costs associated with complications.
Study limitations
Some limitations of this study should be noted. First, participants were all regularly carrying out self‐monitoring of blood glucose and so were familiar with obtaining drops of capillary blood. The wider community with diabetes will have a similar familiarity, so this is a population in which the model derived in the study would need to be validated prior to practical application. Second, only one batch of HemaSpot devices was available to evaluate over the period of the study, so it was not possible to assess the impact of batch to batch variation. Third, the study focussed only on analysis of HbA1c using the Tosoh HbA1c analyser, the method used in the laboratory where routine HbA1c analyses for the Scottish Highlands region are performed. Fourth, the difference observed between clinic‐prepared and home‐prepared DBS remains unexplained and warrants further investigation. Finally, further exploration of the observed laboratory factor effect is needed.
Clinical implications
HbA1c monitoring plays a pivotal role in preventing complications of diabetes and therefore in achieving as good a quality of life as possible. The HemaSpot blood collection device provides an opportunity for improvements in rates of HbA1c monitoring and more effective consultations. Increased participation by the community with diabetes in self‐management would be anticipated.
Acknowledgements
The authors are very grateful to the people with diabetes who participated in the study.
Funding sources
The study was supported by a grant from the Chief Scientist Office (CZH/4/1122).
Competing interests
None declared.
Supporting information
Table S1. The deviation of HbA1c results collected on filter paper from routine dilute blood samples (mmol/mol) and the relationship (with confidence intervals) between filter paper and dilute sample HbA1c results.
Table S2. The effect of the addition of potential covariates on top of the base model of the venous reading (dependent variable) regressed against the home result (the independent variable). Each additional covariate was added separately and as a full interaction with the home result. Each nested pair of models (without covariate and with) were compared using an analysis of variance. For each nested pairwise comparison of models the dataset used was the full dataset, less any observations that had missing data for either the home result or the added covariate.
Diabet. Med. 37: 1463–1470(2020)
References
- 1. Diabetes Control and Complications Trial (DCCT) Research Group . The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent Diabetes Mellitus. N Eng J Med 1993; 329: 977–998. [Google Scholar]
- 2. Hayes A, Arima H, Woodward M, Chalmers J, Poulter N, Hamet P et al Changes in quality of life associated with complications of diabetes: results from the ADVANCE study. Value Health 2016; 19: 36–41. [DOI] [PubMed] [Google Scholar]
- 3. Nielsen HB, Ovesen LL, Mortensen LH, Lau CJ, Joensen LE. Type 1 diabetes, quality of life, occupational status and education level – a comparative population‐based study. Diabetes Res Clin Prac 2016; 121: 62–68. [DOI] [PubMed] [Google Scholar]
- 4. Lenters‐Westra E, Slingerland RJ. Three of 7 hemoglobin A1c point‐of‐care instruments do not meet generally accepted analytical performance criteria. Clin Chem 2014; 60: 1062–1072. [DOI] [PubMed] [Google Scholar]
- 5. Scottish Government Diabetes Action Plan. Scottish Government, 2010.
- 6. Scottish Government Diabetes Improvement Plan. Scottish Government, 2014.
- 7. McDade TW, Williams S, Snodgrass JJ. What a drop can do: dried blood spots as a minimally invasive method for integrating biomarkers into population‐based research. Demography 2007; 44: 899–925. [DOI] [PubMed] [Google Scholar]
- 8. Mei JV, Alexander JR, Adam BW, Hannon WH. Use of filter paper for the collection and analysis of human whole blood specimens. J Nutr 2001; 131: 1631s–1636s. [DOI] [PubMed] [Google Scholar]
- 9. Affan ET, Praveen D, Chow CK, Neal BC. Comparability of HbA1c and lipids measured with dried blood spot versus venous samples: a systematic review and metaanalysis. BMC Clin Pathol 2014; 14: 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mastronardi CA, Whittle B, Tunningley R, Neeman T, Paz‐Filho G. The use of dried blood spot sampling for the measurement of HbA1c: a cross‐sectional study. BMC Clin Pathol 2015; 15: 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maleska A, Hirtz C, Casteleyn E, Villard O, Ducos J, Avignon A, Sultan A et al Comparison of HbA1c detection in whole blood and dried blood spots using an automated ion‐exchange HPLC system. Bioanalysis 2017; 9: 427–434. [DOI] [PubMed] [Google Scholar]
- 12. Clinical and Laboratory Standards Institute Measurement Procedure Comparison and Bias Estimation Using Patient Samples . Approved Guideline—Third Edition. CLSI document EP09‐A3. Wayne, PA: Clinical and Laboratory Standards Institute, 2013. [Google Scholar]
- 13. Fokkema M, Bakker A, de Boer F, Kooistra J, de Vries S, Wolthuis A. HbA1c measurements from dried blood spots: validation and patient satisfaction. Clin Chem Lab Med 2009; 40: 1259–1264. [DOI] [PubMed] [Google Scholar]
- 14. Lakshmy R, Gupta R. Measurement of glycated hemoglobin A1c from dried blood by turbidimetric immunoassay. J Diabetes Sci Technol 2009; 3: 1203–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lomeo A, Bolner A, Scattolo N, Guzzo P, Amadori F, Sartori S et al HPLC analysis of HbA1c in dried blood spot samples (DBS): a reliable future for diabetes monitoring. Clin Lab 2008; 54: 161–167. [PubMed] [Google Scholar]
- 16. Jones TG, Warber KD, Roberts BD. Analysis of hemoglobin A1c from dried blood spot samples with the tina‐quant(®) II immunoturbidimetric method. J Diabetes Sci Technol 2010; 4: 244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anjali S, Geethanjali F, Selva Kumar R, Seshadri M. Accuracy of filter paper method for measuring glycated hemoglobin. J Assoc Physicians India 2007; 55: 115–119. [PubMed] [Google Scholar]
- 18. Tabatabaei O, Heshmat R, Omidfar K, Pasalar P, Delavari A, Keshtkar A et al Glycated hemoglobin measurements from dried blood spots: reliability and relation to results obtained from whole blood samples. J Diabetes Sci Technol 2011; 10: 1–6. [Google Scholar]
- 19. Egier DA, Keys JL, Hall SK, McQueen MJ. Measurement of hemoglobin A1c from filter papers for population‐based studies. Clin Chem 2011; 57: 577–585. [DOI] [PubMed] [Google Scholar]
- 20. Lacher D, Berman L, Chen T‐C, Porter K. Comparison of dried blood spot to venous methods for hemoglobin A1c, glucose, total cholesterol, high‐density lipoprotein cholesterol, and C‐reactive protein. Clin Chim Acta 2013; 422: 54–58. [DOI] [PubMed] [Google Scholar]
- 21. Little RR, McKenzie EM, Wiedmeyer HM, England JD, Goldstein DE. Collection of blood on filter paper for measurement of glycated hemoglobin by affinity chromatogrphy. Clin Chem 1986; 32: 869–871. [PubMed] [Google Scholar]
- 22. Gay EC, Cruickshanks KJ, Chase HP, Klingensmith G, Hamman RF. Accuracy of a filter paper method for measuring glycosylated hemoglobin. Diabetes Care 1992; 15: 108–110. [DOI] [PubMed] [Google Scholar]
- 23. Eross J, Kreutzmann D, Crowell C, Silink M. Glycated haemoglobin measurement in dried blood on filter paper. Clin Chem 1986; 32: 2222. [PubMed] [Google Scholar]
- 24. Buxton OM, Malarick K, Wang W, Seeman T. Changes in dried blood spot HbA1c with varied post‐collection conditions. Clin Chem 2009; 55: 1034–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The deviation of HbA1c results collected on filter paper from routine dilute blood samples (mmol/mol) and the relationship (with confidence intervals) between filter paper and dilute sample HbA1c results.
Table S2. The effect of the addition of potential covariates on top of the base model of the venous reading (dependent variable) regressed against the home result (the independent variable). Each additional covariate was added separately and as a full interaction with the home result. Each nested pair of models (without covariate and with) were compared using an analysis of variance. For each nested pairwise comparison of models the dataset used was the full dataset, less any observations that had missing data for either the home result or the added covariate.


