Abstract
Background and purpose
The diagnosis of rare movement disorders is difficult and specific management programmes are not well defined. Thus, in order to capture and assess care needs, the European Reference Network for Rare Neurological Diseases has performed an explorative care need survey across all European Union (EU) countries.
Methods
This is a multicentre, cross‐sectional study. A survey about the management of different rare movement disorders (group 1, dystonia, paroxysmal dyskinesia and neurodegeneration with brain iron accumulation; group 2, ataxias and hereditary spastic paraparesis; group 3, atypical parkinsonism; group 4, choreas) was sent to an expert in each group of disorders from each EU country.
Results
Some EU countries claimed for an increase of teaching courses. Genetic testing was not readily available in a significant number of countries. Regarding management, patients’ accessibility to tertiary hospitals, to experts and to multidisciplinary teams was unequal between countries and groups of diseases. The availability of therapeutic options, such as botulinum toxin or more invasive treatments like deep brain stimulation, was limited in some countries.
Conclusions
The management of these conditions in EU countries is unequal. The survey provides evidence that a European care‐focused network that is able to address the unmet rare neurological disease care needs and inequalities is highly warranted.
Keywords: disease management, Europe, movement disorders, rare diseases, survey
Introduction
The definition of rare diseases varies in different countries, but generally they are considered as those with a prevalence lower than 5 in 10 000 people [1]. In Europe, it is estimated that more than 500 000 persons are affected by rare neurological diseases (RNDs) [2]. Due to their low prevalence, clinicians are often not familiar with these conditions and making a prompt diagnosis is challenging [3]. Moreover, for many diseases, specific management programmes are not well defined [4]. A global effort is necessary in order to raise awareness, improve knowledge and create specific diagnostic and management guidelines.
As a response to this challenge, which is shared by all rare diseases, European Reference Networks (ERNs) have been launched in 2017 by the European Union (EU) Board of Member States. This is a pan‐European initiative to facilitate access to highly specialized healthcare for patients with rare or low prevalence complex diseases and to support the cooperation of healthcare providers at the European level in the field of rare diseases.
The European Reference Network for Rare Neurological Diseases (ERN‐RND), one of 24 ERNs, is a network of the European RND expertise centres. At present, it has 31 members from 13 countries; however, through a currently ongoing expansion process, the ERN‐RND will very likely have more than 60 members and be covering the vast majority of EU countries by the end of 2020. The huge heterogeneity of RNDs, healthcare systems and socio‐economic situations in Europe, as well as the still limited clinical expert workforce for RNDs, at a time of rapid research‐driven innovations, means that there is a very real risk that not all patients with RNDs across Europe might benefit from improved and emerging opportunities in diagnosis, care and treatment.
Thus, in order to capture and assess care needs that the ERN‐RND can address, taking into account disease‐ as well as country‐specific differences, this explorative care need survey was performed for all rare movement disorders (MDs) covered by the ERN‐RND across all EU countries. Patient representatives have significantly contributed to the design of the study.
Methodology
Design
This is a multicentre, cross‐sectional study. A survey about the management of different rare MDs designed by the ERN‐RND working group on care coordination was sent to an expert in each group of disorders from each EU country.
Groups of movement disorders assessed
Four different groups of rare MDs were studied: group 1 included dystonia, paroxysmal dyskinesia and neurodegeneration with brain iron accumulation (NBIA) (group 1‐DYS); group 2, ataxias and hereditary spastic paraparesis (HSP) (group 2‐ATX/HSP); group 3, atypical parkinsonism such as progressive supranuclear palsy, multiple system atrophy and corticobasal degeneration (group 3‐AP); group 4 included Huntington’s disease (HD) and other choreas (group 4‐C).
Participants
Participants were one expert for each group of disorders and for each EU country. Experts from Norway and Iceland were also asked, as European countries outside the EU are also invited to join the ERN‐RND. The experts were chosen because of their expertise in a specific rare MD and also for the prominent role they play in their management nationally. In cases of countries without specialists in some of these disorders, a university neurology hospital with a good reputation in that country was contacted and the most appropriate neurologist answered the survey.
Questionnaire
A questionnaire was developed based on a modified version of the published survey in the study by Valadas et al. [5], adapted to the current study and tuned to the respective group of rare MDs. The questionnaire was divided into three sections: section I, characterization of participants (i.e. name, qualifications, affiliation and main research area of interest); section II, characterization of the country (i.e. accessibility, training in all kinds of healthcare providers, network, patient associations, tertiary centres, ancillary tests, treatment availability and research); section III was an open question, asking for the three main measures that, as a personal opinion of the participants, should be urgently implemented to improve the management on that particular MD in their specific country. Section II was composed of close‐ended questions (yes/no; easily accessible/satisfactory or accessible with some difficulty/difficult or not available/I don’t know). Specific questions about specific treatments and visits to other specialists for each group of MDs were also included.
In September 2018, the questionnaire was sent by email to the participants, including instructions on how to fill in the form. Up to three reminders were sent and, in the case of no response, a second expert was consulted. Recruitment finished on 1 May 2019. The results were discussed at the ERN‐RND annual meeting in Siena in June 2019.
Ethical statement
Ethical approval was not necessary for this study.
Statistical analysis
A descriptive analysis was performed. Qualitative variables are presented as absolute and relative frequencies. The total of responses by group is indicated for all the items. Data analysis was carried out using Stata 14.0 (Stata Corp, College Station, TX, USA) for Windows.
Results
Of the 28 EU countries plus Norway and Iceland, 26 responses were obtained in group 1‐DYS, 21 responses in group 2‐ATX/HSP, 19 responses in group 3‐AP and 14 responses in group 4‐C (Fig. 1). A total of 80 different respondents with expertise in a specific MD were approached.
Figure 1.
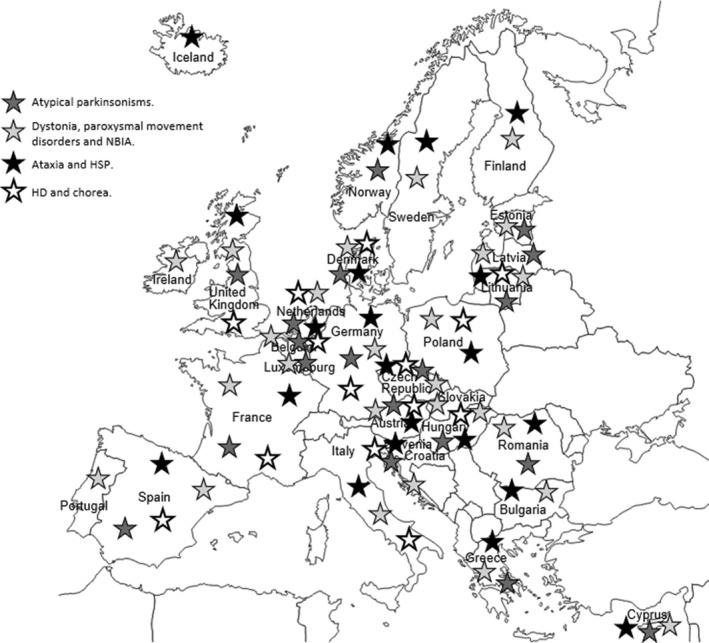
Europe map of the surveyed countries for each group of diseases.
Participants’ characterization
Main research area of interest
The majority of respondents in all the groups were neurologists and their main area of research interest was clinical research (42/80), followed by genetics (10/80) and clinical trials (10/80).
Country’s characterization
Movement disorder global view in each country
Expertise and patients’ accessibility
The accessibility to MD experts in the groups (79/80) was difficult in 27.8%, satisfactory in 55.7% and easy in 16.5%. Specifically, the access to MD experts for patients in group 1‐DYS (26/26) was difficult in 26.9%, satisfactory in 50.0% and easy in 23.1%. The access to experts in group 2‐ATX/HSP (20/21) was difficult in 20.0%, satisfactory in 75.0% and easy in 5.0%. In group 3‐AP (19/19) the access was difficult in 31.6%, satisfactory in 52.6% and easy in 15.8%. Finally, in group 4‐C (14/14), the accessibility was difficult in 35.7%, satisfactory in 42.9% and easy in 21.4%. The main reasons for difficult accessibility were the small number of experts who usually work at university hospitals or in private institutions with expensive consultations, long waiting lists and lack of knowledge amongst clinicians.
Training
All countries had MD experts, except Iceland, and more than 90% had teaching courses or symposia in MDs for residents and general neurologists. Teaching courses in MDs for general practitioners were less common, being present in around 50% of the countries. Specific training in MDs was available for nurses, physiotherapists and speech therapists in 40%, 33% and 28% of the countries, respectively.
Countries that claimed for an increase of teaching courses for neurologists and general medical practitioners were Lithuania, Iceland, Bulgaria, Cyprus, Slovakia, Latvia, Hungary, Romania and Estonia. Almost all the countries requested teaching courses for other healthcare providers.
Scientific societies
More than 80% of the countries had an official MD society or working group.
Answers for each group of disorders
Research
A total of six different types of research (basic, clinical, genetics, brain banks, neurophysiology, imaging) were requested. Clinical research and genetic research were the most frequent type of research in groups 1‐DYS and 2‐ATX/HSP. For group 3‐AP, all kinds of research were similarly present, with a slight predominance of clinical research (68.4%) and neuroimaging studies (52.6%). Finally, in group 4‐C, basic research and clinical studies were the most common. Anatomopathological research was the least in all the groups.
Association
In group 1‐DYS, more than half (65.4%, 17/26) of the countries had patient associations for dystonia but not for paroxysmal dyskinesia or NBIA (7.7% and 15.4%, respectively). In group 2‐ATX/HSP, 52.4% (11/21) of the countries had a national ataxia/HSP patient association and in group 3‐AP the prevalence was lower (21.4%), especially for patients with corticobasal degeneration (11.1%, 2/18). All the responders from group 4‐C said that they had national patient associations.
Management
The proportion of patients who were evaluated in tertiary centres at least once in the course of the disease was 68.4% in group 3‐AP and approximately 50% in the others.
In group 1‐DYS, 100% (26/26) of the countries had experts in the field of dystonia, and to a lesser extent in paroxysmal dyskinesias (88.5%, 23/26) and NBIA (69.2%, 18/26). Over 70% of the countries had tertiary centres for the management of these diseases. As for group 2‐ATX/HSP, 90.5% (19/21) of the countries had ataxia/HSP experts and 61.9% (13/21) had tertiary centres for these diseases. In group 3‐AP, there were experts in all three types of atypical parkinsonism in 72.2% (13/18) of the countries and there were tertiary centres for management of these conditions in 83.3% (15/18) of the countries. Group 4‐C had HD experts in all countries (100%, 14/14) and also tertiary centres for its management.
Networking
In group 1‐DYS, networking was frequent for dystonia (73.1%) but uncommon in paroxysmal dyskinesias (26.9%) or NBIA (42.3%). Around 40% of the respondents said that there was a networking group in ataxia/HSP and in atypical parkinsonism. Almost all experts of group 4‐C answered that there was a networking group (86%) in their countries.
Ancillary tests
In group 1‐DYS, magnetic resonance imaging was available in all countries. Neurophysiological tests were available except in two countries. Genetic testing was accessible in 96.2% (25/26) of the countries, but with some difficulties in 46.2% (12/26) due mainly to economic reasons and too few geneticists. Countries with better accessibility were Belgium, Czech Republic, Denmark, Germany, Ireland, Italy, Sweden, Finland, France, Norway, Netherlands and Portugal. However, some of these countries also had some limitations such as, for example, a fixed number of tests allowed to be done, and others presented difficulties in accessing more sophisticated techniques such as next‐generation sequencing (NGS).
In group 2‐ATX/HSP, genetic and metabolic investigations were accessible with some difficulty in 47.6% (10/21) and 57.1% (12/21) of the countries, respectively. The main problems were economic reasons like no insurance coverage, too few centres performing the tests and long waiting lists. Neurophysiological tests and neuroimaging were 100% available.
In group 3‐AP, magnetic resonance imaging and neuropsychological testing were available in all the countries (19/19). Dopamine transporter single‐photon emission computed tomography and fluorodeoxyglucose positron emission tomography were not available in two countries, with the single‐photon emission computed tomography test more easily accessible than positron emission tomography (68.4%, 13/19 vs. 31.6%, 6/19) in the others. The possibility of consulting a sleep specialist or an ear, nose and throat specialist was available for all countries except Romania, according to the respondents.
In group 4‐C, genetic testing, counselling and prenatal testing were available in all countries. Pre‐implantation genetic diagnosis and exclusion pre‐implantation genetic diagnosis were rarely or not available in Austria and Lithuania.
Treatment availability
In group 1‐DYS, oral drugs such as anticholinergics, antiepileptics, benzodiazepines, antidopaminergic drugs and levodopa were easily available (>85 %) in most countries. The access to dopaminergic blockers (tetrabenazine) was easy in 69.2% (18/26), with some difficulties in 26.9% (7/26) and not available in 3.8% (1/26) of the countries. Botulinum toxin was accessible in all countries but in 30.8% (8/26) with some difficulties. Finally, deep brain stimulation (DBS) was easily accessible in 53.8% (14/26), accessible with some difficulties in 38.5% (10/26) and not available in 7.7% (2/26).
In group 2‐ATX/HSP, anti‐spasticity treatment (e.g. botulinum toxin injections, intrathecal baclofen) was available in all countries but with some difficulties in 47.6% (10/21).
In group 3‐AP, levodopa (100%, 19/19), midodrine (66.7%, 12/18) and fludrocortisone (89.5%, 17/19) were widely available. Botulinum toxin was available in all countries but with limitations in some of them (33.3%, 6/18).
Finally, in group 4‐C, drugs such as antipsychotics, antidepressants, benzodiazepines and antiepileptics were widely and easily available.
Physiotherapy, occupational therapy and language therapy were available in all groups but with some difficulties in 45%–50% of countries. The main problem in terms of accessibility to these therapies were long waiting lists, lack of reimbursement, no possibility of chronic treatment and low number of expert allied healthcare professionals focused on the specific disorders.
Implementation of three main measures to improve the management
Responding to the question ‘which are the three main measures to improve the management of the respective RND patients’, the following seven measures (from a total of 119) were identified to be the most important ones to be implemented in European countries:
Development of multidisciplinary teams composed of neurologists, geneticists, rehabilitation physicians, specialized nurses, psychologists, physiotherapists, speech and language and occupational therapists. Patients should be visited at least one time by these teams. Responders also claimed for better access to rehabilitation therapies (n: 29/119)
Implementation of educational activities to enhance recognition of rare MDs amongst healthcare professionals and in the general population (n: 24/119)
Improvement of the accessibility to standard and advanced genetic testing and to clinical geneticists (n: 16/119)
Development of more dedicated tertiary centres and more expertise in the field (n: 9/119)
Facilitation of access to botulinum toxin and DBS centres, and reimbursement of these treatments (n: 9/119)
Better access to international therapeutic trials and promotion of clinical research (n: 9/119)
Development of national guidelines, which match financial realities (n: 8/119)
Discussion
This study explores the management of different RNDs in the field of MDs and the care needs of European countries, in terms of patient accessibility, networking, availability of therapies and ancillary tests, research and training for different type of healthcare professionals. Overall, the main concerns of the respondents were related to limited education for healthcare professionals, limited numbers of experts and multidisciplinary teams, the need to promote clinical trials, limited access to genetic testing and limited availability of various therapeutic options.
Diagnosing rare MDs is often challenging and requires increased awareness and skilled experts. So, accessibility to these experts and training not only for neurologists but also for residents, general practitioners and other healthcare providers are essential. However, our results brought to light that the accessibility for patients to experts was difficult in 20%–35% of the countries and only about a quarter of the countries considered it easy. The main reasons for difficult accessibility, according to the respondents, were the small number of experts in these disorders and the lack of knowledge amongst non‐expert clinicians. Moreover, some countries did not have experts in certain diseases such as NBIA, paroxysmal dyskinesia, ataxias or atypical parkinsonism. Regarding training in MDs, almost all countries had MD experts and teaching courses or symposia in MDs for residents and general neurologists. However, MD teaching courses for general practitioners, nurses, physiotherapists and speech therapists were rarely provided. In many countries, primary care physicians and general neurologists are part of the first circle of care of the patient. This means that it is important to disseminate knowledge not only to MD experts but also to all other care providers. With technological progress, continuing education and on‐the‐job training are paramount to keep existing skills in line with new developments for all the professionals involved. Patients with RNDs require multidisciplinary management. Multidisciplinary teams should consider the participation of all the professionals aforementioned in point 1 of the implementation measures but the role of other healthcare professionals such as psychiatrists and social workers should also be considered.
The low prevalence of individual RNDs makes networking an important tool to support patients and families and to promote exchange of information, training and research [6]. However, according to the respondents, networking groups were only present in less than 45% of the countries for paroxysmal dyskinesia, NBIA, ataxia/HSP and atypical parkinsonism. In contrast, for dystonia and HD, networking was present in over 70% of the countries.
Regarding ancillary tests, genetics play a key role in RNDs. About 80% of these diseases have a genetic basis [7]. A definitive molecular diagnosis facilitates the access to healthcare resources, reduces uncertainty in patients and families, and provides accurate recurrence risk counselling [3]. However, our results show that the accessibility to genetic testing is not satisfactory in many European countries. In particular, experts from groups 1‐DYS and 2‐ATX/HSP highlighted the need for better access to genetic tests and advanced techniques such as NGS. NGS procedures allow massive‐scale DNA sequencing. In recent years, several studies have shown that NGS is more cost‐effective and can increase diagnostic yield in certain patients compared with other more restricted sequencing technologies [8, 9]. However, a majority of the respondents find it difficult to access these advanced technologies mainly due to financial reasons and to the low number of genetic centres and a shortage of geneticists. Moreover, it is well known that results from NGS are difficult to interpret given the vast quantities of sequencing data generated including variants of unpredictable meaning [10]. Neurologists and residents must familiarize themselves with this advanced technology, but it is crucial to promote the formation of more geneticists who will handle this complex technology and genetic counselling. Guidelines for genetic evaluation and a consensus on when to stop when a mutation is not found are also important to define.
With regard to treatment, the results showed that oral drugs were easily available with the exception of tetrabenazine that was available in most of the countries but with some difficulties. Regarding botulinum toxin in group 1‐DYS, the results were similar to those indicated in the paper published by Valadas et al. in 2016 [5]. This treatment was accessible in all countries but in 30.8% with some difficulties, mainly related to financial issues like reimbursement. More invasive treatments like DBS were more difficult to access or absent in some countries.
Treatment access becomes even more important as advanced therapies such as adeno‐associated virus based gene therapy and antisense oligonucleotides are being developed for a number of RNDs [11]. Given the high costs and complexity of these therapies, the ERN‐RND centres that have been acknowledged to be able to provide highly specialized care for RND patients might thus be considered as a suitable means to provide equal, high quality and diligently monitored access to such advanced therapies.
Neurological rare diseases tend to be chronic and patients present neurological deficits that require a multidisciplinary approach. These RNDs are very heterogeneous and complex, with some symptoms exceeding movement abnormalities, including dysphagia, language problems and nutrition deficits. So it seems that the installation of specific therapies by trained professionals for these problems is necessary. However, the results of the survey showed that accessibility to these experts was difficult in more than a half the countries. Although more studies with proper methodology and long follow‐up for non‐pharmacological therapies are needed, some of them suggest they can provide benefit and improvement of the patient’s quality of life [12, 13, 14]. Acknowledging the importance of non‐pharmacological therapies for RNDs, the ERN‐RND decided to form a specific working group for neurorehabilitation at its last Board meeting in November 2019.
The ERN‐RND has been established as an expertise‐driven and healthcare‐focused network in order to improve (i) access to high quality RND care, (ii) the sharing of RND knowledge and data, as well as (iii) the operation of multidisciplinary teams to provide e‐consultation (through the Clinical Patient Management System, http://www.ern‐rnd.eu/cpms/) to care professionals who seek support for their respective rare and complex RND patients. In fact, a European network is able to address key rarities (i.e. care needs) which are linked to the very problem of rare diseases such as rarity of experts, missing knowledge and data as well as missing training opportunities. However, without a systematic, sustainable and financed integration of the ERN into the national healthcare systems of the EU, achievements, synergies and impacts will not bring about the required effect and will remain dependent on the volatile motivation of participating hospitals and experts.
This study has some limitations. First, the survey did not cover palliative care issues. Taking into account that rare neurological disorders often have very complex needs that are amenable to a palliative approach, this should have been a relevant care need to explore. Secondly, although a large proportion of countries of the EU were included in the study, some of the groups presented a lower rate of respondents. Thirdly, the results were based on a single subject response per country and per group of diseases. This might have biased the results of care needs. However, all respondents were well‐known physicians and they were likely to be well informed about the management of these disorders in their respective country. Furthermore, in particular with focus on the main measures that were identified to address care needs, the commonalities across disease groups and EU countries were striking.
In conclusion, the management of these conditions in EU countries is unequal. Variations in diagnosis, care and treatment may impact directly on patients’ outcomes. This survey offers insights into the current unmet needs of healthcare professionals and patients with these RNDs in Europe and should be considered for healthcare decisions.
The survey has a high informational value for the ERN‐RND and will thus inform planning and operation of the ERN‐RND for the forthcoming years. It provides evidence that a European care‐focused network that is able to address the unmet RND care needs and inequalities is highly warranted.
Disclosure of conflicts of interest
HG: consultancy agreement with Roche. CP: received a grant Rio Hortega from Instituto de Salud Carlos III (CM18/00072). AD: received a grant from Fondation pour la Recherche Medicale (FDM201806005951). vONJH: none. MI: none. MJM: honoraria for consulting and lectures from Merz Pharma and Allergan SA; grants from the MJ Fox Foundation (CP041639), Fundacio la Marato de TV3 (288/C/2014) and Fondo de Investigaciones Sanitarias (FIS)‐ISCIII (PI17/00096). BvdW: receives research support from Radboud University Nijmegen Medical Centre, ZonMW, Hersenstichting, Gossweiler Foundation and uniQure. WGM: consultancy for Lundbeck and Biohaven, teaching honoraria from UCB and Boehringer Ingelheim, and fees for editorial activities with Springer, outside the submitted work. UA: none. RC: none. MAJT: reports grants from the Netherlands Organisation for Health Research and Development ZonMW Topsubsidie (91218013), the European Fund for Regional Development from the EU (01492947), from Dystonia Medical Research Foundation, from Stichting Wetenschapsfonds Dystonie Vereniging, from Fonds Psychische Gezondheid, from Phelps Stichting, and an unrestricted grant from Actelion and AOP Orphan Pharmaceuticals AG.
Acknowledgements
This work is generated within the European Reference Network for Rare Neurological Diseases, Project ID No. 739510. The patient advocates of ERN‐RND contributed to the discussion of the used questionnaires. All the experts that kindly answered the survey are acknowledged.
[Correction added on 08 July 2020 after first online publication: Last author's name has been corrected from 'MAJ Tijssen' to 'M. A. Tijssen' in this current version.]
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. European Union . Regulation (EC) No.141/2000 of the European Parliament and of the Council of 16 December 1999 on orphan medicinal products; 2000. https://eur‐lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2000:018:0001:0005:EN:PDF (accessed 22/11/2019).
- 2. Graessner H, Brunelle A, Reinhard C, Hermanns S, Post A. European reference network for rare neurological diseases – ERN‐RND. Information Brochure 2020. http://www.ern‐rnd.eu/wp‐content/uploads/2020/05/ERN‐RND_info_brochure_2020.pdf (accessed 04/04/2020).
- 3. Boycott KM, Rath A, Chong JX, et al International cooperation to enable the diagnosis of all rare genetic diseases. Am J Hum Genet 2017; 100: 695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Melnikova I. Rare diseases and orphan drugs. Nat Rev Drug Discov 2012; 11: 267–268. [DOI] [PubMed] [Google Scholar]
- 5. Valadas A, Contarino MF, Albanese A, et al Management of dystonia in Europe: a survey of the European network for the study of the dystonia syndromes. Eur J Neurol 2016; 23: 772–779. [DOI] [PubMed] [Google Scholar]
- 6. Jen JC, Ashizawa T, Griggs RC, Waters MF. Rare neurological channelopathies – networks to study patients, pathogenesis and treatment. Nat Rev Neurol 2016; 12: 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nguengang Wakap S, Lambert DM, Olry A, et al Estimating cumulative point prevalence of rare diseases: analysis of the Orphanet database. Eur J Hum Genet 2019; 28: 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang Y, Muzny DM, Xia F, et al Molecular findings among patients referred for clinical whole‐exome sequencing. JAMA 2014; 312: 1870–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nolan D, Carlson M. Whole exome sequencing in pediatric neurology patients: clinical implications and estimated cost analysis. J Child Neurol 2016; 31: 887–894. [DOI] [PubMed] [Google Scholar]
- 10. Sun L, Pfeifer JD. Pitfalls in molecular diagnostics. Semin Diagn Pathol 2019; 36: 342–354. [DOI] [PubMed] [Google Scholar]
- 11. Talbot K, Wood MJA. Wrangling RNA: antisense oligonucleotides for neurological disorders. Sci Transl Med 2019; 11: 2069. [DOI] [PubMed] [Google Scholar]
- 12. Castagna A, Caronni A, Crippa A, et al Sensorimotor Perceptive Rehabilitation Integrated (SPRInt) program: exercises with augmented movement feedback associated to botulinum neurotoxin in idiopathic cervical dystonia – an observational study. Neurol Sci 2020; 41: 131–138. [DOI] [PubMed] [Google Scholar]
- 13. Milne SC, Corben LA, Georgiou‐Karistianis N, Delatycki MB, Yiu EM. Rehabilitation for individuals with genetic degenerative ataxia: a systematic review. Neurorehabil Neural Repair 2017; 31: 609–622. [DOI] [PubMed] [Google Scholar]
- 14. Cruickshank TM, Reyes AP, Penailillo LE, et al Effects of multidisciplinary therapy on physical function in Huntington’s disease. Acta Neurol Scand 2018; 138: 500–507. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


