Abstract
Background and purpose
The use of proton‐pump inhibitors (PPIs) was reported to be associated with increased mortality risk and has been proposed as a potential risk factor for neurodegenerative diseases. We aimed to assess the impact of PPI use on survival in patients with dementia as compared with controls.
Methods
This register‐based control‐matched cohort study included 28 428 patients with dementia ascertained by the prescription of antidementia drugs and two control individuals matched by sex, age and area of residence for each patient with dementia during the study period from 1 January 2005 to 30 June 2016. Cumulative defined daily doses (DDDs) of PPIs were extracted from the health insurance prescription registries. A multivariate Cox regression model for non‐proportional hazards was used to analyse mortality risk in dependence of PPI exposure, which was limited to 1 year preceding the date of cohort entry (index date) in order to avoid immortal time bias.
Results
The PPI exposure of 100 DDDs in the year before the index date was associated with an increased mortality risk in patients with dementia (adjusted hazard ratio, 1.07; 95% confidence intervals, 1.03–1.12), but also in controls (adjusted hazard ratio, 1.47; 95% confidence intervals, 1.31–1.64). The mortality risk in relation to PPI use was significantly lower in patients with dementia as compared with controls (P < 0.0001) and highest in the first 2 years after the index date in both cohorts.
Conclusions
Our findings promote more stringent pharmacovigilance strategies to avoid PPI use in cases lacking a clear indication for therapy or where potential risks outweigh the benefits.
Keywords: dementia, immortal time bias, pharmacoepidemiology, proton‐pump inhibitor
Introduction
Dementia comprises a group of neurodegenerative syndromes with the common features of progressive cognitive decline and deterioration of the ability to maintain social and occupational activities. There is still no cure for dementia but there are a number of symptomatic therapies including the cholinesterase inhibitors rivastigmine, donepezil and galantamine and the partial N‐methyl‐d‐aspartate receptor antagonist memantine. The World Alzheimer Report in 2018 stated that dementia affects around 50 million people worldwide and the total worldwide cost was estimated to be about $1 trillion [1]. Dementia has thus been recognized as a global health challenge [2] and there are emerging trends to face this burden with global action plans as adopted by the World Health Organisation [3].
A number of risk factors have been described as being associated with dementia including low educational attainment, midlife obesity, hypertension, diabetes mellitus, smoking and physical inactivity [4, 5]. In two pharmacoepidemiological cohort studies, the use of proton‐pump inhibitors (PPIs) was shown to constitute another risk factor for dementia [6, 7], which, however, was in contrast to other studies [8, 9]. Methodological differences between the reported studies have been discussed as contributing to the disparity of the findings, highlighting the insufficient bias limitation in studies reporting an association between PPI use and dementia [10].
Proton‐pump inhibitors are among the most widely prescribed medications [11] and associated with a number of adverse effects including renal disease, hypomagnesaemia, infections and osteoporotic fractures [12]. With regard to these adverse events, two recent observational cohort studies reported an increased risk of death associated with the use of PPIs [13, 14]. PPIs have also been suggested to have a specific effect on Alzheimer’s disease pathophysiology [15] and, potentially, Alzheimer’s disease progression. Potential biological mechanisms underlying the detrimental effect of PPIs could be related to an impairment of autophagy and lysosomal acidification by inhibition of vacuolar H+ ATPases and were shown to be associated with telomere erosion and the accelerated senescence of endothelial cells [16].
The use of PPIs might thus represent a frequent and modifiable risk factor in dementia patients with the need to better understand their impact on disease course and survival. For this purpose, we used a large national Austrian prescription database to assess the impact of the cumulative PPI exposure on survival in patients with dementia and matched controls.
Methods
Ethics approval and consent to participate
This study was approved by the Ethical Committee of the Medical University of Vienna (EK 2049/2016). Informed consent was not obtained due to the use of pseudonymized patient information.
Data source and study population
The data in this retrospective control‐matched cohort study are based on health insurance registries of 13 Austrian statutory sickness funds, which together capture >98% of the total Austrian population (a total of 8 506 925 people were covered by the 13 sickness funds in 2015; the total Austrian population was 8 670 690 according to the register‐based census held on 31 October 2015 [17]). The registries are maintained for administrative purposes and the reimbursement of pharmacy claims, and comprise each insurant’s prescription data [coded by the Anatomical Therapeutic Chemical (ATC) code, https://www.whocc.no/atc_ddd_index/], hospital discharge diagnoses (coded according to the International Classification of Disease, 10th revision) and demographic details, but no clinical information.
Ascertainment of patients with dementia and controls
To identify patients with dementia, the registries were searched for individuals who were prescribed an antidementia drug (ADD) during the study period from 1 January 2005 to 30 June 2016 with a follow‐up time of at least 4 years. ADDs included donepezil (ATC code N06DA02), rivastigmine (N06DA03), galantamine (N06DA04) and memantine (N06DX01). As ADDs are only approved for dementia with full coverage of the costs in Austria, the diagnosis could reliably be deduced from ADD prescriptions with the date of cohort entry (index date) defined by the first ADD prescription. Only individuals aged ≥18 years were included. For each patient with dementia, two control individuals without ADD prescriptions during the study period were selected from the registries. Controls with a follow‐up time of at least 4 years were matched for sex, age at index date and area of residence (coded by the zip code). Patients with dementia and controls were pseudonymized with a 40‐figure alphanumeric code before being analysed further. Demographic parameters for patients with dementia and controls included gender, date of birth and date of death. The extraction of prescription data was hypothesis‐driven and included PPIs (ATC code A02BC) and non‐steroidal anti‐inflammatory drugs (NSAIDs) (ATC code M01A), with which PPIs are often coprescribed to avoid or treat NSAID‐associated ulcers and other gastrointestinal complications [18], and the number of distinct drug classes prescribed to an individual person. Survival analyses were adjusted for NSAID exposure in order to avoid confounding by reverse causality [19].
Statistical analysis
In both dementia patients and controls, survival was assessed from the index date until death or censoring. Kaplan–Meier analyses and log‐rank tests were performed to assess survival in relation to PPI use. Exposure to PPIs was defined as a continuous variable by the calculation of the cumulative defined daily dose (cDDD) in the year preceding the index date in order to avoid immortal time bias, with immortal time referring to a span of time in the observation period of a cohort during which the outcome under study could not have occurred [20]. Hazard ratios (HRs) were calculated for a unit of 100 defined daily doses (DDDs) in order to obtain more expressive figures, as it was unlikely that the risk per unit cDDD was substantial. A multivariate Cox regression model for non‐proportional hazards [21] was used to analyse the risk of death in relation to PPI exposure, which was adjusted for sex, age at the index date, NSAID exposure and the number of distinct drug classes prescribed to an individual person. The number of distinct drug classes (defined by different ATC codes) is a validated comorbidity measure predicting mortality and was thus used to adjust for comorbidity in the Cox regression model [22]. To determine particularly vulnerable subgroups in the study population, the multivariate Cox regression model was again applied after stratification of both cohorts by age at the index date and by the presence of cardiovascular disease, which was previously reported to be associated with an excess mortality rate upon PPI exposure [14]. Due to the register‐based nature of the present study, however, we could not identify clinical information on comorbidities including cardiovascular disease. Instead, we assessed whether PPI exposure was associated with an excess of mortality in the subgroups at cardiovascular risk, defined by the prescription of at least two of the following cardiovascular drug classes: (i) drugs used in diabetes (ATC code A10), (ii) antihypertensive drugs (ATC codes C02, C03, C07, C08, C09), (iii) antithrombotic agents (ATC code B01) and (iv) lipid‐modifying agents (ATC code C10). A two‐sided P < 0.05 was considered significant. Data processing was performed using the statistical package SPSS v24 (released 2016, IBM Corp., Armonk, NY, USA) and R version 3.4.3 (www.r‐project.org).
Results
A total of 28 428 individuals with dementia were identified by ADD prescriptions with females constituting 67.8% of the cohort and a median age at the index date of 82.1 years (interquartile range, 76.7–86.6). There were 14 640 deaths (51.5%) in the dementia cohort during the study period, which resulted in a median survival of 1625 days [95% confidence intervals (CI), 1598–1652]. In the control cohort of 56 856 individuals, 20 484 (36.0%) persons died during the study period and survival was significantly longer compared with dementia patients with a median duration of 3356 days (95% CI, 3312–3400; P < 0.0001) (Tables 1 and 2).
Table 1.
Characteristics of patients with dementia and controls: a control‐matched cohort study in Austria (January 2005 to June 2016)
| Patients with dementia | Controls | |||||
|---|---|---|---|---|---|---|
| Total | PPI user | PPI non‐user | Total | PPI user | PPI non‐user | |
| n | 28 428 (100%) | 12 979 (45.7%) | 15 449 (54.3%) | 56 856 (100%) | 21 948 (38.6%) | 34 908 (61.4%) |
| Sex (female) | 19 267 (67.8%) | 8935 (68.8%) | 10 332 (66.9%) | 38 779 (68.2%) | 15 360 (70.0%) | 23 419 (67.1%) |
| Age at index date (years) | 82.1 (76.7–86.6) | 82.2 (76.8–86.6) | 82.1 (76.6–86.6) | 82.3 (77.2–85.9) | 82.8 (77.9–86.5) | 82.0 (76.7–85.6) |
| Age group (years) | ||||||
| <50 | 142 (0.5%) | 58 (0.4%) | 84 (0.5%) | 105 (0.2%) | 25 (0.1%) | 80 (0.2%) |
| 50–59 | 395 (1.4%) | 186 (1.4%) | 209 (1.4%) | 554 (1.0%) | 168 (0.8%) | 386 (1.1%) |
| 60–69 | 1943 (6.8%) | 846 (6.5%) | 1097 (7.1%) | 3883 (6.8%) | 1240 (5.6%) | 2643 (7.6%) |
| 70–79 | 8530 (30.0%) | 3909 (30.1%) | 4621 (29.9%) | 16 161 (28.4%) | 5933 (27.0%) | 10 228 (29.3%) |
| 80–89 | 14 753 (51.9%) | 6796 (52.4%) | 7957 (51.5%) | 31 324 (55.1%) | 12 334 (56.2%) | 18 990 (54.4%) |
| ≥90 | 2665 (9.4%) | 1184 (9.1%) | 1481 (9.6%) | 4829 (8.5%) | 2248 (10.2%) | 2581 (7.4%) |
| ATC | 9.0 (4.0–14.0) | 13 (9–19) | 5 (2–9) | 10.0 (6.0–16.0) | 13 (8–19) | 9 (5–14) |
| ATC groups | ||||||
| ≤2 | 4662 (16.4%) | 74 (0.6%) | 4588 (29.7%) | 2154 (3.8%) | 424 (1.9%) | 1730 (5.0%) |
| 3–7 | 7958 (28.0%) | 2214 (17.1%) | 5744 (37.2%) | 16 939 (29.8%) | 4334 (19.7%) | 12 605 (36.1%) |
| 8–12 | 6859 (24.1%) | 3646 (28.1%) | 3213 (20.8%) | 15 675 (27.6%) | 5943 (27.1%) | 9732 (27.9%) |
| 13–17 | 4224 (14.9%) | 2999 (23.1%) | 1225 (7.9%) | 10 086 (17.7%) | 4593 (20.9%) | 5493 (15.7%) |
| 18–22 | 2414 (8.5%) | 1955 (15.1) | 459 (3.0) | 6023 (10.6) | 3136 (14.3) | 2887 (8.3) |
| ≥23 | 2311 (8.1%) | 2.091 (16.1%) | 220 (1.4%) | 5979 (10.5%) | 3518 (16.0%) | 2461 (7.0%) |
| PPI use (before index date) cDDD | – | 173 ± 152 | – | – | 145 ± 139 | – |
| PPI use (after index date) cDDD | – | 460 ± 595 | 198 ± 421 | – | 603 ± 730 | 219 ± 469 |
| NSAID user | 7862 (27.7%) | 5569 (42.9%) | 2293 (14.8%) | 18 888 (33.2%) | 10 522 (47.9%) | 8366 (24.0%) |
ATC, anatomical therapeutic chemical classification system; cDDD, cumulative defined daily dose; NSAID, non‐steroidal anti‐inflammatory drug; PPI, proton‐pump inhibitor. Data are given as n (%), median (interquartile range) and mean ± SD.
Table 2.
Effect of proton‐pump inhibitor (PPI) use on survival in patients with dementia and controls: a multivariate Cox regression analysis for non‐proportional hazards in a control‐matched cohort study in Austria (January 2005 to June 2016)
| Patients with dementia | Controls | |||||
|---|---|---|---|---|---|---|
| Total | Deaths | Adjusted HR (95% CI) per 100 DDDs | Total | Deaths | Adjusted HR (95% CI) per 100 DDDs | |
| Overall effect a | 28 428 | 19 267 (67.8%) | 1.03 (1.01–1.05) | 56 856 | 20 484 (36.0%) | 1.15 (1.05–1.26) |
| Overall effect b | 10 591 | 5634 (53.2%) | 1.07 (1.03–1.12) | 18 892 | 6436 (34.1%) | 1.47 (1.31–1.64) |
| Time‐dependent effect (years after index date) b | ||||||
| 0 | 10 591 | 1953 (18.4%) | 1.61 (1.50–1.72) | 18 892 | 1248 (6.6%) | 3.45 (3.32–3.58) |
| 1 | 8638 | 1248 (14.4%) | 1.22 (1.17–1.28) | 17644 | 1046 (5.9%) | 2.01 (1.95–2.08) |
| 2 | 7390 | 1104 (14.9%) | 1.00 (0.94–1.06) | 16 598 | 1055 (6.4%) | 1.38 (1.31–1.45) |
| 3 | 5172 | 754 (14.6%) | 0.94 (0.89–1.00) | 13 361 | 850 (6.4%) | 1.27 (1.20–1.33) |
| 4 | 2595 | 336 (12.9%) | 0.98 (0.93–1.03) | 8761 | 630 (7.2%) | 1.40 (1.34–1.46) |
| 5 | 1095 | 162 (14.8%) | 1.02 (0.97–1.08) | 6394 | 436 (6.8%) | 1.55 (1.48–1.63) |
| 6 | 374 | 44 (11.8%) | 1.03 (0.97–1.09) | 5507 | 410 (7.4%) | 1.57 (1.49–1.65) |
| 7 | 174 | 15 (8.6%) | 0.99 (0.91–1.08) | 4710 | 401 (8.5%) | 1.47 (1.40–1.54) |
| 8 | 80 | 15 (18.8%) | 0.94 (0.80–1.08) | 3567 | 301 (8.4%) | 1.30 (1.20–1.39) |
| 9 | 27 | 3 (11.1%) | 0.87 (0.65–1.10) | 1615 | 42 (2.6%) | 1.10 (0.95–1.25) |
| 10 | 7 | 0 (0.0%) | 0.80 (0.49–1.12) | 289 | 17 (5.9%) | 0.92 (0.70–1.14) |
CI, confidence intervals; DDD, defined daily dose; HR, hazard ratio. Data are given as n (%) unless otherwise stated.
Including cases and controls with PPI exposure after the index date.
Excluding cases and controls with PPI exposure after the index date.
Proton‐pump inhibitor exposure is associated with an increased risk of death
In the dementia cohort, 12 979 individual cases (45.7%) were exposed to PPIs with at least one prescription and a mean cDDD of 173 ± 152 in the year preceding the index date (Table 1). The use of PPIs prior to the index date was significantly associated with an increased risk of death (adjusted HR per 100 DDDs of PPIs, 1.03; 95% CI, 1.01–1.05; P = 0.0069) (Table 2). In the control cohort, the proportion of individuals exposed to PPIs in the year before the index date was significantly lower (n = 21 948, 38.6% of the total cohort, P < 0.0001, chi‐squared test) with a mean cDDD of 145 ± 139 (Table 1). PPI exposure was significantly associated with an increased risk of death in the control cohort (adjusted HR per 100 DDDs of PPIs, 1.15; 95% CI, 1.05‐1.26; P = 0.0028) (Table 2).
The association between PPI exposure and risk of death was stronger in controls compared with dementia patients (P < 0.0001), but controls exposed to PPIs in the year before the index date also had a higher PPI usage thereafter (mean cDDD of 603 ± 730 in controls and 460 ± 595 in patients with dementia), which could have contributed to the difference. To account for this bias, Cox regression analyses were repeated in patients with dementia and controls without any PPI use after the index date (37.3% of patients with dementia and 33.2% of controls, respectively) (Table 2). PPI exposure was still associated with an increased risk of death in both patients with dementia (adjusted HR per 100 DDDs of PPIs, 1.07; 95% CI, 1.03‐1.12; P = 0.0008) and the control cohort (adjusted HR per 100 DDDs of PPIs, 1.47; 95% CI, 1.31‐1.64; P < 0.0001) with a significantly stronger detrimental effect of PPI exposure on survival in controls compared with dementia patients (P < 0.0001). As further analysis of our model did not fulfill the proportional hazards assumption, we sought to better describe the time at which the effect was strongest. In both patients with dementia and controls, adjusted HRs were highest at the beginning and decreased over time, plateauing at about 2 years after the index date (Fig. 1 and Table 2). Age at the index date and cardiovascular risk [14] were not associated with an excess of mortality upon PPI exposure (data not shown).
Figure 1.
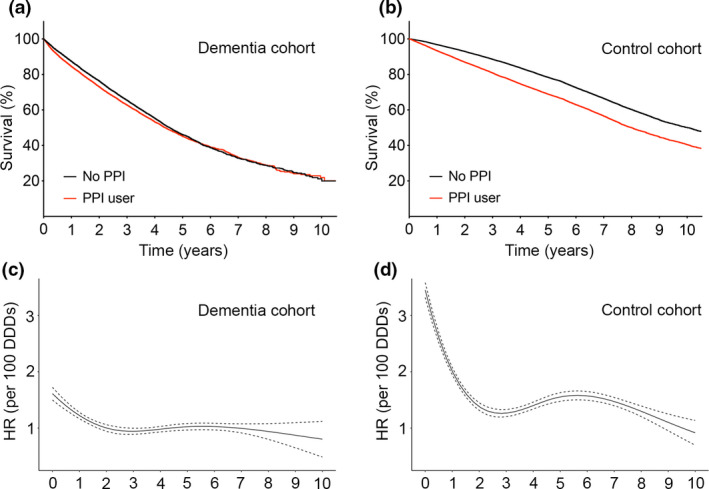
Survival of patients with dementia and controls in relation to the use of proton‐pump inhibitors (PPIs). (a) Median survival in patients with dementia exposed to PPIs before the index date was 1596 days [95% confidence intervals (CI), 1555–1637] and median survival in dementia patients without PPIs was 1655 days (95% CI, 1619–1691) (P = 0.0011). (b) Median survival in controls exposed to PPIs before the index date was 2921 days (95% CI, 2864–2978) and median survival in controls without PPIs was 3657 days (95% CI, 3647–3667) (P < 0.0001). Time‐dependent multivariate Cox regression analysis for non‐proportional hazards in relation to 100 defined daily doses (DDDs) of PPIs in patients with dementia (c) and controls (d). HR, hazard ratio.
Discussion
Proton‐pump inhibitors are among the most widely prescribed drugs with prevalence estimates of over 30% in the aged population [11]. They are often overprescribed and used inappropriately for long‐term duration [23, 24]. As a result, even the association with low mortality risk increments can lead to significant public health implications. In this large control‐matched cohort study, claims data of Austrian health insurance registries were, for the first time, used to investigate the influence of PPI use on survival in both patients with dementia and matched controls. In both groups, PPI use was associated with a detrimental effect on survival, which is in accordance with two other studies revealing an increased risk of death associated with PPI use [13, 14]. In our study, risk of death was highest in the first year after the index date, potentially due to the presence of a particularly susceptible subgroup within both cohorts dying early after PPI exposure.
Potential biological mechanisms underlying the detrimental effect of PPIs have been reported in the literature and have been specifically proposed in dementia models [15]. PPIs have moreover been reported to be associated with profound changes in the gut microbiome and decreased bacterial richness [25]. A combination of these mechanisms could be considered to accelerate the disease process in patients with dementia. However, we intriguingly observed a significantly higher risk of death associated with the use of PPIs in the control cohort compared with dementia patients. One potential explanation for the difference between mortality risks could be a poor adherence of patients with dementia to medication as previously reported [26, 27], which could have resulted in a lower ‘true’ PPI exposure in patients with dementia than predicted from their prescription data. Another potential explanation for the lower risk of death in dependence of PPI use in patients with dementia is based on their reduced life expectancy [28]. In our study, the overall survival of patients with dementia was significantly shorter compared with controls (Table 2). Competing risk factors in the dementia cohort could have obviated the detrimental PPI effect [29], with dementia patients not having lived long enough to be as substantially affected by the detrimental PPI effect as the control group. Finally, different biological effects of PPIs in patients with dementia and controls cannot be ruled out.
A general limitation of the insurance‐based prescription data in our study was the lack of information on over‐the‐counter drugs. However, ADDs are only available on prescription in Austria and as dementia is the only diagnosis for which ADDs are approved and for which the costs are remunerated by the sickness funds, the diagnosis of dementia could reliably be deduced from ADD prescriptions. Most PPIs are also available only on prescription in Austria. Only low‐dose omeprazole and pantoprazole in packaging sizes of 7 or 14 pills have been available over the counter since 2010. As both patients with dementia and controls received drugs for a very low prescription charge in our study, it is unlikely that they would have paid privately to buy over‐the‐counter omeprazole and pantoprazole. Moreover, previous studies established prescription claims as a valid data source for association studies, even though some of the drugs were available over the counter [30, 31]. The insurance registries of the Austrian sickness funds in particular have been shown to be a valid data source for pharmacoepidemiological studies [30, 32]. Based on these factors we do not consider the use of over‐the‐counter drugs as a major limitation in our study.
In conclusion, our findings of an increased risk of death in patients with dementia and controls have significant public health implications. They promote the improvement of standardized guidelines for the prescription and administration of PPIs, and more stringent pharmacovigilance strategies to avoid PPI use in cases lacking clear indication for therapy or where potential risks outweigh the benefits.
Disclosure of conflicts of interest
The authors declare no financial or other conflicts of interest related to this article.
Acknowledgements
We thank the members of the Pharmacoeconomics Advisory Council of the Austrian Sickness Funds for provision of the data.
References
- 1. Patterson C.World Alzheimer Report 2018. The State of the Art of Dementia Research: New Frontiers. Alzheimer's Disease International (ADI), London, 2018.
- 2. GBD 2016 Dementia Collaborators . Global, regional, and national burden of Alzheimer's disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019; 18: 88–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Towards a Dementia Plan: A WHO Guide. World Health Organization, Geneva, 2018.
- 4. Ashby‐Mitchell K, Burns R, Shaw J, Anstey KJ. Proportion of dementia in Australia explained by common modifiable risk factors. Alzheimers Res Ther 2017; 9: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population‐based perspective. Alzheimers Dement 2015; 11: 718–726. [DOI] [PubMed] [Google Scholar]
- 6. Gomm W, von Holt K, Thome F, et al Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol 2016; 73: 410–416. [DOI] [PubMed] [Google Scholar]
- 7. Haenisch B, von Holt K, Wiese B, et al Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci 2015; 265: 419–428. [DOI] [PubMed] [Google Scholar]
- 8. Gray SL, Walker RL, Dublin S, et al Proton pump inhibitor use and dementia risk: prospective population‐based study. J Am Geriatr Soc 2018; 66: 247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taipale H, Tolppanen AM, Tiihonen M, Tanskanen A, Tiihonen J, Hartikainen S. No association between proton pump inhibitor use and risk of Alzheimer's disease. Am J Gastroenterol 2017; 112: 1802–1808. [DOI] [PubMed] [Google Scholar]
- 10. Moayyedi P, Lewis MA. Proton pump inhibitors and dementia: deciphering the data. Am J Gastroenterol 2017; 112: 1809–1811. [DOI] [PubMed] [Google Scholar]
- 11. Halfdanarson OO, Pottegard A, Bjornsson ES, et al Proton‐pump inhibitors among adults: a nationwide drug‐utilization study. Therap Adv Gastroenterol 2018; 11: 1756284818777943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schoenfeld AJ, Grady D. Adverse effects associated with proton pump inhibitors. JAMA Intern Med 2016; 176: 172–174. [DOI] [PubMed] [Google Scholar]
- 13. Xie Y, Bowe B, Li T, Xian H, Yan Y, Al‐Aly Z. Risk of death among users of proton pump inhibitors: a longitudinal observational cohort study of United States veterans. BMJ Open 2017; 7: e015735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xie Y, Bowe B, Yan Y, Xian H, Li T, Al‐Aly Z. Estimates of all cause mortality and cause specific mortality associated with proton pump inhibitors among US veterans: cohort study. BMJ 2019; 365: l1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ortiz‐Guerrero G, Amador‐Munoz D, Calderon‐Ospina CA, Lopez‐Fuentes D, Nava Mesa MO. Proton pump inhibitors and dementia: physiopathological mechanisms and clinical consequences. Neural Plast 2018; 2018: 5257285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yepuri G, Sukhovershin R, Nazari‐Shafti TZ, Petrascheck M, Ghebre YT, Cooke JP. Proton pump inhibitors accelerate endothelial senescence. Circ Res 2016; 118: e36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Statistics Austria: The annual population statistics for the reference date 31 October. https://www.statistik.at/web_en/statistics/index.html (accessed 02/2019).
- 18. Boparai V, Rajagopalan J, Triadafilopoulos G. Guide to the use of proton pump inhibitors in adult patients. Drugs 2008; 68: 925–947. [DOI] [PubMed] [Google Scholar]
- 19. Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf 2006; 15: 291–303. [DOI] [PubMed] [Google Scholar]
- 20. Suissa S. Immortal time bias in pharmaco‐epidemiology. Am J Epidemiol 2008; 167: 492–499. [DOI] [PubMed] [Google Scholar]
- 21. Dunkler D, Ploner M, Schemper M, Heinze G. Weighted Cox regression using the R package coxphw. J Stat Softw 2018; 2: 1–26. [Google Scholar]
- 22. Schneeweiss S, Seeger JD, Maclure M, et al Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol 2001; 154: 854–864. [DOI] [PubMed] [Google Scholar]
- 23. Heidelbaugh JJ, Kim AH, Chang R, Walker PC. Overutilization of proton‐pump inhibitors: what the clinician needs to know. Therap Adv Gastroenterol 2012; 5: 219–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ 2008; 336: 2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Imhann F, Bonder MJ, Vich Vila A, et al Proton pump inhibitors affect the gut microbiome. Gut 2016; 65: 740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Borah B, Sacco P, Zarotsky V. Predictors of adherence among Alzheimer's disease patients receiving oral therapy. Curr Med Res Opin 2010; 26: 1957–1965. [DOI] [PubMed] [Google Scholar]
- 27. Poon I, Lal LS, Ford ME, Braun UK. Racial/ethnic disparities in medication use among veterans with hypertension and dementia: a national cohort study. Ann Pharmacother 2009; 43: 185–193. [DOI] [PubMed] [Google Scholar]
- 28. Brunnstrom HR, Englund EM. Cause of death in patients with dementia disorders. Eur J Neurol 2009; 16: 488–492. [DOI] [PubMed] [Google Scholar]
- 29. Barnes DE, Lee SJ. Predicting Alzheimer's risk: why and how? Alzheimers Res Ther 2011; 3: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cetin H, Klickovic U, Rath J, et al Associations between co‐medications and survival in ALS ‐ a cohort study from Austria. J Neurol 2015; 262: 1698–1705. [DOI] [PubMed] [Google Scholar]
- 31. Yood MU, Campbell UB, Rothman KJ, et al Using prescription claims data for drugs available over‐the‐counter (OTC). Pharmacoepidemiol Drug Saf 2007; 16: 961–968. [DOI] [PubMed] [Google Scholar]
- 32. Jordakieva G, Kundi M, Untersmayr E, Pali‐Scholl I, Reichardt B, Jensen‐Jarolim E. Country‐wide medical records infer increased allergy risk of gastric acid inhibition. Nat Commun 2019; 10: 3298. [DOI] [PMC free article] [PubMed] [Google Scholar]


