Abstract
Objective
Exposure‐response (E‐R) models were developed to provide a description of the time‐course of treatment effect for monthly and quarterly dosing regimens of fremanezumab.
Background
Fremanezumab is a monoclonal antibody for preventive treatment of episodic migraine (EM) and chronic migraine (CM). In phase 2b and 3 clinical studies of fremanezumab, significant reductions in migraine and headache days and other clinical endpoints were observed for patients with EM and patients with CM. Development of E‐R models relating individual‐specific measures of drug exposure to clinical endpoints provides a more granular understanding of the expected effects of different doses on therapeutic outcomes by accounting for variability in pharmacokinetic (PK) properties.
Methods
Data from 2 phase 2b and 2 phase 3 studies of adults with EM or CM were used. Individual exposures were calculated from a population PK model and related to monthly migraine days in EM and moderate‐severe (M/S) headache days in CM. Model‐based stochastic simulations were performed to compare predicted responses for the various treatment regimens.
Results
The effect of average fremanezumab concentration compared to placebo on the reduction in migraine days and M/S headache days was predicted by the models to be similar for 225 mg monthly and 675 mg once quarterly over time for both EM and CM patients. Both regimens were associated with better response than placebo. A similar percent of EM and CM responders was predicted across the range of observed body weights.
Conclusions
Exposure‐response evaluations showed that both monthly (225 mg) and quarterly (675 mg) fremanezumab dosing regimens were appropriate in achieving clinical benefit in adult patients with EM or CM.
Keywords: fremanezumab, episodic migraine, chronic migraine, exposure‐response models, simulation
Abbreviations
- Cav
average fremanezumab concentration
- Cav50
average fremanezumab concentration at which 50% of the maximal response is expected
- CGRP
calcitonin gene‐related peptide
- CM
chronic migraine
- EM
episodic migraine
- E‐R
exposure‐response
- FDA
United States Food and Drug Administration
- IgG2Δa
immunoglobulin G2 delta a
- M/S
moderate‐to‐severe
- mAb
monoclonal antibody
- P
P value
- PK
pharmacokinetic
- sc
subcutaneous
- SD
standard deviation
- TEV‐48125/LBR‐101/RN‐307/Ajovy®
fremanezumab
- VPC
visual predictive check
- VOF
value of the objective function
Introduction
Calcitonin gene‐related peptide (CGRP) is implicated in the pathophysiology of migraine and is a key target in migraine preventive strategies. 1 , 2 Four monoclonal antibodies (mAbs) that target the CGRP pathway have been developed for the prevention of episodic migraine (EM) and/or chronic migraine (CM). 3 , 4 , 5 , 6 , 7 , 8 , 9 Fremanezumab (TEV‐48125, LBR‐101, RN‐307, Ajovy®) is a fully humanized mAb (IgG2Δa) that selectively targets the CGRP ligand.
EM and CM represent different manifestations of the same disease. Clinically, CM typically evolves from EM, meaning that CM is not developed “de novo” in patients without migraine with or without aura. CM evolves from EM at an annual rate of ~2.5%; patients with ≥4 headache days per month are at an increased risk of developing CM. CM may spontaneously revert to EM with rates of reversion estimated at 15 to 26%. 10 Currently, fremanezumab is approved for the preventive treatment of EM and CM in adults in the United States and Australia and was granted marketing authorization in Europe by the European Commission.
Monthly dosing regimens for preventive therapy of migraine have been approved for other mAbs that target the CGRP pathway (erenumab [Aimovig™] and galcanezumab [Emgality™]), however, the use of fremanezumab also allows for quarterly subcutaneous (sc) dosing. 11 In addition, the recommended fremanezumab dose regimens (225 mg sc monthly, 675 mg sc every 3 months) are identical for EM and CM, a harmonization which simplifies use of the product for clinicians.
Exposure‐response (E‐R) analyses are an integral part of clinical drug development and regulatory decision‐making. 12 The United States Food and Drug Administration (FDA) Guidance for Industry states that “exposure‐response information is at the heart of any determination of the safety and effectiveness of drugs” and that knowledge of the relationships of beneficial and adverse effects to a defined drug exposure are critical. 13 Thus, development of E‐R models relating individual‐specific measures of drug exposure to clinical endpoints provides a more granular understanding of the expected effects of different doses on therapeutic outcomes by accounting for variability in pharmacokinetic (PK) properties.
In the phase 2 and 3 clinical studies of fremanezumab, significant reductions in the number of headaches and other clinical endpoints were observed for both patients with EM and patients with CM. The improvement in clinical efficacy endpoints achieved with each of the dosing schedules tested, along with favorable safety results, led to its approval as a migraine treatment. 6 , 7 , 8 , 14 This manuscript describes the development of E‐R models investigating the relationships between differences in fremanezumab exposure resulting from monthly and quarterly dosing schedules and efficacy endpoints of the phase 2 and 3 clinical trials for fremanezumab.
Methods
Data for E‐R analyses were obtained from 2 phase 2b and 2 phase 3 studies 6 , 7 , 8 , 14 of adult men and women with either a history of CM (headaches occurring at least 15 days per month, with at least 8 migraine days per month) or fulfilling the criteria for EM (headaches on 6 [phase 3] or 8 [phase 2b] to 14 days per month, with at least 4 migraine days per month 15 ) as described in Table 1. Monthly fremanezumab doses were administered every 28 days (4 weeks) and quarterly doses were administered every 84 days (12 weeks).
Table 1.
Overview of Studies Included in Exposure‐Response Assessments
| Study Number/Phase/Population | Objectives | Efficacy Endpoints and Sampling Schedules | Dose Administration |
|---|---|---|---|
| LBR‐101‐021/Phase 2b/Patients with CM | Randomized, placebo‐controlled study evaluating the efficacy and safety of sc fremanezumab in comparison to placebo for the preventative treatment of CM | Number of headache days of at least moderate severity during 28‐day monthly periods starting with the 1st dose date | 1 of 3 dose treatments administered sc once monthly for 3 months: (1) monthly dosing 225 mg with a starting dose of 675 mg, (2) monthly dosing of 900 mg, or (3) monthly dosing of placebo |
| LBR‐101‐022/Phase 2b/Patients with EM | Randomized, placebo‐controlled study evaluating the efficacy and safety of sc fremanezumab in comparison to placebo for the preventative treatment of EM | Number of migraine days during 28‐day monthly periods starting with the 1st dose date | 1 of 3 dose treatments administered sc once monthly for 3 months: (1) monthly dosing of 225 mg, (2) monthly dosing of 675 mg, or (3) monthly dosing of placebo |
| TV48125‐CNS‐0049/Phase 3/Patients with CM | Randomized, placebo‐controlled study evaluating the efficacy and safety of sc fremanezumab in comparison to placebo for the preventative treatment of CM | Number of headache days of at least moderate severity during 28‐day monthly periods starting with the 1st dose date | 1 of 3 dose treatments administered sc once monthly for 3 months: (1) monthly dosing: monthly 225 mg (with a starting dose of 675 mg), (2) quarterly dosing: a single dose of 675 mg every 3 months with placebo injections on months in which fremanezumab was not injected to maintain blinding, or (3) monthly dosing of placebo |
| TV48125‐CNS‐0050/Phase 3/Patients with EM | Randomized, placebo‐controlled study evaluating the efficacy and safety of sc fremanezumab in comparison to placebo for the preventative treatment of EM | Number of migraine days during 28‐day monthly periods starting with the 1st dose date | 1 of 3 treatments administered sc once monthly for 3 months: (1) monthly dosing of 225 mg, (2) quarterly dosing: a single dose of 675 mg every 3 months with placebo injections on months in which fremanezumab was not injected to maintain blinding, or (3) monthly dosing of placebo |
| TV48125‐CNS‐0051/Phase 3/Patients with CM or EM | Randomized roll‐over study evaluating long‐term safety, tolerability, and efficacy of sc fremanezumab for the preventive treatment of migraine | Number of headache days of at least moderate severity during 28‐day monthly periods starting with the 1st dose date for patients with CM; Number of migraine days during 28‐day monthly periods starting with the 1st dose date for patients with EM | Patients randomized to active treatment groups in the phase 3 efficacy studies (TV48125‐CNS‐30049 and TV48125‐CNS‐30050) continued to receive the same treatment; patients with CM previously randomized to placebo or “new patients” (patients not rolling over from phase 3) received 1 of 2 treatments for 12 months: monthly dosing of 225 mg (with a starting dose of 675 mg) or quarterly dosing of 675 mg; patients with EM previously randomized to placebo or “new patients” (patients not rolling over from phase 3) received 1 of 2 treatments for 12 months: monthly dosing of 225 mg or quarterly dosing of 675 mg |
CM = chronic migraine; EM = episodic migraine; sc = subcutaneous.
The studies described herein were conducted in accordance with Good Clinical Practice and with the FDA guidelines for safety monitoring of patients. All patients provided written informed consent before enrolling in each of the studies. The study protocols were approved by the institutional review boards for each site.
The analyses of this paper were designed by all authors, some of whom are employees of the funding source, Teva Pharmaceuticals Ltd. All authors had access to all data analyses and were involved in writing the manuscript.
Fremanezumab Exposure Measures
For the E‐R evaluations, individual‐specific measures of fremanezumab exposure following monthly and quarterly dosing were calculated. Using the individual empirical Bayesian PK parameter estimates obtained from the final population PK model, 16 predicted fremanezumab concentration profiles were generated over 28 or 84 days following each monthly or quarterly dose and various exposure measures were calculated. Exposure measures were set to zero for placebo patients.
Efficacy Measures
The primary efficacy endpoint for EM was based on the number of migraine days per month, defined as a day when the patient experienced at least 1 of the following situations: at least 2 consecutive hours of a headache meeting criteria for migraine with or without aura; at least 2 consecutive hours of a headache meeting criteria for probable migraine, a migraine subtype where only 1 migraine criterion is missing; or a headache of any duration that was treated with migraine‐specific medications (triptans and ergot compounds). 7 For CM, the primary efficacy endpoint was based on the number of headache days per month of at least moderate severity, defined according to the Classification Committee of the International Headache Society as a day the patient reported headache pain that lasted ≥4 hours with a peak severity of at least moderate severity or a day when the patient used acute migraine‐specific medication (triptans or ergots) to treat a headache of any severity or duration. 15 , 17 Therefore, for the purposes of exploring E‐R relationships, separate E‐R models were developed for EM and CM based on the following 2 endpoints: (1) monthly number of migraine days for patients with EM, and (2) monthly number of moderate‐to‐severe (M/S) headache days for patients with CM. Patients provided data for daily headaches and use of concomitant medications using electronic headache diary devices. 6 , 7 , 8 , 14
The baseline numbers of monthly migraine days and M/S headache days were defined as the number of migraine days and M/S headache days during the 28‐day run‐in period prior to the 1st dose of fremanezumab.
Exposure‐Response Analysis Methodology
E‐R models were developed using nonlinear mixed effects modeling to address the following:
Time‐course of response for each endpoint and population
Possible effect of fremanezumab exposure on efficacy
Effects of select intrinsic and extrinsic factors (eg, demographics, laboratory tests, concomitant medications) potentially predictive of variability in the time‐course of response or the E‐R relationship
Prediction of the expected response and percent of responders over time, as well as comparison of response with various dosing regimens
Conducted in accordance with the principles stated in the FDA Guidance for Industry, Population PKs and E‐R Relationships, the development of optimal E‐R models in this evaluation was based on selection of the simplest model possible that had reasonable goodness of fit, and that provided a level of predictability appropriate for its use in decision‐making. 13 , 18 In particular, the procedure followed for the development of the E‐R models included:
Generation of individual estimates of exposure based on the population PK model;
Initial E‐R model development for placebo patients, then including all patients to quantify the time‐course of response independent of fremanezumab and evaluation of the effect of fremanezumab exposure on response (in excess of the effect of placebo);
Covariate assessment to explain variability; and
Model refinement and evaluation.
Nonlinear mixed effects modeling, using the computer program NONMEM® Version 7 Level 3.0, 19 was used to describe the E‐R behavior of the drug. For each analysis, NONMEM computes the value of the objective function (VOF), a statistic that is proportional to minus twice the log likelihood of the data. In the case of hierarchical models also applied here, the change in the VOF produced by the inclusion of a parameter is asymptotically χ 2‐distributed, with the number of degrees of freedom equal to the number of parameters added to or deleted from the model.
Goodness of fit was assessed according to criteria and/or considerations, such as the following: convergence of the estimation and covariance routines with ≥3 significant digits and reasonable gradients at the final iteration, reasonableness of parameter estimates based upon the expected relationships, adequate precision of the final parameter estimates, appropriate characteristics of diagnostic goodness‐of‐fit plots, and estimates of interindividual variability and residual variability for a specified model vs comparator models.
Base structural models were 1st established for each endpoint, accounting for the time‐course of placebo response (evaluating appropriate models based on the trends observed in exploratory plots of the data), and the effect of fremanezumab exposure (ie, average fremanezumab concentration [Cav], area under the concentration‐time curve, trough concentration, or maximum concentration) on the endpoint. In each case, previously developed models were used as a starting point and refinements were considered based on apparent misfit or trends observed in the diagnostic plots.
Interindividual variability in model parameters was considered using either exponential or additive forms; residual variability was evaluated using an additive error model. The 1st‐order conditional estimation method in NONMEM was used for all analyses.
The effect of covariates on model parameters was evaluated using a stepwise forward selection (α = 0.01, 1 degree of freedom, change in VOF ≥6.64) followed by backward elimination (α = 0.001, 1 degree of freedom, change in VOF ≥10.83) procedure. Stationary covariates evaluated were age, race, sex, baseline body weight, baseline body mass index, years since disease onset, and baseline value of the endpoint modeled (ie, monthly migraine days or monthly M/S headache days). The evaluation of concomitant use of select medications included: use of analgesic medications (eg, opioids or barbiturates), classified as yes/no; use of other migraine preventive medications, classified as yes/no; and the number of days/month of acute medication use at baseline, specifically triptans or ergot compounds. Functional forms for the effects of covariates on model parameters included linear, power, and exponential models for (centered) continuous covariates and additive and proportional shift models for discrete covariates. The final E‐R models were validated using a simulation‐based, visual predictive check (VPC) methodology to assess concordance between the model‐based simulated data and the observed data.
Simulation Methodology for E‐R Models
To compare the predicted response over time for the various treatment regimens, stochastic simulations (ie, including interindividual variability based on the final population PK and E‐R models) were performed in 5000 virtual patients with EM and 5000 virtual patients with CM. The treatment regimens simulated for 3 months included: fremanezumab administered 225 mg once monthly, 675 mg once quarterly, and 225 mg once monthly with a starting dose of 675 mg, and placebo. The final population PK model was applied to simulate individual‐specific PK parameters for each virtual patient. To predict individual‐specific profiles for the monthly migraine days and M/S headache days, 3 components were required: (1) predicted measures of individual exposure, (2) patient characteristics found to be statistically significant predictors of efficacy, and (3) the final E‐R model parameter estimates. Endpoints were simulated for 3 months. Graphical displays of the change from baseline for each endpoint were created for EM and CM populations separately over time. The mean (± 1 standard deviation [SD]) change from baseline for each endpoint vs time was calculated. Virtual patients who achieved a 50% or greater reduction from baseline in monthly migraine days or M/S headache days were considered responders. The percent of EM and CM responders (95% confidence interval) at each month, and as an average over 3 months, was also calculated.
Results
The data from 1142 patients with EM (4444 migraine day measures) and 1361 patients with CM (5312 M/S headache day measures) were utilized in these analyses. Demographic and disease characteristics along with concomitant medication use are shown in Tables S1 and S2 for the EM and CM patient populations.
Individual Estimates of Fremanezumab Exposure
The population PK of fremanezumab were previously characterized using a 2‐compartment model with 1st ‐order absorption and elimination and allometric weight scaling of clearance and volume of the central compartment. 16 Individual‐specific measures of fremanezumab exposure were calculated using the individual empirical Bayesian PK parameter estimates obtained from the final population PK model. To illustrate the model behavior for the monthly and quarterly dose regimens upon multiple dosing, Figure 1 illustrates the median (90% prediction interval) simulated fremanezumab concentration‐time profiles for the monthly and quarterly dose regimens used in the phase 3 clinical trials over 12 months.
Fig. 1.
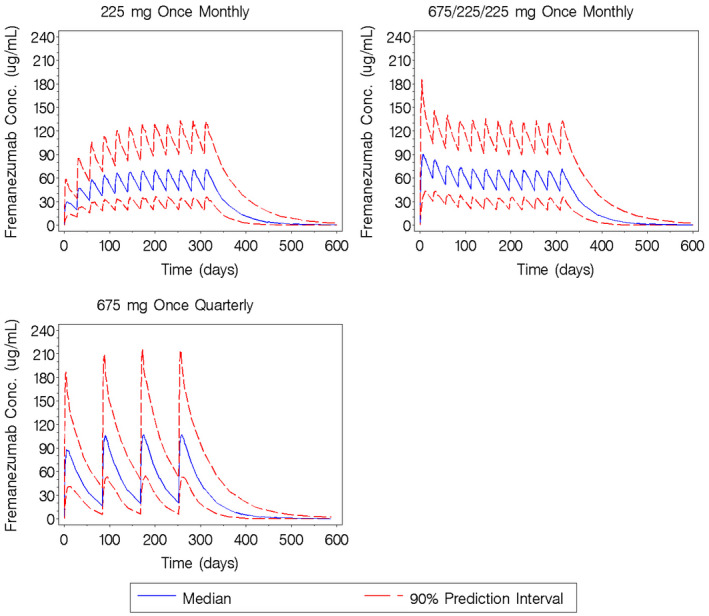
Simulated fremanezumab concentration‐time profiles for the monthly and quarterly subcutaneous dose regimens used in the phase 3 clinical trials administered over 12 months. Conc = concentration.
Exposure‐Response Analysis Results
Monthly Migraine Days in Patients With EM
The model‐predicted effect of Cav and placebo on the reduction in migraine days is shown in Figure 2a. The base structural model describing the time‐course of response with placebo treatment was 1st established in the patients receiving placebo treatment; given that exposure was set to zero for placebo treatment, the placebo treatment arms of all relevant studies included in this analysis were pooled together for this evaluation. The effect of fremanezumab exposure was then evaluated by adding parameters to this model to quantify the effect of drug exposure on the response and estimating the model for the entire population of patients. The only significant covariate included in the model was the number of days per month of acute medication use at baseline, where greater use was associated with a higher number of baseline migraine days (P < .05). The acute medication effect on the baseline migraine days is implemented as a piece‐wise linear model form. For this model, acute medication use of ≤5 days per month is associated with a constant prediction of baseline migraine days, and acute medication use >5 days per month is associated with a linear relationship, whereby baseline migraine days increases as the number of days of acute medication use (at baseline) increases (over 5 days per month). Thus, the estimated typical baseline number of migraine days per month ranged from 9 days for patients receiving acute medications ≤5 days per month to 13 migraine days for patients receiving acute medications for 15 days per month. The final E‐R model for EM predicts a typical reduction from baseline in migraine days with placebo treatment of 3 days per month at 3 months. In excess of the placebo response, treatment with fremanezumab is predicted to contribute an approximately 25% additional maximal reduction (ie, approximately 2 days) in the monthly number of migraine days.
Fig. 2.
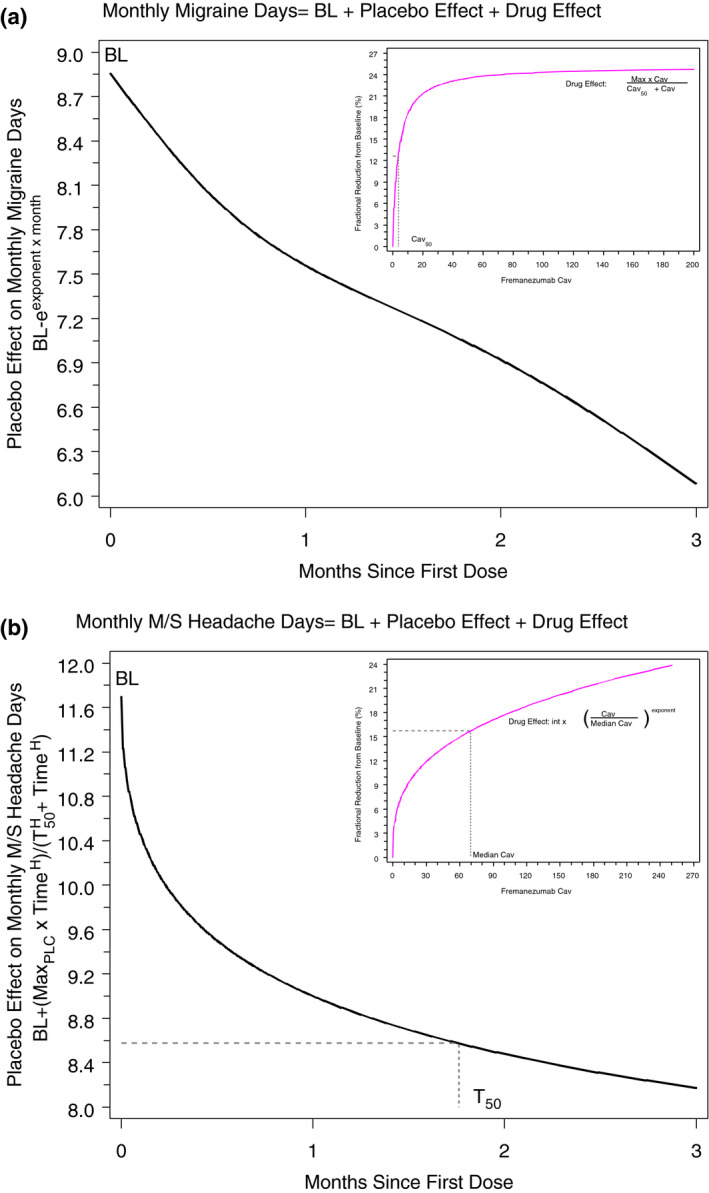
Illustration of exposure‐response models for monthly migraine days (A) and monthly moderate/severe headache days (B). BL = Baseline; Max = maximum effect; MaxPLC = maximum effect due to placebo; M/S = moderate/severe.
The parameter estimates and associated precisions for the final E‐R model of migraine days are presented in Table S3. Goodness‐of‐fit diagnostic plots are provided in Figure S1. The model evaluation results indicated that the model for migraine days adequately characterized the observed data (see Fig. S2). A slight underprediction of the median response was noted at months 1 and 2 in the placebo group.
Monthly Headache Days of at Least Moderate Severity in Patients With CM
The model‐predicted effect of Cav and placebo on the reduction in M/S headache days is shown in Figure 2b. Similar to what was shown for the number of migraine days in EM, the only significant covariate in the model was the number of days/month of acute medication use at baseline, where greater use of acute medications was associated with higher number of baseline M/S headache days (P < .05). The estimated typical baseline number of M/S headache days ranged from 12 days/month for patients receiving acute medications ≤5 days/month to 22 M/S headache days/month for patients receiving acute medications for 28 days per month. The final E‐R model for CM predicts a typical maximal reduction from baseline of approximately 6 days with placebo treatment, with 50% of the response expected by 1.76 months. After 3 months of treatment, the predicted monthly reduction in M/S headache days is approximately 3.5 days with placebo, and the drug effect provides an additional 12% to 16% reduction from baseline in the number of M/S headache days. This 12% to 16% reduction due to fremanezumab treatment amounts to an additional reduction of 1.4 to 1.8 days or 2.7 to 3.6 days per month at the median Cav (28 μg/mL and 70 μg/mL) for the 675 mg once‐quarterly and the 225 mg once‐monthly (starting dose of 675 mg) regimens, respectively. The corresponding predicted reduction in monthly M/S headache days associated with a much higher Cav of 120 μg/mL would range from 2.2 to 4.1 days (for baseline values ranging from 12 to 22 M/S headache days per month). The parameter estimates and associated precisions for the final E‐R model of M/S headache days are presented in Table S4. Goodness‐of‐fit diagnostic plots are provided in Figure S3.
The results from the evaluation of the model for M/S headache days indicated that the observed data were adequately characterized as shown in Figure S4, with the exception of month 1 in the 900‐mg fremanezumab arm where the median drug effect is underpredicted. In the upper 95th percentile of the observed data for the active treatment and placebo groups, several non‐responders (ie, patients with 28 days per month of M/S headache during all months) contribute to the apparent overprediction bias for this percentile.
Simulation Results
Episodic Migraine
Figure 3a,b illustrate the simulated mean (SD) monthly migraine days and mean change from baseline migraine days over time in virtual patients with EM. In these patients, responses to the 225 mg monthly regimen and 675 mg once‐quarterly regimen are shown to be similar.
Fig. 3.
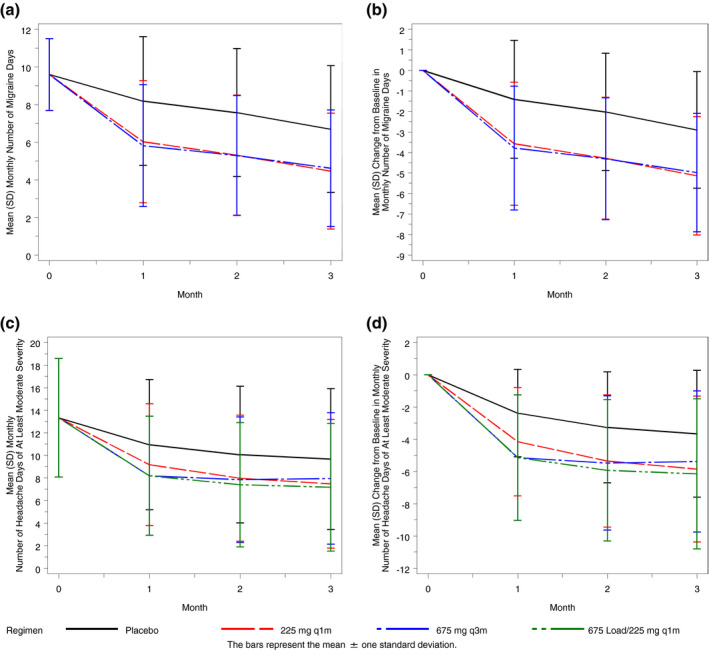
Simulated mean (SD) monthly number of migraine days (A) and change from baseline in monthly migraine days over time in virtual episodic migraine patients (B) and monthly number of headache days of at least moderate severity (C) and change from baseline in monthly headache days of at least moderate severity over time in virtual chronic migraine patients (D). q1m = once monthly; q3m = once quarterly; SD = standard deviation.
In Figure 4a, the predicted percent of virtual EM responders (at least 50% reduction from baseline) at each month is shown for the simulated treatment groups. The percent of responders is substantially higher in the fremanezumab treatment groups compared to placebo by the 1st month and that pattern continues throughout the 3‐month period. As shown in Figure 5a, a similar percent of virtual patient responders (approximately 45%) are predicted (as an average over 3 months) with fremanezumab 675 mg once quarterly and 225 mg once monthly, a response well above that predicted for the placebo group (19%). The observed percent of responders is higher than the predicted percent for each group, with the biggest difference in the placebo group (where the model evaluation results indicated a slight underprediction of placebo effect).
Fig. 4.
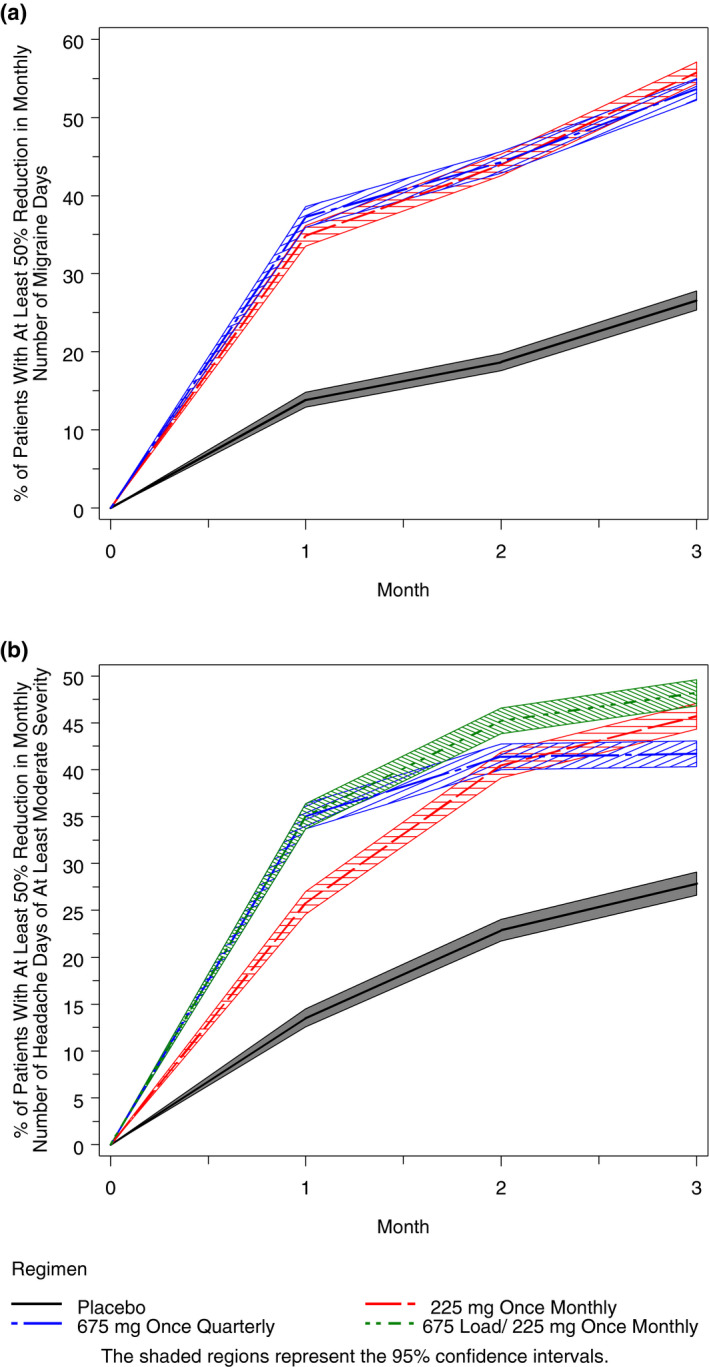
Simulated percent of virtual episodic migraine patients with at least a 50% reduction from baseline in monthly migraine days at each month (A) and simulated percent of virtual chronic migraine patients with at least a 50% reduction from baseline in monthly headache days of at least moderate severity at each month (B).
Fig. 5.
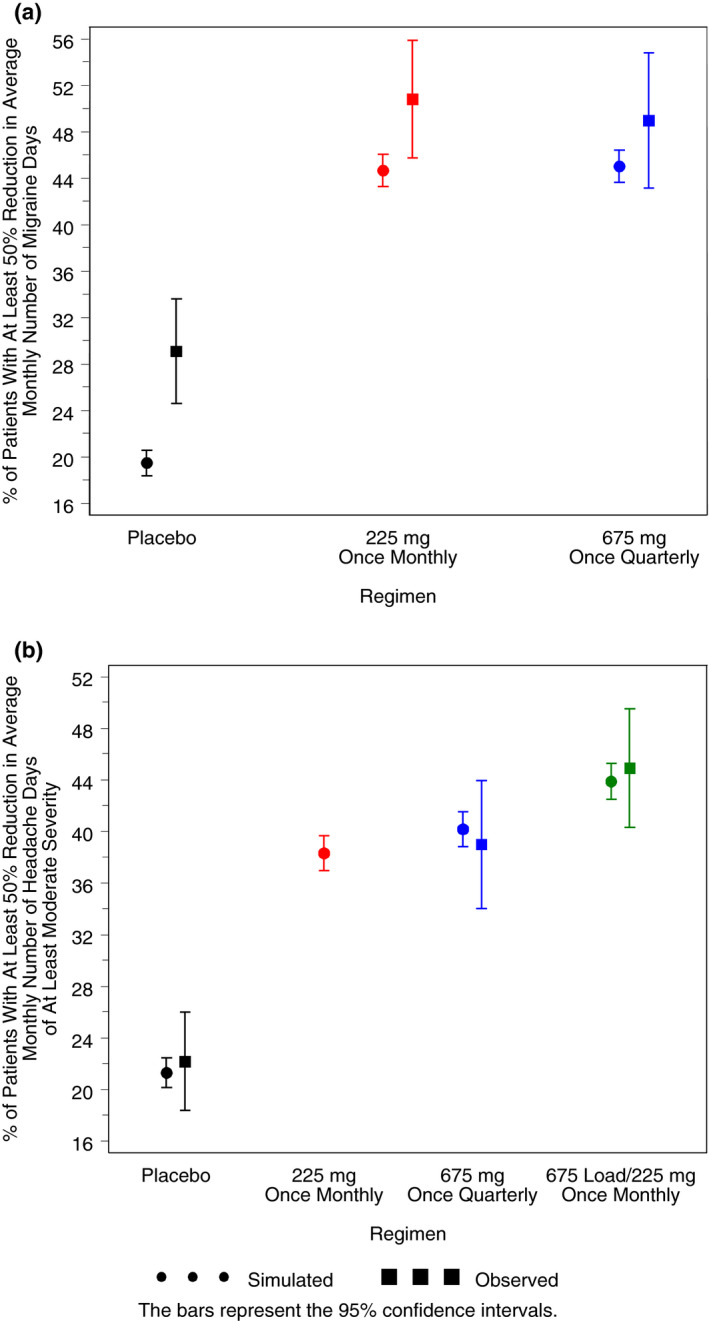
Simulated and observed mean percent of virtual episodic migraine patients with at least a 50% reduction from baseline in average monthly number of migraine days over 3 months (A) and simulated and observed mean percent of virtual chronic migraine patients with at least a 50% reduction from baseline in average monthly number of headache days of at least moderate severity over 3 months (B).
Given the effect of body weight on fremanezumab PK, 16 the impact of fremanezumab exposure across the range of body weight on the predicted efficacy response was investigated in model‐based simulations. The predicted percent of responders (as an average over 3 months) is shown vs body weight quartile and regimen in Figure 6a. A similar percent of responders is expected across the body weight quartiles within a dosing regimen and across the 2 active dosing regimens.
Fig. 6.
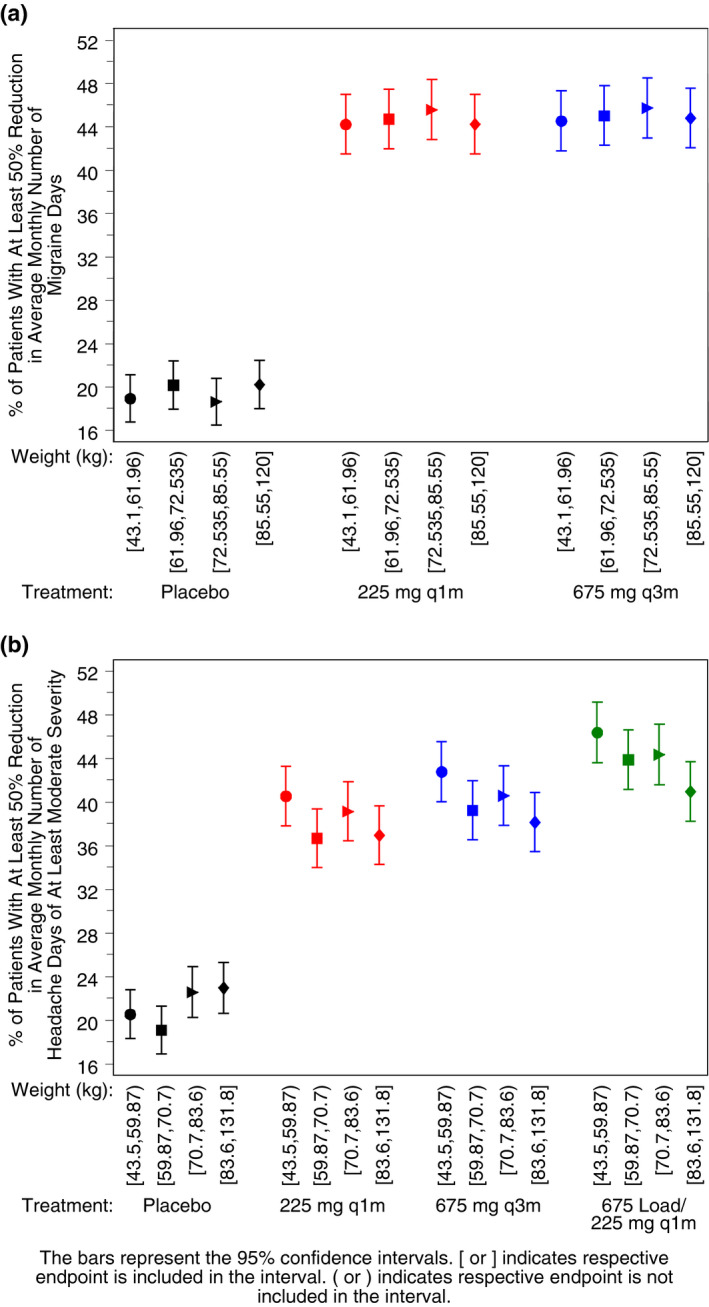
Simulated percent of virtual episodic migraine patients with at least a 50% reduction from baseline in average monthly migraine days, by regimen and body weight quartile (A) and simulated percent of virtual chronic migraine patients with at least a 50% reduction from baseline in average monthly headache days of at least moderate severity, by regimen and body weight quartile (B). q1m = once monthly; q3m = once quarterly.
Chronic Migraine
Figure 3c,d illustrate the mean (SD) monthly number and change from baseline in the number of M/S headache days over time in virtual patients with CM. In this population, the 3 dosing regimens are associated with comparable overall response during the 3‐month study duration. Although the mean ± 1 SD response profiles for all active treatment regimens largely overlap, a smaller response at month 1 is noted for the 225 mg monthly regimen and a slight difference in the response profile is noted for the 675 mg every 3 months regimen, which plateaus after month 1 through month 3, as compared to the 225 mg monthly with 675 mg starting dose regimen, which achieves slightly greater response at months 2 and 3 after initiation. This difference is not unexpected, given the approximately 30‐day half‐life of fremanezumab and the effect of fremanezumab exposure on M/S headache days. Given the estimated half‐life, approximate steady‐state conditions would be expected after about 5 half‐lives: 5 monthly doses or 2 quarterly doses. Thus, as shown in Figure 1, after 3 months of dosing, the regimen with the starting dose achieves exposures slightly in excess of steady‐state conditions, as compared to the other 2 regimens; this difference in exposures results in only a slight difference in clinical response.
The predicted percent of virtual CM responders is plotted vs month in Figure 4b for the simulated treatment groups. A clear trend toward more responders is evident with all fremanezumab dose regimens compared to placebo by the 1st month, and continues throughout the 3‐month period. Of note, despite the lower exposure at month 3 with the quarterly regimen, as compared to the monthly regimens, the response is shown to plateau over the 2 months between doses and is similar in magnitude to that achieved with the monthly regimens. As shown in Figure 5b for the average response over 3 months, a similar percent of virtual patients (approximately 38% to 44%) are predicted to respond to the fremanezumab dosing regimens, and all regimens were well above the percent for the placebo group (21%). The regimen showing numerically the largest percent of responders (95% confidence interval) was 225 mg once monthly with the starting dose of 675 mg regimen (44%; 42% to 45%), followed by the 675 mg quarterly regimen (40%; 39% to 42%) and the 225 mg monthly regimen (38%; 37% to 40%) that demonstrate similar effect. There is very good concordance between the model‐based predictions and the observed percent of patients achieving at least a 50% reduction from baseline in CM for the dose regimens evaluated in the clinic.
While there is a similar percentage of responders in the placebo group for M/S headache days in patients with CM across the weight quartiles (Fig. 6b), in the active treatment groups, the percentage of responders was slightly higher in the lowest weight quartile and slightly lower in the highest weight quartile.
Conclusions
Understanding of the relationships between drug exposure and response is an essential component to support dose regimen selection. Fremanezumab is currently approved for migraine prevention in adults, and is the only monoclonal antibody that targets the CGRP pathway approved for both monthly and quarterly dosing. 20 , 21 , 22 As shown in a recent survey of over 800 physicians and adults in the United States with migraine, a higher proportion of patients favored quarterly dosing (40%) over monthly dosing (35%), indicating that adherence to therapy and, therefore, more favorable outcomes are likely with this regimen. 23
The final E‐R model for monthly migraine days in EM was comprised of 3 components: baseline response, placebo effect, and the additional effect of fremanezumab exposure on response. Greater acute medication use at baseline was associated with higher baseline migraine days, otherwise, no other covariates were found to have a statistically significant effect on the E‐R relationship in EM. The typical placebo response at 3 months was a reduction from baseline of approximately 3 days per month. The effect of fremanezumab exposure on response was found to be an additional 25% reduction from baseline in migraine days, thus, a total decrease of approximately 5 migraine days per month could be expected.
To better understand the implications of the E‐R relationship in relation to the fremanezumab dosing regimens for patients with EM, it is important to note that the estimate of 3.60 μg/mL for Cav50 (the Cav at which 50% of the maximal response is expected; shown in Table S3), the sensitivity of the response to fremanezumab exposure, was well below the median value of the 225 mg once‐monthly regimen at month 1 (24.4 μg/mL) and the median value of the 675 mg once‐quarterly regimen at month 3 (26.9 μg/mL). These results explain the similarity of response predicted for the 225 mg once‐monthly regimen and 675 mg once‐quarterly regimen when the average response over 3 months is considered. Taken together with the half‐life of fremanezumab, the E‐R modeling and simulations illustrate a lack of difference in predicted efficacy between the 225 mg once‐monthly and 675 mg once‐quarterly regimens and, thus, provide support for the use of both regimens in patients with EM.
Similarly, the final E‐R model for the monthly M/S headache days in CM was comprised of 3 components: the baseline response, placebo effect, plus the effect of fremanezumab exposure on response. Again, the only significant baseline covariate was the number of days per month of acute medication use and the typical maximal placebo response was a reduction of 6 days per month.
With respect to the implications of these E‐R findings on comparisons of dosing regimens in CM, the model‐predicted drug effect was an additional reduction of approximately 1.4 to 3.6 days for the 675 mg quarterly and the 225 mg once‐monthly (675 mg starting dose) regimens based on the median Cav values and the baseline number of M/S headache days per month. Indeed, at a higher Cav, that is, the simulated 95th percentile of Cav at month 1 following an initial dose of 675 mg, the predicted reduction in monthly M/S headache days ranged from approximately 2.1 to 4.1 days. Thus, similar to the modeling conclusions for patients with EM, a relatively small difference in predicted response is expected with the 225 mg once‐monthly regimen (with the starting dose of 675 mg) and 675 mg once‐quarterly regimens in patients with CM. Furthermore, model‐based simulations predicting the percent of patients with at least a 50% reduction in the average monthly number of headache days of at least moderate severity (Fig. 5b) illustrate similar results for the 225 mg once‐monthly regimen without the 675 mg starting dose, 675 mg once‐quarterly dosing, and 225 mg once‐monthly regimen with the 675 mg starting dose (38% to 44%). Therefore, the E‐R modeling analysis presented herein provides support for the use of either 225 mg once monthly or 675 mg once quarterly in patients with CM and further suggests that the use of the 675 mg starting dose in this population could be avoided.
Simulations to predict the percent of patients with a reduction of migraine days (EM) or M/S headache days (CM) of at least 50% showed that at month 1, a larger percent of patients who received an initial dose of 675 mg achieved a 50% reduction compared to those given 225 mg, however, both were substantially higher than the group receiving placebo. By the 2nd and 3rd months, the responses in the fremanezumab treatment groups were more similar, with plateauing of response for the quarterly dose regimen, as expected based on the differences in exposures associated with the different frequencies of dosing. The findings were largely consistent with the traditional statistical analyses of the phase 3 trials, 7 , 8 wherein similar magnitudes of response were achieved with the monthly and quarterly regimens (differences of ≤1 migraine day or M/S headache day per month, on average).
The limitations of these E‐R relationship evaluations should also be recognized. Given the extent of between‐ and within‐patient variability in the time‐course of response for monthly migraine days and M/S headache days in the patients receiving placebo, the selected models for the placebo effect provide an adequate representation of the observed reduction in M/S headache days (CM), with slight underprediction of the reduction in migraine days (EM). Based on the nature of the E‐R models, wherein the placebo time‐course is fixed to estimates obtained from the fit to placebo patients and the drug effect is estimated as the portion of response due to drug in excess of the placebo effect, the underprediction in the EM placebo effect was present, although to a lesser extent, in the fremanezumab treatment groups as well.
These analyses also included a thorough evaluation of possible covariate effects on the E‐R relationships in EM and CM. None of the factors tested resulted in a statistically significant effect on E‐R and only the number of days of acute medication use at baseline was found to be predictive of the baseline number of migraine and M/S headache days. As expected, estimated between‐patient variability in all model components was quite high, indicating that unexplained variability in outcomes remained, even after accounting for demographic factors and concomitant medications.
Given that body weight was a significant covariate affecting fremanezumab PK, the impact of body weight on the simulated clinical endpoints was explored. The predicted percent of virtual patients with EM or CM having at least a 50% reduction from baseline to 3 months in the mean number of migraine days or M/S headache days was found to be similar across the body weight quartiles within a dosing regimen. Only small differences were noted for CM, where patients in the lightest quartile of body weight have the largest percent of responders and patients in the heaviest quartile of body weight have the lowest percent of responders in M/S headache days within a dosing regimen. Thus, despite the effect of body weight on fremanezumab exposure, fixed (ie, non‐body weight‐based) dosing options for fremanezumab, administered either monthly (225 mg) or quarterly (675 mg) have been shown to be appropriate in achieving clinical benefit for adult patients with EM or CM.
Another factor to be considered in evaluating the frequency of dose administration is a comparison of the predicted response throughout the dosing interval. Given the approximately 30‐day half‐life of fremanezumab, differences in exposure 90 days after the 1st 675 mg sc dose and 30 days after the 3rd monthly 225 mg sc dose are not unexpected. The effect of exposure on the predicted response then serves to explain the observed pattern of response in virtual patients with CM, as shown in Figures 3c,d, and 4b: the quarterly regimen shows a plateau of response between 1 and 3 months after dosing and the monthly regimen with the same initial dose (675 mg) followed by 225 mg monthly doses thereafter shows a very slight, but continual reduction in M/S headache days after month 1. Thus, the E‐R model predicts the sustained effect of fremanezumab over 90 days and further supports the use of either the 225 mg monthly or 675 mg quarterly sc dosing regimens for fremanezumab.
In conclusion, E‐R relationships, considered by the FDA to be at the heart of any determination of the safety and effectiveness of drugs, 13 were quantitatively characterized to predict the effect of average fremanezumab concentration and placebo on the reduction in migraine days and M/S headache days in adults with EM or CM who received monthly and quarterly dosing regimens of fremanezumab in phase 2b and 3 studies. Based on the E‐R models, similar responses were predicted over time for various dosing regimens in both EM and CM patients, indicating that both monthly (225 mg) and quarterly (675 mg) fremanezumab dosing regimens are appropriate in achieving clinical response in adult patients with EM or CM.
Statement of Authorship
Category 1
(a) Conception and Design
Jill Fiedler‐Kelly, Julie Passarell, Elizabeth Ludwig, Micha Levi, Orit Cohen‐Barak
(b) Acquisition of Data
Micha Levi, Orit Cohen‐Barak
(c) Analysis and Interpretation of Data
Jill Fiedler‐Kelly, Julie Passarell, Elizabeth Ludwig, Micha Levi, Orit Cohen‐Barak
Category 2
(a) Drafting the Manuscript
Jill Fiedler‐Kelly, Julie Passarell, Elizabeth Ludwig, Micha Levi, Orit Cohen‐Barak
(b) Revising It for Intellectual Content
Jill Fiedler‐Kelly, Julie Passarell, Elizabeth Ludwig, Micha Levi, Orit Cohen‐Barak
Category 3
(a) Final Approval of the Completed Manuscript
Jill Fiedler‐Kelly, Julie Passarell, Elizabeth Ludwig, Micha Levi, Orit Cohen‐Barak
Supporting information
Fig S1‐S4
Table S1‐S4
Acknowledgments
The authors would like to thank Pippa S. Loupe, PhD, (Global Research and Development Teva Pharmaceuticals Ltd.) for her valued assistance with this publication’s development.
Conflict of Interest: Jill Fiedler‐Kelly, Julie Passarell, and Elizabeth Ludwig were employed by Cognigen Corporation when the analyses were performed. Cognigen Corporation (a SimulationsPlus company) received financial support from Teva Pharmaceutical Industries Ltd. to perform these analyses. Micha Levi and Orit Cohen‐Barak are employees of Teva Pharmaceutical Industries Ltd. and own stock in Teva Pharmaceutical Industries Ltd.
Funding: This study was funded by Teva Pharmaceuticals Ltd., Netanya, Israel.
Data Availability Statement
Qualified researchers may request access to patient level data and related study documents including the study protocol and the statistical analysis plan. Patient level data will be de‐identified and study documents will be redacted to protect the privacy of trial participants and to protect commercially confidential information. Please email USMedInfo@tevapharm.com to make your request.
REFERENCES
- 1. Edvinsson L, Haanes KA, Warfvinge K, Krause DN. CGRP as the target of new migraine therapies – Successful translation from bench to clinic. Nat Rev Neurol. 2018;14:338‐350. [DOI] [PubMed] [Google Scholar]
- 2. Iyengar S, Johnson KW, Ossipov MH, Aurora SK. CGRP and the trigeminal system in migraine. Headache. 2019;59:659‐681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dodick DW, Goadsby PJ, Silberstein SD, et al. Safety and efficacy of ALD403, an antibody to calcitonin gene‐related peptide, for the prevention of frequent episodic migraine: A randomised, double‐blind, placebo‐controlled, exploratory phase 2 trial. Lancet Neurol. 2014;13:1100‐1107. [DOI] [PubMed] [Google Scholar]
- 4. Monteith D, Collins EC, Vandermeulen C, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of the CGRP binding monoclonal antibody LY2951742 (Galcanezumab) in healthy volunteers. Front Pharmacol. 2017;8:740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: A randomised, double‐blind, placebo‐controlled phase 2 trial. Lancet Neurol. 2017;16:425‐434. [DOI] [PubMed] [Google Scholar]
- 6. Bigal ME, Dodick DW, Rapoport AM, et al. Safety, tolerability, and efficacy of TEV‐48125 for preventive treatment of high‐frequency episodic migraine: A multicentre, randomised, double‐blind, placebo‐controlled, phase 2b study. Lancet Neurol. 2015;14:1081‐1090. [DOI] [PubMed] [Google Scholar]
- 7. Dodick DW, Silberstein SD, Bigal ME, et al. Effect of fremanezumab compared with placebo for prevention of episodic migraine: A randomized clinical trial. JAMA. 2018;319:1999‐2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med. 2017;377:2113‐2122. [DOI] [PubMed] [Google Scholar]
- 9. Bigal ME, Dodick DW, Krymchantowski AV, et al. TEV‐48125 for the preventive treatment of chronic migraine: Efficacy at early time points. Neurology. 2016;87:41‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bigal ME, Lipton RB. Clinical course in migraine: Conceptualizing migraine transformation. Neurology. 2008;71:848‐855. [DOI] [PubMed] [Google Scholar]
- 11. Bigal ME, Rapoport AM, Silberstein SD, Walter S, Hargreaves RJ, Aycardi E. From LBR‐101 to fremanezumab for migraine. CNS Drugs. 2018;32:1025‐1037. [DOI] [PubMed] [Google Scholar]
- 12. Pinheiro J, Duffull S. Exposure response – Getting the dose right. Pharm Stat. 2009;8:173‐175. [DOI] [PubMed] [Google Scholar]
- 13. Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research . Guidance for Industry – Exposure‐Response Relationships – Study Design, Data Analysis, and Regulatory Applications. Silver Spring, MD: United States Food and Drug Administration; 2003. Available at: https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/exposure‐response‐relationships‐study‐design‐data‐analysis‐and‐regulatory‐applications. Accessed November 2019. [Google Scholar]
- 14. Bigal ME, Edvinsson L, Rapoport AM, et al. Safety, tolerability, and efficacy of TEV‐48125 for preventive treatment of chronic migraine: A multicentre, randomised, double‐blind, placebo‐controlled, phase 2b study. Lancet Neurol. 2015;14:1091‐1100. [DOI] [PubMed] [Google Scholar]
- 15. Headache Classification Committee of the International Headache Society (IHS) . The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013;33:629‐808. [DOI] [PubMed] [Google Scholar]
- 16. Fiedler‐Kelly JB, Cohen‐Barak O, Morris DN, et al. Population pharmacokinetic modeling and simulation of fremanezumab in healthy subjects and patients with migraine. Br J Clin Pharmacol. Accepted for Publication: 27 July 2019, Early View: 9 December 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Silberstein S, Tfelt‐Hansen P, Dodick DQ, et al. Guidelines for controlled trials of prophylactic treatment of chronic migraine in adults. Cephalalgia. 2008;28:484‐495. [DOI] [PubMed] [Google Scholar]
- 18. Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research . Guidance for Industry: Population Pharmacokinetics. Silver Spring, MD: United States Food and Drug Administration; 2019. Available at: https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/population‐pharmacokinetics. Accessed March 2020. [Google Scholar]
- 19. Beal SL, Sheiner LB, Boeckmann AJ, Bauer RJ (eds). NONMEM 7.3.0 Users Guides. Hanover, MD: ICON Development Solutions; 1989. –2013. [Google Scholar]
- 20. Ajovy [package insert]. North Wales, PA: Teva Pharmaceuticals USA, Inc.; 2018. [Google Scholar]
- 21. Aimovig [package insert]. Thousand Oaks, CA: Amgen Incorporated; 2018. [Google Scholar]
- 22. Emgality [package insert]. Indianapolis, IN: Eli Lilly and Company; 2019. [Google Scholar]
- 23. Cowan R, Cohen JM, Rosenman E, Iyer R. Physician and patient preferences for dosing options in migraine prevention. J Headache Pain. 2019;20:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S4
Table S1‐S4
Data Availability Statement
Qualified researchers may request access to patient level data and related study documents including the study protocol and the statistical analysis plan. Patient level data will be de‐identified and study documents will be redacted to protect the privacy of trial participants and to protect commercially confidential information. Please email USMedInfo@tevapharm.com to make your request.


