Abstract

Nitrogen dioxide (NO2) is a toxic air pollutant, and efficient abatement technologies are important to mitigate the many associated health and environmental problems. Here, we report the reactive adsorption of NO2 in a redox−active metal–organic framework (MOF), MFM-300(V). Adsorption of NO2 induces the oxidation of V(III) to V(IV) centers in MFM-300(V), and this is accompanied by the reduction of adsorbed NO2 to NO and the release of water via deprotonation of the framework hydroxyl groups, as confirmed by synchrotron X-ray diffraction and various experimental techniques. The efficient packing of {NO2·N2O4}∞ chains in the pores of MFM-300(VIV) results in a high isothermal NO2 uptake of 13.0 mmol g–1 at 298 K and 1.0 bar and is retained for multiple adsorption–desorption cycles. This work will inspire the design of redox-active sorbents that exhibit reductive adsorption of NO2 for the elimination of air pollutants.
Improving air quality is an important global issue. NO2 remains a key outdoor air pollutant, with road transport being a major cause, particularly in areas with high population.1 With increasingly stringent emission standards for vehicles being issued by the World Health Organization (WHO) and other local environmental agencies,2 refinements and improvements to the existing selective-catalytic-reduction (SCR) systems are required urgently to deliver closer-to-zero emissions of NO2. While SCR is based on the reduction of NOx by ammonia or urea at elevated temperatures in the presence of a precious-metal-based catalyst, physio-sorption of gases in porous materials is a well-established method to remove gaseous species. Activated carbons have been long known to remove toxic gases,3 but these often suffer from low adsorption capacity of NO2 and/or rapid structural degradation.4
Metal–organic frameworks (MOFs) show great potential for gas adsorption and storage, and this is attributed to their large surface area and pore functionalities.5 Systems incorporating a redox active metal center are of particular interest in gas separation6 and catalysis.7 However, adsorption of NO2 in MOFs remains largely unexplored,8 and reported studies are based upon breakthrough experiments using gas streams containing NO2 at ppm levels. In most of these cases, the MOF and MOF-containing composites undergo irreversible structural degradation on adsorption of NO2. Infrared spectroscopy has been used to characterize the species that are formed upon contacting the surface with highly reactive NO2.9−14 Additionally, MOFs have also been explored as new sensing materials for NO2 detection with high sensitivity and selectivity.15 Reversible adsorption isotherms for pure NO2 have been measured with MOFs in only two cases, namely with MFM-300(Al)16 and MFM-520(Zn).17 Both materials are highly structurally robust and remain intact throughout the isothermal measurements.
Here we report the reactive adsorption of NO2 in a redox-active MOF, MFM-300(V) [V2(OH)2(C16H6O8)]. MFM-300(V) is redox active, and both VIII and VIV forms, MFM-300(VIII) and MFM-300(VIV), can be obtained as pure phase materials.18 Oxidation of turquoise MFM-300(VIII) leads to conversion of the bridging hydroxyl groups to bridging oxo groups in brown MFM-300(VIV) (Figure 1a,b). The adsorption and binding of CO218 and hydrocarbons19 exhibit notable differences in these two materials with MFM-300(VIII) showing higher adsorption uptakes owing to strong hydrogen bonding between substrate molecules and the bridging hydroxyl group.
Figure 1.

Views along the a-axis of the [VO6]∞ chain in (a) MFM-300(VIII) and (b) MFM-300(VIV) as determined by high-resolution SPXRD data at 298 K. The proton of the bridging hydroxyl group in MFM-300(VIII) is highlighted in purple. Views along the c-axis of the (c) RS and (d) LS models showing the packing of guest molecules. Host–guest interactions are enlarged in (e) and (f) for the RS and LS model, respectively. (g) Extended chain structure of {N2O4·NO2}∞ in the LS model. V, green; O, red; C, gray; H, white; N of NO2, deep blue; N of N2O4, light blue.
We introduced NO2 gas into MFM-300(VIII) and collected a series of high-resolution synchrotron X-ray powder diffraction (SPXRD) data. The first three data sets, noted as rapid scan (RS, RS-1, RS-2; ∼8 min for each scan), were collected immediately after loading of NO2; the final data set, noted as long scan (LS; ∼1.5 h), was collected after adsorption had reached equilibrium, as evidenced by the lack of changes in subsequent SPXRD patterns. Rietveld refinement has allowed the determination of binding domains of adsorbed NO2 molecules and captured key structural changes of the host framework for these models. In the RS model of MFM-300(V)·(NO2)1.67, which represents the kinetically favorable state (Figure 1 c, e), only NO2 was observed and anchored to the pore interior primarily through the hydrogen bonding interaction with the bridging hydroxyl group of the vanadium chain [ONO2···Hhydroxyl = 2.33(2) Å]. The bound NO2 molecule is additionaly stabilized by 8-fold supramolecular interactions with the aromatic ligand of the framework [ONO2···Haromatic = 3.01(4)–3.59(4) Å; NNO2···Caromatic = 3.37(2)–3.76(2) Å]. Unlike NO2@MFM-300(Al),16 which shows coadsorption and settlement of monomers and dimers of NO2 (ratio of ∼1.0:0.9) in the pores, NO2@MFM-300(VIII) shows only monomers of NO2 initially with no N2O4 dimers in the pores. The framework of MFM-300(VIII) [V–O = 1.969(3), 2.005(3), 2.026(4) Å (each appears twice); ∠VO(H)V = 125.6(3)°] is intact in the RS model, and bond valence sum (BVS) calcuations confirm an oxidation state of 3.1 for the V center. The structure of RS-1 [MFM-300(V)·(NO2)1.66] is very similar to that of the RS model.
In the RS-2 model MFM-300(V)·(NO2)1.28·(N2O4)1.23, we observed an increase of the oxidation state of the vanadium centers and dimerization of NO2 molecules in the pore; the overall structure is similar to that of the equilibriated LS model, which is described now in detail. Upon reaching equilibrium for adsorption of NO2 in the LS model for MFM-300(V)·(NO2)1.60·(N2O4)1.53, the coordination environment of the V center changes [V–O = 1.831(1), 1.991(2), 2.056(3)Å (each appears twice); ∠VOV = 136.2(1)°], and BVS calculations confirm the increase of valence of the V centers to 3.7 (Figure 1d). Thus, host–guest charge transfer has occurred via the hydrogen bonds of bridging vanadium hydroxyls, which are deprotonated to bridging oxo groups to accommodate oxidation of the V centers in the LS model. This has been confirmed further by the disappearance of the −OH stretching band at 3627 cm–1 in the FTIR spectrum of the MFM-300(V) on adsorption of NO2 (Figure 2e). The change in oxidation state of V in MFM-300(V) has also been confirmed by in situ EPR spectroscopy where an intense signal centered at g = 1.955 (9.8743 GHz) with a peak-to-peak line width of ca. 75 G appears on adsorption of NO2 (Figure 2f), consistent with the formation of d1 VIV centers.18 This implies that MFM-300(VIII) has undergone redox reaction and the proton of the bridging hydroxyl group is removed upon contacting NO2, yielding weakly adsorbed NO and H2O that are readily displaced by further molecules of NO2. This was confirmed by breakthrough experiments (see below). Interestingly, in contrast to the RS model, 66% of adsorbed NO2 molecules in the LS model form N2O4 dimers, which are stabilized in the pores of MFM-300(VIV) by intermolecular dipole and supramolecular interactions with the aromatic ligands (Figure 1f). This results in a different overall packing of NO2 and N2O4 molecules inside the pores of MFM-300(VIV) compared to that in NO2@MFM-300(Al).16 In the LS model, N2O4 dimers reside closer to the pore wall while the residual NO2 monomer is situated more toward the pore center. The host–guest distances [NN2O4···Caromatic = 3.34(3)–3.97(2) Å; ON2O4···Haromatic = 2.14(4)–3.86(4) Å] are longer in NO2@MFM-300(VIV) than in NO2@MFM-300(Al)16 [3.11(3) Å and 2.62(5)–3.40(5) Å, respectively], indicating an overall weaker host–guest interaction. Alternating dimers and monomers form a 1-D helical chain of {N2O4·NO2}∞ running down the pore of the structure along the c-axis (Figure 1g). The arrangement of these guest molecules is more compact [NNO2···ON2O4 and ONO2···NN2O4 distances of 3.10(2)–3.54(3) Å] than in NO2@MFM-300(Al) [3.33(3)–3.75(3) Å], suggesting that the reduced host–guest interaction in NO2@MFM-300(VIV) is compensated by the stronger guest–guest interactions to give efficient packing of NO2.
Figure 2.
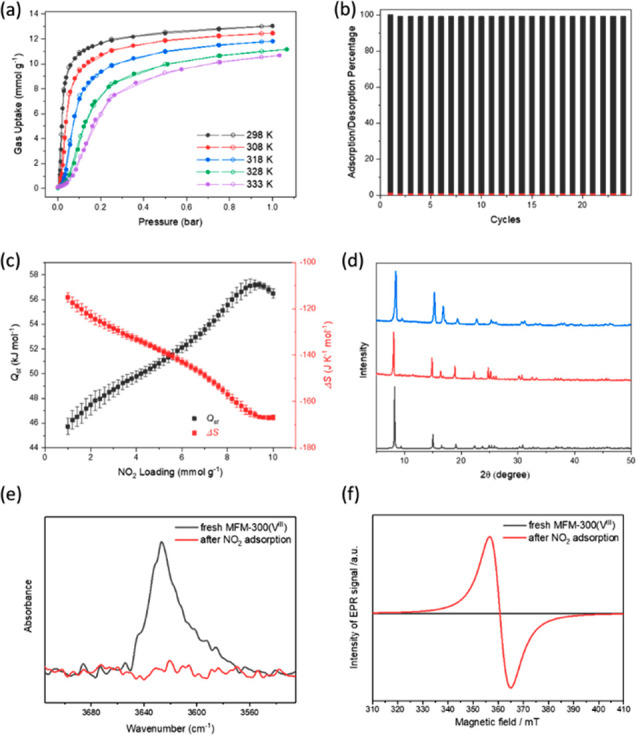
(a) Isotherms for NO2 uptake in MFM-300(VIV) at 298–333 K (adsorption and desorption are marked with solid and open symbols, respectively). (b) Cyclic adsorption–desorption of NO2 in MFM-300(VIV) at 298 K between 0 and 0.5 bar. Red bars show the residual NO2 in the MOF on pressure-swing desorption. (c) Variation of Qst and ΔS for NO2 uptake in MFM-300(VIV). (d) PXRD patterns of MFM-300(V): simulated (black), as-synthesized (red), and after adsorption and desorption of NO2 (blue). Comparison of (e) FTIR spectra and (f) X-band (9.87 GHz) EPR spectra of the freshly activated MFM-300(VIII) and same sample after adsorption and desorption of NO2 at 298 K.
Gravimetric isotherms for uptake of pure NO2 in MFM-300(VIII) were measured at various temperatures (298–333 K) between 0 and 1.0 bar. All isotherms shown in Figure 2a were measured with the same batch of sample, following the sequence of 298 K, 308 K, 318 K, 328 K, and 333 K. This means that although the sample loaded into the instrument was MFM-300(VIII), after initial contact with NO2 the follow-on isotherms are measuring uptake in MFM-300(VIV), as confirmed by SPXRD results. The NO2 uptake of MFM-300(VIV) at 298 K and 1.0 bar is 13.0 mmol g–1 (equivalent to 2.98 NO2/V), which is in good agreement with the LS model and similar to MFM-300(Al) (14.1 mmol g–1 or 2.92 NO2/Al) under the same conditions. Despite the redox host-to-guest charge transfer, the uptake is fully reversible, and no hysteresis was observed at any temperature. The isothermal uptakes decreased with increasing temperature along the entire pressure range, and the isosteric heats of adsorption (Qst) increase from 46 kJ mol–1 steadily to 58 kJ mol–1 (Figure 2c), consistent with the presence of strong guest–guest interactions on increased NO2 loading into MFM-300(VIV). Compared with NO2@MFM-300(Al),16 a more negative entropy of adsorption (ΔS) at higher NO2 loading was observed for MFM-300(VIV), indicating a higher degree of ordering of the N2O4/NO2 molecules in the pore, in excellent agreement with the crystallographic study. The same MFM-300(VIV) sample was then tested across 24 further adsorption–desorption cycles at 298 K, confirming the excellent regeneration of the MOF and no loss of NO2 uptake (Figure 2b). The crystallinity of MFM-300(V) was retained after all isotherm and cycling measurements (Figure 2d).
Although the initial and the repeated isotherms for NO2 in MFM-300(V) at 298 K showed equal uptakes at 1.0 bar, when examined more closely, a clear difference of NO2 uptake can be observed in the low pressure region between these two isotherms. Negligible uptake of NO2 was recorded in fresh MFM-300(VIII) below 10 mbar, whereas once oxidized to MFM-300(VIV) a prominent NO2 uptake of 1.1 mmol g–1 at 10 mbar was observed (Figure S7). We speculated that when NO2 is contacted with a fresh sample MFM-300(VIII), the V center is oxidized and NO2 is then converted to a poorly adsorbing gaseous species, thus leading to the observed low uptake at low pressures. Whereas for the repeated isotherm at 298 K, MFM-300(VIV) cannot convert further NO2 and a notable adsorption of 1.1 mmol g–1 was observed at 10 mbar. This hypothesis was validated by dynamic breakthrough experiments, where a 900 ppm of NO2 balanced flow in He was passed through a glass tubular reactor packed with freshly activated MFM-300(VIII) at 298 K at a total flow rate of 50 mL min–1. The potential exhaust gaseous products (NO2, NO, N2O, N2, H2O, CO2) at the outlet of the fixed-bed were monitored throughout the experiment. After the flow was initiated, a change in the color of the sample from turquoise to brown was clearly observed, starting from the inlet side of the fixed-bed, and this border moved with the gas flow until the entire bed turned brown (Figure 3). This is consistent with the full oxidation of MFM-300(VIII) to MFM-300(VIV). Significantly, NO is almost immediately detected after the NO2 flow was initiated. Thus, upon contact with MFM-300(VIII), NO2 is reduced to NO, which has a much weaker interaction with the framework and is thus readily desorbed and passes rapidly through the fixed bed as confirmed in the dynamic adsorption experiment with NO (Figure S8). This redox reaction is also accompanied by the production of water, which is gradually displaced by molecules of NO2 and is observed at the outlet after NO owing to its stronger interaction with the framework. Finally, NO2 is eluted, while the NO concentration returns to near zero, confirming the completion of the redox reaction at MFM-300(V) and the saturation of the fixed-bed by adsorption of NO2. Throughout the experiment, no other nitrogen-containing species was detected. The breakthrough experiment demonstrates the ability of MFM-300(V) for the dynamic adsorption and reduction of NO2 even at ppm-level concentrations.
Figure 3.

(a) Time-resolved photos of the glass reactor packed with MFM-300(VIII) during breakthrough experiment of the NO2 (900 ppm) in He at 298 K and 1.0 bar showing the color change of sorbent. (b) Breakthrough profiles of species at the outlet of the fixed-bed.
In summary, MFM-300(VIII) exhibits redox conversion to MFM-300(VIV) on adsorption of NO2, which is converted to NO. MFM-300(VIV) shows high capacity and excellent reversibility for NO2 adsorption suggesting future directions for the development of efficient redox-active sorbent materials to realize adsorptive reduction of NO2 into nonharmful species.
Acknowledgments
We thank EPSRC (EP/I011870), the Royal Society, the National Natural Science Foundation of China, Peking University, and University of Manchester for funding, and EPSRC for funding of the EPSRC National EPR Facility at Manchester. This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (Grant Agreement No. 742401, NANOCHEM). We are grateful to ESRF for access to the Beamline ID22 and thank A. Fitch for the help at ESRF. A.M.S. is supported by a Royal Society Newton International Fellowship. Y.M. and J.L. thank the China Scholarship Council (CSC) and the University of Manchester for funding.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.0c06414.
Synthesis procedures, characterization, and additional analysis of crystal structures and adsorption results (PDF)
Crystal data of MFM-300(V)(NO2)1.67 (CIF)
Crystal data of MFM-300(V)·(NO2)1.66 (CIF)
Crystal data of MFM-300(V)·(NO2)1.28·(N2O4)1.23 (CIF)
Crystal data of MFM-300(V)·(NO2)1.60·(N2O4)1.53 (CIF)
The authors declare no competing financial interest.
Notes
Crystal data of MFM-300(V)(NO2)1.67 and MFM-300(V)·(NO2)1.60·(N2O4)1.53 are deposited at Cambridge Crystallographic Data Centre (CCDC 1996818–1996819).
Supplementary Material
References
- Ambient (outdoor) air pollution, 2018. World Health Organization Web site. https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed May 2, 2018).
- Nitrogen dioxide (NO2) pollution, 2019. United States Environmental Protection Agency Website. https://www.epa.gov/no2-pollution (accessed June 13, 2019).
- a Kozhevnikov A. B.Stalin’s great science: the times and adventures of Soviet physicists; Imperial College Press: 2004; pp 10–11. [Google Scholar]; b Ray G. C.; Box E. O. Adsorption of gases on activated charcoal. Ind. Eng. Chem. 1950, 42, 1315–1318. 10.1021/ie50487a021. [DOI] [Google Scholar]
- a Belhachemi M.; Jeguirim M.; Limousy L.; Addoun F. Comparison of NO2 removal using date pits activated carbon and modified commercialized activated carbon via different preparation methods: Effect of porosity and surface chemistry. Chem. Eng. J. 2014, 253, 121–129. 10.1016/j.cej.2014.05.004. [DOI] [Google Scholar]; b Florent M.; Tocci M.; Bandosz T. J. NO2 adsorption at ambient temperature on urea-modified ordered mesoporous carbon. Carbon 2013, 63, 283–203. 10.1016/j.carbon.2013.06.081. [DOI] [Google Scholar]
- Li J. R.; Kuppler R. J.; Zhou H.-C. Selective gas adsorption and separation in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. 10.1039/b802426j. [DOI] [PubMed] [Google Scholar]
- Li L.; Lin R. B.; Krishna R.; Li H.; Xiang S.; Wu H.; Li J.; Zhou W.; Chen B. Ethane/ethylene separation in a metal-organic framework with iron-peroxo sites. Science 2018, 362, 443–446. 10.1126/science.aat0586. [DOI] [PubMed] [Google Scholar]
- Verma P.; Vogiatzis K. D.; Planas N.; Borycz J.; Xiao D. J.; Long J. R.; Gagliardi L.; Truhlar D. G. Mechanism of oxidation of ethane and ethanol at iron(IV)-oxo sites in magnesium-diluted Fe2(dodbc). J. Am. Chem. Soc. 2015, 137, 5770–5781. 10.1021/jacs.5b00382. [DOI] [PubMed] [Google Scholar]
- Han X.; Yang S.; Schröder M. Porous metal-organic-framework as emerging sorbents for clean air. Nat. Rev. Chem. 2019, 3, 108–118. 10.1038/s41570-019-0073-7. [DOI] [Google Scholar]
- Levasseur B.; Petit C.; Bandosz T. J. Reactive adsorption of NO2 on copper-based metal-organic framework and graphite oxide/metal-organic framework composites. ACS Appl. Mater. Interfaces 2010, 2, 3606–3613. 10.1021/am100790v. [DOI] [PubMed] [Google Scholar]
- Petit C.; Levasseur B.; Mendoza B.; Bandosz T. J. Reactive adsorption of acidic gases on MOF/graphite oxide composites. Microporous Mesoporous Mater. 2012, 154, 107–112. 10.1016/j.micromeso.2011.09.012. [DOI] [Google Scholar]
- Ebrahim A. M.; Levasseur B.; Bandosz T. J. Interactions of NO2 with Zr-based MOF: effects of the size of organic linkers on NO2 adsorption at ambient conditions. Langmuir 2013, 29, 168–174. 10.1021/la302869m. [DOI] [PubMed] [Google Scholar]
- Ebrahim A. M.; Bandosz T. J. Effect of amine modification on the properties of zirconium-carboxylic acid based materials and their applications as NO2 adsorbents at ambient conditions. Microporous Mesoporous Mater. 2014, 188, 149–162. 10.1016/j.micromeso.2014.01.009. [DOI] [Google Scholar]
- Peterson G. W.; Mahle J. J.; DeCoste J. B.; Gordon W. O.; Rossin J. A. Extraordinary NO2 removal by the metal-organic framework UiO-66-NH2. Angew. Chem., Int. Ed. 2016, 55, 6235–6238. 10.1002/anie.201601782. [DOI] [PubMed] [Google Scholar]
- Ebrahim A. M.; Bandosz T. J. Ce(III) Doped Zr-based MOFs as excellent NO2 adsorbents at ambient conditions. ACS Appl. Mater. Interfaces 2013, 5, 10565–10573. 10.1021/am402305u. [DOI] [PubMed] [Google Scholar]
- Kumar P.; Kim K. H.; Rarotra S.; Ge L.; Lisak G. The advanced sensing systems for NOx based on metal-organic frameworks: applications and future opportunities. Trends Anal. Chem. 2020, 122, 115730. 10.1016/j.trac.2019.115730. [DOI] [Google Scholar]
- Han X.; Godfrey H. G. W.; Briggs L.; Davies A. J.; Cheng Y.; Daemen L. L.; Sheveleva A. M.; Tuna F.; McInnes E. J. L.; Sun J.; Drathen C.; George M. G.; Ramirez-Cuesta A. J.; Thomas K. M.; Yang S.; Schröder M. Reversible adsorption of nitrogen dioxide within a robust porous metal–organic framework. Nat. Mater. 2018, 17, 691–696. 10.1038/s41563-018-0104-7. [DOI] [PubMed] [Google Scholar]
- Li J.; Han X.; Zhang X.; Sheveleva A. M.; Cheng Y.; Tuna F.; McInnes E. J. L.; McCormick L. J.; Teat S. J.; Daemen L. L.; Ramirez-Cuesta A. J.; Schröder M.; Yang S. Capture of nitrogen dioxide and conversion to nitric acid in a porous metal–organic framework. Nat. Chem. 2019, 11, 1085–1090. 10.1038/s41557-019-0356-0. [DOI] [PubMed] [Google Scholar]
- Lu Z.; Godfrey H. G. W.; da Silva I.; Cheng Y.; Savage M.; Tuna F.; McInnes E. J. L.; Teat S. J.; Gagnon K. J.; Frogley M. D.; Manuel P.; Rudić S.; Ramirez-Cuesta A. J.; Easun T. L.; Yang S.; Schröder M. Modulating supramolecular binding of carbon dioxide in a redox-active porous metal-organic framework. Nat. Commun. 2017, 8, 14212. 10.1038/ncomms14212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z.; Godfrey H. G. W.; da Silva I.; Cheng Y.; Savage M.; Manuel P.; Rudić S.; Ramirez-Cuesta A. J.; Yang S.; Schröder M. Direct observation of supramolecular binding of light hydrocarbons in vanadium(III) and (IV) metal–organic framework materials. Chem. Sci. 2018, 9, 3401–3408. 10.1039/C8SC00330K. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


