Abstract
Objectives
To develop and validate a clinical prediction model (CPM) for survival in hypopharynx cancer, thereby aiming to improve individualized estimations of survival.
Methods
Retrospective cohort study of hypopharynx cancer patients. We randomly split the cohort into a derivation and validation dataset. The model was fitted on the derivation dataset and validated on the validation dataset. We used a Cox's proportional hazard model and least absolute shrinkage and selection operator (LASSO) selection. Performance (discrimination and calibration) of the CPM was tested.
Results
The final model consisted of gender, subsite, TNM classification, Adult Comorbidity Evaluation‐27 score (ACE27), body mass index (BMI), hemoglobin, albumin, and leukocyte count. Of these, TNM classification, ACE27, BMI, hemoglobin, and albumin had independent significant associations with survival. The C Statistic was 0.62 after validation. The model could significantly identify clinical risk groups.
Conclusions
ACE27, BMI, hemoglobin, and albumin are independent predictors of overall survival. The identification of high‐risk patients can be used in the counseling process and tailoring of treatment strategy or follow‐up.
Level of Evidence
4 Laryngoscope, 130:2166–2172, 2020
Keywords: Hypopharynx cancer, survival, total laryngectomy, chemoradiotherapy, clinical prediction model, LASSO
INTRODUCTION
Hypopharynx cancer is a rare disease and has the worst overall survival (OS) of all head and neck squamous cell (SCC) malignancies.1, 2, 3 Due to the “silent” anatomical location of the hypopharynx, tumors can progress relatively far before giving rise to any clinical symptoms. Around 50% of patients with hypopharynx cancer already have lymph node metastases at time of diagnosis.4 Survival rates are gradually improving, but remain low, with 5‐year OS rates of 28% to 41%.1, 4, 5 Oncological management usually consists of either primary radiotherapy (RT), chemoradiotherapy (CRT), laser surgery, or a total laryngectomy with partial or circumferential pharyngectomy (TLP).6
The TNM classification is an important tool to describe tumor characteristics and estimate prognosis on a population level, but does not translate well to survival predictions made on the individual level.7 To estimate prognosis on the individual level, physicians need to consider numerous patient specific variables, such as age, gender, comorbidity, and results from imaging, pathology reports, and possibly peripheral blood tumor markers.8, 9 Based on a combination of all these factors, the best treatment option, usually defined in terms of survival, is selected and discussed with the patient. However, the human cognitive capacity is limited, and capable only of taking into account a few variables at a time when making a decision.10 Several studies have already demonstrated the superiority of statistical decisional models over clinical expertise‐based predictions of physicians.11, 12
In order to improve survival estimations for individual patients with hypopharynx cancer, the objectives of this study are to examine clinical predictors of survival in hypopharynx cancer, and develop and validate a clinical prediction model (CPM) based on these readily available variables. The resulting improved survival estimates might enable tailoring of treatment strategies or follow‐up regimens.
MATERIALS AND METHODS
Study Design and Participants
We performed a retrospective study in which we collected data of patients diagnosed and treated for SCC of the hypopharynx in three dedicated head and neck centers in the Netherlands: the Netherlands Cancer Institute (1990–2013), the University Medical Center Utrecht (1990–2012), and Amsterdam University Medical Center, location VUmc (2003–2010). Data of patients in these cohorts were provided by the research information department of each hospital. We excluded patients with distant metastases at time of diagnosis, patients who were not treated with curative intent, patients who were primarily treated in another hospital, and patients who had revision of diagnosis after additional medical evaluation, mostly because of different tumor localization.
This study does not fall under the scope of the Medical Research Involving Human Subjects Act. The institutional review boards of all hospitals approved this study.
Predictor Variables
We built the model using a limited number of candidate variables, which were preselected based on clinical expertise, scientific evidence, and practical feasibility.13, 14 The preselected variables were age at diagnosis, gender, T classification, N classification, subsite, the Adult Comorbidity Evaluation‐27 (ACE27) score, packyears, alcohol consumption, body mass index (BMI), and the baseline peripheral blood values hemoglobin (mmol/L), albumin (g/L) and leukocyte count (10E9/L). Leukocyte count was dichotomized into low‐normal (<10.5 × 109/L) and high (≥10.5 × 109/L). ACE27 was scored in retrospect by one researcher (J.P.) for all patient cohorts based on the comorbidities registered in the medical files at time of diagnosis. The incidence of HPV positive hypopharynx cancer ranges between 4% to 6%, reason why we did not include this variable into our model.15, 16
Statistical Analysis
To obtain two datasets that were representative of the Dutch population of patients with hypopharynx cancer, we first combined all three cohorts into one dataset, and then randomly split the cohort into two datasets, one of which was used for the model derivation while the other was used for the validation.
Descriptive statistics were used to report patient characteristics and to assess whether there were relevant differences between the derivation dataset and the validation dataset. The samples were compared by means of the independent T‐test (continuous variables), and Linear‐by‐Linear test or Fisher exact test (categorical variables).
Missing data of the predictors under consideration in our cohort was considered to be missing at random. Multiple imputation was used to complete the data in the derivation dataset, using the Multiple Imputation by Chained Equations procedure (MICE package in R).17 We generated 20 imputed datasets from our dataset.
Model Derivation
On each of the 20 imputed datasets, a least absolute shrinkage and selection operator (LASSO) penalized Cox proportional hazards model was fitted using the penalized package in R studio.18 In this method, variables are not selected based on P‐values, but shrinkage is applied to the regression coefficients in such a way that coefficients of the least contributing predictors become exactly zero. Non‐zero coefficients are retained in the model and are therefore considered significant predictors. In this way, LASSO regression strives to balance two competing objectives: optimize the prognostic accuracy versus minimizing the number of predictors contributing to the model, in order to reduce the risk of overfitting. The resulting model thus in part depends on the relative “strength” of these opposing forces, as expressed by a parameter “lambda,” which can be set by the user. In our case, we chose the optimal value of the lambda coefficient (for each imputed dataset separately) for a set of 12 candidate values by internal cross‐validation, using the leave‐one‐out method. Afterwards, the regression coefficients of the models fitted on the imputed datasets were pooled into the final CPM by averaging them.
Model Performance and Validation
Performance of the CPM was assessed in the derivation and validation dataset using discrimination and calibration. Discrimination of the prognostic model is defined as its accuracy to distinguish a patient who died from a patient who survived, and is expressed in the C statistic. A C statistic of 0.5 indicates no discriminative ability, whereas a C statistic of 1.0 indicates perfect discrimination.14 Calibration reflects the similarity between the probabilities of the event for each patient as predicted by the model and the outcomes observed in the sample, and is visually depicted in a calibration plot. A 45° line indicates perfect agreement between predicted and observed outcome.19
After internal validation in the derivation data, we assessed discrimination and calibration of the model in the validation dataset to assess its performance when used in new patients. This step is essential to test the strength of the model before it can be used in clinical practice.14 To assess the model performance in a more clinically interpretable way, we created three risk strata of equal size, based on the distribution of linear predictors in the derivation dataset. Survival rates of these strata in the derivation dataset and the external validation dataset were plotted with Kaplan‐Meier curves, to assess whether the model accurately discriminates between the risk strata. We used a cox proportional hazard analysis to compare hazard ratios (HRs) and 95% confidence intervals (CI) for these risks groups.
To compare the accuracy of the CPM to a model containing only the TNM classification as clinical predictors, we used the C statistic to estimate the performance of a model containing only TNM classification. The TNM classification model was similarly built using a LASSO‐penalized cox proportional hazards model. Furthermore, using a likelihood‐ratio test, we tested whether our model performed significantly better that the model with the TNM classification only. For this test, a P‐value <.05 was considered to indicate statistical significance.
RESULTS
Derivation and Validation Datasets
The research information departments provided us with data on 1077 patients that had been diagnosed with hypopharynx cancer in the respective hospitals. We subsequently excluded 309 patients (Fig. 1). This left us with 768 patients for analysis. Mean age was 63 years, 79% was male and 21% was treated with single modality RT, 42% with CRT (cisplatin), 33% with primary TL, and 4% received another treatment. An analysis of the data revealed that there were no significant differences in all variables between the two datasets, thus the random split could be considered successful. There were predictors without missingness, with a maximum of 29% missing data of the peripheral blood value albumin. Patient characteristics are shown in Table 1.
Figure 1.
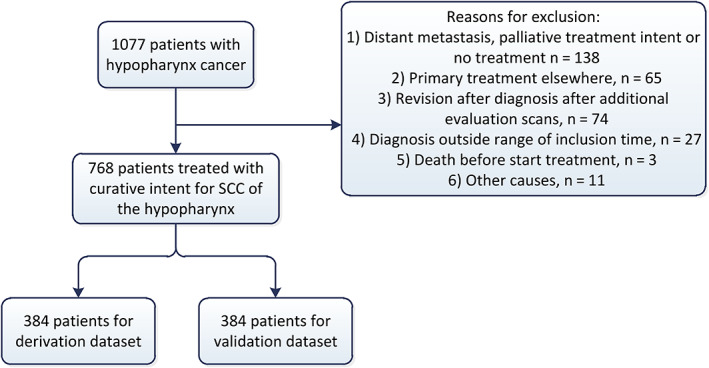
Inclusion of patients [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
Table 1.
Patient Characteristics of the Complete Cases of the Derivation and External Validation Datasets.
| Characteristics | Total cohort (n = 768) | Derivation dataset (n = 384) | Validation dataset (n = 384) | P |
|---|---|---|---|---|
| Age, mean [SD] | 63 [10.1] | 63 [10.1] | 63 [10.2] | .8026* |
| Sex, n (%) | .2874*** | |||
| Female | 161 (21) | 87 (23) | 74 (19) | |
| Male | 607 (79) | 297 (77) | 310 (81) | |
| T‐classification, n (%) | .8375** | |||
| T1 | 81 (10) | 43 (11) | 38 (10) | |
| T2 | 204 (27) | 99 (26) | 105 (27) | |
| T3 | 213 (28) | 109 (28) | 104 (27) | |
| T4 | 268 (35) | 133 (35) | 135 (35) | |
| Missing | 2 | 0 | 2 | |
| N‐classification, n (%) | .8946** | |||
| N0 | 251 (33) | 130 (34) | 121 (32) | |
| N1 | 138 (18) | 59 (15) | 79 (21) | |
| N2 | 305 (40) | 158 (41) | 147 (38) | |
| N3 | 73 (9) | 37 (10) | 36 (10) | |
| Missing | 1 | 0 | 1 | |
| Subsite, n (%) | .8617*** | |||
| Pyriform Sinus | 599 (78) | 298 (78) | 301 (78) | |
| Other | 169 (22) | 86 (22) | 83 (22) | |
| ACE27, n (%) | .511** | |||
| 0 | 261 (34) | 126 (33) | 135 (35) | |
| 1 | 299 (39) | 152 (39) | 147 (38) | |
| 2 | 167 (22) | 84 (22) | 83 (22) | |
| 3 | 41 (5) | 22 (6) | 19 (5) | |
| Packyears, median [IQR] | 37 [25–47] | 38 [26–47] | 36 [25–47] | .4415* |
| Missing (%) | 29 (4) | 16 (4) | 13 (3) | |
| Alcohol consumption, median [IQR] | 21 [14–42] | 21 [14–42] | 21 [14–42] | .4917* |
| Missing (%) | 15 (2) | 7 (2) | 8 (2) | |
| BMI, mean [SD] | 23 [4.3] | 22.9 [4.3] | 22.9 [4.3] | .6171* |
| Missing (%) | 73 (10) | 38 (10) | 35 (9) | |
| Leukocytosis, n (%) | .6089*** | |||
| Yes | 485 (63) | 242 (63) | 243 (63) | |
| No | 190 (25) | 99 (26) | 91 (24) | |
| Missing (%) | 93 (12) | 43 (11) | 50 (13) | |
| Hemoglobin, median [IQR] | 8.6 [7.9–14.5] | 8.6 [7.9–9.1] | 8.6 [7.9–9.2] | .1318* |
| Missing (%) | 19 (2) | 6 (2) | 11 (3) | |
| Albumin, median [IQR] | 40.6 [36.5–44] | 40.1 [37–45] | 40 [34.7–44] | .4227* |
| Missing (%) | 222 (29) | 117 (30) | 85 (27) |
ACE27 = Adult Comorbidity Evaluation‐27; BMI = body mass index; IQR = interquartile range; SD = standard deviation. Leukocytosis was expressed as a level of ≥10.5 10E9/L. Values in parentheses are percentages unless otherwise indicated.
Independent T‐test,
Linear by linear test and
Fisher's exact test
Development and Internal Validation
First, we examined the univariable association of each predictor under consideration with survival.
The regression coefficients for alcohol consumption and the amount of packyears had a wrong sign, showing a small protective effect with increasing exposure. Because of the unreliability of this data, and the biologically implausible association, these variables were excluded as candidate predictors.
Next, we included the remaining candidate predictors in the LASSO model. The distinctions between T4 and T3, between N3 and N2, between N2 and N1, and between all four levels of ACE27, as well as values of BMI, hemoglobin, and albumin contributed significantly to the models on all 20 imputed datasets. In all but one of the datasets there was a significant difference in the survival of T3 versus T2 patients. By contrast, in none of the imputed datasets a significant distinction between N0 and N1 (in their effect on survival) was observed, nor a significant contribution of age. T2 and T1 patients had significantly differing survival in only one dataset. Gender and leukocyte count contributed significantly only in two of 20 cases. Because gender is widely accepted as predictor for survival, we forced this variable into the model. A location in the pyriform sinus proved relevant in 13 out of 20 models. This model had a discrimination (C statistic) of 0.66.
Validation
In the validation, the discriminative ability of the CPM showed a C statistic of 0.62. The CPM is shown in Table 2 and a nomogram of this model is presented in Figure 2. If a patient has less than 154 points on this nomogram, he can be considered low risk, between 155 and 201 medium risk, and above 202 points he can be considered as a patient with a high risk of death. The CPM showed excellent calibration as depicted in Figure 3.
Table 2.
Variables Used in the Final Developed CPM with their Associated Regression Coefficient and Hazard Ratio.
| Variables | Regression coefficient B | HR |
|---|---|---|
| Sex (female vs. male) | −0.00176 | 0.998 |
| Age | 0.00 | 1 |
| Subsite (pyriform sinus vs other) | 0.01385 | 1.014 |
| T‐classification | – | |
| T2 vs T1 | 0.00033 | 1 |
| T3 vs T2 | 0.5807 | 1.060 |
| T4 vs T3 | 0.33530 | 1.398 |
| N‐classification | ||
| N1 vs N0 | 0 | 1 |
| N2 vs N1 | 0.39662 | 1.487 |
| N3 vs N2 | 0.104 | 1.11 |
| ACE27 | – | – |
| 1 vs. 0 | 0.220853 | 1.232 |
| 2 vs. 1 | 0.20235 | 1.224 |
| 3 vs. 2 | 0.11411 | 1.121 |
| BMI | −0.01552 | 0.989 |
| Hemoglobin* | −0.0662 | 0.936 |
| Albumin* | −0.01704 | 0.983 |
| Leukocytosis | 0.00182 | 1.002 |
ACE27 = Adult Comorbidity Evaluation‐27; BMI = body mass index. HR = hazard ratio. Leukocytosis was expressed as a level of ≥10.5 10E9/L
For hemoglobin and albumin expressed coefficients represent the added effect of every mmol/L or g/L increase.
Figure 2.
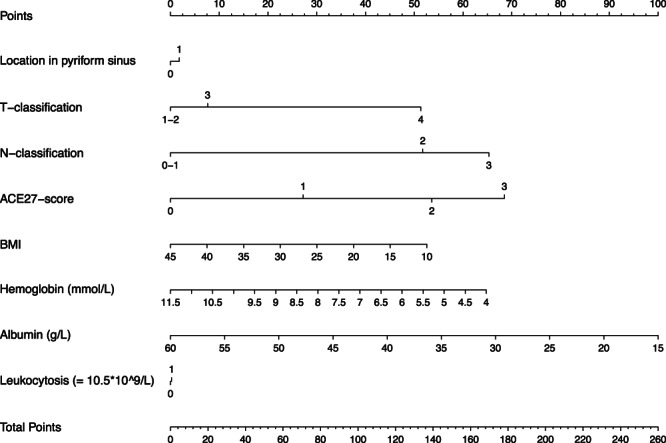
Nomogram of the final model. Combining the amount of points that correspond with each variable on the top scale will lead to a total amount of points. If a patient has less than 154 points on this nomogram, he can be considered low risk, between 155 and 201 medium risk, and above 202 points he can be considered as a patient with a high risk of death.
Figure 3.
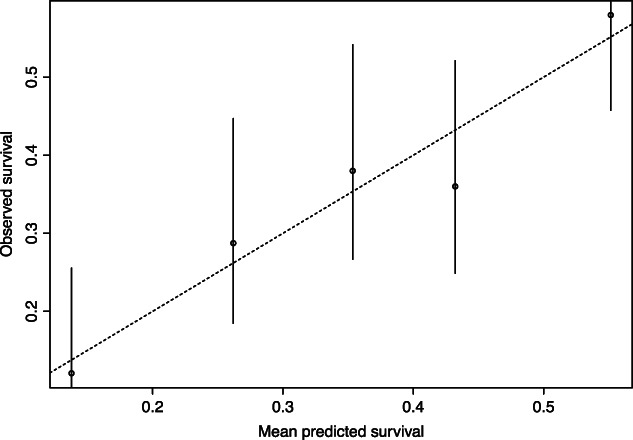
Calibration plot of the CPM model after validation. A 45‐degree line (dashed line) indicates perfect agreement between predicted and observed outcome.19 Calibration of our model is depicted in the straight line, and closely follows the 45‐degree line.
Based on the distribution of the linear predictors in the derivation dataset, we created three risk strata: low‐, medium‐, and high‐risk on death. The Kaplan Meier curves for the three risk strata of the original model showed good discrimination in the derivation dataset. Also, in the validation dataset, discrimination was good for all three risk groups, especially the high‐risk group showed excellent discrimination. The survival curves for medium‐ and low‐risk groups are closer together, but still clearly separated (Fig. 4). The hazard ratio of patients in the low‐risk versus medium‐risk group was 0.76, 95% CI, 0.71–0.81. For the high‐risk versus medium‐risk group this was HR 1.77, 95% CI, 1.67–1.88.
Figure 4.
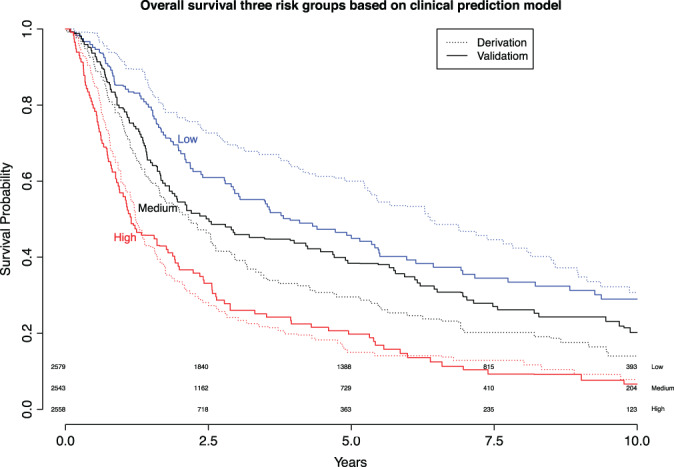
Kaplan Meier curves for three risk groups based on the derivation dataset. The Kaplan Meier curve of the derivation dataset is plotted using the straight line, and the validation dataset with the dashed line. Blue represents low risk, black medium risk, and red represents high risk of death. [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
To test the added effect of our full model to a prediction model based on the TNM classification alone, we constructed another model based on the TNM classification and compared the results of these two models. The CPM using only the TNM classification had a C statistic of 0.61 in both the derivation and validation data, which was lower than the C statistic for our full model. We used a likelihood ratio test to evaluate the model fit of the full model versus the TNM model. This test reported a significant better fit in the full model (P < .0001).
As an example of the added effect of the variables other than TNM classification, we calculated the amount of points and corresponding risk category three fictive patients would obtain with similar TNM classification. The first patient is male, 63 years old, and has a T3N1 pyriform sinus tumor. His BMI is 25, he has an ACE27 score of 2, no leukocytosis, and his peripheral blood tests show a hemoglobin value of 9.0 and albumin value of 40. Based on our model, this patient will obtain 159 points, and falls into a medium risk category. A patient with the exact same values but with an ACE27 score of 1, will obtain 133 points, and be categorized as a low‐risk patient. The third patient has the same age, gender, tumor sublocation and TNM‐classification as the first two patients. His ACE27 score is 3, his BMI 18, he has leukocytosis, and his peripheral blood values show a hemoglobin of 6.0 and albumin of 25. This patient will add up to 244 points and falls into the high‐risk category.
DISCUSSION
This article describes the development and validation of a CPM for hypopharynx cancer containing gender, subsite, TNM classification, ACE27, BMI, albumin, hemoglobin, and leukocyte count. The model showed better discrimination compared to a model based on TNM classification alone and was especially good in distinguishing high‐risk patients that might benefit from more intensive treatment strategies and/or follow‐up regimen. Using the LASSO technique, we identified the variables ACE27, BMI, albumin, and hemoglobin levels to significantly contribute to a predictive model for survival, in addition to the expected TNM classification.
In our model, comorbidity scores, as measured by the ACE27, appeared to be one of the strongest predictors of survival. The ACE27 score is regularly used measure for comorbidity, especially in the setting of (head and neck) cancer and incorporates 27 ailments in nine organ systems plus the presence of a malignancy, substance abuse, and bodyweight. The ACE27 score can range from 0 (no comorbidity) to 3 (severe comorbidity). Although hypopharynx cancer patients are known to have relatively more comorbidity compared to other patients with head and neck cancer, it still has an important prognostic value. The independent prognostic value of comorbidity has indeed been confirmed by other authors and should therefore always be accounted for when estimating prognosis in the frail head and neck cancer patient group.9, 20, 21, 22
In the current ACE27 scoring system, a high BMI is incorporated as one of the 27 ailments.23 However, in our prediction model, a lower BMI and higher ACE27 were both independent predictors of worse OS, a finding that has been reported by others studying patients with head and neck cancer.24, 25 In the current ACE27 scoring system, BMI results in a positive score when a patient has a BMI >38, which is an infrequent finding in our cohort and in other studies on head and neck cancer patients.24, 26 Yet, there was little correlation between the two variables. Thus, since multicollinearity was not a concern, we chose to consider BMI as a separate variable in the CPM, and this variable was retained by the LASSO model.
Besides ACE27 and BMI, the peripheral blood parameters hemoglobin and albumin level both demonstrated an independent prognostic effect on survival. The association between pretreatment anemia and worse OS has been well established in head and neck cancer and is likely a result of the resulting hypoxia of the tumor micro‐environment, which is associated with decreased radiosensitivity.27, 28 Low albumin level as a proxy for reduced nutritional status has similarly been widely reported as a predictor of cancer survival and possibly even functional outcome.29, 30 Sherman et al. used the VA dataset to report on the TALK score, incorporating the variables T‐stage, Albumin, Liquor intake and the comorbidity index Karnofsky score into a prognostic model to estimate functional outcome in terms of larynx preservation.30 In terms of predicting oncological survival, the Glasgow prognostic score, incorporating low albumin and high CRP levels, has been shown to be predictive of outcome in several different tumor sites.31, 32 The original publication on the Glasgow score, however, also investigated other variables such as age, gender, stage, type of tumor (SCC or adenocarcinoma), performance status (ECOG), hemoglobin level, and leukocyte level. The authors analyzed several combinations, each containing two of these variables, and although they reported that the combination of stage and comorbidity was considered to have a comparable prognostic value to the combination albumin and CRP, still they did not include these variables into their scoring system. Possibly, a more extensive model would have led to an increased predictive value.
In recent years, several easy to obtain serum inflammatory markers have been shown to have predictive value in cancer survival.33 Although the inflammatory response should obviously be directed against the tumor, it is clear that inflammatory cells can influence tumor growth, stimulate DNA damage and promote angiogenesis and lymphangiogenesis.34 The association between leukocytosis and OS is a relatively new concept in head and neck cancer, but has gained increasing interest in the past years, especially in the context of neutrophil to lymphocyte ratio (NLR).35, 36, 37 A recent meta‐analysis analyzed by Tham et al. demonstrated that an elevated pretreatment NLR is associated with significant poorer OS and disease‐free survival in head and neck cancer.37 The presence of leukocytosis was however unrelated to OS in our analysis. In our cohort, only leukocyte level was available and further differentiation allowing NLR calculation was lacking. Possibly, the NLR is more informative than leukocytosis alone. If proven to be predictive of survival in hypopharynx, such easy to obtain and inexpensive biological bio‐markers are welcome new discoveries that could improve risk stratification for patients, and possibly tailor (neo)adjuvant treatment.
Apart from tumor‐specific and patient‐specific variables, the choice of treatment obviously also affects survival. However, we did not include treatment in our model, as recommended previously, since the estimate of effect would likely be confounded by indication.14
This is not the first study to report on a prediction model for head and neck cancer but to the best of our knowledge this is the first prediction model including hypopharynx cancer patients only. This distinct subgroup is often analyzed together with larynx cancer, although hypopharynx cancer is known to have 5‐year OS almost half of the expected OS for patients with larynx cancer.4, 38, 39 Thus, when aiming to optimize individual estimations of prognosis through the development of a prediction model, it is important that patients are selected based on type of tumor to create more accurate estimations.
There are certain limitations to this study. Inherent to the retrospective design of our study, there is a weakness in data collection that resulted in a certain degree of missing variables, although we tried to correct this by performing multiple imputation. We were unable to collect variables that might have played an important prognostic role in survival, such tumor volume, the presence of sarcopenia or low skeletal muscle mass, or several peripheral blood tumor markers.25, 37, 40, 41 However, considering the relatively low incidence of this tumor and the lack of prospective databases that can be used to develop or improve prediction models, large retrospective databases like ours still provide valuable information regarding survival. Our clinical prediction model is based on patients treated with curative intent with TLP, RT, or CRT in three dedicated head and neck centers in the Netherlands. One has to be careful in extrapolating survival estimates from this model to a different geographical setting, where possibly different treatment strategies or patient care is delivered. Constructing a model on more heterogeneous group in terms of geographical location and treatment strategies would probably allow for better clinical use around the world.
CONCLUSION
In conclusion we have been able to identify a high ACE27 score, the presence of low BMI, and low hemoglobin and albumin levels as independent prognostic variables for hypopharynx cancer in addition to several patient‐ and tumor‐specific characteristics. The developed clinical model is better for estimating survival, compared to estimations based on TNM classification only. Although predictions at the individual level remain uncertain, the model adequately distinguishes between risk groups. These results can be used during the counseling process and possibly tailor treatment strategies or intensify follow‐up regimens, but should never replace clinicians’ judgment. Further research is needed to investigate which variables, other than those considered to date, can further improve predictions of survival for individual patients with hypopharynx cancer.
Editor's Note: This Manuscript was accepted for publication on September 12, 2018.
The Netherlands Cancer Institute receives a research grant from ATOS Medical Sweden which contributes to the existing infrastructure for health‐related quality of life research of the department of Head and Neck Oncology and Surgery.
The authors have no conflicts of interest to declare.
BIBLIOGRAPHY
- 1. Gatta G, Botta L, Sanchez MJ, Anderson LA, Pierannunzio D, Licitra L. Prognoses and improvement for head and neck cancers diagnosed in Europe in early 2000s: the EUROCARE‐5 population‐based study. Eur J Cancer 2015; 51:2130–2143. [DOI] [PubMed] [Google Scholar]
- 2. Kuo P, Chen MM, Decker RH, Yarbrough WG, Judson BL. Hypopharyngeal cancer incidence, treatment, and survival: temporal trends in the United States. Laryngoscope 2014;124:2064–2069. [DOI] [PubMed] [Google Scholar]
- 3. Carvalho AL, Nishimoto IN, Califano JA, Kowalski LP. Trends in incidence and prognosis for head and neck cancer in the United States: a site‐specific analysis of the SEER database. Int J Cancer 2005;114:806–816. [DOI] [PubMed] [Google Scholar]
- 4. Petersen JF, Timmermans AJ, van Dijk BAC, et al. Trends in treatment, incidence and survival of hypopharynx cancer: a 20‐year population‐based study in the Netherlands. Eur Arch Otorhinolaryngol 2018;275:181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Newman JR, Connolly TM, Illing EA, Kilgore ML, Locher JL, Carroll WR. Survival trends in hypopharyngeal cancer: a population‐based review. Laryngoscope 2015;125:624–629. [DOI] [PubMed] [Google Scholar]
- 6. Takes RP, Strojan P, Silver CE, et al. Current trends in initial management of hypopharyngeal cancer: the declining use of open surgery. Head Neck 2012;34:270–281. [DOI] [PubMed] [Google Scholar]
- 7. Piccirillo JF, Feinstein AR. Clinical symptoms and comorbidity: significance for the prognostic classification of cancer. Cancer 1996;77:834–842. [PubMed] [Google Scholar]
- 8. Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL Jr. Prognostic importance of comorbidity in a hospital‐based cancer registry. JAMA 2004;291:2441–2447. [DOI] [PubMed] [Google Scholar]
- 9. Datema FR, Ferrier MB, van der Schroeff MP, Baatenburg de Jong RJ. Impact of comorbidity on short‐term mortality and overall survival of head and neck cancer patients. Head Neck 2010;32:728–736. [DOI] [PubMed] [Google Scholar]
- 10. Abernethy AP, Etheredge LM, Ganz PA, et al. Rapid‐learning system for cancer care. J Clin Oncol 2010;28:4268–4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ross PL, Gerigk C, Gonen M, et al. Comparisons of nomograms and urologists' predictions in prostate cancer. Semin Urol Oncol 2002;20:82–88. [DOI] [PubMed] [Google Scholar]
- 12. Kattan MW, Yu C, Stephenson AJ, Sartor O, Tombal B. Clinicians versus nomogram: predicting future technetium‐99m bone scan positivity in patients with rising prostate‐specific antigen after radical prostatectomy for prostate cancer. Urology 2013;81:956–961. [DOI] [PubMed] [Google Scholar]
- 13. Moons KG, Altman DG, Reitsma JB, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 2015;162:W1–73. [DOI] [PubMed] [Google Scholar]
- 14. Steyerberg ER. Clinical Prediction Models, A Practical Approach to Development, Validation, and Updating. Switzerland AG: Springer Nature; 2009. [Google Scholar]
- 15. Li H, Torabi SJ, Yarbrough WG, Mehra S, Osborn HA, Judson B. Association of human papillomavirus status at head and neck carcinoma subsites with overall survival. JAMA Otolaryngol Head Neck Surg 2018;144:519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dahm V, Haitel A, Kaider A, Stanisz I, Beer A, Lill C. Cancer stage and pack‐years, but not p16 or HPV, are relevant for survival in hypopharyngeal and laryngeal squamous cell carcinomas. Eur Arch Otorhinolaryngol 2018;275:1837–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Buuren S, Groothuis‐Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. J Stat Software 2011;45(3):1–67. [Google Scholar]
- 18. Goeman JJ. Penalized: L1 (lasso and fused lasso) and L2 (ridge) penalized estimation in GLMs and in the Cox model. Biometrical J 2010;52:1–14. [DOI] [PubMed] [Google Scholar]
- 19. Bleeker SE, Moll HA, Steyerberg EW, et al. External validation is necessary in prediction research: a clinical example. J Clin Epidemiol 2003;56:826–832. [DOI] [PubMed] [Google Scholar]
- 20. Din HA, Zhan KY, Boling C, Nguyen S, Lentsch EJ. Predictors of survival in posterior cricoid squamous cell carcinoma: a study of 248 cases. Laryngoscope 2017;127:1093–1096. [DOI] [PubMed] [Google Scholar]
- 21. Mulcahy CF, Mohamed ASR, Kanwar A, et al. Age‐adjusted comorbidity and survival in locally advanced laryngeal cancer. Head Neck 2018;40:2060–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schimansky S, Lang S, Beynon R, et al. Association between comorbidity and survival in head and neck cancer: Results from Head and Neck 5000. Head Neck 2019;41:1053–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Piccirillo JF, Creech CM, Zequeira R, Anderson S, Johnston AS. Inclusion of comorbidity into oncology data registries. J Reg Manag 1999;26:66–70. [Google Scholar]
- 24. Te Riele R, Dronkers EAC, Wieringa MH, et al. Influence of anemia and BMI on prognosis of laryngeal squamous cell carcinoma: development of an updated prognostic model. Oral Oncol 2018;78:25–30. [DOI] [PubMed] [Google Scholar]
- 25. Fattouh M, Chang GY, Ow TJ, et al. Association between pretreatment obesity, sarcopenia, and survival in patients with head and neck cancer. Head Neck 2018;41:707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gama RR, Song Y, Zhang Q, et al. Body mass index and prognosis in patients with head and neck cancer. Head Neck 2017;39:1226–1233. [DOI] [PubMed] [Google Scholar]
- 27. Prosnitz RG, Yao B, Farrell CL, Clough R, Brizel DM. Pretreatment anemia is correlated with the reduced effectiveness of radiation and concurrent chemotherapy in advanced head and neck cancer. Int J Radiat Oncol Biol Phys 2005;61:1087–1095. [DOI] [PubMed] [Google Scholar]
- 28. Harrison LB, Chadha M, Hill RJ, Hu K, Shasha D. Impact of tumor hypoxia and anemia on radiation therapy outcomes. Oncologist 2002;7:492–508. [DOI] [PubMed] [Google Scholar]
- 29. Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J 2010;9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sherman EJ, Fisher SG, Kraus DH, et al. TALK score: development and validation of a prognostic model for predicting larynx preservation outcome. Laryngoscope 2012;122:1043–1050. [DOI] [PubMed] [Google Scholar]
- 31. Hanai N, Sawabe M, Kimura T, et al. The high‐sensitivity modified Glasgow prognostic score is superior to the modified Glasgow prognostic score as a prognostic predictor for head and neck cancer. Oncotarget 2018;9:37008–37016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non‐small‐cell lung cancer. Br J Cancer 2003;89:1028–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer‐related inflammation and treatment effectiveness. Lancet Oncol 2014;15:e493–503. [DOI] [PubMed] [Google Scholar]
- 34. Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gouw ZAR, Paul de Boer J, Navran A, van den Brekel MWM, Sonke JJ, Al‐Mamgani A. Baseline peripheral blood leukocytosis: biological marker predicts outcome in oropharyngeal cancer, regardless of HPV‐status. Oral Oncol 2018;78:200–206. [DOI] [PubMed] [Google Scholar]
- 36. Schernberg A, Blanchard P, Chargari C, et al. Leukocytosis, prognosis biomarker in locally advanced head and neck cancer patients after chemoradiotherapy. Clin Transl Radiat Oncol 2018;12:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tham T, Bardash Y, Herman SW, Costantino PD. Neutrophil‐to‐lymphocyte ratio as a prognostic indicator in head and neck cancer: a systematic review and meta‐analysis. Head Neck 2018;40:2546–2557. [DOI] [PubMed] [Google Scholar]
- 38. Al‐Mamgani A, Navran A, Walraven I, Schreuder WH, Tesselaar MET, Klop WMC. Organ‐preservation (chemo)radiotherapy for T4 laryngeal and hypopharyngeal cancer: is the effort worth? Eur Arch Otorhinolaryngol 2018;276:575–583. [DOI] [PubMed] [Google Scholar]
- 39. Timmermans AJ, van Dijk BA, Overbeek LI, et al. Trends in treatment and survival for advanced laryngeal cancer: a 20‐year population‐based study in The Netherlands. Head Neck 2016;38 Suppl 1:E1247–1255. [DOI] [PubMed] [Google Scholar]
- 40. Timmermans AJ, Lange CA, de Bois JA, et al. Tumor volume as a prognostic factor for local control and overall survival in advanced larynx cancer. Laryngoscope 2016;126:E60–E67. [DOI] [PubMed] [Google Scholar]
- 41. Bril SI, Pezier TF, Tijink BM, Janssen LM, Braunius WW, de Bree R. Preoperative low skeletal muscle mass as a risk factor for pharyngocutaneous fistula and decreased overall survival in patients undergoing total laryngectomy. Head Neck 2019;41:1745–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]


