Abstract
Background
Precise cannabis treatment dosing remains a major challenge, leading to physicians’ reluctance to prescribe medical cannabis.
Objective
To test the pharmacokinetics, analgesic effect, cognitive performance and safety effects of an innovative medical device that enables the delivery of inhaled therapeutic doses of Δ9‐Tetrahydrocannabinol (THC) in patients with chronic pain.
Methods
In a randomized, three‐arms, double‐blinded, placebo‐controlled, cross‐over trial, 27 patients received a single inhalation of Δ9‐THC: 0.5mg, 1mg, or a placebo.
Δ9‐THC plasma levels were measured at baseline and up to 150‐min post‐inhalation. Pain intensity and safety parameters were recorded on a 10‐cm visual analogue scale (VAS) at pre‐defined time points. The cognitive performance was evaluated using the selective sub‐tests of the Cambridge Neuropsychological Test Automated Battery (CANTAB).
Results
Following inhalation of 0.5 mg or 1mg, Δ9‐THC plasma C max ± SD were 14.3 ± 7.7 and 33.8 ± 25.7 ng/ml. T max ± SD were 3.7 ± 1.4 and 4.4 ± 2.1 min, and AUC0 → infinity±SD were 300 ± 144 and 769 ± 331 ng*min/ml, respectively. Both doses, but not the placebo, demonstrated a significant reduction in pain intensity compared with baseline and remained stable for 150‐min. The 1‐mg dose showed a significant pain decrease compared to the placebo. Adverse events were mostly mild and resolved spontaneously. There was no evidence of consistent impairments in cognitive performance.
Conclusion
This feasibility trial demonstrated that a metered‐dose cannabis inhaler delivered precise and low THC doses, produced a dose‐dependent and safe analgesic effect in patients with neuropathic pain/ complex‐regional pain syndrome (CRPS). Thus, it enables individualization of medical cannabis regimens that can be evaluated pharmacokinetically and pharmacodynamically by accepted pharmaceutical models.
Significance
Evidence suggests that cannabis‐based medicines are an effective treatment for chronic pain in adults. The pharmacokinetics of THC varies as a function of its route of administration. Pulmonary assimilation of inhaled THC causes rapid onset of analgesia. However, currently used routes of cannabinoids delivery provide unknown doses, making it impossible to implement a pharmaceutical standard treatment plan. A novel selective‐dose cannabis inhaler delivers significantly low and precise doses of THC, thus allowing the administration of inhaled cannabis‐based medicines according to high pharmaceutical standards. These low doses of THC can produce safe and effective analgesia in patients with chronic pain.
1. INTRODUCTION
The treatment of chronic pain is the most commonly cited reason for accessing medical cannabis‐based medicines in western countries (Hill, Palastro, Johnson, & Ditre, 2017). Several recent systematic reviews and meta‐analyses examining the evidence for cannabis‐based medicines in chronic pain yielded conflicting results. While some studies have reported minimal or no benefit (Fitzcharles, Baerwald, Ablin, & Häuser, 2016; Stockings et al., 2018), others have reported moderate to large effects (Andreae et al., 2015; Lynch & Campbell, 2011; Romero‐Sandoval, Kolano, & Alvarado‐Vázquez, 2017). Conclusions on the use of cannabis‐based medicines for chronic pain are, therefore, contradictory. On the one hand, in a recent Lancet Psychiatry publication, Hill and colleagues suggested that the evidence base for the therapeutic use of cannabis‐based medicines had not kept pace with the rapid growth of patient interest. They concluded that “we will need to use the same rigorous methods of investigation, when possible, like those used for other pharmacological compounds (Hill, Palastro, & George, 2019). In contrast, the National Academies of Science, Engineering, and Medicine (NASEM) reports on “substantial evidence that cannabis or cannabinoids are an effective treatment for chronic pain in adults” (National Academies of Sciences, Engineering, and Medicine, 2017).
Routes of cannabis‐based medicines use include smoking cannabis flowers, vaporizing oil formulations, vaporizing ethanolic liquids, vaporizing dry herbs, oro‐mucosal administration or oral ingestion of cannabis extracts or edibles. Although smoking cannabis offers a rapid onset of effect, Δ9‐THC plasma concentrations following computer‐controlled cannabis cigarette smoking remained considerably variable, suggesting inconsistent delivery of active compounds (Huestis, 2007). Besides, smoking is not acceptable for therapeutic purposes, mainly because of the health risk caused by noxious pyrolytic by‐products (Abrams et al., 2007). Cannabis oil vaporization presents new potential health risks since it consists of heating liquid formulations with additives of the yet unknown level of pulmonary toxicity (Troutt & DiDonato, 2017). The development of a certified, temperature‐controlled medical device for vaporizing dry flowers, which efficiently decarboxylate inactive acidic cannabinoids at a temperature below the point of combustion, has advanced the safety of delivery. Such a device allows the release of active cannabinoids while avoiding the formation of hazardous combustion products (Gieringer, Laurent, & Goodrich, 2004; Lanz, Mattsson, Soydaner, & Brenneisen, 2016). Oral and oro‐mucosal models of cannabis‐based medicines administration demonstrated even less precise pharmacokinetic profiles, resulting in delayed onset, varying magnitude and duration of desired pharmacodynamic effects (Vandrey et al., 2017).
So far, in most clinical trials, the dose of cannabis‐based medicines was poorly recorded, and data on patients’ actual cannabinoids using was seldom provided. Thus, it is impossible to design an effective and safe rational daily dose regimen, and the administration of cannabis under standard pharmaceutical quality remains a challenge.
In a previous study, we demonstrated that the Syqe Inhaler, a non‐combustion delivery system of multiple small and accurate doses of medical cannabis, produced a Δ9‐THC pharmacokinetic profile with a low inter‐individual variation of plasma concentrations, achieving pharmaceutical standards for inhaled drugs (Eisenberg, Ogintz, & Almog, 2014). Recently, the use of Syqe Inhaler by a cohort of hospitalized patients yielded high levels of patient and staff satisfaction with no complications (Vulfsons, Ognitz, Bar‐Sela, Raz‐Pasteur, & Eisenberg, 2019). The present randomized, placebo‐controlled clinical trial aimed at assessing pharmacokinetics, efficacy and safety aspects of two low doses of cannabis, administered by the novel Syqe Inhaler.
2. METHODS
2.1. Study design and patients
This randomized, three‐arms, double‐blind, placebo‐controlled, cross‐over study was conducted at the Pain Research Unit of Rambam Health Care Campus (Haifa, Israel) between March 2016 and July 2017, following approval by the Rambam Health Care Campus ethics committee (RMB 0131‐13). Due to an administrative error, the study was not registered at the NIH ClinicalTrail.gov website. The investigators provided all subjects with a detailed explanation of the study aims and procedures. All patients signed written informed consent forms prior to undergoing any study‐related procedures. They were enrolled in the study after meeting the following inclusion criteria: adult patients (18 years of age or above), capable of giving informed consent, suffering from chronic pain with a baseline pain intensity of 6 or above on a 10‐cm visual analog scale (VAS), and licensed by the Israeli Ministry of Health to receive cannabis‐based medicines. Active users had to agree to abstain from cannabis‐based medicines 12 hr before study intervention. Women of fertile age had to declare using contraception. Patients were excluded from the study if they had a history of severe, uncontrolled cardiac disease, pulmonary disease, hepatic disease, neurologic dysfunction, personal or family history of psychotic illness, substance abuse, or if they were pregnant, breastfeeding, or did not use adequate birth control.
Patients continued their daily medication routine, including the use of opioids.
After consent, a physician from the investigating team documented details of each patient's demographics, medical history, and medical treatment and conducted a physical examination. Imaging and laboratory tests were reviewed as needed. Patients were given detailed instructions on the use of the Syqe Inhaler. Each patient arrived at the Pain Clinic for three study visits and was equipped with a personal device. The allowed time between two consecutive treatment sessions was at least two days. In each visit, patients were assigned to receive a single inhalation of one of three doses: 0.5, 1.0 mg THC or placebo at random order. The data was recorded on paper Case Report Forms by the trained trial team at the clinical site.
2.2. Study device
The Syqe Inhaler, developed by Syqe Medical, is a single‐patient, portable, hand‐held, battery‐operated, software‐controlled, thermal selective‐dose inhalation (tSDI) medical‐device, designed to precisely aerosolize multiple doses of granulated raw plants. During two‐seconds of heating and aerosolization, initiated by breath‐actuation, 90% of the THC‐acid undergoes a process of decarboxylation to the pharmacologically active THC form. The device engages automatic thermal and flow controllers that ensure the delivery of cannabinoid aerosol to the lungs, independent of the inhalation pattern of the individual patient. Subsequently, the device requires minimal inhalation training. The device electronically controls and logs the entire inhalation process, allowing for storing and uploading of treatment data logs. The Syqe Inhaler is programmed to accurately deliver different doses (0.5–1.5 mg THC aerosol) according to the patient's needs, enabling individualization of THC regimen.
2.3. Study procedures
2.3.1. Preparation and dose uniformity of aerosolized THC
Each cannabis dose unit coupled with a single‐use heating element (dose‐chip) was preloaded with 16 ± 0.5 mg of processed granulated pharmaceutical‐grade cannabis flos (whole, dried female flower; Bedrocan, The Netherlands) containing 22% THC, < 0.1% cannabidiol (CBD), < 0.2% cannabinol (CBN) or a matched placebo. For this study, the Syqe Inhaler was programmed to aerosolize selective doses; 0.50 or 1.00 mg THC, using an algorithm that electronically controls the aerosolization profile (e.g. temperature, duration, airflow). Prior to the study, the inhalers underwent a quality control testing program. For the 0.50 mg session, the amount of aerosolized THC was 0.537 ± 0.052 mg THC and 1.083 ± 0.076 mg for the 1.00 mg session. Thus, the imprecision, inaccuracy and the uncertainty of the 0.5mg dosing measurements were 9.6%, (+)7.4% and 12.1%, and of the 1.0 mg measurements were 7.0%, +8.3% and 10.8%, respectively.
The cannabis flowers were free of pesticides, heavy metals (< 0.2 ppm lead, < 0.02 ppm mercury, and < 0.02 ppm cadmium) and foreign materials. Microbiological purity was confirmed (total aerobic microbial count of < 10 colony‐forming units [CFU]/g, total yeast and mold count of < 10 CFU/g, and absence of Pseudomonas Aeruginosa, Staphylococcus Aureus, and bile‐tolerant gram‐negative bacteria). The placebo was prepared from the same cannabis batch used for the active treatment by ethanolic extraction and sonication, resulting in < 0.01% of cannabinoid compounds. The cannabis flos, as well as the placebo, underwent unique granulation, loaded into the dose‐chip, and packed into the tamper‐proof device, which prevented the identification of the investigated materials by the patients and study team.
Each dose‐chip was preloaded with 16.0 ± 0.5 mg granulated cannabis herb containing either zero (placebo) or 3.52 mg THC.
2.4. Blinding process
To allow proper blinding, doses of 3.52 or 0 mg THC granulated cannabis were assimilated into the device during manufacturing, according to a predetermined randomization order. Hence, the inhalers arrived at the clinical site, ready‐to‐use, charged with the required doses. Neither the clinical trial staff at the site nor the patients were exposed to the content at each inhalation.
2.5. Outcome measures
The primary objectives of the study were (I) studying the Δ9‐THC pharmacokinetics following administration of a single inhalation of 0.5 mg and 1.0 mg aerosolized Δ9‐THC compared to a placebo, using the meter‐dosed Syqe Inhaler, and (II) evaluating their analgesic efficacy (change in pain intensity from baseline) in patients with chronic pain.
For assessing pharmacokinetics, blood samples were collected for plasma levels of THC at baseline; before dose administration, and at 1, 2, 3, 4, 5, 10, 15, 30, 60, 90, 120‐ and 150‐min following inhalation. The plasma samples were stored frozen at −20°C until analysis. The cannabinoid analysis was performed at NMS Labs (Willow Grove, PA, USA), an accredited laboratory by ANAB‐ASCLD/LAB ISO 17025, by a validated high‐performance liquid chromatography/tandem mass spectrometry (HPLC‐MS/MS) method. The reporting limit of THC is 0.5 ng/mL plasma.
The pain intensity was measured using the VAS score before inhalation and at 5, 15, 30, 60, 90, 120 and 150‐min following inhalation. Patients were asked to rank their pain intensity on a 0 (no pain at all) to 10 (worst possible pain) VAS.
The secondary objectives were to study the safety and tolerability of two doses of aerosolized medical cannabis and a placebo, over a post inhalation period of 150 min.
Spontaneous Adverse Events (AEs) were recorded throughout the entire study period. Predetermined AEs, which according to the literature, may appear following the use of medical cannabis, are: “drug‐high,” “dizziness,” “restlessness,” “headache,” “nausea,” “throat irritation,” “dry mouth,” and “general feeling.” Patients were deliberately asked about these predetermined AEs using the VAS scale at baseline and 5, 15, 30, 60, 90, 120‐ and 150‐min following inhalation. An increase of two points and above in the VAS score between two‐time points was recorded as an AE. All AEs were evaluated in terms of severity, seriousness, duration, and relation to study treatment.
Vital signs—blood pressure and heart rate—were recorded at baseline, 15, 30, 60, 90, 120‐ and 150‐min following inhalation.
On each test day, cognitive functions were assessed at baseline, at 15 min, and 75 min post‐dosing. Selected tests from the Cambridge Neuropsychological Test Automated Battery (CANTAB) were used to test the effects of THC on cognition. Domains of interest included processing speed (Reaction Time Test, RTI), episodic memory (Paired Associates Learning Task, PAL), working memory (Spatial Working Memory Test, SWM) and sustained attention (Rapid Visual Information Processing Test, RVP). A cognitive composite score based on key outcome measures from the CANTAB tests was also calculated.
2.6. Statistical analysis
The study was planned as a 3‐period 3‐treatment cross‐over study using a Williams design (3 × 3) resulting in six sequences. The sample size was calculated to detect a difference of 1 unit (in VAS) between any two groups, assuming a maximum standard deviation of 2, with a 5% level of significance and 80% power.
The pharmacokinetics profile—maximum THC plasma concentrations (C max), time to reach C max (T max), and area under the curve (AUC)—was analysed as previously described (Eisenberg et al., 2014).
Safety analysis was performed using the intention‐to‐treat (ITT) population, which included all study patients. The primary and secondary outcome analyses were performed on the per‐protocol (PP) population, which included all the patients who took at least one inhalation using the Syqe Inhaler and did not exhibit any major protocol deviations related to the study drug administration, THC concentration measurements or pain VAS measurements.
Statistical analysis of the efficacy and safety parameters was performed using SAS® V9.4 and was mainly descriptive, where study data were tabulated and summarized using the mean, standard deviation or standard error, median, minimum, maximum, and number of patients by cohort for continuous data. For categorical data, results were summarized via a count and percentage by cohort. Baseline values were defined as the value prior to study drug administration in each session. Confidence intervals, where relevant, are two‐sided with a confidence level of 95% unless otherwise stated. The change in pain, CANTAB parameters (analysed by Cambridge Cognition), and the time course of selected subjective adverse effects were analysed with repeated measures models, which were adjusted for baseline.
3. RESULTS
Between March 2016 and April 2017, a total of 27 patients were found eligible to participate in the current study, signed an informed consent form, and were randomly assigned to study intervention. Patient disposition is illustrated in Figure 1.
FIGURE 1.
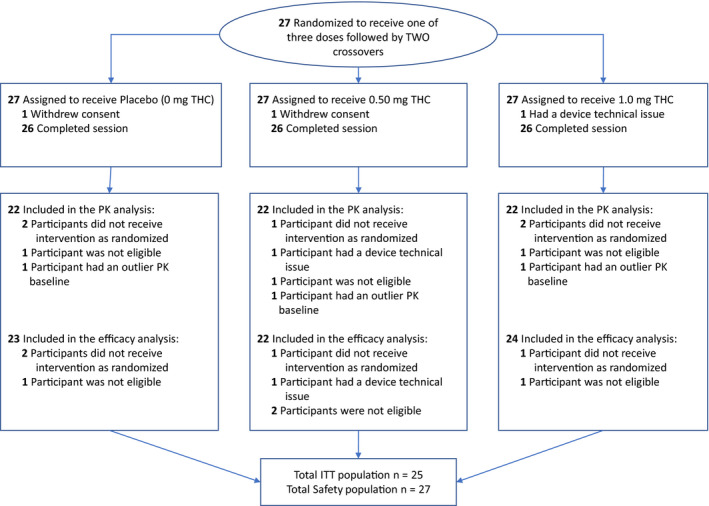
Study design (Consolidated Standards of Reporting Trials [CONSORT]) flow diagram. ITT = intent to treat
Twenty‐five patients completed all study sessions. One patient completed only two study sessions due to a technical problem with the device that was not resolved, and another patient completed only one study session because of consent withdrawal. Eventually, each treatment group included 26 patients. Pharmacokinetics analysis included 22 patients in each treatment group. The efficacy analysis included 23 patients in the placebo, 22 in 0.5 mg, and 24 in 1.0 mg (Figure 1).
Baseline characteristics are presented in Table 1. The mean age was 48.3 (range, 18–67), and most patients (70.37%) were males. Mean weight and body mass index (BMI) was 86.6 ± 19.6 kg and 27.8 ± 5.0 kg/m2, respectively. Twenty‐one patients were suffering from chronic focal or distal symmetric (diabetic) neuropathic pain and six from complex regional pain syndrome (CRPS) (Table 1). The diagnoses of neuropathic pain and CRPS were made by an investigating physician according to IAPS (Treede et al., 2008), and Budapest (Harden et al., 2010) criteria, respectively. The overall pain intensity was 7 and above on a 0–10 scale (92.59%). Cannabis‐based medicines administration prior to the study period was mostly by smoking (77.78%), and the amount of monthly cannabis used was mostly between 16–30 gr per month (74.08%). Most patients used concomitant medications for pain management, including analgesics, antidepressants, anticonvulsants, benzodiazepines and anti‐inflammatory drugs.
TABLE 1.
Baseline characteristics of all patients
| Demographics | Total | |
|---|---|---|
| Gender, n (%) | 27 | |
| Male | 19 (70.37%) | |
| Female | 8 (29.63%) | |
| Age (years), mean (SD) n (%) | 48.3 (11.9) | |
| 18–29 | 1 (3.70%) | |
| 30–39 | 7 (25.93%) | |
| 40–49 | 6 (22.22%) | |
| 50–59 | 7 (25.93%) | |
| 60–69 | 6 (22.22%) | |
| BMI (kg/m2), mean (SD) | 27.8 (5.0) | |
| Pain diagnosis | ||
| Painful radiculopathy | 8 (29.63%) | |
| Painful diabetic neuropathy | 6 (22.22%) | |
| CRPS | 6 (22.22%) | |
| Other focal neuropathies | 4 (14.81%) | |
| Phantom/ stump pain | 3 (11.11%) | |
| Baseline pain score a , n (%) | ||
| VAS Score 6 | 2 (7.41%) | |
| VAS Score 7 | 6 (22.22%) | |
| VAS Score 8 | 9 (33.33%) | |
| VAS Score 9 | 8 (29.63%) | |
| VAS Score 10 | 2 (7.41%) | |
| Previous cannabinoids use, n (%) | ||
| Smoking | 21 (77.78%) | |
| Smoking + cannabis oil | 2 (7.41%) | |
| Smoking + cannabis oil (sublingual) | 1 (3.70%) | |
| Smoking + Vaporization | 1 (3.70%) | |
| Vaporization | 2 (7.41%) | |
| Amount of cannabis used per month, n (%) | ||
| 16−20 g | 7 (25.93%) | |
| 21−30 g | 13 (48.15%) | |
| 31−40 g | 4 (14.81%) | |
| 41−50 g | 3 (11.11%) | |
| Cannabinoids treatment impact b , mean (SD) | 77.29 (2.21) | |
| Use of concurrent medications for pain management, n (%) | ||
| Yes | 22 (81.48%) | |
| No | 5 (18.52%) | |
| Concurrent medications, n (%) | ||
| Simple analgesics | 20 (74.07%) | |
| Anticonvulsants | 5 (18.52%) | |
| NSAIDs | 3 (11.11%) | |
| Opioids | 18 (66.67%) | |
| Antiarrhythmics | 1 (3.70%) | |
| Antidepressants | 12 (44.44%) | |
| Antiepileptic | 3 (11.11%) | |
| Sedatives | 5 (18.52%) | |
| Anticoagulants | 1 (3.70%) | |
Abbreviations: BMI, body mass index; CRPS, complex regional pain syndrome; IBD, inflammatory bowel disease; VAS, visual analogue scale.
Patients were asked to rank their average daily pain on a 0–10 scale.
Patients were asked to rank their subjective feeling regarding the effect of their current cannabinoid's treatment on their pain intensity on a 0–10 scale.
3.1. Pharmacokinetics
Twenty‐two patients were included in the pharmacokinetics analysis for each dose. For each session, at time zero, a non‐detectable concentration of plasma THC (< 0.5 ng/mL) was observed in 16 patients (73%) at the 0.5 mg THC session and in 17 patients (77%) at the 1.0 mg THC session. Mean THC baseline levels of 6.6 ± 4.3 and 8.5 ± 6.4 ng/mL were measured in plasma of 6 (27%) and 5 (23%) patients at sessions of 0.5 and 1.0 mg, respectively, most probably due to previous uses. Following single inhalation of 0.5 mg Δ9‐THC, mean maximum plasma Δ9‐THC concentration (C max) ± SD was 14.3 ± 7.7 ng/ml. The time to peak (T max) was 3.7 ± 1.4 min, and the mean area under the plasma concentration versus. time curve from 0 to infinity (AUC0→infinity) was 300 ± 144 ng*min/mL. Following single inhalation of 1.0 mg Δ9‐THC, C max ± SD was 33.8 ± 25.7 ng/mL, T max was 4.4 ± 2.1 min and the AUC0→infinity was 769 ± 331 ng*min/mL (Figure 2). Doubling the labelled THC dose resulted in nearly doubling the C max and AUC of plasma THC (Figure 2).
FIGURE 2.
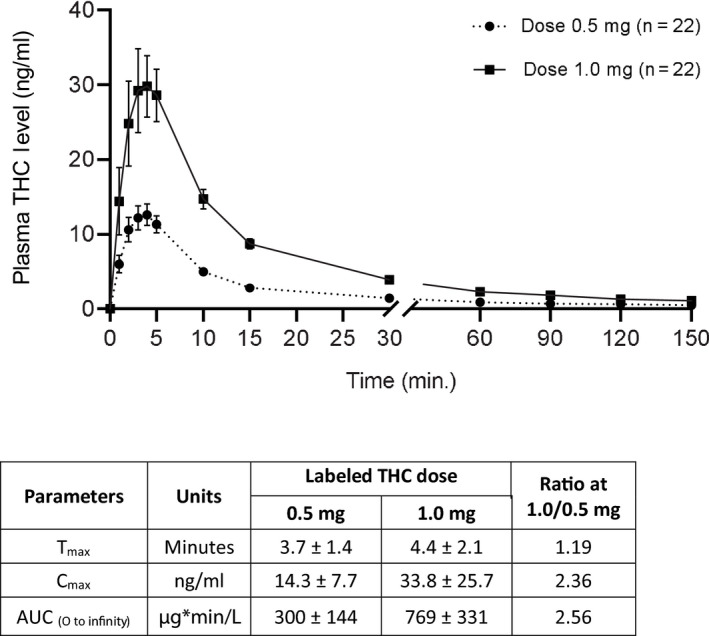
Δ9‐THC plasma levels following a single inhalation of 0.5 mg and 1.0 mg THC doses. Data are presented as means and standard deviations
3.2. Efficacy
Twenty‐four of the twenty‐seven patients were included in the efficacy analysis. The baseline VAS score was determined at each session. Baseline pain intensity at the placebo, 0.5, and 1.0 mg Δ9‐THC was 7.4 ± 1.1, 7.8 ± 1.3 and 7.6 ± 1.1, respectively (mean ± SD). Statistical analysis showed no statistically significant difference between these baseline values (RM ANOVA, p = .4266).
Figure 3 illustrates the effects of the three different interventions on pain intensity. A small and non‐significant decline in pain intensity following placebo administration was noted during the follow‐up period. A larger, statistically significant decline in pain intensity was measured fifteen minutes and onward after inhalation of 0.5 and 1.0 mg Δ9‐THC doses. The reduction in VAS score was statistically significantly larger in the 1.0 mg compared to the placebo and the 0.5 mg dose (RM‐ANOVA, p = .0015 [95% CI, 0.53;2.23], p = .0058 [95% CI, 0.35;2.08], respectively). The mean maximum drop from baseline in the VAS pain score was 1.95 points (24.97%) in the 0.5 mg dose and 2.95 points (39.42%) in the 1.0 mg dose. The number of patients whose pain VAS score reduced by 30% and more, has reached to the maximum 120‐min post inhalation in all study groups. The largest effect was seen in the 1.0 mg dose (n = 14, 60.87%), then the 0.5 mg dose (n = 8, 36.36%) and the placebo (n = 5, 21.74%). The number of patients who demonstrated at least a 2‐point reduction in pain VAS score was higher at this time point while maintaining group order: 1.0 mg dose (n = 16, 69.57%), 0.5 mg dose (n = 14, 63.64%) and placebo (n = 6, 26.09%).
FIGURE 3.
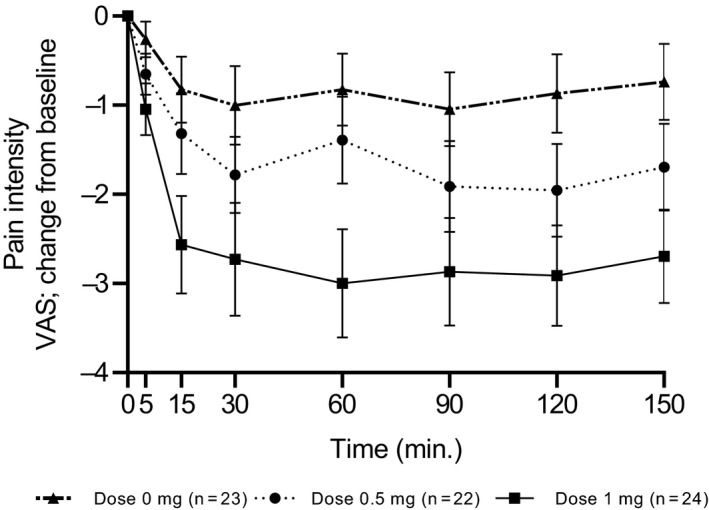
Change in Visual Analogue Score (VAS) pain score from baseline in the three study doses—placebo (0 mg), 0.5 mg and 1.0 mg THC
Figure 3 depicted the changes from baseline in pain intensity at different time points following the three interventions.
3.3. Adverse events
Patients reported AEs for which they were asked, as well as spontaneous AEs. A total of 207 AEs were reported during all study sessions by all patients. Four of the events which were considered serious (SAEs) were not related to the study intervention, but rather to a single patient who was admitted to the hospital due to surgery complications. One patient dropped out of the study due to dizziness caused by the cognitive test. Most of the AEs were classified as mild (95%) and were considered related to the study intervention (80%).
The most common reported AEs were “high” (20.29%) (Figure 4a), “cough” (10.14%), “weakness” (9.17%), “restlessness” (8.2%) (Figure 4b), “dry mouth” (7.24%) (Figure 4c), “dizziness” (6.76%) (Figure 4d), “sleepiness” (6.28%), “nausea” (4.83%) (Figure 4e) and “moderate decrease in blood pressure” (3.86%). In most cases, AEs occurred more frequently in treatment doses than in the placebo treatment (Table 2).
FIGURE 4.
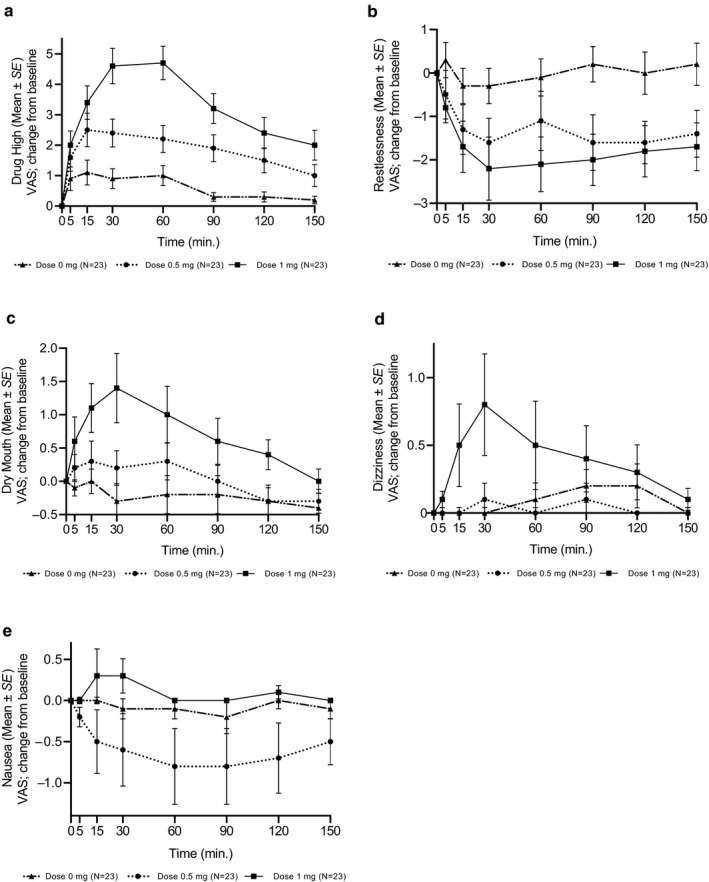
Observed profile of selected subjective adverse effects by inhaling 0, 0.5 and 1.0 mg THC. Adverse events were proactively evaluated and scored on a Visual Analogue Score (VAS) scale of 0–10
TABLE 2.
Most frequently occurring adverse events
| AE term | Placebo | 0.5 mg | 1.0 mg |
|---|---|---|---|
| n (No. of reports) | n (No. of reports) | n (No. of reports) | |
| Drug High | 9 (9) | 12 (13) | 16 (16) |
| Cough | 2 (2) | 4 (6) | 11 (13) |
| Pain | 6 (6) | 7 (7) | 3 (3) |
| Weakness | 5 (5) | 7 (7) | 6 (6) |
| Restlessness | 7 (7) | 7 (7) | 3 (3) |
| Dry mouth | 2 (2) | 8 (8) | 5 (5) |
| Dizziness | 1 (1) | 2 (2) | 8 (8) |
| Sleepiness | 2 (2) | 6 (6) | 4 (4) |
| Nausea | 2 (2) | 2 (2) | 4 (4) |
| BP reduced | 2 (2) | 2 (2) | 2 (2) |
| Hunger | 3 (3) | 2 (2) | |
| Total | 14* (41) | 22* (60) | 20* (66) |
Adverse events sorted by decreasing frequency.
The total number of patients who reported an AE. Each patient could report more than one AE.
Compared to baseline, a significant decrease in the mean diastolic blood pressure was noted 30 min following inhalation of the 0.5 mg dose (from 78.8 ± 9.9 to 75.6 ± 10.4, p = .0452) and 60 min following inhalation of 1.0 mg dose (from 77.7 ± 8.4 to 73.3 ± 9.8, p = .0052). Blood pressure measures returned to baseline after 60 min in the 0.5 mg dose and after 150 min in the 1.0 mg dose. The mean heart rate significantly decreased in the 0.5 mg dose 90 min following inhalation (from 76.2 ± 10.4 to 70.3 ± 10.8, p = .0042), and after 150 min, the heart rate was slightly lower than the baseline. However, a significant increase in heart rate was noticed in the 1.0 mg dose 15 min following inhalation (from 74.7 ± 12.5 to 80.2 ± 13.9, p = .0229), which returned to baseline after 60 min. All AEs were resolved spontaneously up to 150 min following inhalation and required no intervention. There were no tolerability issues related to the study intervention.
The time course of selected subjective adverse effects is depicted in Figure 4; their intensities peaked within 15–30 min and were dose‐dependent. The differences between THC doses over time (5–150 min) are presented in a forest plot graph (Figure S1).
3.4. Cognitive performance
There was no evidence of any consistent impairment in performance in subjects treated with aerosolized THC in the studied doses. In only one test at a one‐time point, there was an indication of an impairment: an increase in the strategy score in the spatial working memory test at the dose of 1.0 mg at the 15‐min time point. There was also an indication of improved performance indicated by faster movement time in RTI, and shorter response latency in RVP of 0.5 mg at 15‐min. However, none of these indications was supported by significant time by dose interactions or an effect of dose in the analysis of variance (Table S1). Examining the correlation between plasma concentration and cognitive performance did not uncover any consistent effects.
4. DISCUSSION
The present study determined the pharmacokinetic and pharmacodynamic profiles of low Δ9‐THC doses inhaled using a novel selective‐dose cannabis inhaler under practical use conditions. It demonstrated a dose‐dependent pharmacokinetic profile, as well as a reduction in pain intensity in response to the inhalation of significantly small doses of cannabis in patients with chronic neuropathic pain/ CRPS. This clinical study aims to find the therapeutic window of medical cannabis for chronic pain patients using the Syqe Inhaler. The current study demonstrated that a single 0.5 mg ∆9‐THC dose yielding a C max of 14.3 ± 7.7 ng/mL and T max of 3.7 ± 1.4 min results in significant analgesia while considerably limiting adverse events. A relatively modest increase to a 1.0 mg ∆9‐THC dose yields a C max of 33.8 ± 25.7 ng/mL and T max of 4.4 ± 2.1 min results in nearly doubling the analgesic effect as compared to the 0.5 mg dose. A clinically meaningful mean pain reduction of 25% and 39% were observed following a single dose inhalation of 0.5 and 1.0 mg THC, respectively. Both doses resulted in a notable reduction in pain intensity: 63.64% of the patients in 0.5 mg dose, and more than 69.57% of the patients in 1.0 mg dose demonstrated at least 2‐points reduction in pain VAS score.
To the best of our knowledge, this is the first time a dose–response effect at such low inhaled doses is demonstrated not only in a pharmacokinetics profile but also in efficacy.
Additionally, there was no evidence of consistent impairments in cognitive performance using the Cambridge cognition battery testing. While no significant objective cognitive impairments were noted in the 1mg dose, the drug “high” intensity doubled compared to the 0.5mg dose, potentially affecting subjective psychoactivity related to quality of life measures. These results may guide the establishment of a therapeutic window of diminishing efficacy returns versus increased psychoactivity. Such subtle microgram dose differences potentially indicate that the ∆9‐THC dose–response effects are extremely sensitive and require very high dosing precision. It is assumed the reasons for such promising drug delivery performance are the bioavailability and adherence‐enhancing technologies of the Syqe Inhaler platform in addition to the electronic selective dosing capabilities.
In a cross‐study comparison of the pharmacokinetic profile, the Syqe Inhaler yielded the highest increase of C max per mg of THC administered (Eisenberg et al., 2014). Among the alternative modes of pulmonary delivery, the Volcano vaporizer (Abrams, Couey, Shade, Kelly, & Benowitz, 2011; Abrams et al., 2007) and smoking cannabis cigarettes (Huestis et al., 1992; Hunault et al., 2008; Hunault et al., 2010), were significantly less effective.
In addition, the pharmacokinetics results of this study were compared to the classical evaporation method (Hartman et al., 2015), in which a larger amount of cannabis was used. The Normalization of the doses reveals significant differences in the blood THC levels given in our study (0.5 and 1 mg), indicating that the Syqe Inhaler is the most effective delivery method for cannabis‐based medicine.
In today's practice, the amount of cannabis used for medical purposes is exceptionally high compared to the amounts used with the Syqe Inhaler. In western countries such as Canada, the UK, Netherlands, and Israel, the average amount of medical cannabis used is 0.67–3 gr per day (Health De Hoop, Heerdink, & Hazekamp, 2018; Canada, 2019; Ware, Adams, & Guy, 2005). Furthermore, we recently reported on 21 hospitalized patients treated with medical cannabis as a part of their ongoing medical care using the device. During hospitalization, they used a median daily dose of 1.5 mg (1.0–2.0) THC, via 3–4 inhalations of 0.5mg THC each per day (Vulfsons et al., 2019). This study demonstrated a 20‐fold reduction in the monthly use of medical cannabis using the Syqe Inhaler compared to patients' regular monthly intake. Overall, our findings (Eisenberg et al., 2014; Vulfsons et al., 2019) using low doses of THC, are consistent with other trials that enrolled a population of patients suffering from chronic neuropathic pain of various etiologies and pointed to relatively low doses of THC as having a favorable risk‐benefit ratio (Wallace, Marcotte, Umlauf, Gouaux, & Atkinson, 2015; Wilsey et al., 2008, 2013). Wilsey et al. reported that inhalation of vaporized 3.03 mg ("low dose") or 5.16 mg ("intermediated dose") THC per day produced equal antinociception at every time point (Wilsey et al., 2013). The authors concluded that "vaporized cannabis, even at low doses, may present an effective option for patients with treatment‐resistant neuropathic pain." Wallace conducted a randomized clinical trial in patients with painful diabetic neuropathy (Wallace et al., 2015) in which each patient was exposed to a single dosing session of a placebo, "low" (2.5 mg), "medium" (10.2 mg THC) or "high" (17.9 mg Δ9‐THC available for inhalation). There was a significant difference in spontaneous pain scores between doses (p < .001). The fact that a substantial percentage of patients with pain are using enormous quantities of THC daily demonstrates a potential gap between evidence and product offerings and warrants reassessment of the cannabis treatment regimen to treat chronic pain.
The current study provides evidence that chronic pain patients population can benefit from low THC doses using the Syqe Inhaler. Since this population is likely characterized by a relative tolerance to medical cannabis, enrolling naive patients in future studies can strengthen the results. Spindle et al. showed cannabinoid concentrations among infrequent users were substantially lower than those observed in prior cannabis administration studies, which enrolled moderate or heavy cannabis users (Spindle et al., 2019). This may imply that naïve subjects may need lower doses of cannabis to get the same effect. At the same time, the naïve population may experience more adverse events (Campbell, Stockings, & Nielsen, 2019), especially in the first days of treatment.
The most frequently reported adverse events related to medical cannabis use involve the central nervous system: fatigue, dizziness, sedation, mental clouding, somnolence, euphoria, confusion, disorientation, dissociation and psychomotor deficits (Ware et al., 2010). Δ9‐THC also impairs cognition involving faculties such as short‐term memory, attention, concentration, executive functioning and visuo‐perception (Nugent et al., 2017; Ware et al., 2015). The question arises whether low doses of cannabis might avoid or at least minimize the adverse effects and psychotomimetic intensity while maintaining the analgesic potency. In the current study, all adverse events, both proactively asked and voluntarily reported, were mostly mild, reversible and receded rapidly. In addition to pharmacokinetic and analgesic dose‐response effect, anticipated AEs demonstrated a dose‐response behavior, suggesting that both desired and undesired cannabis effects can be controlled, delivering low and precise doses in each inhalation. Neurocognitive side effects such as learning, memory and psychomotor deficits are dose‐dependent and may also appear at low doses, but then they are well‐tolerated and self‐resolved. The absence of consistent impairments in cognitive performance provides support for the cognitive safety of single dosing with aerosolized THC using the Syqe Inhaler in patients with chronic pain at the limited range of doses employed in this study.
4.1. Study limitations
Several other points deserve consideration. First, patients in this study were required to have a license to use cannabis‐based medicines; therefore, the results may under‐estimate the potential impact of aerosolized cannabis on drug‐naive patients. Second, the present study tested the effect of single low Δ9‐THC doses only; therefore, we cannot comment on long‐term efficacy and safety issues under the chronic use of medical cannabis. Given that neuropathic pain/ CRPS are chronic conditions, long‐term studies are needed. Third, a limited range of THC doses (0.5–1.0 mg) and one type of medical cannabis (dry flos; high THC/ low CBD) was used. Future studies with additional THC and possibly CBD doses are warranted. Fourth, patients were not asked at the end of each session what treatment they felt they had received, so we cannot be sure that patients were fully blinded to treatment arms. This is particularly important since the absence of the active ingredients in the placebo might have influenced its colour, taste and smell. In contrast, the fact that the inhaled materials were packed inside a cartridge and therefore were not visible, and the similarity in the adverse effect profiles between treatments reduce the likelihood of blinding bias. Finally, possible interactions have been reported between THC and other drugs (i.e. the antiepileptic drug gabapentin; Atwal, Case y, Mitchell, & Vaughan, 2019). Nonetheless, our patients have been using constant doses of their background medications. Since all outcome measures were taken at baseline (while already using these medications) and following medical cannabis inhalation, we believe that these interactions—while important by themselves—are not directly relevant to the findings of this study.
5. CONCLUSIONS
To the best of our knowledge, it is the first time that the delivery of selective, significantly low, and precise therapeutic single doses of inhaled THC demonstrates an analgesic effect. It allows patients to reach the optimum balance between symptom relief and controlled side effects, enabling patients to regain their quality of life. In addition, this metered‐dose cannabis inhaler enables the individualization of medical cannabis regimens that can be evaluated pharmacokinetically and pharmacodynamically using accepted pharmaceutical models.
DISCLOSURE
Dr. Almog is a consultant for Syqe Medical; Prof. Aharon‐Peretz reports no disclosures; Dr. Hayon, Mrs. Ogintz, Mrs. Abalia, and Mr. Lupo are employees of Syqe Medical; Dr. Vulfsons and Prof. Eisenberg received research support from Syqe Medical.
AUTHORS CONTRIBUTION
SA: interpreted PK data; JA‐P: revised the manuscript for intellectual content; SV: revised the manuscript for intellectual content; MO: role in technological execution; HA: drafted the manuscript for intellectual content; TL: role in technological execution; YH: drafted the manuscript for intellectual content; EE: design and conceptualized study; analysed and interpreted of the data; revised the manuscript for intellectual content.
All the authors discussed the results and commented on the manuscript.
Supporting information
Fig S1
Table S1
ACKNOWLEDGEMENT
Supported by Syqe Medical.
Almog S, Aharon‐Peretz J, Vulfsons S, et al. The pharmacokinetics, efficacy, and safety of a novel selective‐dose cannabis inhaler in patients with chronic pain: A randomized, double‐blinded, placebo‐controlled trial. Eur J Pain. 2020;24:1505–1516. 10.1002/ejp.1605
DATA AVAILABILITY STATEMENT
The authors state that any anonymized data not published within the article will be shared on request from any qualified researcher. This statement will be published with the article. If data cannot be shared for legal or ethical reasons or if there are embargoes on datasets, authors must inform the editors at submission and explain the restrictions on the dataset or materials.
REFERENCES
- Abrams, D. I. , Couey, P. , Shade, S. B. , Kelly, M. E. , & Benowitz, N. L. (2011). Cannabinoid‐opioid interaction in chronic pain. Clinical Pharmacology and Therapeutics, 90(6), 844–851. 10.1038/clpt.2011.188 [DOI] [PubMed] [Google Scholar]
- Abrams, D. I. , Vizoso, H. P. , Shade, S. B. , Jay, C. , Kelly, M. E. , & Benowitz, N. L. (2007). Vaporization as a smokeless cannabis delivery system: A pilot study. Clinical Pharmacology and Therapeutics, 82, 572–578. [DOI] [PubMed] [Google Scholar]
- Andreae, M. H. , Carter, G. M. , Shaparin, N. , Suslov, K. , Ellis, R. J. , Ware, M. A. , … Sacks, H. S. (2015). Inhaled cannabis for chronic neuropathic pain: A meta‐analysis of individual patient data. The Journal of Pain, 16, 1221–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwal, N. , Casey, S. L. , Mitchell, V. A. , & Vaughan, C. W. (2019). THC and gabapentin interactions in a mouse neuropathic pain model. Neuropharmacology, 144, 115–121. 10.1016/j.neuropharm.2018.10.006 [DOI] [PubMed] [Google Scholar]
- Campbell, G. , Stockings, E. , & Nielsen, S. (2019). Understanding the evidence for medical cannabis and cannabis‐based medicines for the treatment of chronic non‐cancer pain. European Archives of Psychiatry and Clinical Neuroscience, 269(1), 135–144. 10.1007/s00406-018-0960-9 [DOI] [PubMed] [Google Scholar]
- De Hoop, V. , Heerdink, E. R. , & Hazekamp, A. (2018). Medicinal cannabis on prescription in The Netherlands: Statistics for 2003–2016. Cannabis and Cannabinoid Research, 3, 54–55. 10.1089/can.2017.0059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg, E. , Ogintz, M. , & Almog, S. (2014). The pharmacokinetics, Efficacy, Safety and ease of use of a novel portable metered‐dose inhaler in patients with chronic neuropathic pain: A phase 1a study. Journal of Pain and Palliative Care Pharmacotherapy, 28, 216–225. [DOI] [PubMed] [Google Scholar]
- Fitzcharles, M. A. , Baerwald, C. , Ablin, J. , & Häuser, W. (2016). Efficacy, tolerability and safety of cannabinoids in chronic pain associated with rheumatic diseases (fibromyalgia syndrome, back pain, osteoarthritis, rheumatoid arthritis): A systematic review of randomized controlled trials. Schmerz, 30, 47–61. 10.1007/s00482-015-0084-3 [DOI] [PubMed] [Google Scholar]
- Gieringer, D. , Laurent, J. , & Goodrich, S. (2004). Cannabis vaporizer combines efficient delivery of THC with effective suppression of pyrolytic compounds. Journal of Cannabis Therapeutics, 4, 7–27. 10.1300/J175v04n01_02 [DOI] [Google Scholar]
- Harden, R. N. , Bruehl, S. , Perez, R. S. , Birklein, F. , Marinus, J. , Maihofner, C. , … Vatine, J. J. (2010). Validation of proposed diagnostic criteria (the "Budapest Criteria") for Complex Regional Pain Syndrome. Pain, 150(2), 268–274. 10.1016/j.pain.2010.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman, R. L. , Brown, T. L. , Milavetz, G. , Spurgin, A. , Gorelick, D. A. , Gaffney, G. , & Huestis, M. A. (2015). Controlled cannabis vaporizer administration: Blood and plasma cannabinoids with and without Alcohol. Clinical chemistry, 61(6), 850–869. 10.1373/clinchem.2015.238287. [DOI] [PubMed] [Google Scholar]
- Health Canada . (2019). Data on Cannabis for medical purposes (October 2018‐March 2019). Daily authorized amount associated with client registration, Table 4. Retrieved from https://www.canada.ca/en/health‐canada/services/drugs‐medication/cannabis/research‐data/medical‐purpose.html.
- Hill, K. P. , Palastro, M. D. , & George, T. P. (2019). Therapeutic cannabis use in 2018: Where do we stand? Lancet Psychiatry, 6, 88–89. 10.1016/S2215-0366(18)30417-6 [DOI] [PubMed] [Google Scholar]
- Hill, K. P. , Palastro, M. D. , Johnson, B. , & Ditre, J. W. (2017). Cannabis and pain: A clinical review. Cannabis and Cannabinoid Research, 2, 96–104. 10.1089/can.2017.0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis, M. A. (2007). Human Cannabinoid Pharmacokinetics. Chemistry & Biodiversity, 4, 1770–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis, M. A. , Sampson, A. H. , Holicky, B. J. , Henningfield, J. E. , & Cone, E. J. (1992). Characterization of the absorption phase of marijuana smoking. Clinical Pharmacology and Therapeutics, 52(1), 31–41. [DOI] [PubMed] [Google Scholar]
- Hunault, C. C. , Mensinga, T. T. , de Vries, I. , Kelholt‐Dijkman, H. H. , Hoek, J. , Kruidenier, M. , … Meulenbelt, J. (2008). Delta‐9‐tetrahydrocannabinol (THC) serum concentrations and pharmacological effects in males after smoking a combination of tobacco and cannabis containing up to 69 mg THC. Psychopharmacology (Berl), 201(2), 171–181. 10.1038/clpt.1992.100. [DOI] [PubMed] [Google Scholar]
- Hunault, C. C. , van Eijkeren, J. C. , Mensinga, T. T. , de Vries, I. , Leenders, M. E. , & Meulenbelt, J. (2010). Disposition of smoked cannabis with high Δ(9)‐tetrahydrocannabinol content: A kinetic model. Toxicology and Applied Pharmacology, 246(3), 148–153. 10.1016/j.taap.2010.04.019 [DOI] [PubMed] [Google Scholar]
- Lanz, C. , Mattsson, J. , Soydaner, U. , & Brenneisen, R. (2016). Medicinal cannabis: In vitro validation of vaporizers for the smoke‐free inhalation of cannabis. PLoS One, 11(1), e0147286 10.1371/journal.pone.0147286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch, M. E. , & Campbell, F. (2011). Cannabinoids for treatment of chronic non‐cancer pain; a systematic review of randomized trials. British Journal of Clinical Pharmacology, 72, 735–744. 10.1111/j.1365-2125.2011.03970.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academic of Sciences, Engineering, and Medicine (2017). The health effects of cannabis and cannabinoids: The current state of evidence and recommendations for research. Washington, DC: The National Academies Press. Retrieved from https://www.nap.edu/catalog/24625/the‐health‐effects‐of‐cannabis‐and‐cannabinoids‐the‐current‐state. [PubMed] [Google Scholar]
- Nugent, S. M. , Morasco, B. J. , O'Neil, M. E. , Freeman, M. , Low, A. , Kondo, K. , … Kansagara, D. (2017). The effect of cannabis among adults with chronic pain and an overview of general harms: A Systematic Review. Annals of Internal Medicine, 167, 319–331. [DOI] [PubMed] [Google Scholar]
- Romero‐Sandoval, E. A. , Kolano, A. L. , & Alvarado‐Vázquez, P. A. (2017). Cannabis and cannabinoids for Chronic pain. Current Rheumatology Reports, 19, 67 10.1007/s11926-017-0693-1 [DOI] [PubMed] [Google Scholar]
- Spindle, T. R. , Cone, E. J. , Schlienz, N. J. , Mitchell, J. M. , Bigelow, G. E. , Flegel, R. , … Vandrey, R. (2019). Acute pharmacokinetic profile of smoked and vaporized cannabis in human blood and oral fluid. Journal of Analytical Toxicology, 43, 233–258. 10.1093/jat/bky104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockings, E. , Campbell, G. , Hall, W. D. , Nielsen, S. , Zagic, D. , Rahman, R. , … Degenhardt, L. (2018). Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: A systematic review and meta‐analysis of controlled and observational studies. Pain, 159, 1934–1954. 10.1097/j.pain.0000000000001293 [DOI] [PubMed] [Google Scholar]
- Treede, R. D. , Jensen, T. S. , Campbell, J. N. , Cruccu, G. , Dostrovsky, J. O. , Griffin, J. W. , … Serra, J. (2008). Neuropathic pain: Redefinition and a grading system for clinical and research purposes. Neurology, 70, 1630–1635. 10.1212/01.wnl.0000282763.29778.59 [DOI] [PubMed] [Google Scholar]
- Troutt, W. D. , & DiDonato, M. D. (2017). Carbonyl compounds produced by vaporizing cannabis oil thinning agents. Journal of Alternative and Complementary Medicine, 23, 879–884. 10.1089/acm.2016.0337 [DOI] [PubMed] [Google Scholar]
- Vandrey, R. , Herrmann, E. S. , Mitchell, J. M. , Bigelow, G. E. , Flegel, R. , LoDico, C. , & Cone, E. J. (2017). Pharmacokinetic profile of oral cannabis in humans: Blood and oral fluid Disposition and Relation to Pharmacodynamic Outcomes. Journal of Analytical Toxicology, 41, 83–99. 10.1093/jat/bkx012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulfsons, S. , Ognitz, M. , Bar‐Sela, G. , Raz‐Pasteur, A. , & Eisenberg, E. (2019). Cannabis treatment in hospitalized patients using a Syqe inhaler: Results of a pilot open‐label study. Palliative and Supportive Care, 14, 1–6. [DOI] [PubMed] [Google Scholar]
- Wallace, M. S. , Marcotte, T. D. , Umlauf, A. , Gouaux, B. , & Atkinson, J. H. (2015). Efficacy of inhaled cannabis on painful diabetic neuropathy. Journal of Pain, 16, 616–627. 10.1016/j.jpain.2015.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware, M. A. , Adams, H. , & Guy, G. W. (2005). The medical use of cannabis in the UK: Results of a nationwide survey. International Journal of Clinical Practice, 59, 291–295. [DOI] [PubMed] [Google Scholar]
- Ware, M. A. , Wang, T. , Shapiro, S. , Collet, J.‐P. , Boulanger, A. , Esdaile, J. M. , … O'Connell, C. (2015). Cannabis for the management of pain: Assessment of safety study (COMPASS). The Journal of Pain, 16, 1233–1242. 10.1016/j.jpain.2015.07.014 [DOI] [PubMed] [Google Scholar]
- Ware, M. A. , Wang, T. , Shapiro, S. , Robinson, A. , Ducruet, T. , Huynh, T. , … Collet, J. P. (2010). Smoked cannabis for chronic neuropathic pain: A randomized controlled trial. CMAJ, 182, E694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsey, B. , Marcotte, T. D. , Deutsch, R. , Gouaux, R. , Sakai, S. , & Donaghe, H. (2013). Low dose vaporized cannabis significantly improves neuropathic pain. Journal of Pain, 14, 136–148. 10.1016/j.jpain.2012.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsey, B. , Marcotte, T. , Tsodikov, A. , Millman, J. , Bentley, H. , Gouaux, B. , & Fishman, S. (2008). A randomized, placebo‐controlled, crossover trial of Cannabis Cigarettes in neuropathic pain. The Journal of Pain, 9, 506–521. 10.1016/j.jpain.2007.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1
Data Availability Statement
The authors state that any anonymized data not published within the article will be shared on request from any qualified researcher. This statement will be published with the article. If data cannot be shared for legal or ethical reasons or if there are embargoes on datasets, authors must inform the editors at submission and explain the restrictions on the dataset or materials.


