Abstract
Objective
To examine health behaviours in bladder cancer survivors including physical activity (PA), body mass index, diet quality, smoking and alcohol consumption, and to explore their relationship with health‐related quality of life (HRQoL).
Subjects/Patients and Methods
Cross‐sectional questionnaire packages were distributed to bladder cancer survivors (muscle‐invasive bladder cancer [MIBC] and non‐muscle‐invasive bladder cancer [NMIBC]) aged >18 years, and proficient in English. Lifestyle behaviours were measured using established measures/questions, and reported using descriptive statistics. HRQoL was assessed using the validated Bladder Utility Symptom Scale, and its association with lifestyle behaviours was evaluated using analysis of covariance (ancova) and multivariate regression analyses.
Results
A total of 586 participants completed the questionnaire (52% response rate). The mean (SD) age was 67.3 (10.2) years, and 68% were male. PA guidelines were met by 20% (n = 117) and 22.7% (n = 133) met dietary guidelines. In all, 60.9% (n = 357) were overweight/obese, and the vast majority met alcohol recommendations (n = 521, 92.5%) and were current non‐smokers (n = 535, 91.0%). Health behaviours did not differ between MIBC and NMIBC, and cancer treatment stages. Sufficient PA, healthy diet, and non‐smoking were significantly associated with HRQoL, and the number of health behaviours participants engaged in was positively associated with HRQoL (P < 0.001).
Conclusion
Bladder cancer survivors are not meeting guidelines for important lifestyle behaviours that may improve their overall HRQoL. Future research should investigate the impact of behavioural and educational interventions for health behaviours on HRQoL in this population.
Keywords: health behaviours, health‐related quality of life, patient education, supportive care, #BladderCancer, #blcsm
Introduction
Bladder cancer is a common genitourinary cancer, and with the ageing population, its prevalence is expected to increase significantly 1, 2. Due to older age, high recurrence rates, and repeated surgeries for noninvasive disease and cystectomy for invasive disease, bladder cancer survivors (defined from the point of diagnosis) 3 carry an extensively high disease‐related personal and economic burden 4, 5. Modifiable lifestyle behaviours such as: physical activity (PA), weight management, diet, and smoking cessation, may reduce morbidity and mortality, and have been shown to improve health‐related quality of life (HRQoL) in survivors of multiple other cancers 6, 7, 8. However, to date, there have been few large studies that assess the degree to which bladder cancer survivors are adhering to current lifestyle recommendations across the disease trajectory, and between those with different stage of disease (muscle‐invasive bladder cancer [MIBC] vs non‐muscle‐invasive bladder cancer [NMIBC]). Further, the impact of lifestyle behaviours of bladder cancer survivors on HRQoL remains unclear due to small sample sizes and measurement issues including the use of generic HRQoL questionnaires or bladder cancer‐specific questionnaires that have not been validated 9, 10, 11.
To build upon this literature, we have conducted a large, cross‐sectional study of bladder cancer survivors using validated measures to: (i) describe health behaviours (PA, body mass index [BMI], diet quality, smoking and alcohol consumption) and compare between survivors with MIBC vs NMIBC and across the different phases of the cancer journey (newly diagnosed and undergoing treatment, in follow‐up surveillance, and recurrent/metastatic disease); and (ii) explore the relationship between lifestyle behaviours and HRQoL.
Subjects/Patients and Methods
Study Recruitment and Procedure
This study was approved by the Research Ethics Board at the University Health Network (UHN, 13‐7207). Detailed inclusion/exclusion criteria and recruitment procedures are described in a previous report 12. Briefly, bladder cancer survivors (MIBC and NMIBC) aged >18 years and proficient in English attending regular clinic visits at the Princess Margaret Cancer Centre (PMCC) and The Ottawa Hospital (TOH) were approached in‐person, and an online version of the survey (Appendix S1) was e‐mailed to the registered patient members of Bladder Cancer Canada (BCC).
Outcome Measures
Our previous report describes the initial development and piloting of the questionnaire used for data capture 12. The questionnaire package included the following: (i) Demographic and disease/treatment information; (ii) PA was assessed using the Godin Leisure‐Time Exercise Questionnaire, a three‐item tool that measures the weekly minutes that a participant spends participating in strenuous, moderate, and mild‐intensity exercise 13. Total weekly PA was measured by the total minutes reported engaging in mild/moderate/strenuous exercise. Based on current guidelines for PA, participants were categorised as ‘sufficiently active’ if they met the guideline of ≥150 min of moderate/strenuous exercise, otherwise, they were considered ‘insufficiently active’ 14, 15; (iii) BMI was calculated using the questions, ‘How much do you currently weigh?’ and ‘What is your height?’, and applying the formula: . Each participant was subsequently categorised as: ‘underweight or normal weight’ if their BMI was <25.0 kg/m2, ‘overweight’ if their BMI was 25–29.9 kg/m2, and ‘obese’ if their BMI was ≥30 kg/m2; (iv) Diet quality: was measured by a modified version of the Rapid Eating Assessment for Participants – Short (REAP‐S; 10 items) 16. The REAP‐S contains seven items scored from 1 to 3 that assess intake of fruits, whole grains, and vegetables (FWV), and dairy and meat in a typical week. The summed score estimates dietary quality, with higher scores indicating healthier eating behaviours (range 7–21). In addition to the total sum, we also summed the scores for sufficient consumption of FWV as a marker of healthy dietary patterns. If participants scored a total of 9 points from the three items, they were categorised as having ‘sufficient’ FWV, and <9 points was considered ‘insufficient’ FWV. Lastly, participants were asked how willing they were to make changes in their eating habits in order to be healthier (scale, very willing 1–5 not at all willing); (v) Smoking status: was measured by the question, ‘Have you smoked at least part of a cigarette in the past 7 days?’ – ‘Yes’ indicated current smoker, and ‘No’ indicated that they were a non‐smoker; (vi) Alcohol consumption: was measured using the question, ‘Over the past 12 months, on average how many alcoholic drinks do you have each week?’, and using current guidelines (male ≤15, female ≤10 drinks/week), participants were categorised into ‘Within’ or ‘Exceeding’ alcohol consumption recommendations 17; (vii) HRQoL: was evaluated using the 10‐item, validated Bladder Utility Symptom Scale (BUSS) 18. The BUSS is a 10‐question, global HRQoL questionnaire that evaluates generic and bladder cancer‐specific domains of QoL. The total BUSS for each participant was calculated by summing up the scores from each question (maximum = 100), with a higher score indicating higher HRQoL 12.
Statistical Analysis
Demographic and clinical information on the sample were reported using descriptive statistics.
Description of Health Behaviours
Health behaviours were reported using descriptive statistics across the study cohort. Health behaviours between survivors of MIBC vs NMIBC and across the different phases of the cancer journey (newly diagnosed and undergoing treatment, in follow‐up surveillance, and recurrent/metastatic disease) were compared using chi‐square or Fisher’s exact tests for categorical variables and Student’s t‐test or anova for continuous variables.
Association Between Lifestyle Behaviours and HRQoL
The association of each health behaviour with HRQoL was examined using analysis of covariance (ancova), while adjusting for significant demographic and clinical confounders such as education status, diagnosis with MIBC or NMIBC, and time since diagnosis. To examine whether the cumulative number of net health behaviours would be significantly associated with better HRQoL, health behaviour scores of 0–5 were created by assigning 1 point for each of the following health behaviours: being sufficiently active, consuming sufficient FWV, not smoking, alcohol consumption within recommendation, and BMI of <25 kg/m2 19. Trend analysis was then performed to check the association of health behaviour score with total BUSS score, by controlling for the same confounders used in the aforementioned analysis.
Statistical significance was set as a P < 0.05 (two‐sided). The Statistical Analysis System (SAS), version 9.3 (SAS Institute Inc., Cary, NC, USA) was used for all analyses.
Results
The response rate for the questionnaire was 52% (586/1126, returned/distributed), with 204 (204/308, 66%) from the PMCC, 129 (129/183, 70%) from TOH, and 253 (253/625, 40%) from the BCC network. Data from the three study sites were pooled, as their HRQoL scores did not differ significantly (P = 0.428). Demographic and clinical information of the study cohort are presented in Table 1 and overall health behaviours are shown in Table 2.
Table 1.
Demographic and clinical data of study participants.
| Variable | Value |
|---|---|
| Number of participants | 586 |
| Age, years, mean (sd) | 67.3 (10.2) |
| Sex, n (%) | |
| Male | 401 (68.4) |
| Female | 183 (31.2) |
| Country of birth, n (%) | |
| Canada | 397 (67.7) |
| Outside of Canada | 183 (31.2) |
| Education, n (%) | |
| ≤Post‐secondary | 204 (34.8) |
| >Post‐secondary | 378 (64.5) |
| Residence setting, n (%) | |
| Urban | 319 (54.4) |
| Suburban | 166 (28.3) |
| Rural | 97 (16.6) |
| Time since diagnosis, n (%) | |
| 0–2 years ago | 75 (12.8) |
| 2–5 years ago | 288 (49.1) |
| 5+ years ago | 190 (32.4) |
| Disease type, n (%) | |
| MIBC | 137 (23.4) |
| NMIBC | 324 (55.3) |
| I do not know | 113 (19.3) |
| TNM staging, n (%) | |
| Ta, CIS, T1 | 324 (55.3) |
| T2–T4 | 129 (22.0) |
| N+, M+ | 8 (1.4) |
| I do not know | 113 (19.3) |
| Treatment, n (%) | |
| Surgery only | 170 (29.7) |
| Chemotherapy only | 16 (2.8) |
| Immunotherapy (BCG) | 33 (5.8) |
| Radiation only | 3 (0.5) |
| Surgery + chemotherapy | 108 (21.2) |
| Surgery + immunotherapy (BCG) | 261 (51.2) |
| Surgery + radiation | 35 (6.9) |
BCG, Bacillus Calmette– Guérin.
Table 2.
The number and proportion of participants in each health behaviour group.
| Health behaviour | N (%) of N = 586 |
|---|---|
| PA | |
| Sedentary or insufficiently active | 453 (77.3) |
| Sufficiently active | 117 (20.0) |
| BMI [mean (sd) 27.5 (9.1) kg/m2] | |
| Underweight or normal weight | 211 (36.0) |
| Overweight | 221 (37.7) |
| Obese | 136 (23.2) |
| Diet [mean(sd) REAP‐S score 16.5 (2.9)] | |
| Sufficient FWV | 133 (22.7) |
| Insufficient FWV | 415 (70.8) |
| Smoking | |
| History of smoking | 403 (71.0) |
| Ex‐smoker | 352 (61.9) |
| Current smoker | 51 (9.0) |
| Alcohol | |
| Within recommendation | 521 (92.5) |
| Exceeded recommendation | 42 (7.5) |
Description of Health Behaviours
PA: Participants were engaging in a mean (sd) of 140 (97) min of total PA (mild/moderate/strenuous) per week. Based on current guidelines 15, 20% (n = 117) of respondents were considered sufficiently active. NMIBC vs MIBC and cancer journey groups did not differ significantly in meeting sufficient exercise guidelines (Tables S1 and S2). The MIBC group had a mean (sd) of 61 (119) min of strenuous and moderate exercise and in the NMIBC group it was 75 (117) min (P =0.84). By cancer journey group, the newly diagnosed group had a mean (sd) of 58 (108) min, in the follow‐up group it was 74 (125) min, and in the recurrent/metastatic group it was 45 (82) min (P = 0.60).
BMI
A large portion of participants were overweight (37.7%, n = 221) or obese (23.2%, n = 136). BMI categories did not differ significantly between NMIBC vs MIBC or cancer journey groups (Tables S1 and S2).
Diet Quality
Details of responses to each REAP‐S item are included in Table 3. The mean (sd) REAP‐S score was 16.5 (2.9). In all, 42% (n = 258) of participants were usually/often eating ≥2 servings of fruit, 49.0% (n = 287) vegetables, and 46.9% (n = 275) of whole grains per day and 22.7% (n = 133) did all three. In terms of willingness to make changes in their eating habits, respondents scored a mean (sd, range) of 4.2 (1.0, 1–5). The mean (sd) REAP‐S score between NMIBC [16.8 (2.7)] and MIBC [16.8 (3.0)] did not differ (P = 0.94) and there was no difference in score between newly diagnosed [16.6 (3.0)], follow‐up surveillance [16.5 (3.0)], and recurrent/metastatic disease [17.0 (2.4)] groups (P = 0.65). Furthermore, NMIBC vs MIBC and cancer journey groups did not differ significantly in their FWV consumption (P = 0.18 and P = 0.88, respectively), or their willingness to change their diets (P = 0.31 and P = 0.44, respectively).
Table 3.
Total responses to each item in the REAP‐S measure.
| REAP‐S item (asked in reference to an average week) | N (%) of N = 586 |
|---|---|
| Skip breakfast | |
| Usually/often | 72 (12.8) |
| Sometimes | 84 (14.9) |
| Rarely/never | 407 (72.3) |
| Eat 4 or more meals out | |
| Usually/often | 48 (8.8) |
| Sometimes | 128 (23.4) |
| Rarely/never | 372 (67.9) |
| Eat <2 servings of whole grains a day | |
| Usually/often | 111 (19.9) |
| Sometimes | 173 (30.9) |
| Rarely/never | 275 (49.2) |
| Eat less than 2 servings of fruits a day | |
| Usually/often | 78 (14.0) |
| Sometimes | 193 (34.6) |
| Rarely/never | 287 (51.4) |
| Eat/drink <2 servings of dairy products a day | |
| Usually/often | 122 (21.7) |
| Sometimes | 204 (36.3) |
| Rarely/never | 236 (42.0) |
| Eat <2 servings of vegetables a day | |
| Usually/often | 78 (14.0) |
| Sometimes | 193 (34.6) |
| Rarely/never | 287 (51.4) |
| Eat >227 g (8 oz) of meat a day | |
| Usually/often | 135 (24.3) |
| Sometimes | 192 (34.6) |
| Rarely/never | 228 (41.1) |
Smoking
A large majority of the study participants met smoking guidelines, with only 9.0% (n = 51) being current smokers at the time of the survey. A majority of participants (n = 403, 71.0%) reported a history of smoking. Current smoking status did not differ between MIBC vs NMIBC or between cancer journey groups (P = 0.28 and P = 0.37, respectively).
Alcohol Consumption
Most participants were meeting recommended consumption guidelines for alcohol (n = 521, 92.5%). There were no differences between MIBC vs NMIBC and cancer journey groups based on alcohol consumption (P = 0.82 and P = 0.80, respectively).
Association Between Health Behaviours and HRQoL
For each health behaviour, the unadjusted and adjusted mean ± sd BUSS scores are reported in Table 4. Results from ancova controlling for education status, MIBC or NMIBC diagnosis and time since diagnosis showed that meeting PA recommendations, healthy diet, and non‐smoking were significantly associated with HRQoL. Meeting alcohol recommendations and BMI were not associated with HRQoL (Table 4).
Table 4.
The mean HRQoL for groups of each health behaviour.
| Health behaviour | HRQoL* | |
|---|---|---|
| Unadjusted | Adjusted* | |
| PA, mean (sd) | ||
| Insufficiently active | 76.2 (18.2) | 75.9 (18.0) |
| Sufficiently active | 83.7 (13.6) | 84.1 (13.4) |
| d † | 0.47 | 0.51 |
| BMI, mean (sd) | ||
| Underweight or normal weight | 78.4 (18.2) | 78.3 (18.5) |
| Overweight or obese | 77.2 (17.2) | 77.2 (17.2) |
| d | 0.07 | 0.07 |
| Diet quality, mean (sd) | ||
| Insufficient FWV | 75.2 (18.5) | 76.5 (17.5) |
| Sufficient FWV | 80.0 (16.5) | 81.6 (16.6) |
| d | 0.25 | 0.29 |
| Smoking, mean (sd) | ||
| Current smoker | 69.2 (24.2) | 67.8 (23.5) |
| Non‐smoker | 78.6 (16.6) | 78.5 (16.5) |
| d | 0.45 | 0.53 |
| Alcohol, mean (sd) | ||
| Above recommendation | 81.4 (16.2) | 81.1 (17.1) |
| Within recommendation | 77.5 (17.5) | 77.4 (17.3) |
| d | 0.23 | 0.21 |
Higher scores on the HRQoL measure indicate higher self‐reported HRQoL on the BUSS.
Mean adjusted for education status, MIBC or NMIBC diagnosis and time since diagnosis. † d (Cohen’s d) = Mean1−Mean2/sd pooled; d = 0.2 (small effect); d = 0.5 (moderately large effect); d = 0.8 (large effect).
Health behaviour scores (0–5) were calculated for each participant as described in the statistical analysis section. There were only four participants with a score of 0, and they were combined with individuals with a score of 1 for the purpose of analysis. Similarly, 17 participants with a score of 5 were combined with individuals with a score of 4 to create the score category of 4–5. In total, 42 (7.6%) participants were engaging in 0–1 health behaviours, 224 (40.4%) in 2, 187 (33.6%) in 3, and 102 (18.4%) in 4–5. There was a positive association between the number of health behaviours participants were engaged in and HRQoL, and the linear trend was significant (P < 0.001) (Table 5 and Fig. 1).
Table 5.
Unadjusted and adjusted means of HRQOL by health behaviour scores.
| Score | N (% of population) | Unadjusted, mean (sd) | Adjusted*, mean (sd) |
|---|---|---|---|
| 0–1 | 42 (7.6) | 76.8 (20.4) | 76.7 (18.9) |
| 2 | 224 (40.4) | 74.9 (18.02) | 74.4 (18.0) |
| 3 | 187 (33.6) | 77.7 (17.1) | 77.7 (16.9) |
| 4–5 | 102 (18.4) | 84.2 (13.8) | 85.01 (13.7) |
Mean adjusted for education status, MIBC or NMIBC diagnosis, and time since diagnosis.
Figure 1.
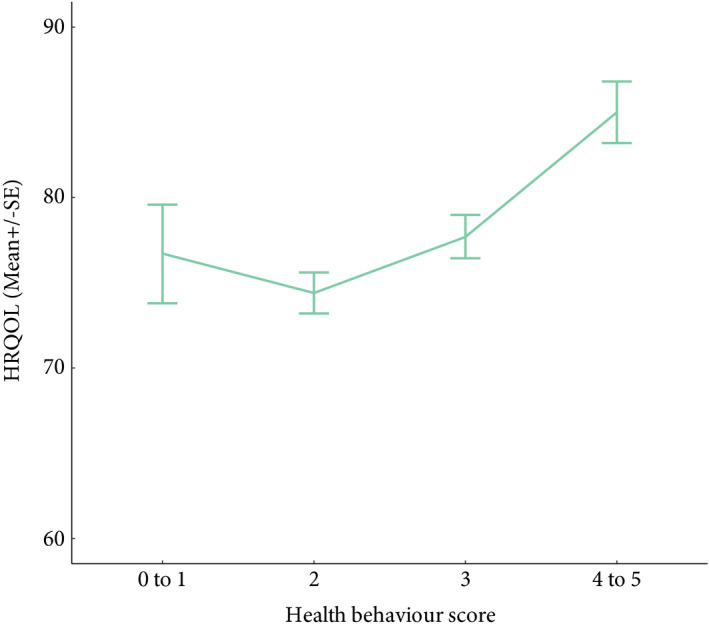
*HRQoL (mean ± se) for total health behaviour. *Mean adjusted for education status, MIBC or NMIBC diagnosis, and time since diagnosis.
Discussion
The present study found that a minority of bladder cancer survivors are meeting PA and dietary recommendations, and the majority are overweight or obese. HRQoL was significantly associated with non‐smoking, meeting PA and dietary recommendations; and they were additive, as revealed by the increasing trend of reported HRQoL with increasing number of met health behaviours. To our knowledge, this is the first study to comprehensively investigate a wide span of health behaviours in a diverse sample of bladder cancer survivors.
More than two‐thirds (77.3%) of study participants were sedentary or insufficiently active according to the American Cancer Society (ACS) guidelines 15. The present study cohort reported lower total PA levels than the general Canadian population, and also compared to other bladder cancer survivor studies 11, 20, 21. This may be due to our exclusion of mild exercise, which is not included in the ACS recommendations. Mild exercise can include domestic work, which was the PA domain that Gopalakrishna et al. 11 found most reported by bladder cancer survivors. PA was positively and strongly associated with HRQoL, a finding that is supported by previous studies of bladder cancer populations 11. Given this, as well as previous work that has demonstrated that regular PA can result in a 50% reduction in risk of functional limitations and disability 19, supervised aerobic and resistance exercise programmes should be recommended to bladder cancer survivors who are not meeting PA recommendations.
In terms of diet quality, we found that 22.7% of the participants were meeting recommendations for FWV intake. This proportion is similar to other reports of patients with cancer (14.8–18.2%), but lower than a recent study of bladder cancer survivors that found 66.8% met fruit and vegetable recommendations 7, 22. These findings warrant concern, given the very low number of bladder cancer survivors who are meeting recommendations in our present cohort, and the emerging evidence that links diet quality to improved health outcomes including mortality, disability and chronic disease in the general population 23. There is a lack of research on the impact of diet quality on the HRQoL of bladder cancer survivors, which has led to mixed evidence of its effects 7, 24, 25. From studies involving other cancer groups, there is evidence suggesting that a poor diet leads to functional decline, worsened overall health outcomes, and recurrence 6, 15, 26. In our present analyses, better diet quality, as measured by consuming sufficient FWV, as well as by the REAP‐S score, was shown to be associated with higher HRQoL. Thus, nutritional screening is recommended to raise awareness regarding diet quality and to identify bladder cancer survivors who may need additional nutrition‐related resources and support services.
The mean (sd) BMI of this study population was 27.5 (9.1) kg/m2, which was comparable to the bladder cancer survivors [27.9 (5.5) kg/m2] in the Gopalakrishna et al. 11 study, and similar to the BMI distribution of the general population of Canadians aged 18–79 years 25, 27. Despite PA and diet quality both being positively correlated with HRQoL, BMI was not associated with HRQoL. This is in contrast to reports from other cancer sites, which have reported that being overweight or obese may result in functional decline and reduced HRQoL 28, 29. Despite this finding, there is emerging evidence to suggest an association between BMI and cancer recurrence, and counselling regarding weight loss is recommended 30.
Encouragingly, while a large proportion of respondents had a history of smoking, which increases their risk of developing bladder cancer 31, very few respondents were current smokers. It is possible that a diagnosis of cancer may have presented an incentive for smoking cessation. Screening for smoking status and the provision of cessation services are important, given that continued smoking can affect disease outcomes 31, and we found that smoking behaviour was also correlated with lower HRQoL. Alcohol consumption among our present participants was quite low, and not associated with HRQoL.
The effect of health behaviours was additive; that is, the more health behaviours bladder cancer survivors engaged in, the higher their HRQoL. Interestingly, we found that none of the health behaviours differed significantly across disease groups (MIBC vs NMIBC) or cancer journey groups (newly diagnosed, follow‐up surveillance, or metastasis/recurrence), which suggests that lifestyle behaviour interventions may not need to target a specific group of bladder cancer survivors and should be offered across the cancer trajectory, although ideally at diagnosis.
A limitation to the present study is its cross‐sectional design, not allowing for definitive conclusions to be made on the benefits of exercise, non‐smoking, and diet quality on HRQoL, and it is uncertain whether HRQoL can conversely influence health behaviours. Also, due to the self‐reported nature of the questionnaire, participants may have overestimated their exercise levels and dietary patterns. Similarly, treatment information was not included in the analysis, as it could have been misreported by patients. Nonetheless, there is scarce research in the field of health behaviours and HRQoL of bladder cancer survivors and our present study provides data to support the importance of conducting more interventional, randomised controlled trials for different health behaviours and their potentially additive effects on HRQoL, and how they can differentially affect different subgroups of bladder cancer survivors 7.
Conclusions
Our present results showed that many MIBC and NMIBC survivors are not engaging in important health behaviours throughout the cancer journey and this is associated with worse HRQoL. Future research should study the impact of lifestyle interventions on behaviour changes and HRQoL in this group of survivors.
Conflict of Interest
There were no conflicts of interest from any of the authors.
Abbreviations
- ACS
American Cancer Society
- BCC
Bladder Cancer Canada
- BMI
body mass indexs
- BUSS
Bladder Utility Symptom Scale
- FWV
fruits, whole grains, and vegetables
- HRQoL
health‐related quality of life
- MIBC
muscle invasive bladder cancer
- NMIBC
non‐muscle invasive bladder cancer
- PA
physical activity
- PMCC
Princess Margaret Cancer Centre
- REAP‐S
Rapid Eating Assessment for Participants – Short
- TOH
The Ottawa Hospital
Supporting information
Table S1. Health behaviours by disease type.
Table S2. Health behaviours by cancer journey.
Appendix S1. Health behaviours questionnaire package.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65: 5–29 [DOI] [PubMed] [Google Scholar]
- 2. Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol 2017; 71: 96–108 [DOI] [PubMed] [Google Scholar]
- 3. Denlinger CS,Carlson RW, Are M et al. Survivorship: introduction and definition. Clinical practice guidelines in oncology. J Natl Compr Canc Netw 2014; 12: 34–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johnson DC, Greene PS, Nielsen ME. Surgical advances in bladder cancer: at what cost? Urol Clin North Am 2015; 42: 235–52, ix [DOI] [PubMed] [Google Scholar]
- 5. Scarpato KR, Morgans AK, Moses KA. Optimal management of muscle‐invasive bladder cancer – a review. Res Rep Urol 2015; 7: 143–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Demark‐Wahnefried W, Jones LW. Promoting a healthy lifestyle among cancer survivors. Hematol Oncol Clin North Am 2008; 22: 319–42, viii [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blanchard CM, Stein KD, Baker F et al. Association between current lifestyle behaviors and health‐related quality of life in breast, colorectal, and prostate cancer survivors. Psychol Health 2004; 19: 1–13. [Google Scholar]
- 8. Beesley VL, Eakin EG, Janda M, Battistutta D. Gynecological cancer survivors' health behaviors and their associations with quality of life. Cancer Causes Control 2008; 19: 775–82 [DOI] [PubMed] [Google Scholar]
- 9. Keimling M, Behrens G, Schmid D, Jochem C, Leitzmann MF. The association between physical activity and bladder cancer: systematic review and meta‐analysis. Br J Cancer 2014; 110: 1862–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Noguchi JL, Liss MA, Parsons JK. Obesity, physical activity and bladder cancer. Curr Urol Rep 2015; 16: 74 [DOI] [PubMed] [Google Scholar]
- 11. Gopalakrishna A, Longo TA, Fantony JJ, Van Noord M, Inman BA. Lifestyle factors and health‐related quality of life in bladder cancer survivors: a systematic review. J Cancer Surviv 2016; 10: 874–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chung J, Kulkarni GS, Morash R et al. Assessment of quality of life, information, and supportive care needs in patients with muscle and non‐muscle invasive bladder cancer across the illness trajectory. Support Care Cancer 2019; 27: 3877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Godin G. The Godin‐Shephard Leisure‐time physical activity questionnaire. Health Fit J Can 2011; 4: 18–22 [Google Scholar]
- 14. Amireault S, Godin G. The Godin‐Shephard leisure‐time physical activity questionnaire: validity evidence supporting its use for classifying healthy adults into active and insufficiently active categories. Percept Mot Skills 2015; 120: 604–22 [DOI] [PubMed] [Google Scholar]
- 15. Rock CL, Doyle C, Demark‐Wahnefried W et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin 2012; 62: 242–74 [DOI] [PubMed] [Google Scholar]
- 16. Johnston CS, Bliss C, Knurick JR, Scholtz C. Rapid Eating Assessment for Participants [shortened version] scores are associated with Healthy Eating Index‐2010 scores and other indices of diet quality in healthy adult omnivores and vegetarians. Nutr J 2018; 17: 89–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Butt P, Beirness D, Gliksman L, Paradis C, Stockwell T. Alcohol and Health in Canada: A Summary of Evidence and Guidelines for Low Risk Drinking. Ottawa, ON: Canadian Centre of Substance Abuse, 2011. Available at: https://www.uvic.ca/research/centres/cisur/assets/docs/report‐alcohol‐and‐health‐in‐canada.pdf. Accessed January 2020. [Google Scholar]
- 18. Perlis N, Krahn MD, Boehme KE et al. Utility symptom scale: a novel patient reported outcome instrument for bladder cancer. J Urol 2018; 200: 283–91 [DOI] [PubMed] [Google Scholar]
- 19. Paterson DH, Warburton DE. Physical activity and functional limitations in older adults: a systematic review related to Canada's Physical Activity Guidelines. Int J Behav Nutr Phys Act 2010; 7: 38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Karvinen KH, Courneya KS, North S, Venner P. Associations between exercise and quality of life in bladder cancer survivors: a population‐based study. Cancer Epidemiol Biomarkers Prev 2007; 16: 984–90 [DOI] [PubMed] [Google Scholar]
- 21. Colley RC, Butler G, Garriguet D, Prince SA, Roberts KC. Comparison of self‐reported and accelerometer‐measured physical activity in Canadian adults. Health Rep 2018; 29: 3–15 [PubMed] [Google Scholar]
- 22. Longo TA, Gopalakrishna A, Fantony JJ, Inman BA. Effect of diet on bladder cancer survivors. J Clin Oncol 2016; 34: 437–437 [Google Scholar]
- 23. Nicklett EJ, Kadell AR. Fruit and vegetable intake among older adults: a scoping review. Maturitas 2013; 75: 305–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jochems SH, Van Osch FH, Bryan RT et al. Impact of dietary patterns and the main food groups on mortality and recurrence in cancer survivors: a systematic review of current epidemiological literature. BMJ Open 2018; 8: e014530. DOI: 10.1136/bmjopen‐2016‐014530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gopalakrishna A, Chang A, Longo TA et al. Dietary patterns and health‐related quality of life in bladder cancer survivors. Urol Oncol 2018; 36: 469.e21–9 [DOI] [PubMed] [Google Scholar]
- 26. Demark‐Wahnefried W, Rogers LQ, Alfano CM et al. Practical clinical interventions for diet, physical activity, and weight control in cancer survivors. CA Cancer J Clin 2015; 65: 167–89. [DOI] [PubMed] [Google Scholar]
- 27. Canada S. Body composition of adults, 2012 to 2013. Health Fact Sheets 2014.
- 28. Chen X, Lu W, Gu K et al. Weight change and its correlates among breast cancer survivors. Nutr Cancer 2011; 63: 538–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fader AN, Frasure HE, Gil KM, Berger NA, von Gruenigen VE. Quality of life in endometrial cancer survivors: what does obesity have to do with it? Obstet Gynecol Int 2011; 2011: 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Westhoff E, Witjes JA, Fleshner NE et al. Body mass index, diet‐related factors, and bladder cancer prognosis: a systematic review and meta‐analysis. Bladder Cancer 2018; 4: 91–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rink M, Crivelli JJ, Shariat SF, Chun FK, Messing EM, Soloway MS. Smoking and bladder cancer: a systematic review of risk and outcomes. Eur Uro Focus 2015; 1: 17–27 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Health behaviours by disease type.
Table S2. Health behaviours by cancer journey.
Appendix S1. Health behaviours questionnaire package.


