Abstract
Background
Increased resting energy expenditure (REE) has been hypothesized to be a potential cause of weight loss in individuals with Crohn's disease (CD). This study aimed to develop and validate new predictive equations for estimating REE in adults with CD.
Methods
Adults, ages 18–65 years, with CD were recruited. Anthropometry, indirect calorimetry, and bioimpedance analysis were performed in all patients. Disease activity was assessed by Crohn's Disease Activity Index. The new predictive equations were generated using different regression models. Prediction accuracy of the new equations was assessed and compared with the most commonly used equations.
Results
A total of 270 CD patients (159 males, 111 females) were included and randomly assigned to the calibration (n = 180) and validation groups (n = 90). REE was directly correlated with weight and bioimpedance index, whereas the relation with both age and disease activity was inverse. The new equations were suitable for estimating REE at population level (bias: −0.2 and −0.3, respectively). Individual accuracy was good in both models (≥80%, respectively), especially in females; and similar results were shown by some of the selected equations. But, when accuracy was set within ±5%, the new equations gave the highest prediction.
Conclusion
The new, disease‐specific, equations for predicting REE in individuals with CD give a good prediction accuracy as far as those proposed in the literature for the general population. However, the new ones performed better at the individual level. Further studies are needed to verify the reliability and usefulness of these new equations.
Keywords: accuracy, Crohn's disease, dietary advice, energy expenditure, predictive equations
Clinical Relevancy Statement
Variations in resting energy expenditure (REE) can be a potential cause of weight loss in patients with Crohn's disease (CD); hence, an accurate estimation of REE is crucial for assessing their energy needs. Indirect calorimetry is recognized as a criterion method for measuring REE, but it is relatively time‐expensive and frequently not available in the clinical setting. Alternatively, REE can be easily and quickly estimated by predictive equations. Thus, this study aims to develop and validate new predictive equations in adult patients with CD, since no specific formula for predicting REE exists in literature.
Introduction
Crohn's disease (CD) is a chronic relapsing, inflammatory condition of the gastrointestinal tract with unpredictable course.1, 2, 3 In patients with CD, disease symptoms like diarrhea, abdominal pain, anorexia, and malabsorption together with increased resting energy expenditure (REE), possibly related to inflammatory response, can be assumed as potential causes of weight loss and secondary malnutrition.4
To date, however, inconsistent evidence has emerged on the relationships between REE and disease activity in patients with CD,4, 5 since comparisons among studies are hampered by differences in data reporting. Sasaki et al6 found that REE measured by indirect calorimetry (IC) was higher in CD patients with moderate disease activity compared with healthy controls. Similarly, Kushner et al7 observed that measured REE (MREE) increased with increasing disease activity. On the contrary, Stokes et al8 showed a mean REE value of 33 kcal/kg/d in patients with CD, which was similar to that observed for healthy subjects even when clinically active patients were included.9 Still, adjusting REE per unit of lean mass (REE/kg), it was found to be higher10 or not different11 in comparison with controls.
The assessment of REE provides basic information for energy requirements in both health and disease statuses.12 Currently, IC is recognized as a criterion method for measuring REE, but it is relatively time‐expensive and frequently not available in the clinical setting. Hence, REE is often estimated by predictive equations, which includes variables that can be easily collected, such as age, stature, and body weight. Among patients with CD, predicted REE (PREE) by equations based on anthropometric variables, for instance the Harris and Benedict (HB) equation, showed lower6 or similar results4, 10 compared with MREE, unrelated to disease activity. However, these results could be affected by small sample size, differences in study population, or inadequate evaluation of disease activity.5
From a practical point of view, bioimpedance analysis (BIA) is a commonly used tool for body‐composition assessment in the clinical setting13 that might be valuable in estimating REE, since the major determinant of REE is fat‐free mass (FFM). However, the interpretation of BIA results is highly dependent on the equations used to estimate FFM.14, 15 Alternatively, raw BIA data, such as bioimpedance index (BI‐Index) and phase angle (PhA), might be taken into consideration for evaluating the relationship of FFM with energy expenditure.16, 17
To our knowledge, previous studies have not proposed specific predictive equations for estimating REE in individuals with CD. Therefore, the primary aim of the present study was to develop and validate predictive equations for patients with CD, using both anthropometric and raw bioimpedance variables as predictors. As an additional aim, we assessed the accuracy of selected predictive equations of REE (for general population) and compared them with the formulas developed in this paper.
Materials and Methods
Patients and Study Design
Inclusion criteria consisted of the following: male and female adult patients, ages 18–65 years, with a diagnosis of CD. Exclusion criteria were untreated dysthyroidism and type 2 diabetes mellitus; use of corticosteroids in the last 3 months; history of acute or chronic liver or kidney disease; current enteral and parenteral nutrition; presence of fistulae, ileostomy, or colostomy; presence of extensive small‐bowel resections (residual small bowel of <2 m); pregnancy or lactation; unstable body weight in the last month; and inability or unwillingness to give informed consent.
Disease activity was clinically defined using the Crohn's Disease Activity Index (CDAI), classifying patients in the active and quiescent phases (≥150 and <150, respectively).18
The present study was a retrospective analysis of data collected between 2005 and 2018 from patients undergoing procedures to evaluate nutrition status at the Department of Clinical Medicine and Surgery, Federico II University Hospital, in Naples, Italy. The study was conducted in accordance with the Declaration of Helsinki and received the approval of the Ethical Committee of Federico II University Hospital. Informed consent was obtained from all patients recruited.
All measurements were performed early in the morning after a fasting period of 8–10 hours, according to standardized conditions—abstention from alcohol, smoking, and vigorous physical activity for 24 hours prior to the assessment. As previously reported,16 smoking was not allowed for occasional and current smokers on the morning of the test until the end of measurements; however, current smokers were asked to maintain their smoking habits on the day before. Data were excluded from analysis if the respiratory quotient (RQ) was outside the expected range (0.71–1.00) and when MREE was ±3 SDs outside the mean REE.
Anthropometry and Bioimpedance Analysis
Body weight and stature were measured to the nearest 0.1 kg and 0.5 cm, respectively. Measurements were taken while the subject wore light clothes and no shoes, using a platform beam scale with a built‐in stadiometer (Seca 709, Seca, Hamburg Germany). Body mass index (BMI) (kg/m²) was calculated as body weight divided by squared stature.
BIA15 was performed at 50 kHz (Human Im Plus II, DS Medica, Milan, Italy) at room temperature (22°C–25°C). Measurements were carried out on the nondominant side of the body, in the postabsorptive state, after voiding, and with the participant in the supine position for 20 minutes. The BIA variables considered (data produced by the device) were resistance (R), reactance, and PhA. BI‐index was calculated as the ratio stature²/resistance (cm²/ohm).
Measurements of Resting Energy Expenditure
REE was measured by IC19 using a canopy system, V max29 (Sensor Medics, Anaheim, CA, USA). The instrument was routinely checked by burning ethanol, whereas oxygen and carbon dioxide analyzers were calibrated on the test day using nitrogen and standardized gases (mixtures of nitrogen, carbon dioxide, and oxygen).
Measurement conditions for IC were defined following the suggestions made by Compher et al20 and Fullmer et al.21 REE was assessed at an ambient temperature of 22°C–25°C and, in fertile women, during the follicular phase to avoid any potential effects of the menstrual cycle. Participants lay down on a bed in a quiet environment for a 15‐minute adaptation period. Afterward, oxygen consumption and carbon dioxide production were measured for 45 minutes, discarding the first 5 minutes. Energy expenditure was calculated using the abbreviated Weir formula, neglecting protein oxidation.22
Predictive Equations
REE was also estimated using the following predictive equations: HB,23 Food and Agriculture Organization (FAO),24 Schofield,25 Owen,26, 27 Muller,28 and De Lorenzo.29 Thus, accuracy of the new predictive equations at the population and individual levels was calculated and then compared with those equations.
Statistical Analysis
Statistical analyses were performed using IBM SPSS (version 24, Chicago, IL, USA). All data are presented as mean ± SD, unless otherwise specified, and significance was defined as P < .05. As highlighted in Tables 1 and 2, participants were randomly assigned to a calibration or a validation subset in a way that the ratio between them remained constant.16 To examine whether variables were normally distributed, the Kolmogorov‐Smirnov test and the Shapiro‐Wilk test were used.
Table 1.
Characteristics of the Study Sample for the Calibration Group
| Males (n = 104) | Females (n = 76) | All (n = 180) | |
|---|---|---|---|
| Age, y | 37.7 ± 12.8 | 38.1 ± 14.0 | 37.9 ± 13.3 |
| Weight, kg | 66.6 ± 10.5a | 55.4 ± 9.0 | 61.8 ± 11.3 |
| Stature, cm | 171.9 ± 6.2a | 159.7 ± 6.0 | 166.7 ± 8.6 |
| BMI, kg/m2 | 22.5 ± 3.4 | 21.7 ± 3.4 | 22.2 ± 3.4 |
| BI‐Index, cm²/Ω | 58.5 ± 8.0a | 41.6 ± 6.4 | 51.4 ± 11.1 |
| PhA (degrees) | 6.7 ± 0.9a | 5.6 ± 0.7 | 6.3 ± 1.0 |
| MREE, kcal/d | 1641 ± 195a | 1340 ± 162 | 1514 ± 235 |
| RQ | 0.816 ± 0.076 | 0.812 ± 0.081 | 0.814 ± 0.078 |
Data are expressed as mean ± SD.
BI‐Index, bioimpedance index; BMI, body mass index; MREE, measured resting energy expenditure; PhA, phase angle; RQ, respiratory quotient.
P < .05 between sexes.
Table 2.
Characteristics of the Study Sample for the Validation Group
| Males (n = 55) | Females (n = 35) | All (n = 90) | |
|---|---|---|---|
| Age, y | 37.9 ± 12.5 | 39.5 ± 15.1 | 38.5 ± 13.5 |
| Weight, kg | 68.8 ± 11.4a | 55.9 ± 10.6 | 63.8 ± 12.7 |
| Stature, cm | 172.0 ± 6.1a | 159.4 ± 5.6 | 167.1 ± 8.5 |
| BMI, kg/m2 | 23.2 ± 3.7 | 21.9 ± 3.7 | 22.7 ± 3.7 |
| BI‐Index, cm²/Ω | 60.0 ± 7.7a | 41.8 ± 6.9 | 53.0 ± 11.6 |
| PhA (degrees) | 6.7 ± 0.9a | 5.6 ± 0.6 | 6.3 ± 1.0 |
| MREE, kcal/d | 1679 ± 182a | 1360 ± 151 | 1555 ± 231.1 |
| RQ | 0.810 ± 0.075 | 0.790 ± 0.075 | 0.802 ± 0.075 |
Data are expressed as mean ± SD.
BI‐Index, bioimpedance index; BMI, body mass index; MREE, measured resting energy expenditure; PhA, phase angle; RQ, respiratory quotient.
P < .05 between sexes.
Data were compared between genders using unpaired t‐tests, whereas linear correlation was applied for evaluating associations between variables. Multivariate linear regression analysis was performed to develop the new predictive equations, with REE measured by IC as the dependent variable. We generated models as follows: in Model 1, age, sex, weight, stature, and CDAI were set as predictors, whereas in Model 2, we added the raw BIA variables (BI‐Index and PhA). Coefficient of determination (R²) and standard error of the estimate (SEE) were considered for assessing the predictive power of formulas. The regression equations derived from the calibration subset were applied to the validation subset.
Mean difference between PREE and MREE, as well as bias (that is, the average percent difference), were both used as a measure of accuracy at the group level. Bias was found acceptable if within ±5%.30, 31 Concurrently, the percentage of patients with a PREE within 90%–110% and the percentage of patients with an MREE within 95%–105% were both used as measures of accuracy at the individual level. According to the range, values lower than 90% and 95% were classified as underprediction, whereas values higher than 105% and 110% were classified as overprediction. The root mean squared error (RMSE) was used to define the predictions obtained with these models. Finally, comparisons of PREE‐MREE differences vs mean PREE‐MREE values were performed by Bland‐Altman plots to estimate the limits of agreement.32
Results
Two hundred eighty‐four patients with CD were selected for this study. Ten were excluded for not fulfilling the inclusion criteria, and 4 were excluded for taking corticosteroids. Therefore, a total of 270 patients with CD (159 males and 111 females) were included in the analysis (Supplementary material). Anthropometric, raw BIA variables, and MREE data are summarized for the calibration and validation groups in Tables 1 and 2, respectively. Age, BMI, and RQ did not vary between genders, whereas body weight, stature, MREE, and raw BIA variables significantly differed. Thirty‐six percent of patients were in active phase (CDAI 225 ± 58; median = 215, min‐max = 152–472), whereas 64% were in quiescent phase (CDAI 71 ± 41; median = 77, min‐max = 2–140). The proportion of patients in the active phase was similar in males and females.
Pearson linear correlation showed that in patients with CD, MREE was directly correlated with individual characteristics and raw BIA variables except for age and CDAI, which displayed an inverse relation. Overall, BI‐Index had the strongest correlation with MREE (r = .762, P < .001), followed by body weight (r = 0.733, P < .001), stature (r = 0.681, P < .001), and PhA (r = 0.464, P < .001). On the contrary, neither age (r = −0.102, P = .175) nor CDAI (r = −0.113, P = .165) was significantly correlated with MREE.
Next, multiple regression analysis was performed to assess the relationship between MREE and various combinations of potential predictors. Age, basic anthropometric measures (stature, weight, and BMI), and CDAI were considered first (Model 1) to create the following equation:
| (1) |
When raw BIA variables were added to the model (Model 2), BI‐Index and PhA were both included in the equation:
| (2) |
Validation of the New Predictive Equations
To evaluate the accuracy of the new predictive equations, 90 individuals with CD (55 males and 35 females) were randomly assigned to the validation group, using the statistical software. Prediction accuracy at the group level (assessed by the difference between PREE and MREE, percent bias, and the RMSE in kcal/d) was reported for the new equations and selected equations from the literature in the Table 3. We found that the newly developed predictive equations were accurate at the group level in both sexes, since mean bias was <1% (Equation (1): −0.2% and Equation (2): −0.3% in males; Equation (1): −1% and Equation (2): −0.7% in females). When REE was predicted using selected equations from the literature, bias was acceptable for the HB (−3.0%), FAO (−0.1%), Schofield (0.2%), and De Lorenzo equations (−2.0%) in males and for the HB (−2.7%), FAO (−2.8%), and Schofield equations (−4.4%) in females.
Table 3.
Evaluation of New and Selected Predictive Equations in Crohn's Disease Patients, According to Sex
| Males (n = 55) | Females (n = 35) | |||||
|---|---|---|---|---|---|---|
| Difference PREE‐MREE, kcal/d | Biasa | Difference PREE‐MREE, kcal/d | Biasa | |||
| REE predictive equations | Mean (SD) | % | RMSE, kcal/d | Mean (SD) | % | RMSE, kcal/d |
| Equation (1) | −12 (123) | −0.2 | 93 | −19 (98) | −1 | 80 |
| Equation (2), b | −13 (117) | −0.3 | 88 | −15 (92) | −0.7 | 69 |
| HB | −58 (129) | −3.0 | 111 | −43 (109) | −2.7 | 96 |
| FAO | −11 (127) | −0.1 | 101 | −47 (113) | −2.8 | 99 |
| De Lorenzo | −41 (128) | −2.0 | 104 | −74 (109) | −5.1 | 109 |
| Schofield | −5 (128) | 0.2 | 102 | −60 (115) | −3.8 | 105 |
| Muller | 170 (145) | 11 | 189 | 150 (117) | 12 | 160 |
| Owen | −98 (133) | −5.2 | 135 | −164 (110) | −11.4 | 166 |
HB, Harris and Benedict; FAO, Food and Agriculture Organization; MREE, measured resting energy expenditure; PREE, predicted resting energy expenditure; REE, resting energy expenditure; RMSE, root mean square error.
Mean percentage error between predicted and measured REE.
Including bioimpedance index and phase angle.
As far as the accuracy at the individual level is concerned, the percentage of participants with a PREE within ±10% of MREE is presented for the new and other predictive equations in Figure 1. The new equations gave the highest accuracy in males (Equation (1): 80%; Equation (2): 82%) and females (Equation (1): 80%; Equation (2): 83%). The HB, FAO, Schofield, and De Lorenzo equations were also accurate (≈80%) in male patients, whereas the HB (74%) and FAO (80%) equations performed well in female patients.
Figure 1.
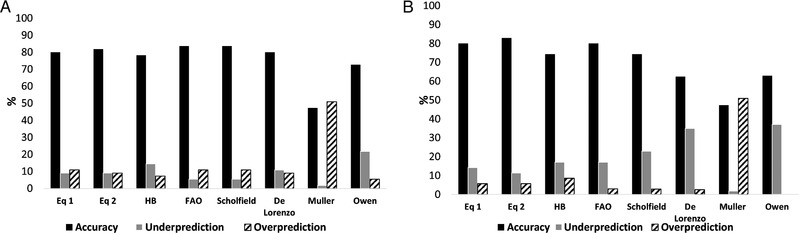
Prediction accuracy within ±10%. Accuracy of prediction equations for measurements of resting energy expenditure within ±10% using each equation in 55 males and 35 females with Crohn's disease (A and B), respectively. HB, Harris and Benedict; FAO, Food and Agriculture Organization; Eq 1, equation generated by Model 1; Eq 2, equation generated by Model 2.
Conversely, by setting the accuracy range within ±5% of MREE, all equations considered showed low prediction accuracy (<50%) at the individual level in both genders, as presented in Figure 2. Specifically, most of them tended to underpredict REE, except for Muller equation, which overpredicted REE. On the contrary, the new equations provided the best accuracy in males (Equation (1): 58%; Equation (2): 64%), whereas the one including raw BIA variables gave the highest prediction in females (Equation (2): 63%).
Figure 2.
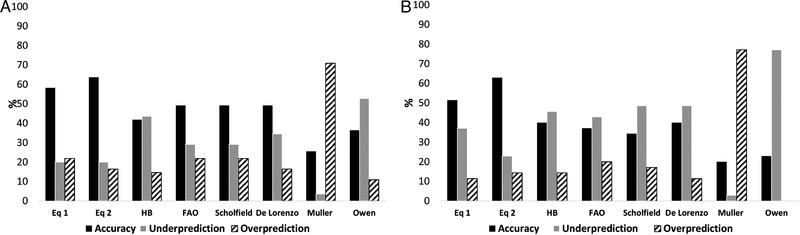
Prediction accuracy within ±5%. Accuracy of prediction equations for measurements of resting energy expenditure within ±5% using each equation in 55 males and 35 females with Crohn's disease (A and B), respectively. HB, Harris and Benedict; FAO, Food and Agriculture Organization; Eq 1, equation generated by Model 1; Eq 2, equation generated by Model 2.
Bland‐Altman Plots of PREE‐MREE Differences
Finally, the Bland‐Altman plots of PREE‐MREE differences vs mean PREE‐MREE values were shown in Figure 3 for the new equations, since those selected plots highlight the best agreement.
Figure 3.
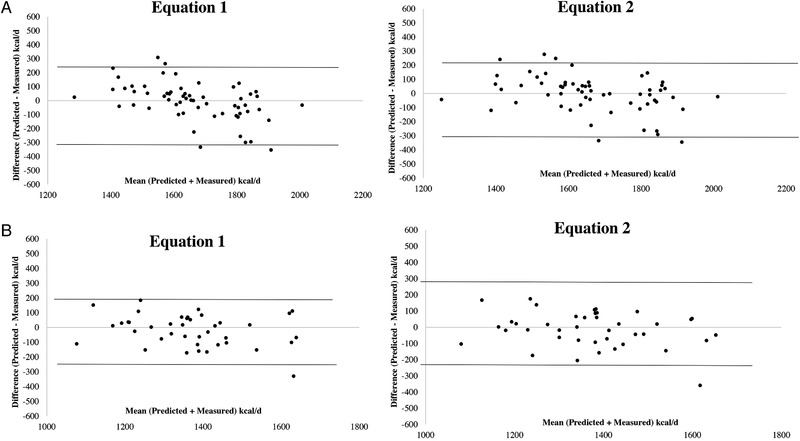
Bland‐Altman plots. Bland‐Altman plots between differences and mean predicted‐measured resting energy expenditure using the new predictive equations (Equations (1) and (2)) in 55 males (A) and in females (B) with Crohn's disease. The dotted lines represent 2 SDs from the mean (limits of agreement).
Discussion
This study aimed to develop and cross‐validate, in individuals with CD, specific predictive equations for estimating REE and to explore the relationships of REE with age, disease activity,and main anthropometric and raw BIA variables. Our results showed that REE is largely predicted by BI‐Index and body weight. The new equations have a good prediction power, providing by far the best results when accuracy was set within ±5%.
The role of increased REE in the worsening of nutrition status in patients with CD5 is still unclear. So far, only a few studies have evaluated the use of predictive equations of REE in participants with CD, showing contrasting results. Three papers have given results on the HB equation, whereas no data are available on other predictive equations proposed in the literature for the general population. Barot et al33 did not find any significant difference between MREE and PREE in 9 CD patients undergoing nutrition supplementation. Similarly, Chan et al4 showed that REE measured by IC in 54 CD patients did not significantly differ from PREE. On the contrary, Stokes and Hill8 found that MREE was 14% higher than that predicted. The great variability among those studies can be explained by different inclusion criteria, small sample size, and several methodological shortfalls.8, 33
Theoretically, disease‐specific equations could ensure a better predictive accuracy. Hence, in the present study, we developed new predictive equations based on age and basic anthropometric parameters (age, weight, stature, and BMI) (Model 1) or also including raw BIA variables in the model (Model 2). Our results showed that weight and BI‐Index were the best predictors of REE, whereas (not surprisingly) we found an inverse association between REE and age. In the calibration groups, the new formula based on individual parameters (Model 1) led to similar SEEs in the 2 genders.
We have opted for including raw BIA variables in the regression model. Previously, we found that both BI‐Index and PhA can estimate REE in individuals with obesity16 and anorexia nervosa17; and so they are expected to be potential predictors of REE in patients with CD as well. Moreover, we recently found that BIA‐derived PhA is a valid indicator of nutrition status in these patients, and its values were impaired with increasing disease activity.34 First, we evaluated predictive equations of REE including raw BIA variables alone (R² = 0.614; SEE: 147 kcal/d) or in combination with age (R² = 0.624, SEE: 145 kcal/d), but the prediction power was lower compared with those including age and basic anthropometric variables. Secondly, we included age and basic anthropometric variables plus raw BIA variables in the model, finding an increase of R² and a decrease in SEE values. In such a case, both sex and age were not identified as significant predictors, as occurred in both models also for disease activity (assessed by CDAI). However, 64% of patients recruited were in clinical remission, whereas those clinically active showed from mild to moderate disease activity, likely without having any influence on REE prediction. Nowadays, although CDAI is easy to get, simple, and applicable to large populations, it is no longer the “gold standard” tool for assessing disease severity among patients with CD. Alternatively, the use of the endoscopy‐based scores, which address mucosal healing, might be more suitable for classifying disease severity, with potential effects on REE prediction, but this option needs to be further investigated.
In the validation group, the accuracy at the population level was very good for our equations, since it ranged within ±1%, and the accuracy at individual level (within ±10%) was also reasonable with equivalent figures in the 2 models (≈80%). When we set the accuracy range within ±5%, we found that the new equations gave by far the highest accuracy (Equation (1): ≈60% in males and ≈51% in females; Equation (2): ≈64% in males and ≈63% in females).
On the contrary, the bias at the population level was similarly within ±5% for the HB, FAO, and Schofield equations in both genders, confirming previous results on the use of the HB equation in CD.4, 33 At the individual level, accuracy within ±10% was good and close to 80% for different equations, whereas, by setting the range within ±5%, prediction accuracy sharply decreased to <50% in both genders. The choice of analyzing and reporting prediction accuracy within ±5% will be useful for providing specific equations that are able to enhance REE prediction in the clinical setting. Surprisingly, we noted that the inclusion of raw BIA variables (Equation (2)) has slightly improved REE prediction compared with the equation based on anthropometric variables (Equation (1)); but, unfortunately, BIA is often not used or available in clinical settings. However, in the absence of BIA, we can reasonably opt for Equation (1), since its accuracy achieved almost 60% in males, which is good, and gave the best result (>51%) among females, being higher than other available equations based on anthropometric variables.
To the best of our knowledge, this is the first study that develops and cross‐validates disease‐specific equations to predict REE in participants with CD, also considering raw BIA variables as potential predictors. Overall, we performed a cross‐sectional study in a reasonable sample of individuals, using known and documented methods and in line with previous studies that derived predictive equations for REE in healthy as well as ill participants. However, some limitations need to be considered. Firstly, as this is a single‐center study including adult patients with CD showing from mild to moderate disease activity, our findings need to be substantiated in other clinical subgroups or in different clinical settings. Secondly, although conventionally accepted, the use of CDAI might not be the best choice for defining disease activity because it is based on subjective criteria symptoms, resulting in a measure of severity of illness rather than of mucosal inflammatory activity.
In conclusion, the new, disease‐specific equations proposed here to predict REE in individuals with CD give a good prediction accuracy at population level. Raw BIA variables are significant predictors of REE, but their inclusion in the model improves the prediction power by only a small extent. The new, disease‐specific equations ensure a good accuracy also at the individual level and perform much better than the equations proposed in the literature for the general population. Further studies are needed to verify the reliability and usefulness of these new equations and to explore their role in estimating REE in clinically active patients.
Statement of Authorship
M. Marra and I. Cioffi equally contributed to the conception and design of the research; F. Pasanisi contributed to the design of the research; O. Di Vincenzo, D. Morlino, I. Cioffi, N. Imperatore, M. C. Pagano, L. Alfonsi, L. Santarpia, and F. Castiglione contributed to the acquisition of the data; M. Marra contributed to the analysis and interpretation of the data; I. Cioffi and L. Scalfi drafted the manuscript. All authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
Acknowledgments
We gratefully acknowledge the hospital staff for their assistance during the study, all participants enrolled, and the Italian Society of Parenteral and Enteral Nutrition (SINPE, Società Italiana di Nutrizione Artificiale e Metabolismo), which provided a young investigator research grant to Dr Iolanda Cioffi for the project, “Valutazione del dispendio energetico in pazienti con malattia di Crohn (assessment of resting energy expenditure in patients with Crohn's disease).”
Financial disclosure: None declared.
Conflicts of interest: None declared.
References
- 1. Castiglione F, Imperatore N, Testa A, et al. One‐year clinical outcomes with biologics in Crohn's disease: transmural healing compared with mucosal or no healing. Aliment Pharmacol Ther. 2019;49(8):1026‐1039. [DOI] [PubMed] [Google Scholar]
- 2. Rispo A, Imperatore N, Testa A, et al. Bowel damage in Crohn's disease: direct comparison of ultrasonography‐based and magnetic resonance‐based Lemann index. Inflamm Bowel Dis. 2017;23(1):143‐151. [DOI] [PubMed] [Google Scholar]
- 3. Torres J, Mehandru S, Colombel J‐F, Peyrin‐Biroulet L. Crohn's disease. Lancet Lond Engl. 2017;389(10080):1741‐1755. [DOI] [PubMed] [Google Scholar]
- 4. Chan ATH, Fleming CR, O'Fallon WM, Huizenga KA. Estimated versus measured basal energy requirements in patients with Crohn's disease. Gastroenterology. 1986;91(1):75‐78. [DOI] [PubMed] [Google Scholar]
- 5. Forbes A, Escher J, Hébuterne X, et al. ESPEN guideline: clinical nutrition in inflammatory bowel disease. Clin Nutr. 2017;36(2):321‐347. [DOI] [PubMed] [Google Scholar]
- 6. Sasaki M, Johtatsu T, Kurihara M, et al. Energy metabolism in Japanese patients with Crohn's disease. J Clin Biochem Nutr. 2010;46(1):68‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kushner RF, Schoeller DA. Resting and total energy expenditure in patients with inflammatory bowel disease. Am J Clin Nutr. 1991;53(1):161‐165. [DOI] [PubMed] [Google Scholar]
- 8. Stokes MA, Hill GL. Total energy expenditure in patients with Crohn's disease: measurement by the combined body scan technique. J Parenter Enter Nutr. 1993;17(1):3‐7. [DOI] [PubMed] [Google Scholar]
- 9. Schneeweiss B, Lochs H, Zauner C, et al. Energy and substrate metabolism in patients with active Crohn's disease. J Nutr. 1999;129(4):844‐848. [DOI] [PubMed] [Google Scholar]
- 10. Capristo E, Addolorato G, Mingrone G, Greco AV, Gasbarrini G. Effect of disease localization on the anthropometric and metabolic features of Crohn's disease. Am J Gastroenterol. 1998;93(12):2411‐2419. [DOI] [PubMed] [Google Scholar]
- 11. Zoli G, Katelaris PH, Garrow J, Gasbarrini G, Farthing MJ. Increased energy expenditure in growing adolescents with Crohn's disease. Dig Dis Sci. 1996;41(9):1754‐1759. [DOI] [PubMed] [Google Scholar]
- 12. Pinheiro Volp AC, Esteves de Oliveira FC, Duarte Moreira Alves R, Esteves EA, Bressan J. Energy expenditure: components and evaluation methods. Nutr Hosp. 2011;26(3):430‐440. [DOI] [PubMed] [Google Scholar]
- 13. Day K, Kwok A, Evans A, et al. Comparison of a bioelectrical impedance device against the reference method dual energy X‐ray absorptiometry and anthropometry for the evaluation of body composition in adults. Nutrients. 2018;10(10):1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Das SK. Body composition measurement in severe obesity. Curr Opin Clin Nutr Metab Care. 2005;8(6):602‐606. [DOI] [PubMed] [Google Scholar]
- 15. Kyle UG, Bosaeus I, De Lorenzo AD, et al. Bioelectrical impedance analysis‐part II: utilization in clinical practice. Clin Nutr Edinb Scotl. 2004;23(6):1430‐1453. [DOI] [PubMed] [Google Scholar]
- 16. Marra M, Cioffi I, Sammarco R, et al. Are raw BIA variables useful for predicting resting energy expenditure in adults with obesity? Nutrients. 2019;11(2). 10.3390/nu11020216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marra M, Sammarco R, De Filippo E, et al. Prediction of body composition in anorexia nervosa: results from a retrospective study. Clin Nutr Edinb Scotl. 2018;37(5):1670‐1674. [DOI] [PubMed] [Google Scholar]
- 18. Rispo A, Imperatore N, Testa A, et al. Combined endoscopic/sonographic‐based risk matrix model for predicting one‐year risk of surgery: a prospective observational study of a tertiary centre severe/refractory Crohn's disease cohort. J Crohns Colitis. 2018;12(7):784‐793. [DOI] [PubMed] [Google Scholar]
- 19. Elia M, Livesey G. Theory and validity of indirect calorimetry during net lipid synthesis. Am J Clin Nutr. 1988;47(4):591‐607. [DOI] [PubMed] [Google Scholar]
- 20. Compher C, Frankenfield D, Keim N, Roth‐Yousey L; Evidence Analysis Working Group . Best practice methods to apply to measurement of resting metabolic rate in adults: a systematic review. J Am Diet Assoc. 2006;106(6):881‐903. [DOI] [PubMed] [Google Scholar]
- 21. Fullmer S, Benson‐Davies S, Earthman CP, et al. Evidence analysis library review of best practices for performing indirect calorimetry in healthy and non–critically ill individuals. J Acad Nutr Diet. 2015;115(9):1417‐1446.e2. [DOI] [PubMed] [Google Scholar]
- 22. Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. 1949. Nutr Burbank Los Angel Cty Calif. 1990;6(3):213‐221. [PubMed] [Google Scholar]
- 23. Harris JA, Benedict FG. A biometric study of human basal metabolism. Proc Natl Acad Sci U S A. 1918;4(12):370‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Energy and protein requirements. Report of a joint FAO/WHO/UNU expert consultation. World Health Organ Tech Rep Ser. 1985;724:1‐206. [PubMed] [Google Scholar]
- 25. Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr. 1985;39(suppl 1):5‐41. [PubMed] [Google Scholar]
- 26. Owen OE, Kavle E, Owen RS, et al. A reappraisal of caloric requirements in healthy women. Am J Clin Nutr. 1986;44(1):1‐19. [DOI] [PubMed] [Google Scholar]
- 27. Owen OE, Holup JL, D'Alessio DA, et al. A reappraisal of the caloric requirements of men. Am J Clin Nutr. 1987;46(6):875‐885. [DOI] [PubMed] [Google Scholar]
- 28. Müller MJ, Bosy‐Westphal A, Klaus S, et al. World Health Organization equations have shortcomings for predicting resting energy expenditure in persons from a modern, affluent population: generation of a new reference standard from a retrospective analysis of a German database of resting energy expenditure. Am J Clin Nutr. 2004;80(5):1379‐1390. [DOI] [PubMed] [Google Scholar]
- 29. De Lorenzo A, Di Renzo L, Morini P, de Miranda RC, Romano L, Colica C. New equations to estimate resting energy expenditure in obese adults from body composition. Acta Diabetol. 2018;55(1):59‐66. [DOI] [PubMed] [Google Scholar]
- 30. Frankenfield D, Roth‐Yousey L, Compher C. Comparison of predictive equations for resting metabolic rate in healthy nonobese and obese adults: a systematic review. J Am Diet Assoc. 2005;105(5):775‐789. [DOI] [PubMed] [Google Scholar]
- 31. Frankenfield DC, Rowe WA, Smith JS, Cooney RN. Validation of several established equations for resting metabolic rate in obese and nonobese people. J Am Diet Assoc. 2003;103(9):1152‐1159. [DOI] [PubMed] [Google Scholar]
- 32. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet Lond Engl. 1986;1(8476):307‐310. [PubMed] [Google Scholar]
- 33. Barot LR, Rombeau JL, Feurer ID, Mullen JL. Caloric requirements in patients with inflammatory bowel disease. Ann Surg. 1982;195(2):214‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cioffi I, Marra M, Imperatore N, et al. Assessment of bioelectrical phase angle as a predictor of nutritional status in patients with Crohn's disease: a cross sectional study [published online July 4, 2019]. Clin Nutr. In press. [DOI] [PubMed] [Google Scholar]


