Abstract
Aim
To investigate the efficacy and safety of evogliptin compared with linagliptin in patients with type 2 diabetes.
Materials and Methods
In this 12‐week, multicentre, randomized, double‐blind, active‐controlled, and 12‐week open‐label extension study, a total of 207 patients with type 2 diabetes who had HbA1c levels of 7.0%‐10.0% were randomized 1:1 to receive evogliptin 5 mg (n = 102) or linagliptin 5 mg (n = 105) daily for 12 weeks. The primary efficacy endpoint was the change from baseline HbA1c at week 12. The secondary endpoint was the change in the mean amplitude of glycaemic excursion (MAGE) assessed by continuous glucose monitoring. In the extension study conducted during the following 12 weeks, evogliptin 5 mg daily was administered to both groups: evogliptin/evogliptin group (n = 95) and linagliptin/evogliptin group (n = 92).
Results
After 12 weeks of treatment, the mean change in HbA1c in the evogliptin group and in the linagliptin group was −0.85% and −0.75%, respectively. The between‐group difference was −0.10% (95% CI: −0.32 to 0.11), showing non‐inferiority based on a non‐inferiority margin of 0.4%. The change in MAGE was −24.6 mg/dL in the evogliptin group and −16.7 mg/dL in the linagliptin group. These values were significantly lower than the baseline values in both groups. However, they did not differ significantly between the two groups. In the evogliptin/evogliptin group at week 24, HbA1c decreased by −0.94%, with HbA1c values of <7.0% in 80.2% of the patients. The incidence and types of adverse events were comparable between the two groups for 24 weeks.
Conclusion
In this study, the glucose‐lowering efficacy of evogliptin was non‐inferior to linagliptin. It was maintained at week 24 with a 0.94% reduction in HbA1c. Evogliptin therapy improved glycaemic variability without causing any serious adverse events in patients with type 2 diabetes.
Keywords: continuous glucose monitoringdipeptidyl peptidase‐4 inhibitorevogliptinglycaemic variabilitylinagliptintype 2 diabetes
1. INTRODUCTION
Glycaemic control in patients with diabetes has been regarded as controlling HbA1c to lower the average glucose level. 1 However, HbA1c itself does not reflect interpersonal differences in glucose levels, and poorly predicts the risk of hypoglycaemia, which is the main impediment to intensive treatment for diabetes. 2 Thus far, not only lowering HbA1c levels, but also reducing glycaemic variability, which is derived from treatment‐related hypoglycaemia and postprandial hyperglycaemia, have become major therapeutic goals in diabetes management. 3 Continuous glucose monitoring (CGM) is now regarded as an accurate method for the assessment of glycaemic variability. 4 Several studies have shown that increased glycaemic variability assessed by CGM was associated with diabetic complications, such as diabetic retinopathy, cardiovascular complications and mortality. 5 , 6 , 7 Moreover, increased glycaemic variability is closely associated with oxidative stress, which can result in the development of cardiovascular disease. 8 , 9
Dipeptidyl peptidase‐4 (DPP‐4) inhibitors are widely used for patients with type 2 diabetes (T2D), mostly as a second‐line treatment after metformin treatment or as a first‐line treatment in patients who cannot tolerate metformin. 10 , 11 Incretin‐based DPP‐4 inhibitors are associated with a low risk of hypoglycaemia. They can improve β‐cell function with a neutral effect on body weight. In addition, they have significant and clinically meaningful glucose‐lowering effects. 12 A previous study reported that DPP‐4 inhibitors were associated with fewer fluctuations in blood glucose levels in both hypoglycaemic and hyperglycaemic status in a glucose‐dependent manner. 13 Evogliptin is a selective DPP‐4 inhibitor, forming interactions with the S2‐extensive subsite of the DPP‐4 active site. 14 Evogliptin was shown to inhibit >80% of the plasma DPP‐4 activity within 60 minutes and the inhibitory effect was sustained over 24 hours. 15 Evogliptin increased postprandial active glucagon‐like peptide‐1 (GLP‐1) levels by 1.5‐ to 2.4‐fold, resulting in reduced postprandial glucose levels by 20% to 35% compared with placebo. In addition, dose adjustments of evogliptin are not required in patients with renal impairment because it is not metabolized through the kidneys. 16 Evogliptin also showed beneficial effects on the kidneys, including reductions in albuminuria and the attenuation of renal fibrosis in preclinical studies. 17 , 18 , 19 Previously, several clinical trials of evogliptin have shown strong glucose‐lowering effects by reducing HbA1c levels compared with placebo 20 , 21 and sitagliptin. 22
Based on these findings, we hypothesized that evogliptin, a novel DPP‐4 inhibitor, would have potent glucose‐lowering effects by reducing glucose fluctuation and tolerability with a low risk of adverse events (AEs). Linagliptin, another DPP‐4 inhibitor, is only partially excreted by the kidneys (6%), and mostly through the bile (85%) into the gastrointestinal tract. Therefore, dose adjustments are not required for patients with renal impairment. 18 As favourable effects of linagliptin on glycaemic variability and renal outcomes, in addition to its glucose‐lowering effects, have been revealed, 23 , 24 , 25 the aim of this study was to evaluate the efficacy and safety of evogliptin compared with linagliptin as an active comparator, showing changes in HbA1c, CGM and renal variables for 12 weeks and an extension period of 12 weeks in inadequately controlled patients with T2D.
2. METHODS
This was a 12‐week, multicentre, randomized, double‐blind, active‐controlled study, with a 12‐week open‐label extension study. It was conducted at 19 different sites in Korea from September 2016 through March 2018. All participants provided written informed consent. The protocol of this study was approved by the institutional review board at each participating site. This study adhered to the tenets of the Declaration of Helsinki. It was conducted in accordance with the principles of Good Clinical Practice and was registered at ClinicalTrials.gov (NCT02974504).
2.1. Study participants
Eligible patients were aged ≥20 years, diagnosed with T2D with an HbA1c level of ≥7.0% and ≤10.0%, with a body mass index (BMI) of ≥20 and ≤40 kg/m2, and had not been prescribed any hypoglycaemic medication within the last 8 weeks. Details of the exclusion criteria are described in the Supplementary Methods (see the supporting information for this article).
2.2. Study design
This trial consisted of the following three periods: a 2‐week, single‐blind, run‐in period; a 12‐week, double‐blind, randomized, treatment period; and a 12‐week, open‐label, extension period (Figure S1). At visit 1 (week –2), the subjects who met the inclusion criteria without meeting any exclusion criteria were administered evogliptin placebo and linagliptin placebo for 14 days starting from the day of visit 1 until the day before visit 3 in a single‐blind manner. At visit 3 (week 0), the participants were assigned randomly at a 1:1 ratio, depending on whether their HbA1c was <8% or ≥8% in clinical laboratory test results performed at visit 1, to receive evogliptin 5 mg and linagliptin placebo (evogliptin group) or evogliptin placebo and linagliptin 5 mg (linagliptin group) in a double‐blind manner for 12 weeks. At visit 2 (3 days before visit 3) and visit 5 (3 days before visit 6), a CGM device (iPro2, Medtronic MiniMed, Northridge, CA, USA) was attached to the subject for 3 days. During the extension period, for 12 weeks from the day of visit 6 (week 12) to the day before visit 9 (week 24), evogliptin 5 mg was administered to both groups (evogliptin/evogliptin and linagliptin/evogliptin) in an open‐label manner. At visit 8 (3 days before visit 9), a CGM device was attached to the subject for 3 days. At visit 9 (week 24), the CGM device was removed from the subject. To ensure the safety of subjects, metformin prescription was permitted as a rescue therapy for subjects with fasting plasma glucose (FPG) values of >270 mg/dL at visits 3, 4 and 6, subjects with FPG > 240 mg/dL at visits 7 and 9, and subjects who were deemed necessary to receive a rescue drug at the investigator's discretion.
2.3. Outcomes
The primary efficacy endpoint of this study was the change in the mean level of HbA1c from baseline to 12 weeks after treatment with evogliptin or linagliptin. The secondary efficacy endpoints included the proportions of patients with HbA1c of <6.5% or <7.0% at 12 weeks, changes in FPG from baseline to 12 weeks after treatment, and the CGM variables at 12 and 24 weeks. Using the CGM system, the intra‐day variation and inter‐day variability were assessed with changes in the mean amplitude of glucose excursions (MAGE), the coefficient of variance (CV), the standard deviation (SD), and the mean of daily differences (MODD). The MAGE variable allows the estimation of major intra‐day glucose swings while minor ones are not taken into account. 26 This variable was calculated as the arithmetic average of the absolute value differences between consecutive glucose peaks and nadirs when it was greater than one SD from the mean value. 27 The CV was defined as the ratio of SD to the mean and describes the magnitude of the glucose values. The variation within them is useful for comparing the degree of variation in subjects with different mean glucose levels. 28 The MODD is a sole index for estimating the day‐to‐day glycaemic variability, which was calculated as the mean of the absolute value differences between the glucose levels measured at the same time of day on two consecutive days. 29 The details of the CGM variables are described in the Supplementary Methods (see the supporting information).
The exploratory endpoints included nitrotyrosine and 2‐thiobarbituric acid‐reactive substances (TBARS) as inflammatory and oxidative stress markers. N‐acetyl‐β‐D‐glucosaminidase (NAG), a marker of proximal tubular damage, and nephrin, a marker for podocyte injury, were also measured. Biochemical variables, including creatinine, the estimated glomerular filtration rate (eGFR), and the urinary albumin‐to‐creatinine ratio (UACR), were determined at baseline and 12 and 24 weeks after treatment. The details of the laboratory measurements are described in the Supplementary Methods (see the supporting information).
AEs, serious AEs (SAEs) and adverse drug reactions (ADRs) were recorded during the study period. Lipid variables (total cholesterol, low‐density lipoprotein [LDL] cholesterol, high‐density lipoprotein [HDL] cholesterol, and triglycerides) and liver enzymes (aspartate aminotransaminase [AST] and alanine aminotransaminase [ALT]) at baseline and 12 and 24 weeks after treatment were measured. Additional details of the safety evaluation are described in the Supplementary Methods (see the supporting information).
2.4. Statistical analysis
The target number of subjects to prove the non‐inferiority between evogliptin and linagliptin at a one‐sided significance level of 0.025 with a power of 90% was 85 per group, assuming a SD of 0.8 and a non‐inferiority margin of 0.40%. Considering a drop‐out rate of 15%, the number of subjects required for this study was estimated to be 100 per group. Therefore, this trial aimed to recruit a total of 200 subjects with the goal of allocating 100 subjects to each treatment group. The efficacy endpoints were analysed using the full analysis (FA) set population (i.e. all randomized participants were treated with at least one dose of the study medication and the participants had baseline measurements and at least one measurement during the study period). The 95% two‐sided confidence intervals (CIs) for the mean difference in the 12‐week changes from baseline between the treatment groups are presented, and whether the upper limit of the CI was below the non‐inferiority margin of 0.40% was determined. The secondary analysis population was the per‐protocol (PP) set defined as a group of subjects who met the inclusion/exclusion criteria and completed the main study without the occurrence of any major protocol violations during the main study. Those who received rescue therapy during the clinical trial were excluded from the PP set. The efficacy data obtained from a subject who received rescue therapy were processed as missing values for the analysis from the time point at which the rescue therapy was given to rule out the effect of the rescue drug on the efficacy endpoints. The details of how drop‐outs or missing data and type 1 errors in the presence of multiple comparisons were handled are described in the Supplementary Methods (see the supporting information).
All continuous variables are presented as means ± SDs, and categorical variables are expressed as frequencies with percentages. A paired t‐test or Wilcoxon signed‐rank test was performed, depending on the normality satisfaction status to compare the results obtained at baseline, week 12, and week 24 within the treatment groups. For comparisons between the two groups, a two‐sample t‐test or a Wilcoxon rank‐sum test was used for continuous efficacy variables and a Chi‐squared test or a Fisher's exact test was used for categorical data, as appropriate. In addition, we performed subgroup analyses defined by HbA1c (<8.0%, ≥8.0%). In principle, all statistical analyses were performed by the two‐sided test at a significance level of 5% using SAS version 9.4 (SAS Institute, Cary, NC, USA).
3. RESULTS
3.1. Disposition of patients
Of the 246 patients screened, 207 eligible patients were enrolled, and 102 and 105 patients were randomized to the evogliptin group and the linagliptin group, respectively. Of these randomized patients, 96 (94%) in the evogliptin group and 98 (93%) in the linagliptin group completed the 12‐week main study. Among them, 95 patients in the evogliptin 5 mg group and 92 patients in the linagliptin 5 mg group participated in the extension study as the evogliptin/evogliptin group and the linagliptin/evogliptin group. Finally, 92 patients (90%) in the evogliptin/evogliptin group and 88 patients (84%) in the linagliptin/evogliptin group completed the 12‐week extension study.
The baseline demographics and clinical characteristics of the randomized patients are summarized in Table 1. The mean age of the study participants was 56.1 ± 10.4 years and 115 (55.6%) of subjects were men. The mean baseline BMI was 26.1 ± 3.4 kg/m2 and the mean baseline HbA1c level was 7.82% ± 0.67% in both groups. There were no significant differences in the baseline characteristics between the two groups.
TABLE 1.
Baseline characteristics of study population
| Evogliptin (n = 102) | Linagliptin (n = 105) | P‐value | |
|---|---|---|---|
| Age (years) | 56.6 (10.7) | 55.6 (10.2) | .690 a |
| Sex (men), n (%) | 61 (59.8) | 54 (51.4) | .225 b |
| BMI (kg/m2) | 26.0 (3.3) | 26.2 (3.5) | .559 a |
| Obesity c , n (%) | 59 (57.8) | 60 (57.1) | .919 b |
| Duration of diabetes (years) | 4.1 (4.4) | 3.7 (4.1) | .564 a |
| Duration of diabetes ≤4 weeks, n (%) | 26 (25.5) | 23 (21.9) | .544 b |
Abbreviation: BMI, body mass index.
P‐values were derived from a Wilcoxon rank‐sum test.
P‐values were derived from a Chi‐square test.
BMI > 25 kg/m2.
There was no statistically significant difference in the proportion of subjects who were administered rescue drugs at least once between the two treatment groups during the 12‐week main study: 0 (0%) in the evogliptin group and three (2.9%) in the linagliptin group (P = .247).
3.2. Efficacy
Figure 1 shows that the change in HbA1c from baseline to week 12 was −0.85% ± 0.67% for the evogliptin and −0.75% ± 0.87% for the linagliptin group. A significant reduction from the baseline HbA1c was observed at week 12 in both the evogliptin and the linagliptin group (both P < .0001). The between‐group difference was −0.10% [95% CI: −0.32, 0.11] and the upper limit of the two‐sided 95% CI was 0.11%, which was below the non‐inferiority margin of 0.40%, indicating non‐inferiority of the evogliptin to the linagliptin group (Table 2). In the PP set, the between‐group difference in HbA1c at week 12 was −0.03% [95% CI: −0.25, 0.20], similar to that in the FA set. The change in the mean HbA1c level from baseline to week 12, the primary endpoint of this study, was analysed using a mixed‐effects model with repeated measures (MMRM) for sensitivity analysis. The MMRM included the fixed categorical effects of treatment, visit, and treatment‐by‐visit interaction. We found that the between‐group difference was −0.08% [95% CI: −0.30, 0.14], which was similar to that in the FA set.
FIGURE 1.
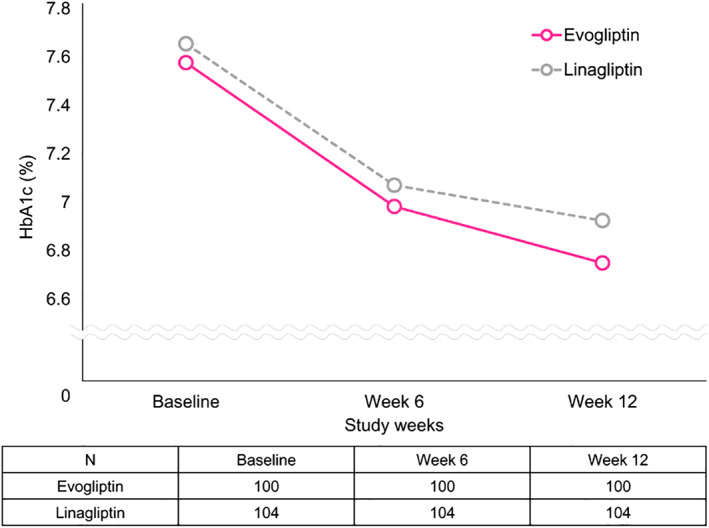
Changes in HbA1c from baseline to week 12 after treatment
TABLE 2.
Baseline, week 12, and changes in the outcomes from baseline
| Evogliptin | Linagliptin | Difference [95% CI] or P‐value | |
|---|---|---|---|
| (n = 100) | (n = 104) | ||
| HbA1c (%) | |||
| Baseline | |||
| Mean ± SD | 7.55 ± 0.78 | 7.63 ± 0.78 | |
| At week 12 | |||
| Mean ± SD | 6.70 ± 0.73 | 6.88 ± 0.91 | |
| Change from baseline to week 12 (mean ± SD) | −0.85 ± 0.67 | −0.75 ± 0.87 | −0.10 [−0.32, 0.11] |
| P‐value for mean difference from baseline at week 12 | <.0001 a | <.0001 a | |
| HbA1c response rate | n (%) | n (%) | |
| <7.0% | 68 (68.0) | 7 0(67.3) | 1.0000 c |
| ≥7.0% | 32 (32.0) | 34 (32.7) | |
| <6.5% | 44 (44.0) | 38 (36.5) | .3182 c |
| ≥6.5% | 56 (56.0) | 66 (63.5) | |
| Fasting plasma glucose (mg/mL) | |||
| Baseline | |||
| Mean ± SD | 144.1 ± 24.7 | 151.1 ± 26.9 | |
| At week 12 | |||
| Mean ± SD | 131.3 ± 23.0 | 135.7 ± 33.5 | |
| Change from baseline to week 12 (mean ± SD) | −12.8 ± 19.5 | −15.4 ± 34.1 | .3253 d |
| P‐value for mean difference from baseline at week 12 | <.0001 b | <.0001 a | |
Abbreviations: CI, confidence interval, SD, standard deviation.
P‐values were derived from a Wilcoxon signed‐rank test.
P‐values were derived from a paired t‐test.
P‐values were derived from a Fisher's exact test.
P‐values were derived from a Wilcoxon rank‐sum test.
In the extension study, evogliptin treatment resulted in a sustained decrease in the HbA1c levels. The change in HbA1c from baseline to week 24 was −0.94% ± 0.75% in the evogliptin/evogliptin group and −0.83% ± 0.75% in the linagliptin/evogliptin group (Table S1).
Treatment with evogliptin and linagliptin for 12 weeks decreased the FPG by 12.8 ± 19.5 and 15.4 ± 34.1 mg/dL (both P < .0001), respectively. However, the FPG values were not significantly different between the two treatment groups (P = .325). The PP set showed significant changes in FPG of −12.8 ± 18.8 mg/dL in the evogliptin (n = 93) and −16.5 ± 36.6 mg/dL in the linagliptin group (n = 84) (both P < .0001), with no significant difference between the groups (P = .162). These results were similar to the results of the FA set.
In the extension study, the change in FPG from baseline to week 24 was −11.7 ± 25.1 mg/dL in the evogliptin/evogliptin and −14.3 ± 24.6 mg/dL in the lnagliptin/evogliptin group (both P < .0001).
In subgroup analyses, significant reductions in the HbA1c and FPG levels from baseline to week 12 were seen in patients with HbA1c values of <8.0% and ≥8.0% (Table S2). Patients with HbA1c values of ≥8.0% showed greater changes in HbA1c and FPG values from baseline to week 12 compared with patients with HbA1c values of <8.0%, without significant between‐group differences.
Regarding the HbA1c target goal achievement rate, after week 12 administrations, a comparable proportion of patients achieved HbA1c levels of <6.5% in each treatment group. Forty‐four per cent of the patients in the evogliptin and 36.5% of those in the linagliptin group had HbA1c levels of <6.5% (P = .318 by Fisher's exact test). When an HbA1c of <7.0% was used, 68.0% in the evogliptin and 67.3% in the linagliptin group met this target goal, without significant differences between the groups (P = 1.000). The results in the PP set were similar to those in the FA set. In the evogliptin group, 46.2% (n = 93) achieved an HbA1c of <6.5% versus 38.1% (n = 84) in the linagliptin group, without significant between‐group differences (P = .290).
In the extension study, among 91 subjects in the evogliptin/evogliptin group, 73 (80.2%) and 44 (48.4%) had HbA1c values of <7.0% and <6.5%, respectively. Among 86 subjects in the linagliptin/evogliptin group, 61 (70.9%) and 35 (40.7%) had HbA1c values of <7.0% and <6.5%, respectively. The proportion of subjects with HbA1c values of <6.5% or <7.0% at week 24 was similar between the two groups.
As shown in Table 3, after 12 weeks of treatment, the CGM variables, including the mean glucose over 0‐24 hours and glycaemic variability values, such as SD, MAGE, CV and MODD, significantly decreased in both groups. The PP set showed similar results.
TABLE 3.
Summary of changes in continuous glucose monitoring (CGM) variables from baseline at week 12
| Evogliptin | Linagliptin | P‐value | |||
|---|---|---|---|---|---|
| (n = 100) | (n = 104) | ||||
| Mean glucose for 0‐24 h (mg/dL) | |||||
| Baseline (n, mean ± SD) | 82 a | 164.2 ± 34.2 | 87 a | 165.2 ± 32.3 | |
| At week 12 (n, mean ± SD) | 77 b | 146.6 ± 28.2 | 68 b | 142.8 ± 24.8 | |
| Change from baseline to week 12 (n, mean ± SD) | 68 | −17.3 ± 27.5 | 62 | −19.0 ± 29.9 | .660 e |
| P‐value for mean difference | <.0001 c | <.0001 f | |||
| SD (mg/dL) | |||||
| Baseline (n, mean ± SD) | 82 a | 36.8 ± 12.0 | 87 a | 36.2 ± 12.6 | |
| At week 12 (n, mean ± SD) | 77 b | 27.8 ± 10.4 | 68 b | 27.0 ± 9.7 | |
| Change from baseline to week 12 (n, mean ± SD) | 68 | −9.8 ± 12.7 | 62 | −8.3 ± 11.6 | .482 d |
| P‐value for mean difference | <.0001 c | <.0001 c | |||
| Mean amplitude of glucose excursions | |||||
| Baseline (n, mean ± SD) | 82 a | 95.1 ± 29.4 | 87 a | 90.0 ± 28.5 | |
| At week 12 (n, mean ± SD) | 77 b | 71.6 ± 25.9 | 68 b | 70.2 ± 25.0 | |
| Change from baseline to week 12 (n, mean ± SD) | 68 | −24.6 ± 34.5 | 62 | −16.7 ± 29.7 | .166 d |
| P‐value for mean difference | <.0001 c | <.0001 c | |||
| Coefficient of variance (%) | |||||
| Baseline (n, mean ± SD) | 82 a | 22.4 ± 5.6 | 87 a | 21.9 ± 6.2 | |
| At week 12 (n, mean ± SD) | 77 b | 19.0 ± 6.2 | 68 b | 18.8 ± 5.7 | |
| Change from baseline to week 12 (n, mean ± SD) | 68 | −3.9 ± 7.1 | 62 | −3.0 ± 6.0 | .464 d |
| P‐value for mean difference | <.0001 c | .0002 c | |||
| Mean of daily differences (mg/dL) | |||||
| Baseline (n, mean ± SD) | 82 a | 32.6 ± 16.0 | 87 a | 31.9 ± 11.6 | |
| At week 12 (n, mean ± SD) | 77 b | 25.5 ± 11.1 | 68 b | 24.9 ± 9.6 | |
| Change from baseline to week 12 (n, mean ± SD) | 68 | −7.2 ± 15.6 | 62 | −6.4 ± 13.0 | .759 d |
| P‐value for mean difference | .0003 c | .0002 c | |||
| Proportion of time in hypoglycaemia (< 54 mg/dL) | |||||
| Baseline (n, mean ± SD) | 82 a | 0.08 ± 0.58 | 87 a | 0.07 ± 0.44 | |
| At week 12 (n, mean ± SD) | 77 b | 0.22 ± 1.15 | 68 b | 0.24 ± 1.10 | |
| Change from baseline to week 12 (n, mean ± SD) | 68 | 0.06 ± 1.23 | 62 | 0.17 ± 1.28 | .522 e |
| P‐value for mean difference | .8750 f | .4688 f | |||
| Proportion of time in hypoglycaemia (<70 mg/dL) | |||||
| Baseline (n, mean ± SD) | 82 a | 0.45 ± 2.04 | 87 a | 0.69 ± 2.30 | |
| At week 12 (n, mean ± SD) | 77 b | 0.42 ± 1.73 | 68 b | 0.65 ± 2.37 | |
| Change from baseline to week 12 (n, mean ± SD) | 68 | −0.19 ± 2.66 | 62 | −0.05 ± 2.71 | .927 e |
| P‐value for mean difference | .5342 f | .4729 f | |||
| Proportion of time in range (70‐180 mg/dL) | |||||
| Baseline (n, mean ± SD) | 82 a | 67.7 ± 26.0 | 87 a | 68.1 ± 22.6 | |
| At week 12 (n, mean ± SD) | 77 b | 81.6 ± 22.3 | 68 b | 84.6 ± 18.6 | |
| Change from baseline to week 12 (n, mean ± SD) | 68 | 13.9 ± 21.8 | 62 | 15.0 ± 23.7 | .390 e |
| P‐value for mean difference | <.0001 f | <.0001 f | |||
| Proportion of time in hyperglycaemia (>180 mg/dL) | |||||
| Baseline (n, mean ± SD) | 82 a | 31.9 ± 26.2 | 87 a | 31.2 ± 23.1 | |
| At week 12 (n, mean ± SD) | 77 b | 18.0 ± 22.5 | 68 b | 14.7 ± 18.4 | |
| Change from baseline to week 12 (n, mean ± SD) | 68 | −13.7 ± 21.8 | 62 | −14.9 ± 23.8 | .413 e |
| P‐value for mean difference | <.0001 f | <.0001 f | |||
| Proportion of time in hyperglycaemia (>250 mg/dL) | |||||
| Baseline (n, mean ± SD) | 82 a | 7.2 ± 11.0 | 87 a | 6.6 ± 12.7 | |
| At week 12 (n, mean ± SD) | 77 b | 2.6 ± 6.7 | 68 b | 1.6 ± 5.9 | |
| Change from baseline to week 12 (n, mean ± SD) | 68 | −4.9 ± 9.6 | 62 | −3.0 ± 10.0 | .805 e |
| P‐value for mean difference | <.0001 f | .0014 f | |||
Abbreviation: SD, standard deviation.
Subjects whose measurement data of 0‐24 h on day −2 and day −1 at week 0 were all available.
Subjects whose measurement data of 0‐24 h on day ‐2 and day ‐1 at week 12 were all available.
P‐values were derived from a paired t‐test.
P‐values were derived from a two‐sample t‐test.
P‐values were derived from a Wilcoxon rank‐sum test.
P‐values were derived from a Wilcoxon signed‐rank test.
The proportion of time in the 70‐180 mg/dL range and the time in hyperglycaemia (>180 and >250 mg/dL) were also significantly improved after 12 weeks of treatment in both groups. In the evogliptin group, the proportion of time in the 70‐180 mg/dL range increased from 67.7% at baseline to 81.6% at week 12 (P < .0001) and the proportion of time in hyperglycaemia (>180 mg/dL) decreased by 13.7% (P < .0001) at week 12 from baseline. The linagliptin group showed similar results, leading to no significant differences in these CGM variables between the two groups.
The mean daily glucose level obtained from CGM at week 24 decreased by 24.60 ± 30.61 mg/dL in the evogliptin/evogliptin group and by 15.2 ± 27.2 mg/dL in the linagliptin/evogliptin group (both P < .0001; Table S3). The SD at week 24 decreased by 10.29 ± 11.91 mg/dL in the evogliptin/evogliptin group and by 6.62 ± 10.78 mg/dL in the linagliptin/evogliptin group (both P < .0001). Similarly, the change in MAGE from baseline to week 24 in the extension study was −26.86 ± 33.73 mg/dL in the evogliptin/evogliptin group (P < .0001) and −13.37 ± 29.67 mg/dL in the linagliptin/evogliptin group (P = .0023). The CV and MODD showed similar patterns.
The eGFR and nephrin levels did not change significantly in either group after the original 12 weeks of treatment (Table S4). The percentage change from baseline in the UACR at week 24 significantly decreased by 47.27 ± 262.24% in the evogliptin/evogliptin group and by 35.28 ± 258.85% in the linagliptin/evogliptin group (Table S5). Significant reductions in urinary NAG at week 12 (P = .0289) and week 24 (P = .0026) were only observed in the evogliptin group, although the inter‐group difference was not significant. Only the evogliptin group showed significantly greater reductions in nitrotyrosine and TBARS (both P < .01) at week 12, but there was no significant difference between the two groups.
3.3. Safety
As shown in Table 4, after 12 weeks of administration, 30 (29.7%) patients in the evogliptin and 42 (40.0%) patients in the linagliptin group reported AEs, regardless of study drug causality. ADRs were observed in 4.0% of the evogliptin and 4.8% of the linagliptin group. Two patients in the evogliptin group and three patients in the linagliptin group had SAEs during the 12‐week main study period. All SAEs were considered to have no causal relationship with the investigational product.
TABLE 4.
Incidence of adverse events (AEs) during 12 weeks of treatment
| Evogliptin (n = 101) | Linagliptin (n = 105) | |
|---|---|---|
| AEs | 30 (29.7%) | 42 (40.0%) |
| Infections | 4 (4.0%) | 10 (9.5%) |
| Gastrointestinal disorders | 4 (4.0%) | 3 (2.9%) |
| Renal and urinary disorders | 0 (0.0%) | 4 (3.8%) |
| Hypoglycaemia | 13 (12.9%) | 14 (13.3%) |
| Adverse drug reactions | 4 (4.0%) | 5 (4.8%) |
| Serious adverse events | 2 (2.0%) | 3 (2.9%) |
| Documented symptomatic hypoglycaemia | 0 (0.0%) | 1 (1.0%) |
| Asymptomatic hypoglycaemia | 13 (12.9%) | 14 (13.3%) |
The hypoglycaemic events were categorized as severe hypoglycaemia, asymptomatic hypoglycaemia, documented symptomatic hypoglycaemia, probable symptomatic hypoglycaemia and relative hypoglycaemia. Asymptomatic hypoglycaemia occurred in 28 patients (29.8%, 35 events) in the evogliptin/evogliptin group and 26 subjects (28.9%, 41 events) in the linagliptin/evogliptin group. Documented symptomatic hypoglycaemia occurred in two patients (2.20%, three events) in the linagliptin/evogliptin group. Probable symptomatic hypoglycaemia occurred in one subject (1.10%, one event) in the evogliptin/evogliptin group. Total hypoglycaemia occurred in 28 patients (29.8%, 36 events) in the evogliptin/evogliptin group and 26 patients (28.9%, 44 events) in the linagliptin/evogliptin group.
The LDL cholesterol levels significantly decreased at week 12 in both groups (Table S6). The AST and ALT levels significantly decreased at week 12 only in the evogliptin group, but no significant between‐group difference was seen.
4. DISCUSSION
In this 12‐week, multicentre, randomized, double‐blind, active‐controlled study, evogliptin treatment significantly decreased HbA1c by 0.85% ± 0.67%, which was non‐inferior to linagliptin treatment. After 24 weeks of treatment, the evogliptin group showed a persistent decrease in HbA1c levels. Regarding the glycaemic target goal, in the evogliptin group, 80.2% of subjects had HbA1c levels of <7.0% at week 24. Evogliptin treatment also resulted in improved glycaemic variability with increased glucose target ranges and decreased time in hypoglycaemia and hyperglycaemia investigated by the CGM system. Evogliptin was well tolerated during the entire clinical trial period of 24 weeks.
In the present study, the glucose‐lowering efficacy of evogliptin was found to be robust. HbA1c was decreased by 0.85% at week 12 and by 0.94% at week 24. During the entire 24‐week study period, FPG decreased significantly and most of the CGM variables improved markedly from baseline to week 24.
DPP‐4 inhibitors are known to cause glucose‐dependent insulin secretion and inhibit glucagon production by increasing GLP‐1 levels. 30 In this manner, both fasting and postprandial glucose levels were markedly decreased by evogliptin treatment without increasing the risk of treatment‐induced hypoglycaemia. This was related to significant improvements in glycaemic variability by evogliptin treatment assessed by CGM.
Of note, we evaluated various measures of glycaemic variability representing day‐to‐day variability and intra‐day variation assessed by CGM (SD, CV, MAGE and MODD) in this study. 29 , 31 , 32 All these glycaemic variability values showed great reductions in the evogliptin group that were comparable with the linagliptin group. Evogliptin treatment resulted in significantly reduced peak daily glucose levels, area under the curve for 2 hours after each meal (data not shown), and the proportion of time spent in hyperglycaemia, representing its potent effect in controlling postprandial hyperglycaemia. Several studies have shown that postprandial glucose levels had a greater effect on HbA1c control, as well as on glycaemic variability, than fasting glucose. 33 , 34
A large glycaemic burden after a meal appears important in inducing oxidative stress, which has a direct toxic effect on the vascular endothelium. It can lead to the development and progression of diabetic complications. 35 In this study, evogliptin treatment showed significant decreases in oxidative stress markers, nitrotyrosine and TBARS, accompanied by an attenuation of glycaemic variability mainly derived from postprandial glucose control. The presence of nitrotyrosine in the plasma of diabetic patients is considered indirect evidence of oxidative stress resulting from an imbalance in the ratio of nitric oxide to peroxynitrite production. 36 Nitrotyrosine concentrations were positively correlated with plasma glucose levels, but not with HbA1c levels. 37 Also, serum levels of TBARS have been found to be significantly increased in patients with T2D in many studies. 38 , 39 Diabetic patients with angiopathy showed significantly higher TBARS levels than those without angiopathy, which suggests enhanced lipid peroxidation. 40 Taken together, evogliptin treatment can be used as an effective therapeutic strategy to control postprandial glucose excursions and fluctuations in glucose levels and its effects might be related to the prevention of vascular complications.
In this study, significant reductions in NAG levels at week 12 and week 24 were only observed in the evogliptin group, although no significant difference was found between the treatment groups. NAG, a lysosomal enzyme present in the proximal tubular cells, is eliminated in the urine and appears to be a sensitive biomarker of early renal tubular injury and is linked to glycaemic excursion in patients with T2D. 41 , 42 In addition, the UACR significantly decreased after evogliptin treatment for 24 weeks. These results suggest that evogliptin may have a protective effect on the kidneys by alleviating proximal renal tubular damage, which may be combined with the glucose‐lowering effect of DPP‐4 inhibition.
During the entire 24‐week study period, the safety evaluation results showed that the frequency of AEs was comparable between the two treatment groups. Also, the incidence of SAEs that occurred during the entire study period was very low. No serious ADRs were observed. In particular, the evogliptin group had no documented symptomatic hypoglycaemia during the 24‐week study period. These data support the safety and tolerability of evogliptin.
This study has several strengths. First, this was a head‐to‐head study compared with linagliptin, which has substantial data regarding its efficacy and safety. Second, this study adopted 3‐day CGM to assess glycaemic variability. We also evaluated various measures of glycaemic variability assessed by CGM. In addition, the number of study participants was comparatively large and the 24‐week treatment completion rate was high. Lastly, an extension study was conducted to confirm the findings obtained from the original study.
In conclusion, in the first 12‐week original study and the 12‐week extension study, treatment with evogliptin showed robust glucose‐lowering efficacy and significant improvement in glycaemic variability with excellent safety and tolerability in patients with T2D that were similar to linagliptin treatment.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
All authors participated in the design of the study. All authors conducted the study and contributed to data acquisition. G.K and S.L. interpreted the data and drafted and revised the manuscript for important intellectual content. M.K.L. interpreted the data and reviewed the manuscript for important intellectual content. All authors reviewed and approved the final manuscript.
Supporting information
Supplementary Figure S1 Study flow chart. CGMS: continuous glucose monitoring system
Supplementary Figure S2 Subject disposition.
Supplementary Figure S3 Changes in glycated hemoglobin (HbA1c) level from baseline to week 24 after treatment.
Supplementary Figure S4 Percentage of patients with HbA1c < 6.5% and < 7.0% at week 24.
HbA1c: glycated hemoglobin
Appendix S1: Supporting information
ACKNOWLEDGMENTS
Gyuri Kim and Soo Lim contributed equally to this work. This study was funded by Dong‐A ST, Co., Ltd., Seoul, Republic of Korea. The funding source had no role in the study design, data collection, data analysis, decision to publish, or preparation of the manuscript.
Kim G, Lim S, Kwon H‐S, et al. Efficacy and safety of evogliptin treatment in patients with type 2 diabetes: A multicentre, active‐controlled, randomized, double‐blind study with open‐label extension (the EVERGREEN study). Diabetes Obes Metab. 2020;22:1527–1536. 10.1111/dom.14061
Funding information This study was funded by Dong‐A ST, Co., Ltd., Seoul, Republic of Korea.
REFERENCES
- 1. American Diabetes Association . 6. Glycemic targets: standards of medical care in diabetes‐2018. Diabetes Care. 2018;41:S55‐S64. [DOI] [PubMed] [Google Scholar]
- 2. Rama Chandran S, Tay WL, Lye WK, et al. Beyond HbA1c: comparing glycemic variability and glycemic indices in predicting hypoglycemia in type 1 and type 2 diabetes. Diabetes Technol Ther. 2018;20:353‐362. [DOI] [PubMed] [Google Scholar]
- 3. Kovatchev BP. Metrics for glycaemic control ‐ from HbA1c to continuous glucose monitoring. Nat Rev Endocrinol. 2017;13:425‐436. [DOI] [PubMed] [Google Scholar]
- 4. Cappon G, Vettoretti M, Sparacino G, Facchinetti A. Continuous glucose monitoring sensors for diabetes management: a review of technologies and applications. Diabetes Metab J. 2019;43:383‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lu J, Ma X, Zhou J, et al. Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care. 2018;41:2370‐2376. [DOI] [PubMed] [Google Scholar]
- 6. Cavalot F. Do data in the literature indicate that glycaemic variability is a clinical problem? Glycaemic variability and vascular complications of diabetes. Diabetes Obes Metab. 2013;15(Suppl 2):3‐8. [DOI] [PubMed] [Google Scholar]
- 7. Spallone V. Update on the impact, diagnosis and management of cardiovascular autonomic neuropathy in diabetes: what is defined, what is new, and what is unmet. Diabetes Metab J. 2019;43:3‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681‐1687. [DOI] [PubMed] [Google Scholar]
- 9. Costantino S, Paneni F, Battista R, et al. Impact of glycemic variability on chromatin remodeling, oxidative stress, and endothelial dysfunction in patients with type 2 diabetes and with target HbA1c levels. Diabetes. 2017;66:2472‐2482. [DOI] [PubMed] [Google Scholar]
- 10. Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of diabetes (EASD). Diabetes Care. 2018;41:2669‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim MK, Ko SH, Kim BY, et al. 2019 clinical practice guidelines for type 2 diabetes mellitus in Korea. Diabetes Metab J. 2019;43:398‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goossen K, Graber S. Longer term safety of dipeptidyl peptidase‐4 inhibitors in patients with type 2 diabetes mellitus: systematic review and meta‐analysis. Diabetes Obes Metab. 2012;14:1061‐1072. [DOI] [PubMed] [Google Scholar]
- 13. Ahren B, Landin‐Olsson M, Jansson PA, Svensson M, Holmes D, Schweizer A. Inhibition of dipeptidyl peptidase‐4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab. 2004;89:2078‐2084. [DOI] [PubMed] [Google Scholar]
- 14. Nabeno M, Akahoshi F, Kishida H, et al. A comparative study of the binding modes of recently launched dipeptidyl peptidase IV inhibitors in the active site. Biochem Biophys Res Commun. 2013;434:191‐196. [DOI] [PubMed] [Google Scholar]
- 15. Gu N, Park MK, Kim TE, et al. Multiple‐dose pharmacokinetics and pharmacodynamics of evogliptin (DA‐1229), a novel dipeptidyl peptidase IV inhibitor, in healthy volunteers. Drug Des Devel Ther. 2014;8:1709‐1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deacon CF, Lebovitz HE. Comparative review of dipeptidyl peptidase‐4 inhibitors and sulphonylureas. Diabetes Obes Metab. 2016;18:333‐347. [DOI] [PubMed] [Google Scholar]
- 17. Kim MJ, Kim NY, Jung YA, et al. Evogliptin, a dipeptidyl peptidase‐4 inhibitor, attenuates renal fibrosis caused by unilateral ureteral obstruction in mice. Diabetes Metab J. 2020;44:186‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oh J, Kim AH, Lee S, et al. Effects of renal impairment on the pharmacokinetics and pharmacodynamics of a novel dipeptidyl peptidase‐4 inhibitor, evogliptin (DA‐1229). Diabetes Obes Metab. 2017;19:294‐298. [DOI] [PubMed] [Google Scholar]
- 19. Eun Lee J, Kim JE, Lee MH, et al. DA‐1229, a dipeptidyl peptidase IV inhibitor, protects against renal injury by preventing podocyte damage in an animal model of progressive renal injury. Lab Invest. 2016;96:547‐560. [DOI] [PubMed] [Google Scholar]
- 20. Jung CH, Park CY, Ahn KJ, et al. A randomized, double‐blind, placebo‐controlled, phase II clinical trial to investigate the efficacy and safety of oral DA‐1229 in patients with type 2 diabetes mellitus who have inadequate glycaemic control with diet and exercise. Diabetes Metab Res Rev. 2015;31:295‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Park J, Park SW, Yoon KH, et al. Efficacy and safety of evogliptin monotherapy in patients with type 2 diabetes and moderately elevated glycated haemoglobin levels after diet and exercise. Diabetes Obes Metab. 2017;19:1681‐1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hong SM, Park CY, Hwang DM, et al. Efficacy and safety of adding evogliptin versus sitagliptin for metformin‐treated patients with type 2 diabetes: a 24‐week randomized, controlled trial with open label extension. Diabetes Obes Metab. 2017;19:654‐663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. von Eynatten M, Gong Y, Emser A, Woerle HJ. Efficacy and safety of linagliptin in type 2 diabetes subjects at high risk for renal and cardiovascular disease: a pooled analysis of six phase III clinical trials. Cardiovasc Diabetol. 2013;12:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takahashi H, Nishimura R, Tsujino D, Utsunomiya K. Which is better, high‐dose metformin monotherapy or low‐dose metformin/linagliptin combination therapy, in improving glycemic variability in type 2 diabetes patients with insufficient glycemic control despite low‐dose metformin monotherapy? A randomized, cross‐over, continuous glucose monitoring‐based pilot study. J Diabetes Investig. 2019;10:714‐722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spencer NY, Yang Z, Sullivan JC, Klein T, Stanton RC. Linagliptin unmasks specific antioxidant pathways protective against albuminuria and kidney hypertrophy in a mouse model of diabetes. PLoS One. 2018;13:e0200249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Monnier L, Colette C, Owens DR. Glycemic variability: the third component of the dysglycemia in diabetes. Is it important? How to measure it? J Diabetes Sci Technol. 2008;2:1094‐1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rawlings RA, Shi H, Yuan LH, Brehm W, Pop‐Busui R, Nelson PW. Translating glucose variability metrics into the clinic via continuous glucose monitoring: a graphical user Interface for diabetes evaluation (CGM‐GUIDE[c]). Diabetes Technol Ther. 2011;13:1241‐1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Le Floch JP, Kessler L. Glucose variability: comparison of different indices during continuous glucose monitoring in diabetic patients. J Diabetes Sci Technol. 2016;10:885‐891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Molnar GD, Taylor WF, Ho MM. Day‐to‐day variation of continuously monitored glycaemia: a further measure of diabetic instability. Diabetologia. 1972;8:342‐348. [DOI] [PubMed] [Google Scholar]
- 30. Park SE, Lee BW, Kim JH, et al. Effect of gemigliptin on glycaemic variability in patients with type 2 diabetes (STABLE study). Diabetes Obes Metab. 2017;19:892‐896. [DOI] [PubMed] [Google Scholar]
- 31. McDonnell CM, Donath SM, Vidmar SI, Werther GA, Cameron FJ. A novel approach to continuous glucose analysis utilizing glycemic variation. Diabetes Technol Ther. 2005;7:253‐263. [DOI] [PubMed] [Google Scholar]
- 32. Jin SM, Kim TH, Bae JC, et al. Clinical factors associated with absolute and relative measures of glycemic variability determined by continuous glucose monitoring: an analysis of 480 subjects. Diabetes Res Clin Pract. 2014;104:266‐272. [DOI] [PubMed] [Google Scholar]
- 33. Monnier L, Colette C, Dunseath GJ, Owens DR. The loss of postprandial glycemic control precedes stepwise deterioration of fasting with worsening diabetes. Diabetes Care. 2007;30:263‐269. [DOI] [PubMed] [Google Scholar]
- 34. Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care. 2003;26:881‐885. [DOI] [PubMed] [Google Scholar]
- 35. Ceriello A, Hanefeld M, Leiter L, et al. Postprandial glucose regulation and diabetic complications. Arch Intern Med. 2004;164:2090‐2095. [DOI] [PubMed] [Google Scholar]
- 36. Halliwell B. What nitrates tyrosine? Is nitrotyrosine specific as a biomarker of peroxynitrite formation in vivo? FEBS Lett. 1997;411:157‐160. [DOI] [PubMed] [Google Scholar]
- 37. Ceriello A, Mercuri F, Quagliaro L, et al. Detection of nitrotyrosine in the diabetic plasma: evidence of oxidative stress. Diabetologia. 2001;44:834‐838. [DOI] [PubMed] [Google Scholar]
- 38. Turk HM, Sevinc A, Camci C, et al. Plasma lipid peroxidation products and antioxidant enzyme activities in patients with type 2 diabetes mellitus. Acta Diabetol. 2002;39:117‐122. [DOI] [PubMed] [Google Scholar]
- 39. Kesavulu MM, Giri R, Kameswara Rao B, Apparao C. Lipid peroxidation and antioxidant enzyme levels in type 2 diabetics with microvascular complications. Diabetes Metab. 2000;26:387‐392. [PubMed] [Google Scholar]
- 40. Griesmacher A, Kindhauser M, Andert SE, et al. Enhanced serum levels of thiobarbituric‐acid‐reactive substances in diabetes mellitus. Am J Med. 1995;98:469‐475. [DOI] [PubMed] [Google Scholar]
- 41. Kim SR, Lee YH, Lee SG, et al. Urinary N‐acetyl‐beta‐D‐glucosaminidase, an early marker of diabetic kidney disease, might reflect glucose excursion in patients with type 2 diabetes. Medicine. 2016;95:e4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gluhovschi C, Gluhovschi G, Petrica L, et al. Urinary biomarkers in the assessment of early diabetic nephropathy. J Diabetes Res. 2016;2016:4626125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1 Study flow chart. CGMS: continuous glucose monitoring system
Supplementary Figure S2 Subject disposition.
Supplementary Figure S3 Changes in glycated hemoglobin (HbA1c) level from baseline to week 24 after treatment.
Supplementary Figure S4 Percentage of patients with HbA1c < 6.5% and < 7.0% at week 24.
HbA1c: glycated hemoglobin
Appendix S1: Supporting information


