Abstract
A wealth of evidence supports the role of tumor immunotherapy as a vital therapeutic option in cancer. In recent decades, accumulated studies have revealed the anticancer activities of natural products and their derivatives. Increasing interest has been driven toward finding novel potential modulators of tumor immunotherapy from natural products, a hot research topic worldwide. These works of research mainly focused on natural products, including polyphenols (e.g., curcumin, resveratrol), cardiotonic steroids (e.g., bufalin and digoxin), terpenoids (e.g., paclitaxel and artemisinins), and polysaccharide extracts (e.g., lentinan). Compelling data highlight that natural products have a promising future in tumor immunotherapy. Considering the importance and significance of this topic, we initially discussed the integrated research progress of natural products and their derivatives, including target T cells, macrophages, B cells, NKs, regulatory T cells, myeloid‐derived suppressor cells, inflammatory cytokines and chemokines, immunogenic cell death, and immune checkpoints. Furthermore, these natural compounds inactivate several key pathways, including NF‐κB, PI3K/Akt, MAPK, and JAK/STAT pathways. Here, we performed a deep generalization, analysis, and summarization of the previous achievements, recent progress, and the bottlenecks in the development of natural products as tumor immunotherapy. We expect this review to provide some insight for guiding future research.
Keywords: cardiotonic steroids, polyphenolics, polysaccharides, terpenoids, Tregs, tumor immunotherapy
This review aims to summarize the integrated research progress of natural products (e.g. curcumin) on the tumor immunotherapy and key intracellular pathways.
Abbreviations
- BMDMs
bone marrow‐derived macrophages
- CGs
cardiac glycosides
- CRT
calreticulin
- CTLs
CD8+ cytotoxic T cells
- DCs
dendritic cells
- DTH
delayed‐type hypersensitivity
- EGCG
(−)‐Epicatechin‐3‐gallate
- ICD
immunogenic cell death
- IDO
indoleamine 2,3dioxygenase
- MDSCs
myeloid‐derived suppressor cells
- PBMCs
peripheral blood mononuclear cells
- PD‐1
programmed cell death‐1
- PD‐L1
programmed death ligand‐1
- ROS
reactive oxygen species
- SMMT
spontaneous mouse mammary tumor
- TAMs
tumor‐associated macrophages
- tBregs
regulatory B cells
- TME
tumor microenvironment
- Tregs
CD4+CD25+Foxp3+ regulatory T cells
- VEGF
vascular endothelial growth factor
- VES
vitamin E succinate
1. INTRODUCTION
It is considered that 18,100,000 new cancer cases and 9,600,000 cancer deaths reported worldwide in 2018. 1 In recent years, unsatisfactory results in cancer treatment have been partly due to a condition of systemic immune unresponsiveness or immunosuppression against cancers. 2 , 3 Many immune cells, including macrophages, CD4+ T cells, CD8+ cytotoxic T cells (CTLs), NKs, B cells, CD4+CD25+Foxp3+ regulatory T cells (Tregs), myeloid‐derived suppressor cells (MDSCs), and dendritic cells (DCs) play key roles in the pathologic process of tumor growth and development, as well as in response to chemotherapies. 2 , 3 In addition to these immune cells, several other inflammatory cytokines and chemokines, such as TGF‐β, ILs (e.g., IL‐10), IFN‐γ, and TNF‐α, can be triggered, enhanced, or reduced. 2 , 4 , 5 , 6 Moreover, immune checkpoints pathways, such as programmed cell death‐1 (PD‐1) and CTLA‐4, and several key pathways including the NF‐κB, PI3K/Akt, STAT, and MAPK pathways, are associated with anticancer effects of immune system. 7 , 8 , 9 , 10 , 11 , 12 The main function of immune system in tumor microenvironment (TME) is to monitor tissue homeostasis, protect against invading pathogens, and interfere with the detection and clearance of malignant cells. 13 , 14 However, immune system dysfunction in tumors results in malignant cells escaping immune surveillance, immune recognition, and eradication. As a developing approach, tumor immunotherapy has transferred focus from tumor itself to the host's immune system, mobilizing immune cells, recognizing and eventually destroying the tumor cells. 15
Recently, the role of several immune checkpoint inhibitors targeting tumor immunity has become increasingly important, 16 for example, programmed death ligand‐1 (PD‐L1) inhibitor durvalumab and CTLA4 inhibitor ipilimumab. 12 High‐dose IL‐2 and IFN α‐2b have been used to treat multiple advanced cancers. 17 Adoptive T cell therapy has been investigated in several kinds of solid tumors. 18 However, these tumor immunotherapies also result in unique adverse effects owing to their mechanisms of actions, distinct from those caused by other cancer therapies, such as the organ‐specific inflammatory side effects (or immune‐related adverse events), cytokine release syndrome, and immune effector cell‐associated neurotoxicity syndrome. 17 , 19 , 20 , 21 The management of these adverse effects often requires close monitoring and specific treatment, including steroids and immunoregulatory therapy, thus further reducing the patient's quality of life and increasing their financial burden. Therefore, tumor immunotherapy needs to be explored from a novel perspective.
Natural products and their derivatives possess characteristics of structural diversity, diverse biologic activities, low toxicity and side effects, and availability of a wide range of sources; their role in the development of new anticancer drugs and lead drug compounds is increasing in importance. 22 , 23 Notably, there has been a growing interest for discovering novel natural product‐derived potential modulators of tumor immunotherapy, a hot research topic worldwide. In fact, several well‐known natural products including polyphenols (e.g., curcumin, resveratrol), cardiotonic steroids (e.g., digoxin and bufalin), terpenoids (e.g., paclitaxel, artemisinin, and triptolide), polysaccharides (e.g., lentinan), saponins, and capsaicin have potential immunomodulatory effects. 24 , 25 , 26 , 27 , 28 , 29 In this review, we aimed to summarize the integrated research progress of several representative classes of natural products on tumor immunotherapy and key intracellular pathways, highlighting the increased potential of natural products in immunotherapy.
2. MOLECULAR MECHANISMS ON NATURAL PRODUCTS IN TUMOR IMMUNOTHERAPY
2.1. Polyphenols
Polyphenols are a large group of compounds, possessing more than 8000 structural variants. Most polyphenols contribute to pharmacologic activities, such as anti‐inflammatory, immunemodulatory, and anticancer actions. 30 , 31 For instance, curcumin, 32 resveratrol, 33 apigenin, 34 wogonin, 35 epigallocatechin‐3‐gallate, 36 and icariin 37 have demonstrated efficacy as anticancer compounds. Polyphenols are commonly found in vegetables, fruits, and cereals as glycoside esters or free aglycones; they are mainly classified into two groups: nonflavonoids and flavonoids. 38
2.1.1. Nonflavonoids
Nonflavonoids include phenolic acids (e.g., curcumin), stilbenoids (e.g., resveratrol), and phenolic amides. The medicinal nonflavonoids mainly comprise phenolic acids and stilbenes. 30 , 31 , 38 Hence, we only reviewed the role of curcumin and resveratrol in tumor immunity.
Curcumin
Curcumin (Fig. 1) is abundantly present in the plant Curcuma longa. 39 Curcumin has a long history of use as an edible spice and in the medical treatment of inflammatory diseases. 39 Here, we summarized previous achievements in the development of curcumin as a modulator of the tumor immune system. The plasticity, polarization, and function of tumor‐associated macrophages (TAMs), 40 modulation of Tregs, 26 and modulation of the immunogenicity of dying cancer cells, 27 along with the TME 41 in response to curcumin, have provided a wealth of knowledge on the tumor immunotherapy potential of curcumin against various cancers. Recently, Hung et al. demonstrated that TNF‐α triggered cancer immunosuppression through CSN5‐mediated stabilization of PD‐L1, which can be inhibited by curcumin in several types of cancer cells. Moreover, curcumin alone or in combination with anti‐CTLA4 therapy effectively suppressed tumor growth in 4T1 breast cancer, B16 melanoma, and CT26 colon cancer, which could be attributed to the decrease of CSN5‐mediated stabilization of PD‐L1, inhibition of PD‐L1 expression through the NF‐κB pathway, and the increase of CD8+ T cell population. 42 Immune dysfunction, such as the depletion of T cells, and a switch from Th1 to Th2 response, along with augmentation of CD4+CD25+FoxP3+ Tregs population, was restored by curcumin in tumor‐bearing hosts. 43 , 44 Furthermore, curcumin prevented tumor‐induced inhibition of T cell proliferation, suppressed Tregs activity by inhibiting TGF‐β and IL‐10, as well as enhanced the ability of T cells to eliminate tumor cells. 45 Similarly, curcumin treatment improved the antitumor immune response in Cal 27 and FaDu oral cancer cells in vitro and in a 4‐nitroquinoline‐oxide‐induced mouse model in vivo, accompanied by the inhibition of PD‐L1 and p‐STAT3Y705 expression, increased of CD8+ T cells, and decreased Tregs and MDSCs. 46 Curcumin also acts as an immunorestorer to protect against thymic atrophy in tumor‐bearing mice, as evidenced by the neutralization of tumor‐induced oxidative stress and restoration of NF‐κB activity, along with the reformation of the TNF‐α signaling pathway. 47 These evidences indicate that curcumin can successfully reverse tumor‐induced immunosuppression. Hou et.al observed that curcumin inhibited tumor growth in a Lewis lung carcinoma isogenic tumor model by suppressing MDSCs, which is characterized by a decrease of MDSCs in the spleen and tumor tissues, promotion of MDSCs maturation, and differentiation, and down‐regulation of ARG1, reactive oxygen species (ROS), and IL‐6 in tumor‐bearing mice. 48 Notably, curcumin at a dose of 100 mg/kg/d was less effective but decreased T cells in 3LL tumor‐bearing mice. Interesting, 50 mg/kg curcumin significantly inhibited tumor growth by promoting the cytotoxicity of CD8+ T cells against tumor cells and IFN‐γ secretion. 49 Thus, curcumin dose should be carefully considered when used as an immunomodulator. Evidence of the immune modulation properties of curcumin in vitro and in vivo is summarized in Table 1.
FIGURE 1.
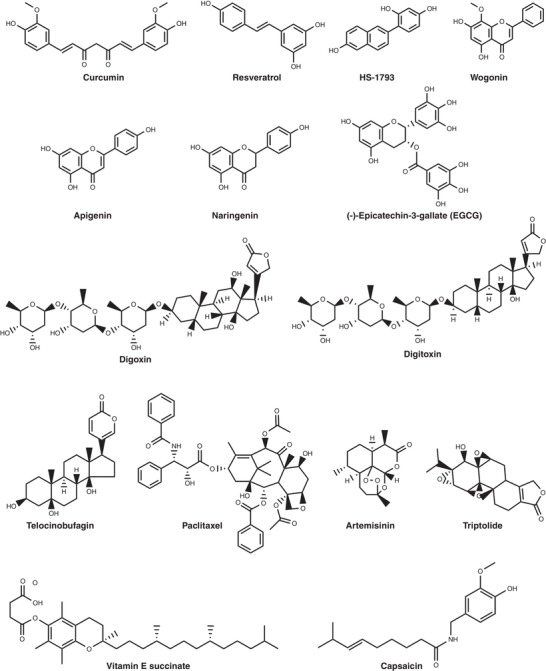
The chemical structure of representative natural products
TABLE 1.
In vitro and in vivo evidence of typical natural products on immune cells
| Cell type | Natural products | Dose | Experimental model | Type of cancer | References |
|---|---|---|---|---|---|
| T cells | Curcumin | 50 mg/kg, every alternate day, p.o. | Tumor‐bearing Swiss albino mice, healthy human volunteers | Any tumor | 45 |
| / | 4‐nitroquinoline‐oxide‐induced ♀C57BL/6 mice | Tongue carcinoma | 46 | ||
| 5, 10 μM | Cal 27 and FaDu cell lines | ||||
| 25, 50, or 100 mg/kg/d × 10 d, i.p. | 3LL tumor‐bearing ♀C57BL/6 or nude mice | Lewis lung carcinoma | 49 | ||
| 0.8 mg/mice | fLuc‐4T1 tumor‐bearing BALB/c mice | Breast cancer | 50 | ||
| 1, 5, 10, 20, 40 μM | 4T1 cells | ||||
| 2 mg/kg at days 6, 9, 12, 15 | B16F10 tumor‐bearing C57BL/6 mice | Melanoma | 51 | ||
| Resveratrol | 100 μM | JIMT‐1 cells | Breast cancer | 52 | |
| 10 μmol/mouse, paint | ♀C3H/HeN and ♀C3H/HeJ mice | Cutaneous carcinogenesis | 53 | ||
| 4 mg/kg, i.p. | EG7 tumor‐bearing ♀C57BL/6 mice | Lymphoma | 54 | ||
| 1, 2.5, 5 mg/kg, i.p. | Renca tumor‐bearing ♀BALB/c mice | Renal cell carcinoma | 55 | ||
| 50 μM | Nalm‐6.DR4 and Ramos.DR4 cells | Lymphoma | 56 | ||
| HS‐1793 | 0.5, 1 1.5 mg/kg, i.p. | FM3A tumor‐bearing ♀C3H/He mice | Breast cancer | 57 | |
| 0.3–2.5 μM, 72 h | Tumor‐bearing mice isolated splenocytes | Breast cancer | 58 | ||
| Scutellaria ocmulgee leaf extract | 100 mg/kg, p.o. | F98 tumor‐bearing F344 rats | Malignant gliomas | 59 | |
| Apigenin | 25 mg/kg, i.p. | TC‐1 tumor‐bearing ♀C57BL/6 mice | Cervical cancer | 60 | |
| Baicalein | 50 mg/kg | H22 tumor‐bearing ♀BALB/c mice or BALB/c‐nu/nu mice | Hepatocellular carcinoma | 61 | |
| Baicalin | 80 mg/kg | H22 tumor‐bearing ♀BALB/c mice or BALB/c‐nu/nu mice | Hepatocellular carcinoma | ||
| EGCG | ‐ | ♀SKH‐1 hairless mice | Cutaneous carcinogenesis | 62 | |
| 3 mg/mouse/200 μL acetone | ♀C3H/HeN mice | Cutaneous carcinogenesis | 63 | ||
| 0.1, 0.5, or 2.5 mg/mL | TC‐1 tumor‐bearing ♀C57BL/6 mice | Cervical cancer | 64 | ||
| Naringenin | 200 mg/kg p.o. | 4T1 tumor‐bearing ♀BALB/c mice | Breast cancer | 65 | |
| Procyanidin | 1.2 mg/mice | B16F10 tumor‐bearing ♀C57BL/6 mice | Melanoma | 66 | |
| Digoxin | 2 mg/kg, i.t. | B16F10 tumor‐bearing ♀C57BL/6 mice | Melanoma | 67 | |
| Telocinobufagin | 5, 25, 125 mg/L | ♂BALB/c mice isolated lymphocyte cell | / | 68 | |
| Cinobufagin | 0.5, 1, 2.5 mg/L | ♂BALB/c mice isolated lymphocyte cell | / | 69 | |
| Gamabufotalin | 8, 16 ng/mL | Human peripheral blood mononuclear cells | Glioblastoma and pancreatic cancer | 70 | |
| Paclitaxel | 0.04, 0.4, 4, 40 nM | OVCAR‐3 cells | Ovarian carcinoma | 71 | |
| 5 mg/kg, i.p. | MCA102 tumor‐bearing ♀C57BL/6 mice | Fibrosarcoma | 72 | ||
| 135 mg/m2, i.v. | Patients | Cervical cancer | 73 | ||
| 6.5, 13 mg/kg, i.v. | B16F10 tumor‐bearing ♂C57BL/6 mice | Melanoma | 74 | ||
| Artemether | 10 mg/kg, i.p. | Spontaneous mouse mammary tumor (SMMT)‐bearing ♀BALB/c mice | Breast cancer | 75 | |
| Dihydroartemisinin | 12.5, 25, 50,100, 200 mM | SW1990, BxPC‐3, PANC‐1 cells | Pancreatic cancer | 76 | |
| Artesunate | 0.03125, 0.125, 0.5, 2, 8 mg/L | HepG2 cells | Hepatocellular carcinoma | 77 | |
| Artemisinin | 100 mg/kg, i.p. | 4T1 tumor‐bearing ♀BALB/c mice | Breast cancer | 78 | |
| Triptolide | 100 nM | U251‐MG, T98G, U87‐MG, A172, LN229 and LN18 cells | Glioma | 79 | |
| 10 mg/kg, i.p. | B16F10 tumor‐bearing ♂C57BL/6 mice | Melanoma | 80 | ||
| 5 or 10 μg/kg | ♂Sprague‐Dawley rats | / | 81 | ||
| Platycodin D | 10 μM | NCI‐H1975 cells | Lung cancer | 82 | |
| POL‐P3b | 250 mg/mL | U14 cells | Cervical cancer | 83 | |
| Vitamin E succinate | 5, 10, 20 mg/mL | MKN28 cells | Gastric cancer | 84 | |
| Vitamin E | 2 mg/kg, i.p. | TC‐1 tumor‐bearing ♀C57BL/6 mice | Cervical cancer | 85 | |
| Capsaicin | 100, 200 μg, i.p. | Meth A. and CT26 tumor‐bearing BALB/cJ, BALB/cJ nu/nu mice | Fibrosarcomas | 86 | |
| B cells | Resveratrol | 20, 50 mg/mouse, i.p. | 4T1 tumor‐bearing ♀BALB/c mice | Breast cancer | 87 |
| 50, 500 mg/mouse, i.p. | B16F10 tumor‐bearing ♀C57BL/6 mice | Melanoma | |||
| Artesunate | 200 mg/kg, i.p. | BL‐41 tumor‐bearing NOD.Cg‐Prkdcscid Il2rgtm1Wjl/SzJ mice | Lymphoma | 88 | |
| MDSCS | Curcumin | 50 mg/kg | LLC cells tumor‐bearing ♀C57BL/6 mice | Lewis lung carcinoma | 48 |
| Polyphenon E | 0.3% in drinking water | Tumor‐bearing SCID mice | Neuroblastomas | 89 | |
| Silibinin | 150 mg/kg, s.c. | 4T1 tumor‐bearing ♀BAlB/c mice | Breast cancer | 90 | |
| NKs | Asiatic acid | 10 mg/kg, i.p. | B16F10 ang LCC tumor‐bearing ♂C57BL/6 mice | Melanoma and Lung carcinoma | 91 |
| Naringenin | 50 mg/kg i.p. | B16F10 ang LCC tumor‐bearing ♂C57BL/6 mice | Melanoma and Lung carcinoma | ||
| Ouabain | 0.75, 1.5, 3 mg/kg, p.o. | WEHI‐3 tumor‐bearing ♂BALB/c mice | Leukemia | 92 | |
| Artemisinin | 0.1 μM | K562 cells | Leukemia | 93 | |
| Artesunate | 6.25 mg/L | Colon26 cells | Colorectal cancer | 94 | |
| 12.5 mg/L | Colorectal cancer RKO cells | Colorectal cancer | |||
| Ginsenoside F1 | 25 mg/kg, i.p. | B16F10 tumor‐bearing C57BL/6 mice | Melanoma | 95 | |
| Macrophages | Hydrazinocurcumin | 100 μM 3 d intervals × 5 times, i.v. | 4T1 tumor‐bearing ♀BALB/c mice | Breast cancer | 96 |
| Dendrosomal curcumin | 40, 80 mg/kg, 35 consecutive days | 4T1 tumor‐bearing ♀BALB/c mice | Breast cancer | 97 | |
| Resveratrol | 100 mg/kg, i.p. | LCCs tumor‐bearing C57BL/6 mice | Lung cancer | 98 | |
| Bufalin | 0.1, 0.2 or 0.4 mg/kg, p.o. | WEHI‐3 tumor‐bearing ♂BALB/c mice | Leukemia | 99 | |
| Cinobufagin | 0.0125‐0.05 g/mL | BALB/C mice | / | 100 | |
| G. atrum polysaccharide (PSG‐1) | 50, 100, 200 mg/kg | CT26 tumor‐bearing mice | Colon cancer | 101 | |
| 50, 100, 200 mg/kg | S180 tumor‐bearing BALB/c mice | Sarcoma | 102 | ||
| Capsaicin | 100 μg | CT26 tumor‐bearing BALB/cJ or nu/nu mice | Colon cancer | 103 | |
| DCs | Paclitaxel | 75 mg/m2 | Patients | Prostate cancer | 104 |
| POL‐P3b | 50, 100, 200 mg/kg | U14‐bearing ♀Kunming mice | Cervical cancer | 105 | |
| Capsaicin | 32 μg/mL | MG‐63 cells | Osteosarcoma | 106 | |
| IDO | Epigallocatechin‐3‐Gallate (EGCG) | 10, 50, 100 μM | Caco2, HCT116, HT29, SW480 and SW837 cells | Colorectal cancer | 107 |
| Paclitaxel | 25 mg/kg, i.v. | 4T1.2 tumor‐bearing ♀BALB/c mice | Breast cancer | 108 | |
| Cytokines | Wogonin | 100 μM | Mouse gastric carcinoma MFC cells tumor‐bearing mice | Gastric carcinoma | 109 |
| Immune checkpoints | EGCG | 0.3% in drinking water | 4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanone treated A/J mice | Nonsmall‐cell lung cancer | 110 |
| Splenocytes | Resveratrol | 15, 30, 60 mg/kg | Specific‐pathogen‐free mice | / | 111 |
Note: oral administration, p.o.; intratumoral injection, i.t.
Curcumin derivatives, such as hydrazinocurcumin encapsulated nanoparticles or dendrosomal curcumin, have been found to exert immunomodulatory effects via macrophages. Curcumin derivatives induced TAMs repolarization from tumor‐promoting M2 phenotype toward the more antitumor M1 phenotype by inhibiting STAT3 when cocultured with 4T1 cells, and subsequently inhibiting breast tumor growth, angiogenesis, and metastasis, as well as prolonging tumor‐bearing mice survival in vivo. 96 , 97 Recently, novel drug loading strategies were applied to increase the efficiency of tumor immunotherapy. For example, a lipid‐encapsulated formulation of curcumin caused tumor remission in 50% of GL261‐implanted glioblastoma mice through changing the polarity of tumor‐associated microglia, inhibiting the tumor‐promoting Arginase1high, iNOSlow M2‐type tumor‐associated microglia population while activating the Arginase1low, iNOShigh M1‐type tumoricidal microglia. 112 The combination immunotherapy strategy containing curcumin efficiently induced the immunogenic cell death (ICD) of residual cancer cells, and consequently enhanced the tumor immunogenicity and sensitized the tumor to antitumor T cell immunity. 50 Another effective antitumor immunotherapy involved a core‐shell structural nanodrug, loading NF‐κB inhibitor curcumin, and anti‐PD‐1 antibody, which was pH‐sensitive, which markedly improved the antitumor immunotherapeutic effect both in vitro and in vivo. 51
Resveratrol
Resveratrol (Fig. 1) exists in a variety of plants, such as berries, grapes, mulberries, and pomegranates. 33 , 113 Several review articles have described the antitumor potential of resveratrol in preclinical and clinical studies against various cancers, such as leukemia, breast and lung cancer, and hepatocellular carcinoma, as well as the antitumor mechanisms of resveratrol, including induction of apoptosis and autophagy, cell cycle disruption, inhibition of angiogenesis, and epithelial‐mesenchymal transition. 33 , 114 , 115 , 116 , 117 Although the utility of resveratrol as tumor immunotherapy has been reported in several published reviews, 29 , 118 , 119 , 120 we reviewed the relevant studies of considerable significance in recent years. As the PD‐1/PD‐L1 signaling pathway plays a significant role in immune tumor evasion, 121 new inhibitors of the PD‐1/PD‐L1 signaling pathways are urgently needed. Menendez's group demonstrated that resveratrol enhanced cytotoxic T lymphocytes against tumor cells, probably by targeting the N‐linked glycan decoration of PD‐L1 through GSK3β activation. 52 In their research, resveratrol was predicted to bind PD‐L1 dimerization using molecular docking and molecular dynamics simulations. However, direct evidence of the interaction between resveratrol and PD‐L1 dimerization is lacking, such as a cocrystallization of resveratrol directly bound to PD‐L1 dimerization. Elmets's group observed that the TLR4 activation plays a protective role in 7,12‐dimethylbenz(a)anthracene‐induced cutaneous carcinogenesis, which can be prevented by resveratrol via TLR4 targeting to enhance the cell‐mediated immune response. 53 , 122 The above conclusion could be assumed owing to the fewer tumors formed, smaller tumor size, and higher levels of IFN‐γ and IL‐12 in the TLR4 competent mice treated with resveratrol, and not TLR4 deficient mice. Additionally, resveratrol inhibited angiogenesis and increased the cell‐mediated immune response to a considerable extent in the TLR4 competent mice than in TLR4 deficient mice. 53 In EG7 tumor‐bearing C57BL/6 mice, resveratrol treatment induced changes in the Tregs population and tumor‐associated immunomodulatory cytokines, as evidenced by the decrease of the CD4+CD25+ cell population among CD4+ cells, FoxP3+ expression, as well as secretion of TGF‐β ex vivo and in vivo, and the increase of IFN‐γ expression in CD8+ T cells ex vivo and in vivo. 54 Xiong et al. observed that the inhibition of Renca tumor growth by resveratrol depended on CD8+ T cells. Resveratrol switched the expression of Th2 cytokines (e.g., IL‐6 and IL‐10) to Th 1 cytokines with modulation of Fas expression through IFN‐γ and inhibited angiogenesis via the decreased level of vascular endothelial growth factor (VEGF) in the TME. 55 Additionally, resveratrol, at low and noncytotoxic doses for immune cells, efficiently inhibited tumor growth and lung metastasis in 4T1 tumor‐bearing mice through the inactivation of STAT3, as well as by preventing the generation and function of regulatory B cells (tBregs). Furthermore, resveratrol inhibited TGF‐β production from tBregs. 87 Similarly, in a mouse lung cancer xenograft, STAT3 inactivation was also observed in resveratrol inhibited M2 polarization. 98 Interesting, low doses of resveratrol treatment (50 μM) up‐regulated the expression of Rab 4B, resulting in increased HLA class II protein recycling and presentation, as well as restoring the CD4+ T cell recognition of B‐cell lymphomas, 56 indicating that resveratrol can be used for restoring the immune defense to prevent cancer recurrence. Similar to levamisole, resveratrol also demonstrated potent immune enhancing activity through the activation of NF‐κB in immunosuppressive mice. 111 Evidence of the immune modulation properties of resveratrol in vitro and in vivo is summarized in Table 1 and Figure 2.
FIGURE 2.
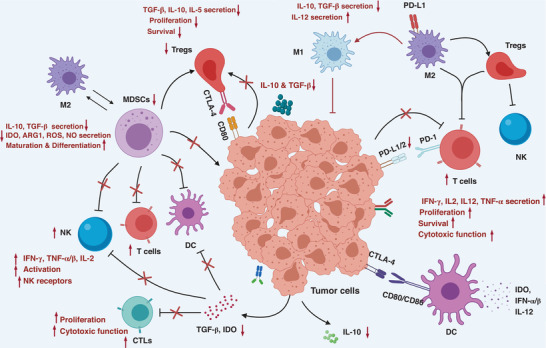
Proposed models for the molecular mechanisms of immunomodulatory effects by natural productions. Inhibitory effects of natural products on tumors through enhancing the cytotoxic function of effect T cells and NKs, attenuating CD4+CD25+Foxp3+ regulatory T cells (Tregs), reducing myeloid‐derived suppressor cells, decreasing TGF‐β, IL‐10, IL‐5, indoleamine 2,3‐dioxygenase, ARG1 levels, inducing IL‐12, IFN‐γ, and TNF‐α/β production, and preventing cell‐cell interaction of Tregs via the inhibition of CTLA‐4 and PD‐L1
HS‐1793 (Fig. 1), a resveratrol derivative with better stability and more effective tumoricidal activity, has been synthesized. 123 HS‐1793 induced an in vivo antitumor effect in FM3A tumor‐bearing mice through the suppression of CD4+/CD25+/Foxp3+ Tregs and production of TGF‐β, the increase of IFN‐γ‐expressing CD8+ T cells, and the up‐regulation of IFN‐γ production. 57 The similar molecular mechanisms of HS‐1793 induced the modulation of tumor‐derived T cells. 58
2.1.2. Flavonoids
Most plant‐based foods are rich in flavonoids, especially the dark fruits and vegetables, as well as dark chocolate, red wine, and tea. They consist of 15 carbon atoms, 2 phenyl rings, and 1 heterocyclic ring. Flavonoids such as flavones (e.g., apigenin, wogonin, baicalein, and baicalin), flavanes (e.g., catechins), flavonols (e.g., icariin), flavanones (e.g., naringenin), isoflavones, and anthocyanidins (procyanidin) have anti‐inflammatory effects and anticancer activities. 124 , 125 In this review, we only discussed flavones, flavanes, flavonols, and flavanones.
Flavones
Wogonin (Fig. 1) is one of the main flavonoids in the extract of Scutellaria baicalensis Georgi. It possesses antioxidant, anti‐inflammatory, immunomodulatory, and antitumor activities. 126 Wogonin can reverse the viability of chronic lymphocytic leukemia cells in vitro and prevent the development of leukemia in mice after the adoptive transfer of Eμ‐T‐cell leukemic cells through the regulation of TNF‐α‐induced NF‐κB activity. 35 Wogonin demonstrated a strong antitumor immune effect in vivo by inducing the ROS‐mediated endoplasmic reticulum stress response, and then activated the PI3K pathway to induce the translocation of calreticulin (CRT) and annexin A1, along with the release of high‐mobility group protein 1 and ATP. 109 It has also been reported that wogonin can reverse tumor‐induced immune inhibition through the suppression of Treg cell function, as evidenced by the decreased secretion of TGF‐β1 and IL‐10 in Tregs culture, and activation of p38 MAPK pathway. 59 In vivo studies have shown that wogonin has a significant inhibitory effect on early chronic lymphoid leukemia, mouse gastric cancer xenografts, and gliomas. 35 , 59 , 109
Apigenin (Fig. 1), is a bioavailable flavonoid, derived from a variety of fruits, vegetables, and drinks. Apigenin has anti‐inflammatory, antioxidant, and anticancer characteristics. 127 , 128 , 129 Apigenin also inhibited the expression of IFN‐L1‐induced PD‐L1 protein in breast cancer patients, inh6ibited the phosphorylation of STAT1 tyrosine 701 site to inhibit the up‐regulation of IFN‐L1 induced PD‐L1, and induced more effective T cells killing, 130 or apoptosis of human cervical cancer cells induced by the p53 dependent pathway. 131 In vivo studies have shown that it has a significant inhibitory effect against A375 melanoma xenografts. 132 Similarly, baicalein and baicalin inhibited STAT3 activity, down‐regulated PD‐L1 expression, and enhanced the killing ability of T cells. 61 Furthermore, apigenin enhanced the anticancer effect toward cervical cancer in vitro and in vivo, by enhancing the ability of HPV DNA vaccination to trigger IFNγ‐inducing CD8+ T cell. 60
Flavanes
Flavanes, also named catechins, are the major constituents of green tea, such as (−)‐Epicatechin‐3‐gallate (EGCG, Fig. 1), (−)‐Epigallocatechin, and (−)‐Epicatechin, exerting anticancer activities in various tumors. Flavanes These effects are partially attributed to their antioxidant, antiangiogenic and antimutagenic effects, as well as their anti‐inflammatory activities. 133 Reportedly, in an in vivo tumor model, increased numbers of CD8+ T cells were observed in (−)‐epigallocatechin‐3‐gallate‐treated tumors when compared with the non‐EGCG‐treated tumors. 62 Katiyar et al. reported that EGCG increased the number of CD8+ CTLs infiltrating the TME, inducing a direct cytotoxicity on tumor cells, enhancing the release of IL‐12, and thereby inducing a Th1 response against tumors. 63 EGCG also improved the antitumor effects of DNA vaccination through the enhanced tumor‐specific T cell immune response, as evidenced by a significant increase in E7‐specific CD8+/CD4+ T cell‐mediated immune responses when co‐treated with Sig/E7/LAMP‐1 DNA vaccination and EGCG. 134 EGCG restored the killing ability of T cells by suppression of immune checkpoint PD‐L1/PD‐1 signaling and, finally, inhibition of lung cancer growth. The underlying mechanisms include IFN‐γ, EGF, and JAK2/STAT1 signaling. 110 In colorectal cancer cells, EGCG down‐regulated the expression and enzymatic activity of IFN‐γ‐induced indoleamine 2,3‑dioxygenase (IDO) through the suppression of STAT1 activation. 107
Flavonols
Icariin (Fig. 1) is isolated from Epimedium plants and possesses anti‐inflammatory effects, immunologic regulation, and anticancer potency. 135 Furthermore, it has immunoadjuvant effects on enhancing the Th1‐immune response, suggesting that icariin may serve as an adjuvant for tumor immunotherapy. Icariin could increase the CTLs response toward the P815AB peptide in tumor‐bearing DBA/2J mice. 136 Reportedly, icariin and its derivative reduced the proportion of MDSCs in the spleen of tumor‐bearing mice, decreased the production of NO and ROS, and finally delayed the development of tumors. 137
Flavanones
Naringenin (Fig. 1), mainly isolated from citrus, functions as an effective inhibitor of Smad3 and potential immunomodulator. 138 , 139 Treatment with asiatic acid and naringenin generated an synergistic effect on the inactivation of TGF/Smad3 signaling, suppressed melanoma, and lung carcinoma growth by enhancing NKs killing against cancer via a mechanism associated with Id2 and IRF2. 91 Naringenin administration decreased the number of MDSCs in the spleen and lungs of tumor‐bearing 4T1/TGF‐β1 mice overexpressing the immunosuppressive TGF‐β1 cytokine. Furthermore, naringenin increased the level of activated T cells. 65 Additionally, procyanidin and silibinin enhances the function of T cells and reduces the number of circulating MDSCs in mice bearing cancer cells, respectively. 66 , 90
2.2. Cardiac glycosides (CGs)
CGs are compounds containing a steroid‐like structure and an unsaturated 5‐ or 6‐membered lactone ring as the common parent nucleus, including one or more sugar moieties located at C3 (Fig. 1). Usually, CGs are divided into two main categories: bufadienolides bearing the lactone 2‐furanone at C17 (e.g., bufalin and telocinobufagin) and cardenolides bearing the lactone 2‐pyrone at C17 (e.g., ouabain, digitoxin, and digoxin). 140 Most CGs have been isolated from plants, including Digitalis purpurea, Digitalis lanata, Strophanthus gratus, Kalanchoe, Helleborus, Cotyledon, and Nerium oleander. Additionally, some CGs have also been observed in amphibians (e.g., Bufonidae) and snakes (e.g., Rhabdophis tigrinus). 141 Traditionally, CGs (e.g., digitoxin and digoxin) have been approved by the FDA for the treatment of heart failure and atrial arrhythmias (www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/default.htm). The specific inhibition of the Na+/K+‐ATPase pump is the mechanism by which CGs affect myocardial contraction. 142 Recently, preclinical experiments revealed that CGs (e.g., bufalin and digitoxin) exhibited potent anticancer effects. 140 , 143 Additionally, the Huachansu injection containing bufadienolides (e.g., bufalin, cinobufagin, cinobufotalin, and resibufogenin) was approved by the Chinese FDA to treat cancer, and several CGs (e.g., Digitalis, Anvirzel and UNBS1450) entered clinical trials for the treatment of solid tumors, such as liver, lung, and breast cancer (see http://clinicaltrials.gov/ and the literature 140 , 144 , 145 , 146 , 147 ).
2.2.1. Cardenolides
An ICD inducer is a compound that possesses cytotoxicity while triggering an immune response toward dead cell‐associated antigens. 148 To identify novel inducers of ICD, Kroemer's group used a fluorescence microscopy platform to screen the U.S. FDA‐approved 120 anticancer drugs and a library encompassing 879 chemicals in human osteosarcoma U2OS cells. They identified cardenolides, including digoxin, digitoxin, ouabain, and digitoxigenin as potent ICD inducers. 149 , 150 They found cardenolides (e.g., digoxin and digitoxin)‐induced ICD was associated with the suppression of Na+/K+‐ATPase in cell membranes. In immunocompetent mice, digoxin enhanced the antitumor effect of DNA‐damaging agents cisplatin and mitomycin C. A retrospective analysis (145 cancer patients treated with digoxin and 290 cancer patients untreated with digoxin) demonstrated an improved 5 yr survival rate in patients treated with digoxin. 150 Huang et al. reported that the combination strategy of cisplatin prodrug coadministration with digoxin effectively induced ICD by promoting DC maturation, activating CD8+ T cell responses, and completely eradicating residual tumors in B16F10 tumor‐bearing mice. 67 Furthermore, these two research groups demonstrated that tumor cells succumbing to chemotherapeutic drugs (cisplatin and cisplatin prodrug) and digoxin could vaccinate syngeneic mice against the subsequent challenge with living cells of the same type in vivo. 67 , 150 These combination strategies suggest that digitoxin can ameliorate the efficacy of nonimmunogenic anticancer therapies and generate vaccine‐like functions for improved immunochemotherapy.
2.2.2. Bufadienolides
For the past few years, several researchers have focused on the anticancer effects of bufadienolides, partly by enhancing the immune system function. For example, Chung's group observed that both cardenolide ouabain and bufadienolide bufalin promoted immune responses in murine WEHI‐3 leukemia cells in mice in vivo, evidenced by the decreased liver and spleen weights, modulation of immune‐associated leukocyte markers, such as CD3 (T cells), CD19 (B cells), Mac‐3 (macrophages), and CD11b (monocytes), restoration of glutamic‐oxaloacetic transaminase, glutamic‐pyruvic transaminase, and lactate dehydrogenase levels, and the activation of macrophage phagocytosis. 92 , 99 Furthermore, telocinobufagin and cinobufagin activated the immunologic system in vitro, including stimulate splenocyte proliferation, enhance the activation of NKs and the phagocytosis ability of macrophage, and increase the percentage of CD4+/CD8+ cells in splenocytes and the ratio of Th1/Th2 (the levels of Th1 cytokines such as IL2, IL12, IFN‐γ, and TNF‐α are up‐regulated while the levels of Th2 cytokines such as IL4 and IL10 are down‐regulated after these two agents treatment). 68 , 69 , 100 Moreover, γbufotalin, at a concentration of 8 ng/mL (IC50 value in human pancreatic cancer cell line SW1990), almost nontoxic to peripheral blood mononuclear cells (PBMCs) (IC50 value is 44.1 ± 2.4 ng/mL), efficiently decreased the ratio of CD4+CD25+Foxp3+ Tregs in mitogen‐stimulated PBMCs, suggesting that γbufotalin not only exerts a killing effect on tumor cells, but also enhances the antitumor immunity by inhibiting the expansion and function of Tregs. 70 In addition to the immunomodulatory effects mentioned earlier, Yang et al. reported that bufalin inhibited the proliferation and migration of HCC Bel‐7404 and SK‐Hep1 cells, which may be attributed to its ability to suppress the APOBEC3F‐induced intestinal immune network. 151
2.3. Terpenoids
Terpenoids are a class of compounds derived from mevalonic acid, possessing a basic carbon structure with two or more isoprene units (C5 units), such as monoterpenes (comprised of 10 carbon atoms, C10), sesquiterpenes (C15), diterpenes (C20), and so on. It is widely present in plants and has extensive pharmacologic actions, including anti‐inflammatory, immunomodulatory, antibacterial, antitumor, and neuroprotective effects. 152 These natural compounds are widely used in medicine, for example, paclitaxel and its derivatives as common antitumor drugs were used for the treatment of nonsmall‐cell lung cancer, ovarian cancer, and breast cancer. 153 Artemisinin and its derivatives are currently the most effective treatments for malarial parasite infection. 154 Here, we highlighted the role of these compounds in tumor immunity.
2.3.1. Paclitaxel
Paclitaxel (Taxol, Fig. 1) is a well‐known natural anticancer drug in clinical use. It is a tricyclic diterpenoid compound, isolated from the bark and needles of Taxus brevifolia. 153 There have been several published reviews on the antitumor effects of paclitaxel and its analogs, as well as its role in tumor immunotherapy. 153 , 155 , 156 Tumor‐infiltrating DCs are considered potent antigen‐presenting cells. DCs can activate the CTLs or tolerogenic DCs to suppress the immune reaction against tumor cells to escape. Reportedly, a low and nontoxic concentration of paclitaxel stops the pre‐DCs to be tolerogenic DCs and maintain DC functions, thus suppressing the immune reaction against tumors to escape. 71 Moreover, DCs can induce antigen‐specific CTLs, and an injection of paclitaxel induces tumor‐specific cytotoxic T lymphocyte response and acquisition of prolonged tumor immunity. 72 Jordanova et al. showed that paclitaxel treatment lead to a markable reduction in proliferating (Ki67+) CD3+CD8− T cells and FoxP3+ (CD3+CD8−) Tregs, with increased rates of CTLs, providing a theoretical basis for paclitaxel in the treatment of tumor stroma. 73 In chemotherapy, a high concentration of paclitaxel is used for antitumor treatment but is toxic to immune cells. Therefore, many vectors have been used to study the reduction of paclitaxel toxicity. Many studies demonstrated that systemic delivery of paclitaxel using a nanocarrier or receptor resulted in a significantly improved antitumor response. 108 , 157 For example, paclitaxel derivative‐loaded nanoparticles showed lower cytotoxicity toward bone marrow‐derived macrophages (BMDMs) than free paclitaxel, up‐regulating the CD11b expression in BMDMs. This nanoparticle polarized macrophages toward M1 and inhibited their M2 differentiation, both on phenotypic and functional levels. Accordingly, they also observed similar results in B16F10 melanoma tumor inhibition in vivo, which was associated with immune system stimulation. 158 Moreover, paclitaxel is used not only as a chemotherapeutic agent for the treatment of tumors, but also in combination with immunotherapeutic agents, such as IL‐2, IDO inhibitor NLG919, and a DC‐based cancer vaccine. 74 , 104 , 159 Recently, novel drug loading strategies were applied to increase the efficiency of tumor immunotherapy. For example, an effective antitumor immunotherapy composed of a nanodrug loading paclitaxel and anti‐PD‐1 antibody, which was pH and matrix metalloproteinase dual sensitive, markedly improved the antitumor immunotherapeutic effect both in vitro and in vivo. 160 Although the direct anticancer effects of paclitaxel on various cancer cells remain well known, paclitaxel also acts as an adjuvant drug to regulate immune cells, and its mechanism of action is becoming increasingly evident. However, cancer is difficult to cure as the tumor‐associated microenvironment is an extremely complicated system that involves several kinds of multifunctional immunizing cells and molecules, as well as tumor cells. The function of paclitaxel alone or in combination with other immunotherapies in tumor interventions need to be further investigated.
2.3.2. Artemisinins
Artemisinin (Fig. 1) is a natural compound derived from the Chinese herb Artemisia annua, also known as Qinghao or sweet wormwood. Artemisinin and its synthetic derivatives (e.g., dihydroartemisinin, artesunate, artemether, artether) have demonstrated immunotherapeutic effects against a range of cell lines and are also effective against drug‐resistant cancer cell lines. 161 , 162 Artemisinin enhanced the human NK cell line NK‐92MI, and primary NK cell cytotoxicity against leukemia K562 cells via the stimulation of granule exocytosis, mediated by the activation of Vav‐1 and ERK1/2 signaling. 93 In addition to NKs killing and lymphocyte proliferation, the antitumor mechanisms of artesunate in vitro also involved blocking the secretion of immunosuppressive factors, such as TGF‐β1 and IL‐10, in colorectal cancer colon26 (murine) and RKO (human) cells. 94 Artemether not only promoted the delayed‐type hypersensitivity (DTH) response and hemagglutination antibody production in normal mice, but also inhibited tumor growth in spontaneous mouse mammary tumor‐bearing BALB/c mice, which may be attributed to the depletion of immunosuppressive cells CD4+CD25+Foxp3+ Tregs in the spleen. 75 The enhanced DTH response was also observed in dihydroartemisinin‐treated BALB/c mice against sheep red blood cells. 163 γδ T cells play an important role in the antitumor activity of dihydroartemisinin and artesunate against pancreatic cancer cells (SW1990, BxPC‐3, and PANC‐1) and liver cancer cells (HepG2), respectively. 164 , 165 Mechanistically, dihydroartemisinin increased the expression of intracellular perforin, granulase B, and the production of IFN‐γ, and then enhanced the γδ T cell‐mediated killing activity. 164 In contrast, elevating the expression of GraB and Fas, as well as reducing the secretion of TGFβ1, may be important artesunate mechanisms promoting the antitumor activity of γδ T cells. 165 Furthermore, artemisinin enhanced the antitumor immune response in 4T1 breast cancer cells in vivo by promoting T cell function and quelling immunosuppression from Tregs and MDSCs in the tumor. 78 A recent study has also shown that the topical administration of artemisinin could suppress contact the hypersensitivity response and Con A‐induced T cell proliferation. 166 Notably, a novel immune regulatory function of dihydroartemisinin involves the reciprocal regulation of Th and Tregs generation by modulating mTOR signal. 167 Furthermore, artesunate, a semisynthetic analog of artemisinin, demonstrated potent apoptosis‐inducing effects across a broad range of B‐cell lymphoma cell lines in vitro, and prominent antilymphoma activity in vivo, indicating its relevance for the treatment of B cell lymphoma. 88
2.3.3. Triptolide
Triptolide (Fig. 1) is a biologically active diterpene triepoxide extracted from Celastraceae, and possesses antiproliferative properties against several types of cancers. 168 Recently, a study demonstrated that triptolide may be used to reverse CD4+ T cell inhibition caused by glioma cells and is an alternative candidate for targeting PD‐L1, one of the checkpoint inhibitors for the treatment of glioma. 79 Triptolide treatment also induces the down‐regulation of CD4+CD25+Foxp3+ Tregs, and up‐regulation of IL‐10, TGF‐β, and VEGF in melanoma‐bearing mice. 80 Interestingly, triptolide can treat tumors, as well as cancer‐related pain by regulating the functions of other immune cells, such as T cells. 81 , 169
2.4. Saponins
Saponin is a type of glycoside whose aglycon is a triterpene or a spiral sterane compound. It is mainly distributed in higher terrestrial plants and is also observed in small amounts in marine organisms, such as starfish and sea cucumber. 170 Saponin exhibits a wide range of biologic and pharmacologic properties, including analgesic, antipyretic, anti‐inflammatory, immunomodulatory, and antitumor activities. 170 Saponin mainly includes steroidal saponin and triterpenoid saponin, of which triterpenoid saponin plays an important role in tumor immunotherapy. Reportedly, quillaja saponin‐21, a highly purified saponin extracted from the Chilean tree Quillaja saponaria Molina, is among the most potent immunologic adjuvants. 171 It has been shown that saponin‐based ISCOMATRIX vaccines could treat tumors by activating the immune response of CD4+ and CD8+ T cells. 172 , 173 The saponin‐based vaccine, chitosan hydrogel, generated effector CD8+ T cells in a mouse model. Additionally, other triterpenoid saponins also have tumor immunotherapeutic effects. 174 Platycodin D, a triterpenoid saponin isolated from Platycodon grandiflorus (Jacq.) A., triggered the extracellular release of PD‐L1 and PD‐treated cancer cells, restoring Jurkat T cells activation. 82 Ginsenoside F1, a triterpenoid saponin component of ginseng, enhanced NK function and may possess chemotherapeutic potential in NK‐based immunotherapy. 95
2.5. Other natural products
In addition to the earlier mentioned natural products, there are some several others that have not mentioned, such as polysaccharides (e.g., mushroom extract), anthracyclines, polyunsaturated fatty acids, ginger, which have been previously described. 26 , 27 Here, the parent review just briefly summarized part natural products, reported in previous reviews.
2.5.1. Polysaccharides
Polysaccharides are a kind of polymers formed by the connection of aldose or ketose through glycoside bonds and are found mainly in plants, animals, and microorganisms. Studies have shown that polysaccharides have a wide range of pharmacologic activities, such as antitumor, immune regulation, antiviral, antiaging, hypoglycemic, and blood lipid activities, especially in antitumor immunity with minimal adverse reactions. 175 , 176 , 177 , 178 For example, Portulaca oleracea L. polysaccharides (POL‐P3b), derived from the monodentate plant, demonstrated tumor growth inhibition and immunoregulation, and can be used for treating cervical cancer and for enhancing immunity. 83 , 105 As a biologic response regulator, polysaccharides promote cellular humoral and immune responses to inhibit and eliminate tumor cells. POL‐P3b can increase the level of Th1‐type cytokines (IL‐12) and decrease the level of Th2‐type cytokines (IL‐10). 83 Additionally, POL‐P3b can also induce apoptosis of intestinal DC by stimulating the TLR4‐PI3K/AKT‐NF‐κB signaling pathway. In vivo studies have shown that POL‐P3b possess a significant inhibitory effect on cervical cancer xenografts. 105 Xie's group reported the immunoregulatory activity of the polysaccharide from Gganoderma atrum (PGA) in CT26 and S180 tumor‐burdened mouse models, as evidenced by the activation of macrophage phagocytosis and inhibition of tumor growth by PGA through the inhibition of the MAPK signal pathway, induced by TLR4. 101 , 102 Several studies indicated that the antitumor immune mechanism of lentinan polysaccharide, especially β‐glucans, is associated with immune cells, activating immune cells through signaling pathways, such as CR3‐Syk‐PI3K signaling, and NF‐κB, to enhance the antitumor activity, as well as cytokines (e.g., IFN‐γ). 179 , 180 , 181 , 182 , 183 , 184 The immunomodulatory mechanisms of these compounds are also summarized in reviews. 185 , 186 Furthermore, clinical studies indicated that lentinan showed effective tumor growth inhibition in gastric, nonsmall‐cell lung cancer, ovarian, or colorectal cancers. 187 , 188 , 189 Reportedly, polysaccharides from Plantago asiatica, aloe, ginseng, and achyranthes also improved the immune cell activity in tumor‐bearing mice. 190
2.5.2. Vitamin E succinate (VES)
VES is one of the eight isomers of vitamin E, 191 and possesses strong anticancer properties, including inhibition of tumor cell proliferation, the introduction of apoptosis, as well as suppression of tumor progression in tumor‐bearing mice in multiple malignant tumors, such as prostate, breast and gastric cancer, and melanoma, lymphoma, and leukemia. 192 , 193 , 194 , 195 , 196 , 197 VES selectively stimulates the expression of TRAIL and TRAIL receptors in human CD4+ T cells and enhances the expression of TRAIL in nonstimulated CD4+ T cells. The binding of VES to human CD4T cells enhances the anticancer effect of VES on Human gastric cancer cell line MKN28. 84 Vitamin E demonstrated potent antitumor effects against the HPV16 E7‐expressing TC‐1 tumor model by reducing the immunosuppression mediated by MDSCs and CD8+ T cells. Moreover, vitamin E therapy can be combined with the active immunity of antigen‐specific tumor vaccine, resulting in a strong antitumor effect. 85 Furthermore, the effect of natural vitamins, such as vitamins A–E have been systematically reviewed. 28 , 198
2.5.3. Capsaicin
Capsaicin (Fig. 1), a derivative of vanillyl amide, is the pungent component of red peppers. 199 The pharmacologic effects of capsaicin include enhancing immunity, resisting inflammation, lowering blood pressure, reducing excessive blood coagulation, and lowering blood sugar levels. 200 Capsaicin could target TAMs, accompanied by the apoptosis and destruction of tumor‐associated stromal cells, which is tumor‐specific T cells dependent, depleting the immunosuppressive host cell population at the tumor site, and thereby altering the cytokine profile at the microenvironment. 103 Another mechanism by which tumors escape the body's immune attack is by increasing the intratumoral population of Tregs and depleting CD8+ CTLs. Intratumoral administration of capsaicin has been shown to increase the CTL population while decreasing the numbers of Tregs. Furthermore, capsaicin demonstrated in vivo inhibition in the Meth A fibrosarcoma and CT26 xenograft tumors. 86 In human osteosarcoma cells, capsaicin can induce the translocation of a large quantity of CRT from intracellular compartments to the cell surface. Additionally, CRT on the human OS cell surface can be used as specific signaling molecules to promote the phagocytosis of tumor cells, thereby mediating tumor cell immunogenic death. 106 Capsaicin induced the pre‐ and early apoptotic cell surface exposure of CRT, HSP90, and HSP70, as well as ATP release. 201
3. CONCLUSION AND FUTURE PERSPECTIVE
Compared with tumor immunity targeted therapies, such as durvalumab and ipilimumab, natural products (e.g., polysaccharide) have an extensive range of immunomodulatory activities, abundant sources, and fewer adverse reactions. In the last decades, series of studies have revealed that natural products exert anticancer activities based on immunoregulation in vitro and in vivo. Most recently, some natural product derivatives with enhanced stability and efficacy have been developed through chemical modification, indicating an attractive prospect for the development of natural products as novel tumor immunotherapy. For example, natural vitamins C, D, and E have been tested in clinical trials. Curcumin alone is not clinically effective due to its absorption and metabolism issues, but its derivatives (e.g., demethoxycurcumin, bis‐demethoxycurcumin) are undergoing clinical trials evaluations. Currently, more than 100 ongoing trials on resveratrol are listed on www.clinicaltrials.gov. These findings provide a new approach for the development of novel and effective tumor immunotherapies. Moreover, the combination of natural products with traditional antitumor therapies targeting tumor cells can further exert their advantages in targeted therapies, with synergistic effects and reduced toxicity. They could provide a safer and more effective strategy for clinical interventions. It is expected that natural products will demonstrate promising breakthroughs in tumor immunotherapy in the future.
The immunoregulation mechanisms of natural products are complex and involve multiple signal transduction pathways. Although natural products and their derivatives, as modulators of tumor immunotherapy have made encouraging progress and appear promising in in vitro and in vivo preclinical studies in this review, the following problems still exist in this field: (i) Due to the characteristics of individual patient differences, tumor heterogeneity, and TME differences, the overall effective rate of tumor immunotherapy is still relatively low, and the target population is relatively small. (ii) Most natural products possess extensive pharmacologic effects, but their targets and molecular mechanisms have not been fully elucidated, especially those related to tumor immunity, and there is still a considerable lag in developing natural products as tumor immunotherapy.
AUTHORSHIP
J.‐X.C. and L.‐J.D. designed the manuscript. L.‐J.D., M.Q., and N.L. drafted the manuscript. Y.‐H.L. and D.‐M.Z. participated in the procedures. J.‐X.C. and D.‐M.Z. helped to design the manuscript and with its revision. Li‐Juan Deng, Ming Qi, and Nan Li contributed equally to this work.
DISCLOSURES
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by the key research project supported by National Natural Science Foundation of China (81973748, 81630104, 81803790, and 81904077), National Natural Science Foundation of Guangdong (2020A1515011090 and 2018A0303130112) and the Huang Zhendong Research Fund for Traditional Chinese Medicine of Jinan University (201911).
Deng L‐J, Qi M, Li N, Lei Y‐H, Zhang D‐M, Chen J‐X. Natural products and their derivatives: promising modulators of tumor immunotherapy. J Leukoc Biol. 2020;108:493–508. 10.1002/JLB.3MR0320-444R
REFERENCES
- 1. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: gLOBOCAN sources and methods. Int J Cancer. 2019;144:1941‐1953. [DOI] [PubMed] [Google Scholar]
- 2. Galon J, Bruni D. Tumor immunology and tumor evolution: intertwined histories. Immunity. 2020;52:55‐81. [DOI] [PubMed] [Google Scholar]
- 3. Garner H, de Visser KE. Immune crosstalk in cancer progression and metastatic spread: a complex conversation. Nat Rev Immunol. 2020. [DOI] [PubMed] [Google Scholar]
- 4. Ouyang W, O'Garra A. IL‐10 family cytokines IL‐10 and IL‐22: from basic science to clinical translation. Immunity. 2019;50:871‐891. [DOI] [PubMed] [Google Scholar]
- 5. Batlle E, Massague J. Transforming growth factor‐beta signaling in immunity and cancer. Immunity. 2019;50:924‐940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ivashkiv LB. IFNgamma: signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat Rev Immunol. 2018;18:545‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bilanges B, Posor Y, Vanhaesebroeck B. PI3K isoforms in cell signalling and vesicle trafficking. Nat Rev Mol Cell Biol. 2019;20:515‐534. [DOI] [PubMed] [Google Scholar]
- 8. Clara JA, Monge C, Yang Y, Takebe N. Targeting signalling pathways and the immune microenvironment of cancer stem cells—a clinical update. Nat Rev Clin Oncol. 2019;17(4):204‐232. [DOI] [PubMed] [Google Scholar]
- 9. Huynh J, Chand A, Gough D, Ernst M. Therapeutically exploiting STAT3 activity in cancer—using tissue repair as a road map. Nat Rev Cancer. 2019;19:82‐96. [DOI] [PubMed] [Google Scholar]
- 10. Peluso I, Yarla NS, Ambra R, Pastore G, Perry G. MAPK signalling pathway in cancers: olive products as cancer preventive and therapeutic agents. Semin Cancer Biol. 2019;56:185‐195. [DOI] [PubMed] [Google Scholar]
- 11. Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH. Coinhibitory pathways in immunotherapy for cancer. Annu Rev Immunol. 2016;34:539‐573. [DOI] [PubMed] [Google Scholar]
- 12. Boutros C, Tarhini A, Routier E, et al. Safety profiles of anti‐CTLA‐4 and anti‐PD‐1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13:473‐486. [DOI] [PubMed] [Google Scholar]
- 13. Missiaen R, Mazzone M, Bergers G. The reciprocal function and regulation of tumor vessels and immune cells offers new therapeutic opportunities in cancer. Semin Cancer Biol. 2018;52:107‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Villani AC, Sarkizova S, Hacohen N. Systems immunology: learning the rules of the immune system. Annu Rev Immunol. 2018;36:813‐842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest. 2015;125:3335‐3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069‐1086. [DOI] [PubMed] [Google Scholar]
- 17. Thompson JA. New NCCN guidelines: recognition and management of immunotherapy‐related toxicity. J Natl Compr Canc Netw. 2018;16:594‐596. [DOI] [PubMed] [Google Scholar]
- 18. D'Aloia MM, Zizzari IG, Sacchetti B, Pierelli L, Alimandi M. CAR‐T cells: the long and winding road to solid tumors. Cell Death Dis. 2018;9:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin. 2020;70(2):86‐104. [DOI] [PubMed] [Google Scholar]
- 20. Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T‐cell therapy—assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15:47‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Postow MA, Sidlow R, Hellmann MD. Immune‐related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158‐168. [DOI] [PubMed] [Google Scholar]
- 22. Deng LJ, Wang LH, Peng CK, et al. Fibroblast activation protein alpha activated tripeptide bufadienolide antitumor prodrug with reduced cardiotoxicity. J Med Chem. 2017;60:5320‐5333. [DOI] [PubMed] [Google Scholar]
- 23. Agarwal G, Carcache PJB, Addo EM, Kinghorn AD. Current status and contemporary approaches to the discovery of antitumor agents from higher plants. Biotechnol Adv. 2020;38:107337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pan P, Huang YW, Oshima K, et al. The immunomodulatory potential of natural compounds in tumor‐bearing mice and humans. Crit Rev Food Sci Nutr. 2019;59:992‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kepp O, Menger L, Vacchelli E, et al. Anticancer activity of cardiac glycosides: at the frontier between cell‐autonomous and immunological effects. Oncoimmunology. 2012;1:1640‐1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bahrami A, Fereidouni M, Pirro M, Bianconi V, Sahebkar A. Modulation of regulatory T cells by natural products in cancer. Cancer Lett. 2019;459:72‐85. [DOI] [PubMed] [Google Scholar]
- 27. Diederich M. Natural compound inducers of immunogenic cell death. Arch Pharm Res. 2019;42:629‐645. [DOI] [PubMed] [Google Scholar]
- 28. Grudzien M, Rapak A. effect of natural compounds on NK cell activation. J Immunol Res. 2018;2018:4868417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schnekenburger M, Dicato M, Diederich MF. Anticancer potential of naturally occurring immunoepigenetic modulators: a promising avenue. Cancer‐Am Cancer Soc. 2019;125:1612‐1628. [DOI] [PubMed] [Google Scholar]
- 30. Yahfoufi N, Alsadi N, Jambi M, Matar C. The immunomodulatory and anti‐inflammatory role of polyphenols. Nutrients. 2018;10(11):1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mileo AM, Nistico P, Miccadei S. Polyphenols: immunomodulatory and therapeutic implication in colorectal cancer. Front Immunol. 2019;10:729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Banerjee S, Chakravarty AR. Metal complexes of curcumin for cellular imaging, targeting, and photoinduced anticancer activity. Acc Chem Res. 2015;48:2075‐2083. [DOI] [PubMed] [Google Scholar]
- 33. Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493‐506. [DOI] [PubMed] [Google Scholar]
- 34. Zhao M, Ma J, Zhu HY, et al. Apigenin inhibits proliferation and induces apoptosis in human multiple myeloma cells through targeting the trinity of CK2, Cdc37 and Hsp90. Mol Cancer. 2011;10:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Durr C, Hanna BS, Schulz A, et al. Tumor necrosis factor receptor signaling is a driver of chronic lymphocytic leukemia that can be therapeutically targeted by the flavonoid wogonin. Haematologica. 2018;103:688‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hsieh DS, Wang H, Tan SW, et al. The treatment of bladder cancer in a mouse model by epigallocatechin‐3‐gallate‐gold nanoparticles. Biomaterials. 2011;32:7633‐7640. [DOI] [PubMed] [Google Scholar]
- 37. Li S, Dong P, Wang J, et al. Icariin, a natural flavonol glycoside, induces apoptosis in human hepatoma SMMC‐7721 cells via a ROS/JNK‐dependent mitochondrial pathway. Cancer Lett. 2010;298:222‐230. [DOI] [PubMed] [Google Scholar]
- 38. Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010;2:1231‐1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gupta SC, Patchva S, Koh W, Aggarwal BB. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin Exp Pharmacol Physiol. 2012;39:283‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mohammadi A, Blesso CN, Barreto GE, Banach M, Majeed M, Sahebkar A. Macrophage plasticity, polarization and function in response to curcumin, a diet‐derived polyphenol, as an immunomodulatory agent. J Nutr Biochem. 2019;66:1‐16. [DOI] [PubMed] [Google Scholar]
- 41. Focaccetti C, Izzi V, Benvenuto M, et al. Polyphenols as immunomodulatory compounds in the tumor microenvironment: friends or foes. Int J Mol Sci. 2019;20(7):1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lim SO, Li CW, Xia W, et al. Deubiquitination and Stabilization of PD‐L1 by CSN5. Cancer Cell. 2016;30:925‐939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xu B, Yu L, Zhao LZ. Curcumin up regulates T helper 1 cells in patients with colon cancer. Am J Transl Res. 2017;9:1866‐1875. [PMC free article] [PubMed] [Google Scholar]
- 44. Zou JY, Su CH, Luo HH, et al. Curcumin converts Foxp3+ regulatory T cells to T helper 1 cells in patients with lung cancer. J Cell Biochem. 2018;119:1420‐1428. [DOI] [PubMed] [Google Scholar]
- 45. Bhattacharyya S, Md Sakib Hossain D, Mohanty S, et al. Curcumin reverses T cell‐mediated adaptive immune dysfunctions in tumor‐bearing hosts. Cell Mol Immunol. 2010;7:306‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liao F, Liu L, Luo E, Hu J. Curcumin enhances anti‐tumor immune response in tongue squamous cell carcinoma. Arch Oral Biol. 2018;92:32‐37. [DOI] [PubMed] [Google Scholar]
- 47. Bhattacharyya S, Mandal D, Sen GS, et al. Tumor‐induced oxidative stress perturbs nuclear factor‐kappaB activity‐augmenting tumor necrosis factor‐alpha‐mediated T‐cell death: protection by curcumin. Cancer Res. 2007;67:362‐370. [DOI] [PubMed] [Google Scholar]
- 48. Liu D, You M, Xu Y, et al. Inhibition of curcumin on myeloid‐derived suppressor cells is requisite for controlling lung cancer. Int Immunopharmacol. 2016;39:265‐272. [DOI] [PubMed] [Google Scholar]
- 49. Luo F, Song X, Zhang Y, Chu Y. Low‐dose curcumin leads to the inhibition of tumor growth via enhancing CTL‐mediated antitumor immunity. Int Immunopharmacol. 2011;11:1234‐1240. [DOI] [PubMed] [Google Scholar]
- 50. Liu X, Feng Z, Wang C, et al. Co‐localized delivery of nanomedicine and nanovaccine augments the postoperative cancer immunotherapy by amplifying T‐cell responses. Biomaterials. 2020;230:119649. [DOI] [PubMed] [Google Scholar]
- 51. Xiao Z, Su Z, Han S, Huang J, Lin L, Shuai X. Dual pH‐sensitive nanodrug blocks PD‐1 immune checkpoint and uses T cells to deliver NF‐kappaB inhibitor for antitumor immunotherapy. Sci Adv. 2020;6:eaay7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Verdura S, Cuyas E, Cortada E, et al. Resveratrol targets PD‐L1 glycosylation and dimerization to enhance antitumor T‐cell immunity. Aging (Albany NY). 2020;12:8‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yusuf N, Nasti TH, Meleth S, Elmets CA. Resveratrol enhances cell‐mediated immune response to DMBA through TLR4 and prevents DMBA induced cutaneous carcinogenesis. Mol Carcinog. 2009;48:713‐723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yang Y, Paik JH, Cho D, Cho JA, Kim CW. Resveratrol induces the suppression of tumor‐derived CD4+CD25+ regulatory T cells. Int Immunopharmacol. 2008;8:542‐547. [DOI] [PubMed] [Google Scholar]
- 55. Chen L, Yang S, Liao W, Xiong Y. Modification of antitumor immunity and tumor microenvironment by resveratrol in mouse renal tumor model. Cell Biochem Biophys. 2015;72:617‐625. [DOI] [PubMed] [Google Scholar]
- 56. Radwan FF, Zhang L, Hossain A, Doonan BP, God JM, Haque A. Mechanisms regulating enhanced human leukocyte antigen class II‐mediated CD4 + T cell recognition of human B‐cell lymphoma by resveratrol. Leuk Lymphoma. 2012;53:305‐314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jeong MH, Yang KM, Choi YJ, et al. Resveratrol analog, HS‐1793 enhance anti‐tumor immunity by reducing the CD4+CD25+ regulatory T cells in FM3A tumor bearing mice. Int Immunopharmacol. 2012;14:328‐333. [DOI] [PubMed] [Google Scholar]
- 58. Choi YJ, Yang KM, Kim SD, et al. Resveratrol analogue HS‐1793 induces the modulation of tumor‐derived T cells. Exp Ther Med. 2012;3:592‐598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dandawate S, Williams L, Joshee N, et al. Scutellaria extract and wogonin inhibit tumor‐mediated induction of T(reg) cells via inhibition of TGF‐beta1 activity. Cancer Immunol Immunother. 2012;61:701‐711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chuang CM, Monie A, Wu A, Hung CF. Combination of apigenin treatment with therapeutic HPV DNA vaccination generates enhanced therapeutic antitumor effects. J Biomed Sci. 2009;16:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ke M, Zhang Z, Xu B, et al. Baicalein and baicalin promote antitumor immunity by suppressing PD‐L1 expression in hepatocellular carcinoma cells. Int Immunopharmacol. 2019;75:105824. [DOI] [PubMed] [Google Scholar]
- 62. Mantena SK, Roy AM, Katiyar SK. Epigallocatechin‐3‐gallate inhibits photocarcinogenesis through inhibition of angiogenic factors and activation of CD8+ T cells in tumors. Photochem Photobiol. 2005;81:1174‐1179. [DOI] [PubMed] [Google Scholar]
- 63. Katiyar SK, Challa A, McCormick TS, Cooper KD, Mukhtar H. Prevention of UVB‐induced immunosuppression in mice by the green tea polyphenol (‐)‐epigallocatechin‐3‐gallate may be associated with alterations in IL‐10 and IL‐12 production. Carcinogenesis. 1999;20:2117‐2124. [DOI] [PubMed] [Google Scholar]
- 64. Kang TH, Lee JH, Song CK, et al. Epigallocatechin‐3‐gallate enhances CD8+ T cell‐mediated antitumor immunity induced by DNA vaccination. Cancer Res. 2007;67:802‐811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhang F, Dong W, Zeng W, et al. Naringenin prevents TGF‐beta1 secretion from breast cancer and suppresses pulmonary metastasis by inhibiting PKC activation. Breast Cancer Res. 2016;18:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhang L, Wang S, Liu Z, Zhang L, Wang S, Wang B. Procyanidin, a kind of biological flavonoid, induces protective anti‐tumor immunity and protects mice from lethal B16F10 challenge. Int Immunopharmacol. 2017;47:251‐258. [DOI] [PubMed] [Google Scholar]
- 67. Xiang Y, Chen L, Li L, Huang Y. Restoration and enhancement of immunogenic cell death of cisplatin by coadministration with digoxin and conjugation to HPMA copolymer. ACS Appl Mater Interfaces. 2020;12:1606‐1616. [DOI] [PubMed] [Google Scholar]
- 68. Cao Y, Song Y, An N, et al. The effects of telocinobufagin isolated from Chan Su on the activation and cytokine secretion of immunocytes in vitro. Fundam Clin Pharmacol. 2009;23:457‐464. [DOI] [PubMed] [Google Scholar]
- 69. Wang XL, Zhao GH, Zhang J, et al. Immunomodulatory effects of cinobufagin isolated from Chan Su on activation and cytokines secretion of immunocyte in vitro. J Asian Nat Prod Res. 2011;13:383‐392. [DOI] [PubMed] [Google Scholar]
- 70. Yuan B, He J, Kisoh K, et al. Effects of active bufadienolide compounds on human cancer cells and CD4+CD25+Foxp3+ regulatory T cells in mitogen‐activated human peripheral blood mononuclear cells. Oncol Rep. 2016;36:1377‐1384. [DOI] [PubMed] [Google Scholar]
- 71. Matsuhashi T, Shimizu M, Negishi Y, Takeshita T, Takahashi H. A low, non‐toxic dose of paclitaxel can prevent dendritic cell‐precursors from becoming tolerogenic dendritic cells with impaired functions. Biomed Res. 2014;35:369‐380. [DOI] [PubMed] [Google Scholar]
- 72. Byun JW, Lee HS, Song SU, et al. Combined treatment of murine fibrosarcoma with chemotherapy (Paclitaxel), radiotherapy, and intratumoral injection of dendritic cells. Ann Dermatol. 2014;26:53‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Heeren AM, van Luijk IF, Lakeman J, et al. Neoadjuvant cisplatin and paclitaxel modulate tumor‐infiltrating T cells in patients with cervical cancer. Cancer Immunol Immunother. 2019;68:1759‐1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Meng X, Du G, Ye L, et al. Combinatorial antitumor effects of indoleamine 2,3‐dioxygenase inhibitor NLG919 and paclitaxel in a murine B16‐F10 melanoma model. Int J Immunopathol Pharmacol. 2017;30:215‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Farsam V, Hassan ZM, Zavaran‐Hosseini A, Noori S, Mahdavi M, Ranjbar M. Antitumor and immunomodulatory properties of artemether and its ability to reduce CD4+ CD25+ FoxP3+ T reg cells in vivo. Int Immunopharmacol. 2011;11:1802‐1808. [DOI] [PubMed] [Google Scholar]
- 76. Zhou ZH, Chen FX, Xu WR, et al. Enhancement effect of dihydroartemisinin on human gammadelta T cell proliferation and killing pancreatic cancer cells. Int Immunopharmacol. 2013;17:850‐857. [DOI] [PubMed] [Google Scholar]
- 77. Qian P, Zhang YW, Zhou ZH, et al. Artesunate enhances gammadelta T‐cell‐mediated antitumor activity through augmenting gammadelta T‐cell function and reversing immune escape of HepG2 cells. Immunopharmacol Immunotoxicol. 2018;40:107‐116. [DOI] [PubMed] [Google Scholar]
- 78. Cao Y, Feng YH, Gao LW, et al. Artemisinin enhances the anti‐tumor immune response in 4T1 breast cancer cells in vitro and in vivo. Int Immunopharmacol. 2019;70:110‐116. [DOI] [PubMed] [Google Scholar]
- 79. Zhang L, Yu JS. Triptolide reverses helper T cell inhibition and down‐regulates IFN‐gamma induced PD‐L1 expression in glioma cell lines. J Neurooncol. 2019;143:429‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Liu B, Zhang H, Li J, et al. Triptolide downregulates Treg cells and the level of IL‐10, TGF‐beta, and VEGF in melanoma‐bearing mice. Planta Med. 2013;79:1401‐1407. [DOI] [PubMed] [Google Scholar]
- 81. Hu JY, Li CL, Wang YW. Intrathecal administration of triptolide, a T lymphocyte inhibitor, attenuates chronic constriction injury‐induced neuropathic pain in rats. Brain Res. 2012;1436:122‐129. [DOI] [PubMed] [Google Scholar]
- 82. Huang MY, Jiang XM, Xu YL, et al. Platycodin D triggers the extracellular release of programed death ligand‐1 in lung cancer cells. Food Chem Toxicol. 2019;131:110537. [DOI] [PubMed] [Google Scholar]
- 83. Zhao R, Zhang T, Zhao H, Cai Y. Effects of Portulaca oleracea L. polysaccharides on phenotypic and functional maturation of murine bone marrow derived dendritic cells. Nutr Cancer. 2015;67:987‐993. [DOI] [PubMed] [Google Scholar]
- 84. Hou L, Zhang H, Xu P, et al. Effect of vitamin E succinate on the expression of the tumor necrosis factor‐related apoptosis‐inducing ligand (TRAIL) receptor in gastric cancer cells and CD4(+) T cells. Mol Biosyst. 2015;11:3119‐3128. [DOI] [PubMed] [Google Scholar]
- 85. Kang TH, Knoff J, Yeh WH, et al. Treatment of tumors with vitamin E suppresses myeloid derived suppressor cells and enhances CD8+ T cell‐mediated antitumor effects. PLoS One. 2014;9:e103562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Beltran J, Ghosh AK, Basu S. Immunotherapy of tumors with neuroimmune ligand capsaicin. J Immunol. 2007;178:3260‐3264. [DOI] [PubMed] [Google Scholar]
- 87. Lee‐Chang C, Bodogai M, Martin‐Montalvo A, et al. Inhibition of breast cancer metastasis by resveratrol‐mediated inactivation of tumor‐evoked regulatory B cells. J Immunol. 2013;191:4141‐4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Vatsveen TK, Myhre MR, Steen CB, et al. Artesunate shows potent anti‐tumor activity in B‐cell lymphoma. J Hematol Oncol. 2018;11:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Santilli G, Piotrowska I, Cantilena S, et al. Polyphenon [corrected] E enhances the antitumor immune response in neuroblastoma by inactivating myeloid suppressor cells. Clin Cancer Res. 2013;19:1116‐1125. [DOI] [PubMed] [Google Scholar]
- 90. Forghani P, Khorramizadeh MR, Waller EK. Silibinin inhibits accumulation of myeloid‐derived suppressor cells and tumor growth of murine breast cancer. Cancer Med. 2014;3:215‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lian GY, Wang QM, Tang PM, Zhou S, Huang XR, Lan HY. Combination of Asiatic acid and naringenin modulates NK cell anti‐cancer immunity by rebalancing Smad3/Smad7 signaling. Mol Ther. 2018;26:2255‐2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Shih YL, Shang HS, Chen YL, et al. Ouabain promotes immune responses in WEHI‐3 cells to generate leukemia mice through enhancing phagocytosis and natural killer cell activities in vivo. Environ Toxicol. 2019;34:659‐665. [DOI] [PubMed] [Google Scholar]
- 93. Houh YK, Kim KE, Park S, et al. The effects of artemisinin on the cytolytic activity of natural killer (NK) cells. Int J Mol Sci. 2017;18:1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Cui C, Feng H, Shi X, et al. Artesunate down‐regulates immunosuppression from colorectal cancer Colon26 and RKO cells in vitro by decreasing transforming growth factor beta1 and interleukin‐10. Int Immunopharmacol. 2015;27:110‐121. [DOI] [PubMed] [Google Scholar]
- 95. Kwon HJ, Lee H, Choi GE, et al. Ginsenoside F1 promotes cytotoxic activity of NK cells via insulin‐like growth factor‐1‐dependent mechanism. Front Immunol. 2018;9:2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhang X, Tian W, Cai X, et al. Hydrazinocurcumin Encapsuled nanoparticles “re‐educate” tumor‐associated macrophages and exhibit anti‐tumor effects on breast cancer following STAT3 suppression. PLoS One. 2013;8:e65896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Shiri S, Alizadeh AM, Baradaran B, et al. Dendrosomal curcumin suppresses metastatic breast cancer in mice by changing m1/m2 macrophage balance in the tumor microenvironment. Asian Pac J Cancer Prev. 2015;16:3917‐3922. [DOI] [PubMed] [Google Scholar]
- 98. Sun L, Chen B, Jiang R, Li J, Wang B. Resveratrol inhibits lung cancer growth by suppressing M2‐like polarization of tumor associated macrophages. Cell Immunol. 2017;311:86‐93. [DOI] [PubMed] [Google Scholar]
- 99. Shih YL, Chou JS, Chen YL, et al. Bufalin enhances immune responses in leukemic mice through enhancing phagocytosis of macrophage in vivo. In Vivo. 2018;32:1129‐1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Yu Y, Wang H, Meng X, et al. Immunomodulatory effects of cinobufagin on murine lymphocytes and macrophages. Evid Based Complement Alternat Med. 2015;2015:835263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zhang S, Nie S, Huang D, Li W, Xie M. Immunomodulatory effect of Ganoderma atrum polysaccharide on CT26 tumor‐bearing mice. Food Chem. 2013;136:1213‐1219. [DOI] [PubMed] [Google Scholar]
- 102. Zhang S, Nie S, Huang D, Huang J, Wang Y, Xie M. Polysaccharide from Ganoderma atrum evokes antitumor activity via Toll‐like receptor 4‐mediated NF‐kappaB and mitogen‐activated protein kinase signaling pathways. J Agric Food Chem. 2013;61:3676‐3682. [DOI] [PubMed] [Google Scholar]
- 103. Ghosh AK, Basu S. Tumor macrophages as a target for Capsaicin mediated immunotherapy. Cancer Lett. 2012;324:91‐97. [DOI] [PubMed] [Google Scholar]
- 104. Kongsted P, Borch TH, Ellebaek E, et al. Dendritic cell vaccination in combination with docetaxel for patients with metastatic castration‐resistant prostate cancer: a randomized phase II study. Cytotherapy. 2017;19:500‐513. [DOI] [PubMed] [Google Scholar]
- 105. Zhao R, Shao X, Jia G, et al. Anti‐cervical carcinoma effect of Portulaca oleracea L. polysaccharides by oral administration on intestinal dendritic cells. BMC Complement Altern Med. 2019;19:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Jin T, Wu H, Wang Y, Peng H. Capsaicin induces immunogenic cell death in human osteosarcoma cells. Exp Ther Med. 2016;12:765‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ogawa K, Hara T, Shimizu M, et al. (2012) (‐)‐Epigallocatechin gallate inhibits the expression of indoleamine 2,3‐dioxygenase in human colorectal cancer cells. Oncol Lett;4:546‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Chen Y, Xia R, Huang Y, et al. An immunostimulatory dual‐functional nanocarrier that improves cancer immunochemotherapy. Nat Commun. 2016;7:13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Yang Y, Li XJ, Chen Z, et al. Wogonin induced calreticulin/annexin A1 exposure dictates the immunogenicity of cancer cells in a PERK/AKT dependent manner. PLoS One. 2012;7:e50811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Rawangkan A, Wongsirisin P, Namiki K, et al. Green tea catechin is an alternative immune checkpoint inhibitor that inhibits PD‐L1 expression and lung tumor growth. Molecules. 2018;23:2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lai X, Pei Q, Song X, et al. The enhancement of immune function and activation of NF‐kappaB by resveratrol‐treatment in immunosuppressive mice. Int Immunopharmacol. 2016;33:42‐47. [DOI] [PubMed] [Google Scholar]
- 112. Mukherjee S, Baidoo J, Fried A, et al. Curcumin changes the polarity of tumor‐associated microglia and eliminates glioblastoma. Int J Cancer. 2016;139:2838‐2849. [DOI] [PubMed] [Google Scholar]
- 113. Huang XT, Li X, Xie ML, et al. Resveratrol: review on its discovery, anti‐leukemia effects and pharmacokinetics. Chem Biol Interact. 2019;306:29‐38. [DOI] [PubMed] [Google Scholar]
- 114. Sinha D, Sarkar N, Biswas J, Bishayee A. Resveratrol for breast cancer prevention and therapy: preclinical evidence and molecular mechanisms. Semin Cancer Biol. 2016;40–41:209‐232. [DOI] [PubMed] [Google Scholar]
- 115. Bishayee A, Politis T, Darvesh AS. Resveratrol in the chemoprevention and treatment of hepatocellular carcinoma. Cancer Treat Rev. 2010;36:43‐53. [DOI] [PubMed] [Google Scholar]
- 116. Carter LG, D'Orazio JA, Pearson KJ. Resveratrol and cancer: focus on in vivo evidence. Endocr Relat Cancer. 2014;21:R209‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Rauf A, Imran M, Butt MS, Nadeem M, Peters DG, Mubarak MS. Resveratrol as an anti‐cancer agent: a review. Crit Rev Food Sci. 2018;58:1428‐1447. [DOI] [PubMed] [Google Scholar]
- 118. Ghiringhelli F, Rebe C, Hichami A, Delmas D. Immunomodulation and anti‐inflammatory roles of polyphenols as anticancer agents. Anti‐Cancer Agent Me. 2012;12:852‐873. [DOI] [PubMed] [Google Scholar]
- 119. Chen CY, Kao CL, Liu CM. The cancer prevention, anti‐inflammatory and anti‐oxidation of bioactive phytochemicals targeting the TLR4 signaling pathway. Int J Mol Sci. 2018;19:2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Trung LQ, An DTT. Is resveratrol a cancer immunomodulatory molecule. Front Pharmacol. 2018;9:1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Zhang J, Dang F, Ren J, Wei W. Biochemical aspects of PD‐L1 regulation in cancer immunotherapy. Trends Biochem Sci. 2018;43:1014‐1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Yusuf N, Nasti TH, Long JA, et al. Protective role of Toll‐like receptor 4 during the initiation stage of cutaneous chemical carcinogenesis. Cancer Res. 2008;68:615‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Jeong SH, Song IS, Kim HK, et al. An analogue of resveratrol HS‐1793 exhibits anticancer activity against MCF‐7 cells via inhibition of mitochondrial biogenesis gene expression. Mol Cells. 2012;34:357‐365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Karabin M, Hudcova T, Jelinek L, Dostalek P. Biotransformations and biological activities of hop flavonoids. Biotechnol Adv. 2015;33:1063‐1090. [DOI] [PubMed] [Google Scholar]
- 125. Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22:19‐34. [DOI] [PubMed] [Google Scholar]
- 126. Li‐Weber M. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat Rev. 2009;35:57‐68. [DOI] [PubMed] [Google Scholar]
- 127. Meyer H, Bolarinwa A, Wolfram G, Linseisen J. Bioavailability of apigenin from apiin‐rich parsley in humans. Ann Nutr Metab. 2006;50:167‐172. [DOI] [PubMed] [Google Scholar]
- 128. Shukla S, Gupta S. Apigenin: a promising molecule for cancer prevention. Pharm Res. 2010;27:962‐978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Wang YC, Huang KM. In vitro anti‐inflammatory effect of apigenin in the Helicobacter pylori‐infected gastric adenocarcinoma cells. Food Chem Toxicol. 2013;53:376‐383. [DOI] [PubMed] [Google Scholar]
- 130. Rezai‐Zadeh K, Ehrhart J, Bai Y, et al. Apigenin and luteolin modulate microglial activation via inhibition of STAT1‐induced CD40 expression. J Neuroinflammation. 2008;5:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Zheng PW, Chiang LC, Lin CC. Apigenin induced apoptosis through p53‐dependent pathway in human cervical carcinoma cells. Life Sciences. 2005;76:1367‐1379. [DOI] [PubMed] [Google Scholar]
- 132. Xu L, Zhang Y, Tian K, et al. Apigenin suppresses PD‐L1 expression in melanoma and host dendritic cells to elicit synergistic therapeutic effects. J Exp Clin Cancer Res. 2018;37:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429‐439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Kang TH, Lee JH, Song CK, et al. Epigallocatechin‐3‐gallate enhances CD8(+) T cell‐mediated antitumor immunity induced by DNA vaccination. Cancer Res. 2007;67:802‐811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Indran IR, Liang RL, Min TE, Yong EL. Preclinical studies and clinical evaluation of compounds from the genus Epimedium for osteoporosis and bone health. Pharmacol Ther. 2016;162:188‐205. [DOI] [PubMed] [Google Scholar]
- 136. Zhang X, Kang Z, Li Q, et al. Antigen‐adjuvant effects of icariin in enhancing tumor‐specific immunity in mastocytoma‐bearing DBA/2J mice. Biomed Pharmacother. 2018;99:810‐816. [DOI] [PubMed] [Google Scholar]
- 137. Zhou J, Wu J, Chen X, et al. Icariin and its derivative, ICT, exert anti‐inflammatory, anti‐tumor effects, and modulate myeloid derived suppressive cells (MDSCs) functions. Int Immunopharmacol. 2011;11:890‐898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Liu X, Wang W, Hu H, et al. Smad3 specific inhibitor, naringenin, decreases the expression of extracellular matrix induced by TGF‐beta1 in cultured rat hepatic stellate cells. Pharm Res. 2006;23:82‐89. [DOI] [PubMed] [Google Scholar]
- 139. Du G, Jin L, Han X, Song Z, Zhang H, Liang W. Naringenin: a potential immunomodulator for inhibiting lung fibrosis and metastasis. Cancer Res. 2009;69:3205‐3212. [DOI] [PubMed] [Google Scholar]
- 140. Prassas I, Diamandis EP. Novel therapeutic applications of cardiac glycosides. Nat Rev Drug Discov. 2008;7:926‐935. [DOI] [PubMed] [Google Scholar]
- 141. Gao H, Popescu R, Kopp B, Wang Z. Bufadienolides and their antitumor activity. Nat Prod Rep. 2011;28:953‐969. [DOI] [PubMed] [Google Scholar]
- 142. Laursen M, Gregersen JL, Yatime L, Nissen P, Fedosova NU. Structures and characterization of digoxin‐ and bufalin‐bound Na+,K+‐ATPase compared with the ouabain‐bound complex. Proc Natl Acad Sci U S A. 2015;112:1755‐1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Menger L, Vacchelli E, Kepp O, et al. Trial watch: cardiac glycosides and cancer therapy. Oncoimmunology. 2013;2:e23082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Meng Z, Yang P, Shen Y, et al. Pilot study of huachansu in patients with hepatocellular carcinoma, nonsmall‐cell lung cancer, or pancreatic cancer. Cancer‐Am Cancer Soc. 2009;115:5309‐5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Meng Z, Garrett CR, Shen Y, et al. Prospective randomised evaluation of traditional Chinese medicine combined with chemotherapy: a randomised phase II study of wild toad extract plus gemcitabine in patients with advanced pancreatic adenocarcinomas. Br J Cancer. 2012;107:411‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Xie X, Huang X, Li J, et al. Efficacy and safety of Huachansu combined with chemotherapy in advanced gastric cancer: a meta‐analysis. Med Hypotheses. 2013;81:243‐250. [DOI] [PubMed] [Google Scholar]
- 147. Frankel AE, Eskiocak U, Gill JG, et al. Digoxin plus trametinib therapy achieves disease control in BRAF wild‐type metastatic melanoma patients. Neoplasia. 2017;19:255‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Diederich M, Muller F, Cerella C. Cardiac glycosides: from molecular targets to immunogenic cell death. Biochem Pharmacol. 2017;125:1‐11. [DOI] [PubMed] [Google Scholar]
- 149. Sukkurwala AQ, Adjemian S, Senovilla L, et al. Screening of novel immunogenic cell death inducers within the NCI mechanistic diversity set. Oncoimmunology. 2014;3:e28473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Menger L, Vacchelli E, Adjemian S, et al. Cardiac glycosides exert anticancer effects by inducing immunogenic cell death. Sci Transl Med. 2012;4:143ra99. [DOI] [PubMed] [Google Scholar]
- 151. Yang Z, Tao Y, Xu X, Cai F, Yu Y, Ma L. Bufalin inhibits cell proliferation and migration of hepatocellular carcinoma cells via APOBEC3F induced intestinal immune network for IgA production signaling pathway. Biochem Biophys Res Commun. 2018;503:2124‐2131. [DOI] [PubMed] [Google Scholar]
- 152. Tholl D. Biosynthesis and biological functions of terpenoids in plants. Adv Biochem Eng Biotechnol. 2015;148:63‐106. [DOI] [PubMed] [Google Scholar]
- 153. Zhu L, Chen L. Progress in research on paclitaxel and tumor immunotherapy. Cell Mol Biol Lett. 2019;24:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Tu Y. Artemisinin—a gift from traditional Chinese medicine to the world (Nobel lecture). Angew Chem Int Ed Engl. 2016;55:10210‐10226. [DOI] [PubMed] [Google Scholar]
- 155. Cragg GM, Pezzuto JM. Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Med Princ Pract. 2016;25(Suppl 2):41‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Naaz F, Haider MR, Shafi S, Yar MS. Anti‐tubulin agents of natural origin: targeting taxol, vinca, and colchicine binding domains. Eur J Med Chem. 2019;171:310‐331. [DOI] [PubMed] [Google Scholar]
- 157. Khayrani AC, Mahmud H, Oo AKK, et al. Targeting ovarian cancer cells overexpressing CD44 with immunoliposomes encapsulating glycosylated paclitaxel. Int J Mol Sci. 2019;20:1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Tang W, Yang J, Yuan Y, Zhao Z, Lian Z, Liang G. Paclitaxel nanoparticle awakens immune system to fight against cancer. Nanoscale. 2017;9:6529‐6536. [DOI] [PubMed] [Google Scholar]
- 159. Song Q, Yin Y, Shang L, et al. Tumor microenvironment responsive nanogel for the combinatorial antitumor effect of chemotherapy and immunotherapy. Nano Lett. 2017;17:6366‐6375. [DOI] [PubMed] [Google Scholar]
- 160. Su Z, Xiao Z, Wang Y, et al. Codelivery of anti‐PD‐1 antibody and paclitaxel with matrix metalloproteinase and pH dual‐sensitive micelles for enhanced tumor chemoimmunotherapy. Small. 2020;16:e1906832. [DOI] [PubMed] [Google Scholar]
- 161. Efferth T. From ancient herb to modern drug: artemisia annua and artemisinin for cancer therapy. Semin Cancer Biol. 2017;46:65‐83. [DOI] [PubMed] [Google Scholar]
- 162. Konstat‐Korzenny E, Ascencio‐Aragon JA, Niezen‐Lugo S, Vazquez‐Lopez R. Artemisinin and its synthetic derivatives as a possible therapy for cancer. Med Sci (Basel). 2018;6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Noori S, Hassan ZM, Taghikhani M, Rezaei B, Habibi Z. Dihydroartemisinin can inhibit calmodulin, calmodulin‐dependent phosphodiesterase activity and stimulate cellular immune responses. Int Immunopharmacol. 2010;10:213‐217. [DOI] [PubMed] [Google Scholar]
- 164. Zhou ZH, Chen FX, Xu WR, et al. Enhancement effect of dihydroartemisinin on human gamma delta T cell proliferation and killing pancreatic cancer cells. Int Immunopharmacol. 2013;17:850‐857. [DOI] [PubMed] [Google Scholar]
- 165. Qian P, Zhang YW, Zhou ZH, et al. Artesunate enhances gamma delta T‐cell‐mediated antitumor activity through augmenting gamma delta T‐cell function and reversing immune escape of HepG2 cells. Immunopharm Immunot. 2018;40:107‐116. [DOI] [PubMed] [Google Scholar]
- 166. Li T, Chen H, Wei N, et al. Anti‐inflammatory and immunomodulatory mechanisms of artemisinin on contact hypersensitivity. Int Immunopharmacol. 2012;12:144‐150. [DOI] [PubMed] [Google Scholar]
- 167. Zhao YG, Wang Y, Guo Z, et al. Dihydroartemisinin ameliorates inflammatory disease by its reciprocal effects on Th and regulatory T cell function via modulating the mammalian target of rapamycin pathway. J Immunol. 2012;189:4417‐4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Noel P, Von Hoff DD, Saluja AK, Velagapudi M, Borazanci E, Han H. Triptolide and its derivatives as cancer therapies. Trends Pharmacol Sci. 2019;40:327‐341. [DOI] [PubMed] [Google Scholar]
- 169. Hang LH, Li SN, Shao DH, Chen Z, Chen YF, Shu WW. Evidence for involvement of spinal RANTES in the antinociceptive effects of triptolide, a diterpene triepoxide, in a rat model of bone cancer pain. Basic Clin Pharmacol Toxicol. 2014;115:477‐480. [DOI] [PubMed] [Google Scholar]
- 170. Guclu‐Ustundag O, Mazza G. Saponins: properties, applications and processing. Crit Rev Food Sci Nutr. 2007;47:231‐258. [DOI] [PubMed] [Google Scholar]
- 171. Ragupathi G, Gardner JR, Livingston PO, Gin DY. Natural and synthetic saponin adjuvant QS‐21 for vaccines against cancer. Expert Rev Vaccines. 2011;10:463‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Martinez P, Sundling C, O'Dell S, Mascola JR, Wyatt RT, Karlsson Hedestam GB. Primate immune responses to HIV‐1 Env formulated in the saponin‐based adjuvant AbISCO‐100 in the presence or absence of TLR9 co‐stimulation. Sci Rep. 2015;5:8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Chen JL, Dawoodji A, Tarlton A, et al. NY‐ESO‐1 specific antibody and cellular responses in melanoma patients primed with NY‐ESO‐1 protein in ISCOMATRIX and boosted with recombinant NY‐ESO‐1 fowlpox virus. Int J Cancer. 2015;136:E590‐601. [DOI] [PubMed] [Google Scholar]
- 174. Highton AJ, Kojarunchitt T, Girardin A, Hook S, Kemp RA. Chitosan hydrogel vaccine generates protective CD8 T cell memory against mouse melanoma. Immunol Cell Biol. 2015;93:634‐640. [DOI] [PubMed] [Google Scholar]
- 175. Kim HS, Shin BR, Lee HK, et al. A polysaccharide isolated from Pueraria lobata enhances maturation of murine dendritic cells. Int J Biol Macromol. 2013;52:184‐191. [DOI] [PubMed] [Google Scholar]
- 176. Sunila ES, Hamsa TP, Kuttan G. Effect of Thuja occidentalis and its polysaccharide on cell‐mediated immune responses and cytokine levels of metastatic tumor‐bearing animals. Pharm Biol. 2011;49:1065‐1073. [DOI] [PubMed] [Google Scholar]
- 177. Zhan X, Jia L, Niu Y, et al. Targeted depletion of tumour‐associated macrophages by an alendronate‐glucomannan conjugate for cancer immunotherapy. Biomaterials. 2014;35:10046‐10057. [DOI] [PubMed] [Google Scholar]
- 178. Cheng J, Zhou ZW, Sheng HP, et al. An evidence‐based update on the pharmacological activities and possible molecular targets of Lycium barbarum polysaccharides. Drug Des Devel Ther. 2015;9:33‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Deng S, Zhang G, Kuai J, et al. Lentinan inhibits tumor angiogenesis via interferon gamma and in a T cell independent manner. J Exp Clin Cancer Res. 2018;37:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Ning Y, Xu D, Zhang X, et al. beta‐glucan restores tumor‐educated dendritic cell maturation to enhance antitumor immune responses. Int J Cancer. 2016;138:2713‐2723. [DOI] [PubMed] [Google Scholar]
- 181. Kikete S, Luo L, Jia B, Wang L, Ondieki G, Bian Y. Plant‐derived polysaccharides activate dendritic cell‐based anti‐cancer immunity. Cytotechnology. 2018;70:1097‐1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Li B, Allendorf DJ, Hansen R, et al. Yeast beta‐glucan amplifies phagocyte killing of iC3b‐opsonized tumor cells via complement receptor 3‐Syk‐phosphatidylinositol 3‐kinase pathway. J Immunol. 2006;177:1661‐1669. [DOI] [PubMed] [Google Scholar]
- 183. Kwon HK, Jo WR, Park HJ. Immune‐enhancing activity of C. militaris fermented with Pediococcus pentosaceus (GRC‐ON89A) in CY‐induced immunosuppressed model. BMC Complement Altern Med. 2018;18:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Tian J, Ma J, Ma K, et al. beta‐Glucan enhances antitumor immune responses by regulating differentiation and function of monocytic myeloid‐derived suppressor cells. Eur J Immunol. 2013;43:1220‐1230. [DOI] [PubMed] [Google Scholar]
- 185. Wasser SP. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl Microbiol Biot. 2002;60:258‐274. [DOI] [PubMed] [Google Scholar]
- 186. Pandya U, Dhuldhaj U, Sahay NS. Bioactive mushroom polysaccharides as antitumor: an overview. Nat Prod Res. 2019;33:2668‐2680. [DOI] [PubMed] [Google Scholar]
- 187. Wang XE, Wang YH, Zhou Q, et al. Immunomodulatory effect of lentinan on aberrant T subsets and cytokines profile in non‐small cell lung cancer patients. Pathol Oncol Res. 2020;26:499‐505. [DOI] [PubMed] [Google Scholar]
- 188. Yoshino S, Nishikawa K, Morita S, et al. Randomised phase III study of S‐1 alone versus S‐1 plus lentinan for unresectable or recurrent gastric cancer (JFMC36‐0701). Eur J Cancer. 2016;65:164‐171. [DOI] [PubMed] [Google Scholar]
- 189. Higashi D, Seki K, Ishibashi Y, et al. The effect of lentinan combination therapy for unresectable advanced gastric cancer. Anticancer Res. 2012;32:2365‐2368. [PubMed] [Google Scholar]
- 190. Liu LQ, Nie SP, Xie MY. Tumor microenvironment as a new target for tumor immunotherapy of polysaccharides. Crit Rev Food Sci. 2016;56:S85‐S94. [DOI] [PubMed] [Google Scholar]
- 191. Constantinou C, Papas A, Constantinou AI. Vitamin E and cancer: an insight into the anticancer activities of vitamin E isomers and analogs. Int J Cancer. 2008;123:739‐752. [DOI] [PubMed] [Google Scholar]
- 192. Qian M, Sanders BG, Kline K. RRR‐alpha‐tocopheryl succinate induces apoptosis in avian retrovirus‐transformed lymphoid cells. Nutr Cancer. 1996;25:9‐26. [DOI] [PubMed] [Google Scholar]
- 193. Malafa MP, Fokum FD, Mowlavi A, Abusief M, King M. Vitamin E inhibits melanoma growth in mice. Surgery. 2002;131:85‐91. [DOI] [PubMed] [Google Scholar]
- 194. Yamamoto S, Tamai H, Ishisaka R, et al. Mechanism of alpha‐tocopheryl succinate‐induced apoptosis of promyelocytic leukemia cells. Free Radic Res. 2000;33:407‐418. [DOI] [PubMed] [Google Scholar]
- 195. Zhang Y, Ni J, Messing EM, Chang E, Yang CR, Yeh S. Vitamin E succinate inhibits the function of androgen receptor and the expression of prostate‐specific antigen in prostate cancer cells. Proc Natl Acad Sci U S A. 2002;99:7408‐7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Kline K, Yu W, Sanders BG. Vitamin E and breast cancer. J Nutr. 2004;134:3458S‐3462S. [DOI] [PubMed] [Google Scholar]
- 197. Wu K, Zhao Y, Liu BH, et al. RRR‐alpha‐tocopheryl succinate inhibits human gastric cancer SGC‐7901 cell growth by inducing apoptosis and DNA synthesis arrest. World J Gastroenterol. 2002;8:26‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Alizadeh F, Bolhassani A, Khavari A, Bathaie SZ, Naji T, Bidgoli SA. Retinoids and their biological effects against cancer. Int Immunopharmacol. 2014;18:43‐49. [DOI] [PubMed] [Google Scholar]
- 199. Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143‐201. [PubMed] [Google Scholar]
- 200. Surh YJ, Lee SS. Capsaicin, a double‐edged sword: toxicity, metabolism, and chemopreventive potential. Life Sci. 1995;56:1845‐1855. [DOI] [PubMed] [Google Scholar]
- 201. D'Eliseo D, Manzi L, Velotti F. Capsaicin as an inducer of damage‐associated molecular patterns (DAMPs) of immunogenic cell death (ICD) in human bladder cancer cells. Cell Stress Chaperon. 2013;18:801‐808. [DOI] [PMC free article] [PubMed] [Google Scholar]


