Abstract
Successful integrated pest management in protected crops implies an evaluation of the compatibility of pesticides and natural enemies (NE), as control strategies that only rely on one tactic can fail when pest populations exceed NE activity or pests become resistant to pesticides. Nowadays in Almería (Spain), growers release NE prior to transplanting or early in the crop cycle to favor their settlement before pest arrival because this improves biocontrol efficacy, although it extends pesticide exposure periods. The purpose of this research was to evaluate the compatibility of two applications of pesticides with key NE in 2‐year trials inside tomato and sweet pepper commercial greenhouses: Nesidiocoris tenuis (Reuter) (Hemiptera: Miridae), Orius laevigatus (Say) (Hemiptera: Anthocoridae) and Amblyseius swirskii (Athias‐Henriot) (Acari: Phytoseiidae). In tomato, flubendiamide and chlorantraniliprole (IOBC category 1) were compatible with N. tenuis, but chlorpyrifos‐methyl and spinosad (IOBC categories 2–3), which effectively reduced Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) density, compromised its predatory activity. In sweet pepper, chlorantraniliprole (IOBC category 1) was the only pesticide compatible with O. laevigatus while chlorantraniliprole, emamectin benzoate, spirotetramat and pymetrozine were harmless (IOBC category 1) to Amblyseius swirskii, and sulfoxaflor slightly harmful (IOBC category 2) to this phytoseiid predator.
Keywords: Amblyseius swirskii, chemical control; Frankliniella occidentalis, Nesidiocoris tenuis, Orius laevigatus, Tuta absoluta
Introduction
The region Almería, in Southeastern Spain, has developed an intensive protected crop industry (48 000 ha), which has been the driving force of the socioeconomic development in recent decades. The two main crops are sweet pepper and tomato (MAPAMA, 2018a). Currently, one of the most aggressive pests affecting tomato crops is the South American tomato pinworm Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae), present in European, African, Asian, and South American tomato‐producing areas (Biondi et al., 2018; Mansour et al., 2018; Han et al., 2019). This invasive pest favors secondary pathogen infections and reduces the photosynthetic activity of the host plant and thus its production (Desneux et al., 2010; Tropea Garzia et al., 2010). The Western flower thrips, Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae) and the tobacco whitefly, Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) are other important pests for both tomato and pepper. They cause economic losses by feeding when populations reach high levels and interfere with crop physiological processes, by transmission of plant viruses and by production of vast amounts of honeydew, which promotes sooty mold growth (De Barro, 2011; CABI, 2018).
Successful Integrated Pest Management (IPM) systems, mandatory in the European Union (EU) since the implementation of the directive on the sustainable use of pesticides, 2009/128/EC (OJEU, 2009), came into effect in 2014 along with a variety of control measures (Ehler, 2006). IPM programs based on biological control had been practicing in the region for many years since campaign 2005–2006, undoubtedly in parallel with the increase in crop area under this strategy; from 129 ha in the 2005–2006 campaign to 26 590 ha in 2016–2017 (Biocolor, 2018). In the region of Almería, due to the high presence of insecticide resistance (Elbert & Nauen, 2000; Espinosa et al., 2002; Bielza et al., 2007; Fernández et al., 2009; Grávalos et al., 2014), virtually all greenhouses follow IPM programs primarily based on biological strategies occasionally supported with selective chemical treatments (Robledo et al., 2009; Glass & Egea González, 2012). Several polyphagous predators are commercialized to control sweet pepper and tomato pests. The phytoseiid mite Amblyseius swirskii (Athias‐Henriot) (Acari: Phytoseiidae) is the most widely used NE in the region to control B. tabaci, and it is also a very efficient predator of F. occidentalis even at low pest densities (Chow et al., 2008; Colomer et al., 2011; Amor et al., 2012; Calvo et al., 2012a). Two other interesting NE available are the anthocorid Orius laevigatus (Say) (Hemiptera: Anthocoridae), widely used in inoculative releases against F. occidentalis (Sánchez et al., 2000; Brodsgaard, 2004), and the generalist zoophytophagous mirid Nesidiocoris tenuis (Reuter) (Hemiptera: Miridae). The latter species often appears spontaneously in the Mediterranean region (Calvo et al., 2012a), feeds on thrips, mites, aphids, leafminers, and whiteflies (Sánchez et al., 2009; Pérez‐Hedo & Urbaneja, 2015; Perdikis & Arvaniti, 2016; Bouagga et al., 2018), and can regulate T. absoluta by feeding on eggs and young larvae (Urbaneja et al., 2012; Biondi et al., 2018).
Pest control strategies that rely on one approach seem easy and adequate yet often fail when pest populations exceed NE activity or pests become resistant to pesticides (Glass & Egea González, 2012). Therefore, despite the ability of NE to control several pests with simultaneous outbreaks in the crop, chemical treatments are often sometimes needed to maintain key and secondary pest populations under economic thresholds and the ascertaining of their compatibility is crucial for success. Thus, toxicity and sublethal effects of pesticides on NE of horticultural pests has been extensively studied under laboratory or semifield conditions (Amor et al., 2012; Bengochea et al., 2012; Abraham et al., 2013; Döker et al., 2014; Garzón et al., 2015; Maia et al., 2016; Wanumen et al., 2016; De Bortoli et al., 2017; Fernández et al., 2017, Madbouni et al., 2017). In general, much less information is available on the compatibility of novel pesticides and NE inside commercial greenhouses. Some formulations and concentrations of methoxyfenozide, chlorantraniliprole, flonicamid, spiromesifen, and sulfur have been found to be compatible with A. swirskii and O. laevigatus (Bielza et al., 2009; Colomer et al., 2011; Gázquez et al., 2011; Gradish et al., 2011). Emamectin benzoate was only compatible in semifield when applied before the NE introduction (Amor et al., 2012).
Nowadays, the presence of NE in crops in sufficient numbers before the arrival of pests is considered determinant for biological control efficacy (Sánchez et al., 2014; Bouagga et al., 2018) and NE producers offer long duration products, which are well suited to a preventive approach (Koppert, 2018). Therefore, A. swirskii or O. laevigatus are introduced in crops shortly after transplanting while N. tenuis is released in the nursery so when the plants are transplanted they already contain the eggs in their tissue, which accelerates NE colonization and establishment (Calvo et al., 2012a,b). However, the early introduction of NE in crops makes its coincidence with any necessary pesticide applications unavoidable; thus NE are subjected to the action of the applied active substances for longer time periods.
The objective of this research was to determine whether the preplant introduction of N. tenuis in tomato and early introductions of A. swirskii and O. laevigatus in sweet pepper commercial multispan greenhouses could be compatible with repeated applications of some of the most frequently used pesticides in the region of Almería.
Materials and methods
Study sites and growing conditions
The trials took place in 2016 and 2017 in El Ejido (Almería, Spain) inside representative tomato and sweet pepper commercial multispan plastic greenhouses of 1–1.5 hectares, with 100 mm artificial sand mulch soil, and drip irrigation and fertilization carried out according to standard practices in the area.
Crops were transplanted from nursery seedlings and handled according to good standard agricultural practices. Solanum lycopersicum L. var. Delizia in 2016 and var. Rambo in 2017 were transplanted on August 18–19 and August 22–23, respectively, at a density of 1.5 plants per 0.4 m2. Capsicum annuum L. var. Palermo was transplanted on August 23–25, 2016, at a density of 1.5 plants per 0.5 m2.
Natural enemies
Natural enemies were introduced at the initial commercial rates recommended by the manufacturer (Koppert España SL, La Mojonera, Almería, Spain; Koppert, 2018). The mirid N. tenuis (Nesibug®, 500 mL bottles, 500 adults + nymphs with vermiculite) was released in the nursery at a rate of 0.5 individual per plant, 5 d before transplanting, using Dibox® application boxes on the top leaves of the tomato seedlings with three leaves from the main stem unfolded (BBCH 13; Meier, 2001). To ensure NE survival from its introduction until the arrival of the pests, Entofood® (Ephestia kuehniella Zeller eggs + Artemia sp. Cysten; 500 mL bottles) was added at a rate of 60 g per row every 7 d, three to four times, using a Mini‐Airbug® device. In sweet pepper, the two NE were early released, 19 d after transplanting, when the first inflorescence opened (BBCH 61; Meier, 2001). The anthocorid bug O. laevigatus (Thripor‐L®, 100 mL bottles, 2000 adults + nymphs mixed with vermiculite and buckwheat husks) was released at a rate of 4 individuals per m2 using Dibox® boxes on the top leaves. The predatory mite A. swirskii (Swirskii‐mite LD®, paper sachets with 125 mobile forms mixed with wheat bran, various developmental stages of the mite Carpoglyphus lactis L. and other acari as a food source) was released at a rate of 75 individuals per m2 by hanging the sachets on the middle leaves.
Pesticide application
Pesticides with distinct modes of action (FRAC, 2018; IRAC, 2018) were selected among the most frequently used inside Almería greenhouses for the control of key pests, based on lack of information on the compatibility with NE according to field technicians and manufacturers. All active ingredients (a.i.) were registered in the EU (MAPAMA, 2018b) and tested at their maximum field recommended concentrations (MFRC) in accordance with the Spanish registration (Table 1). Following regular farmer's pest control practices, plots in both crops were sprayed with the broad‐spectrum and systemic fungicide tebuconazole in order to control Botrytis cinerea Pers.:Fr (anamorphic form) powdery mildew and other fungal diseases.
Table 1.
Active ingredients (a.i.), trademark names, IRAC/FRAC modes of action, applied concentrations, target pests, and crops
| Active ingredient | Commercial trademark names in Spain | IRAC/FRAC† modes of action | MFRC‡(g a.i./ha) | Pests | Crop |
|---|---|---|---|---|---|
| Chlorantraniliprole | Altacor® | Ryanodine receptor modulator | 40 | Caterpillars | Sweet pepper, |
| DuPont, Madrid | T. absoluta | tomato | |||
| Chlorpyrifos‐methyl | Reldan E® | Acetylcholinesterase (AChE) inhibitor | 900 | Caterpillars thrips | Tomato |
| Dow AgroSciences, Madrid | |||||
| Emamectin benzoate | Affirm® 0.85% SG | Glutamate‐gated chloride channel (GluCl) allosteric modulator | 12.75 | Caterpillars | Sweet pepper, tomato |
| Syngenta Agro S.A., Madrid | T. absoluta | ||||
| Flubendiamide | Fenos® 24% WG | Ryanodine receptor | 90 | Caterpillars | Tomato |
| Bayer Cropscience S.L., Valencia | modulator | T. absoluta | |||
| Metaflumizone | Alverde® 24% SC | Voltage‐dependent sodium channel blocker | 240 | Caterpillars, | Tomato |
| BASF Española S.L., Madrid | T. absoluta | ||||
| Pymetrozine | Plenum® | Chordotonal organ TRPV (transient receptor potential vanilloid) channel modulator | Aphids, whiteflies | Sweet pepper | |
| Syngenta, Madrid | |||||
| Spinosad | Spintor® | Nicotinic acetylcholine receptors (nAChRs) | 110 | Caterpillars, thrips | Tomato |
| Dow AgroSciences, Madrid | |||||
| Spirotetramat | Movento® 15% SC | Inhibitor of acetyl CoA | 75 | Aphids, whiteflies, scales | Sweet pepper |
| Bayer Cropscience S.L., Valencia | carboxylase | ||||
| Sulfoxaflor | Isoclast® | nAChR agonist | 24 | Aphids, whiteflies | Sweet pepper |
| Dow AgroSciences, Madrid | |||||
| Tebuconazole§ | Folicur® 25 WG | C14‐demethylase in sterol biosynthesis | 600 | Fungal diseases | Sweet pepper, tomato |
| Bayer Cropscience S.L., Valencia | 1500 |
†
IRAC = Insecticide Resistance Action Committee; FRAC = Fungicide Resistance Action Committee.
‡
Maximum field recommended concentration.
§
Control, following farmers’ regular practices.
Two trials with two pesticide applications were carried out in tomato (September 30 and October 11 in 2016; October 11 and 21 in 2017) and one trial in sweet pepper (October 6 and 18 in 2016), with same or different pesticides based on farmers’ interest. Because in our greenhouses the soil is artificial, a strip trial design (Milliken & Johnson, 1984) with five to six insecticide treatments and two controls randomly distributed was performed in an area per treatment of 72–100 m2 with six rows of plants 2 m apart oriented North (N)–South (S). Treatment distribution from East to West was as follows, in tomato 2016: chlorantraniliprole, flubendiamide, control 1, metaflumizone, chlorpyrifos‐methyl, spinosad, control 2 and emamectin benzoate; in tomato 2017: flubendiamide, metaflumizone, control 1, chlorpyrifos‐methyl, spinosad, control 2 and emamectin benzoate; in sweet pepper 2016: emamectin benzoate, control 1, sulfoxaflor, spirotetramat, control 2, pymetrozine and chlorantraniliprole.
In every treatment, four replicates (18–25 m2) from the open sides covered with anti‐pest nets (N) to the central corridor (S) were established because pest distribution could be biased, which in turn could have an influence on the natural enemy density.
Sampling
Direct visual data collection of mobile forms of pests and natural enemies was carried out weekly, with the aid of a small magnifier (6 ×) from 9:00 a.m. to 2:00 p.m., on plants located in the two central rows of each replicate and treatment to avoid spray drift contamination from adjacent pesticides, and on the preferred loci of each species. In tomato, in 2016 we examined 60 leaves per treatment (15 per replicate) on the upper part of the plants to monitor N. tenuis and T. absoluta; in 2017, only 30 leaves per treatment (7–8 per replicate) because populations were very homogenous. On sweet pepper, we examined 60 leaves per treatment (15 per replicate) from the middle part of flowering plants to monitor mobile forms of A. swirskii, and 40 flowers per treatment (10 per replicate) for O. laevigatus and F. occidentalis.
Statistical analysis
Statistical analyses of data (presented as mean ± SEM) were carried out using IBM Statistics SPSS v.23.0 package (IBM Corp., 2015). The weighted mean numbers of insects per replicate (n = 4; dependent variable), very homogeneous among samples of the same replicate, were used for statistical analyses (Crawley, 2013). Initially, homogeneity of control plots in every crop and year (P < 0.05) was studied with a Student's t‐test or a nonparametric Mann–Whitney U‐test when neither raw nor transformed data followed criteria, and because no statistically significant differences were obtained, an average control was calculated in every case (Colomer et al., 2011). The data were analyzed with a Lineal Mixed–effect Model (LMM) (P < 0.05), very appropriate for our approach (Wang & Goonewardene, 2004; Crawley, 2013). The lowest value of Akaike's Information Criterion (AIC) was used to select the best covariance structure in each model (Wang & Goonewardene, 2004). The significance of the effects was determined by the F‐test statistic and the estimated marginal means were compared with the LSD test. Because pesticides changed with the crop and year, separately analyses were carried out. The different insect species and pesticides were considered as fixed factors and the sampling dates as a repeated measures factor. Based on the NE density on the last sampling date compared to the control, the pesticide effect was categorized according to the four IOBC (International Organization for Biological and integrated Control) toxicity categories for semifield conditions because NE were released in the crops: 1 = harmless: <25% mortality; 2 = slightly harmful: 25%–50% mortality; 3 = moderately harmful: 51%–75% mortality; or 4 = harmful: >75% mortality (Hassan, 1994).
Results
Pests T. absoluta in tomato and F. occidentalis in sweet pepper infested the crops. The whitefly B. tabaci was not present in either of the crops. The predatory mite Balaustium hernandezi von Heyden (Acari: Erythraeidae) was also found in sweet pepper but at a very low density; thus it was not considered. The numbers of pests and NE were found to be homogeneous among the two control replicates of each trial (Table 2). When pooling insect density data of the same year and crop together, there were statistically significant interactions among factors (Table 3); therefore, we proceeded to analyze each insect separately in every crop each year.
Table 2.
Mean ± SEM population density of N. tenuis, Orius laevigatus, Amblyseius swirskii, Tuta absoluta, and Frankliniella occidentalis per leaf in two control plots in tomato and sweet pepper with corresponding sampling dates
| Tomato 2016 | Sept 29 | Oct 6 | Oct 13 | Oct 20 | Oct 27 | |||
|---|---|---|---|---|---|---|---|---|
| N. tenuis | ||||||||
| C1 | 3.55 ± 0.23 | 4.55 ± 0.22 | 4.88 ± 0.17 | 5.70 ± 0.23 | 6.00 ± 0.17 | |||
| C2 | 3.63 ± 0.18 | 4.95 ± 0.17 | 5.00 ± 0.13 | 5.88 ± 0.24 | 5.95 ± 0.17 | |||
| T = −0.258 | U = 3.500 | U = 6.000 | U = 6.000 | U = 7.500 | ||||
| df = 6 | Z = −1.307 | Z = −0.584 | Z = −0.577 | Z = −0.146 | ||||
| P = 0.805 | P = 0.191 | P = 0.559 | P = 0.564 | P = 0.884 | ||||
| T. absoluta | ||||||||
| C1 | 0.15 ± 0.09 | 0.15 ± 0.03 | 0.13 ± 0.06 | 0.15 ± 0.06 | 0.10 ± 0.04 | |||
| C2 | 0.20 ± 0.04 | 0.18 ± 0.05 | 0.20 ± 0.04 | 0.10 ± 0.04 | 0.10 ± 0.07 | |||
| U = 5.000 | U = 7.000 | U = 4.500 | U = 6.000 | U = 7.000 | ||||
| Z = −0.0.893 | Z = −0.319 | Z = −1.049 | Z = −0.599 | Z = −0.303 | ||||
| P = 0.486 | P = 0.886 | P = 0.343 | P = 0.686 | P = 0.886 |
| Tomato 2017 | Oct 10 | Oct 17 | Oct 24 | Oct 31 | Nov 7 | |||
|---|---|---|---|---|---|---|---|---|
| N. tenuis | ||||||||
| C1 | 3.90 ± 0.23 | 4.93 ± 0.24 | 5.23 ± 0.14 | 6.05 ± 0.24 | 6.33 ± 0.21 | |||
| C2 | 3.98 ± 0.19 | 5.25 ± 0.19 | 5.33 ± 0.11 | 5.93 ± 0.23 | 6.33 ± 0.17 | |||
| t = −0.249 | U = 4.500 | U = 6.500 | t = −0.374 | t = −0.000 | ||||
| df = 6 | Z = −1.016 | Z = −0.447 | df = 6 | df = 6 | ||||
| P = 0.812 | P = 0.309 | P = 0.655 | P = 0.721 | P = 1.000 | ||||
| T. absoluta | ||||||||
| C1 | 1.33 ± 0.06 | 1.33 ± 0.05 | 1.25 ± 0.09 | 1.20 ± 0.11 | 1.23 ± 0.09 | |||
| C2 | 1.35 ± 0.06 | 1.30 ± 0.04 | 1.35 ± 0.06 | 1.20 ± 0.07 | 0.68 ± 0.17 | |||
| t = −0.277 | U = 6.500 | U = 5.500 | U = 7.500 | U = 0.500 | ||||
| df = 6 | Z = −0.458 | Z = −0.744 | Z = −0.149 | Z = −2.178 | ||||
| P = 0.791 | P = 0.686 | P = 0.486 | P = 0.886 | P = 0.029 |
| Sweet pepper 2016 | Oct 5 | Oct 12 | Oct 19 | Oct 26 | Nov 3 | Nov 9 | Nov 16 | Nov 23 |
|---|---|---|---|---|---|---|---|---|
| O. laevigatus | ||||||||
| C1 | 1.03 ± 0.30 | 0.73 ± 0.13 | 0.70 ± 0.04 | 0.88 ± 0.13 | 0.78 ± 0.15 | 0.63 ± 0.12 | 0.95 ± 0.16 | 0.75 ± 0.16 |
| C2 | 1.05 ± 0.13 | 1.13 ± 0.19 | 1.18 ± 0.05 | 1.35 ± 0.09 | 1.33 ± 0.11 | 0.85 ± 0.12 | 0.83 ± 0.08 | 0.93 ± 0.06 |
| t = −0.077 | t = −1.712 | t = −7.592 | t = −3.017 | t = −2.874 | t = −1.342 | t = −0.724 | t = −1.043 | |
| df = 6 | df = 6 | df = 6 | df = 6 | df = 6 | df = 6 | df = 6 | df = 6 | |
| P = 0.941 | P = 0.138 | P < 0.001 | P = 0.023 | P = 0.028 | P = 0.228 | P = 0.496 | P = 0.337 | |
| A. swirskii | ||||||||
| C1 | 3.35 ± 0.34 | 3.25 ± 0.43 | 2.85 ± 0.34 | 2.58 ± 0.39 | 2.80 ± 0.15 | 2.30 ± 0.19 | 2.38 ± 0.11 | 2.75 ± 0.13 |
| C2 | 3.63 ± 0.64 | 2.88 ± 0.52 | 3.08 ± 0.06 | 2.25 ± 0.23 | 2.53 ± 0.19 | 2.45 ± 0.21 | 2.90 ± 0.36 | 2.93 ± 0.13 |
| U = 7.500 | t = −0.555 | t = −0.655 | t = −0.240 | t = −1.133 | t = −0.533 | t = −1.419 | t = −0.938 | |
| Z = −0.145 | df = 6 | df = 6 | df = 6 | df = 6 | df = 6 | df = 6 | df = 6 | |
| P = 0.886 | P = 0.599 | P = 0.537 | P = 0.819 | P = 0.301 | P = 0.613 | P = 0.206 | P = 0.384 | |
| F. occidentalis | ||||||||
| C1 | 1.40 ± 0.45 | 1.53 ± 0.49 | 2.15 ± 0.13 | 1.50 ± 0.65 | 1.10 ± 0.28 | 1.83 ± 0.34 | 1.13 ± 0.24 | 1.30 ± 0.20 |
| C2 | 1.40 ± 0.31 | 2.35 ± 0.58 | 1.43 ± 0.23 | 1.08 ± 0.35 | 0.73 ± 0.17 | 1.35 ± 0.10 | 1.05 ± 0.09 | 1.25 ± 0.19 |
| t = 0.000 | t = −1.085 | t = −2.621 | t = −0.0.576 | U = 5.000 | t = −1.413 | t = −0.193 | t = −0.182 | |
| df = 6 | df = 6 | df = 6 | df = 6 | Z = −1.155 | df = 6 | df = 6 | df = 6 | |
| P = 1.000 | P = 0.320 | P = 0.059 | P = 0.585 | P = 0.343 | P = 0.207 | P = 0.854 | P = 0.862 |
Note: Data within the same column was analyzed through Student's t‐test or Mann–Whitney U‐test to compare homogeneity of control plots (P < 0.05).
Table 3.
Statistics and interactions among factors in the tomato and sweet pepper trials according to a Linear Mixed Model using insect and pesticide as fixed factors, and sampling dates as the repeated measures factor (5 weeks in tomato and 8 weeks in sweet pepper) (P < 0.05)
| Tomato 2016; Nesidiocoris tenuis, Tuta absoluta | |
|---|---|
| Insect | F 1,174.530 = 9048.358; P < 0.001 |
| Pesticide | F 6,174.530 = 138.829; P < 0.001 |
| Insect × Pesticide | F 6,174.530 = 133.346; P < 0.001 |
| Tomato 2017; Nesidiocoris tenuis, Tuta absoluta | |
|---|---|
| Insect | F 1,150.319 = 5139.886; P < 0.001 |
| Pesticide | F 5,150.319 = 233.893; P < 0.001 |
| Insect × Pesticide | F 5,150.319 = 81.817; P < 0.001 |
| Sweet pepper 2016; Amblyseius swirskii, Orius laevigatus, Frankliniella occidentalis | |
|---|---|
| Insect | F 2,529.434 = 788.333; P < 0.001 |
| Pesticide | F 5,529.434 = 4.368; P = 0.001 |
| Insect × Pesticide | F 10,529.434 = 40.181; P < 0.001 |
Nesidiocoris tenuis and T. absoluta in tomato
The pattern of weighted average numbers of N. tenuis was cohesive both years and significant differences among treatments accentuated with time (2016: F 6,94.955 = 162.134; P < 0.001; 2017: F 5,79.356 = 168.984; P < 0.001) (Fig. A and B). Control plots had the highest density of mirids (up to 6 individuals per leaf the last evaluation date, both years). Under the two diamides treatments (flubendiamide and chlorantraniliprole, the latter only used in 2016), mirid density was similar to control and increased over time even after the insecticide applications. On the last sampling date of each year, numbers of mirids under metaflumizone, emamectin benzoate and spinosad treatments were close to the initial density registered before treatments and significantly lower than those registered for the two diamides and the control. Chlorpyrifos‐methyl was the most aggressive pesticide both years and its application decreased the mirid population approximately by 35% since the starting of the trial.
Figure 1.
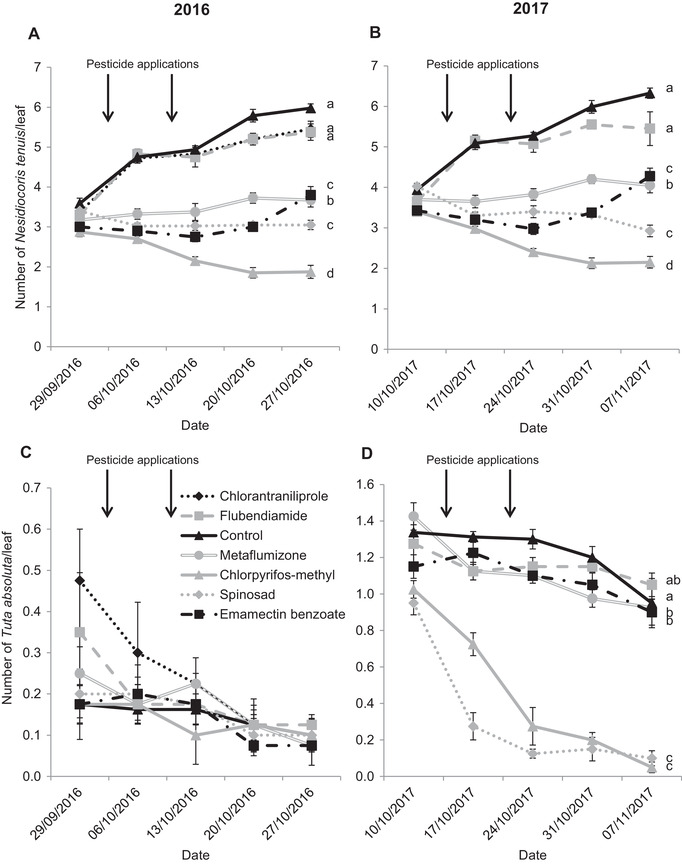
Mean ± SEM population of Nesidiocoris tenuis and Tuta absoluta per leaf inside tomato commercial greenhouses in 2016 (A and C) and 2017 (B and D) after two pesticide applications on September 30 and October 11, 2016, and on October 11 and 21, 2017. Arrows point out the moment of pesticide applications. Different letters stand for statistical differences among treatments according to a Linear Mixed Model test using pesticide as the fixed factor and sampling dates as the repeated measures factor, followed by LSD pairwise comparisons (P < 0.05).
The pesticide impact on T. absoluta population varied each year (Fig. C and D). In 2016, density was extremely low (from 0.48 ± 0.13 to 0.08 ± 0.05 individual per leaf) and no statistically significant differences were observed among treatments (F 6,131.379 = 0.947; P = 0.464) (Fig. C). However, in 2017 the initial population density was 3‐fold higher (up to 1.43 ± 0.08 individuals per leaf; Fig. D). Chlorpyrifos‐methyl and spinosad significantly decreased population over time, which reached values near zero in the last monitoring date. Flubendiamide behaved like the control, and metaflumizone and emamectin benzoate only caused punctual significant decreases (F 5,74.664 = 162.591; P < 0.001) (Fig. D).
Orius laevigatus, A. swirskii, and F. occidentalis in sweet pepper
The presence of F. occidentalis over the different sampling dates was low and pesticide treatments were not very successfully effective (Table 4). The thrip population significantly grew in plots treated with pymetrozine, spirotetramat, sulfoxaflor, and chlorantraniliprole compared to control and emamectin benzoate (F 5,177.538 = 31.549; P < 0.001). Both NE also settled in the crop at low densities, which decreased in all treatments over time (Table 4). Sulfoxaflor was the most toxic pesticide to O. laevigatus although statistically similar to pymetrozine, followed by spirotetramat and emamectin benzoate (F 5,178.159 = 30.725; P < 0.001). Chlorantraniliprole was harmless and statistically similar to control and emamectin benzoate. For A. swirskii, density in control and emamectin benzoate was significantly different to rest of pesticides (F 5,157.977 = 10.262; P < 0.001). Sulfoxaflor was slightly harmful, and emamectin benzoate, spirotetramat, pymetrozine, and chlorantraniliprole harmless, based on IOBC toxicity categories.
Table 4.
Mean ± SEM population of Orius laevigatus, Amblyseius swirskii, and Frankliniella occidentalis per leaf in sweet pepper commercial greenhouses with pesticide applications on October 6 and 18, 2016
| Sweet pepper 2016 | Oct 5 | Oct 12 | Oct 19 | Oct 26 | Nov 3 | Nov 9 | Nov 16 | Nov 23 | |
|---|---|---|---|---|---|---|---|---|---|
| O. laevigatus | |||||||||
| Control | 1.04 ± 0.15 | 0.93 ± 0.13 | 0.94 ± 0.09 | 1.11 ± 0.12 | 1.05 ± 0.14 | 0.74 ± 0.09 | 0.89 ± 0.08 | 0.84 ± 0.08 | a |
| Chlorantraniliprole | 1.08 ± 0.10 | 0.90 ± 0.07 | 0.95 ± 0.23 | 1.05 ± 0.10 | 1.23 ± 0.12 | 0.85 ± 0.13 | 0.73 ± 0.09 | 0.65 ± 0.06 | ab |
| Emamectin benzoate | 1.15 ± 0.35 | 1.08 ± 0.10 | 0.85 ± 0.12 | 0.90 ± 0.11 | 0.78 ± 0.08 | 0.88 ± 0.16 | 0.75 ± 0.10 | 0.55 ± 0.10 | b |
| Spirotetramat | 1.25 ± 0.10 | 0.93 ± 0.12 | 0.65 ± 0.09 | 0.53 ± 0.13 | 0.45 ± 0.03 | 0.43 ± 0.09 | 0.45 ± 0.06 | 0.53 ± 0.05 | c |
| Pymetrozine | 0.95 ± 0.13 | 1.00 ± 0.16 | 0.53 ± 0.07 | 0.33 ± 0.05 | 0.48 ± 0.09 | 0.30 ± 0.04 | 0.33 ± 0.05 | 0.53 ± 0.06 | cd |
| Sulfoxaflor | 1.33 ± 0.15 | 0.95 ± 0.09 | 0.70 ± 0.07 | 0.40 ± 0.04 | 0.48 ± 0.06 | 0.15 ± 0.03 | 0.25 ± 0.10 | 0.23 ± 0.08 | d |
| A. swirskii | |||||||||
| Control | 3.49 ± 0.34 | 3.06 ± 0.32 | 2.96 ± 0.16 | 2.41 ± 0.22 | 2.66 ± 0.12 | 2.38 ± 0.13 | 2.64 ± 0.20 | 2.84 ± 0.08 | a |
| Chlorantraniliprole | 3.35 ± 0.44 | 2.70 ± 0.29 | 1.60 ± 0.11 | 2.48 ± 0.32 | 2.33 ± 0.17 | 2.18 ± 0.14 | 2.28 ± 0.13 | 3.35 ± 0.17 | b |
| Emamectin benzoate | 3.93 ± 0.26 | 3.70 ± 0.40 | 2.45 ± 0.36 | 2.65 ± 0.26 | 2.53 ± 0.27 | 2.50 ± 0.11 | 2.55 ± 0.14 | 2.48 ± 0.11 | a |
| Spirotetramat | 3.13 ± 0.40 | 2.80 ± 0.34 | 2.05 ± 0.21 | 1.78 ± 0.12 | 1.70 ± 0.20 | 2.13 ± 0.09 | 2.15 ± 0.21 | 2.28 ± 0.13 | b |
| Pymetrozine | 2.93 ± 0.33 | 2.68 ± 0.09 | 2.58 ± 0.46 | 2.20 ± 0.44 | 2.00 ± 0.15 | 1.98 ± 0.11 | 2.55 ± 0.12 | 2.18 ± 0.11 | b |
| Sulfoxaflor | 4.03 ± 0.39 | 3.08 ± 0.32 | 1.95 ± 0.24 | 1.88 ± 0.13 | 2.10 ± 0.11 | 2.20 ± 0.21 | 2.33 ± 0.19 | 1.63 ± 0.26 | b |
| F. occidentalis | |||||||||
| Control | 1.40 ± 0.25 | 1.94 ± 0.38 | 1.79 ± 0.18 | 1.29 ± 0.35 | 0.91 ± 0.17 | 1.59 ± 0.19 | 1.09 ± 0.12 | 1.28 ± 0.13 | c |
| Chlorantraniliprole | 1.68 ± 0.13 | 2.18 ± 0.41 | 1.93 ± 0.37 | 1.53 ± 0.28 | 1.28 ± 0.22 | 1.85 ± 0.20 | 2.83 ± 0.98 | 2.33 ± 0.37 | b |
| Emamectin benzoate | 1.00 ± 0.07 | 1.05 ± 0.13 | 1.85 ± 0.18 | 1.70 ± 0.25 | 2.03 ± 0.43 | 1.73 ± 0.24 | 1.58 ± 0.28 | 1.18 ± 0.24 | c |
| Spirotetramat | 2.13 ± 0.36 | 2.28 ± 0.35 | 2.68 ± 0.39 | 3.73 ± 0.54 | 4.55 ± 0.62 | 4.13 ± 0.71 | 3.33 ± 0.48 | 2.48 ± 0.25 | a |
| Pymetrozine | 2.10 ± 0.18 | 1.95 ± 0.48 | 4.28 ± 0.73 | 4.93 ± 0.36 | 4.15 ± 1.03 | 3.65 ± 0.72 | 3.18 ± 0.35 | 2.73 ± 0.44 | a |
| Sulfoxaflor | 1.53 ± 0.22 | 1.55 ± 0.32 | 1.45 ± 0.34 | 4.23 ± 0.35 | 3.68 ± 0.70 | 2.83 ± 0.44 | 2.63 ± 0.23 | 2.40 ± 0.21 | b |
Note: Different letters for each insect stand for statistical differences among treatments throughout the whole duration of the experiment according to a Linear Mixed Model test using pesticide as the fixed factor and sampling dates as the repeated measures factor, followed by LSD pairwise comparisons (P < 0.05).
Final IOBC pesticide toxicity categories in both crops are shown in Table 5.
Table 5.
Final IOBC toxicity categories† based on the mortality with two pesticide applications to the natural enemies in the commercial tomato and sweet pepper greenhouses
| Nesidiocoris tenuis | Orius laevigatus | Amblyseius swirskii | |||
|---|---|---|---|---|---|
| Pesticide | MFRC‡ (g a.i./ha) | 2016 | 2017 | 2016 | 2016 |
| Chlorantraniliprole | 40 | 1 | Nontested | 1 | 1 |
| Chlorpyrifos‐methyl | 900 | 3 | 3 | Nontested | Nontested |
| Emamectin benzoate | 12.75 | 2 | 2 | 2 | 1 |
| Flubendiamide | 90 | 1 | 1 | Nontested | Nontested |
| Metaflumizone | 240 | 2 | 2 | Nontested | Nontested |
| Spinosad | 75 | 2 | 3 | Nontested | Nontested |
| Spirotetramat | 75 | Nontested | Nontested | 2 | 1 |
| Pymetrozine | 250 | Nontested | Nontested | 2 | 1 |
| Sulfoxaflor | 24 | Nontested | Nontested | 3 | 2 |
†
IOBC toxicity categories for field test: 1 = harmless (<25% mortality); 2 = slightly harmful (25%–50% mortality); 3 = moderately harmful (51%–75% mortality); and 4 = harmful (>75% mortality).
‡
Maximum field recommended concentration.
Discussion
The use of NE is the key control strategy in the greenhouses of Almería. At present, omnivorous predatory species are the most recommended because pest pressure is high from the beginning of the crop cycle and NE can establish early, which is essential to their success. In our trials, the three released NE successfully established in the crops before pest arrival. Amblyseius swirskii can feed on other available prey species (e.g., the mite B. hernandezi was present in the crop), pollen and preys provided in the formulation. Nesidiocoris tenuis can prey exclusively on T. absoluta eggs and larvae (Biondi et al., 2018), plant sap and the alternative food provided. Orius laevigatus has a marked preference for thrips but the genus can also feed on pollen, xylem and mesophyll contents (Armer et al., 1998).
Biological control is nowadays applied in Almería in more than 50% of the total greenhouse surface (Biocolor, 2018; MAPAMA, 2018a), encouraged by pressure from supply chain and consumers (Glass & Egea González, 2012), pesticide resistance (Bielza & Gillén, 2014; Grávalos et al., 2014; Roditakis et al., 2018), and the EU legislation making IPM obligatory (OJEU, 2009). Ideally, the adoption of biological control alone in protected cultures should be possible because of the emphasis on sustainable production systems and the great deal of NE commercially available worldwide. However, the adoption can sometimes be difficult because the risk tolerance of farmers is usually very low, especially during harvest; consumers demand aesthetic products; the governments not always give support to this strategy and there is dominance of the pesticide industry (van Lenteren, 2011). Besides, crops are threatened by key and secondary pests that can coexist, invasive pest species or emergent virus diseases, as it has unfortunately happened in the region during the last decades (Robledo et al., 2009; Parrella & Lewis, 2018).
Therefore, there is still reliance on pesticide applications in punctual moments when NE are not sufficiently efficient. Pesticides applied can negatively affect parasitoids and predators even when releases are carefully timed (Bielza et al., 2009; Colomer et al., 2011; Gázquez et al., 2011; Amor et al., 2012); therefore, it is essential to ascertain their compatibility prior use (IOBC, 2018).
Multiple exposure routes enhance the pesticide risk to NE, as reported by Madbouni et al. (2017) for N. tenuis. In our trials, as NE were introduced in the nursery or early in the crop, possible sources of contamination are contact with residues on leaves or with droplets during application, and feeding on contaminated preys or alternative food. Multiple pesticide applications or pesticide mixtures also entail a greater risk to NE than single applications (Panizzi et al., 2017), but in literature there is scarce information.
In our commercial greenhouses, the harmfulness of the tested pesticides for our NE agree with results published in several pesticide databases, even though it is difficult to know exactly how these data were generated (Biobest, 2019; Koppert, 2019), except for IOBC database, where references are added (IOBC, 2019). The fungicide tebuconazole did not affect the populations of N. tenuis, O. laevigatus and A. swirskii in control plots, which were higher than under the majority of pesticide‐treated plots. Tebuconazole is selective for phytoseiid mites (Fountain & Med, 2015; Put et al., 2016), Orius spp. (Biobest, 2019) and other NE in semifield and field (Sterk et al., 1999). Besides, the fungicide seemed not to have synergized effects with any of our pesticides despite they are reported with neonicotinoid thiacloprid (Willow et al., 2019).
The tomato crop was only attacked by the leafminer T. absoluta. In the second year (2017), the initial population on the first monitoring date was threefold higher than in 2016 and decreased over time, especially with the spinosad and chlorpyrifos‐methyl treatments (89% and 95% reduction in final monitoring compared to the initial, respectively). In contrast, flubendiamide, emamectin benzoate, and metaflumizone did not control our population. This pest exhibits widespread or moderate resistance to diamides and spinosyns in several world areas (Biondi et al., 2018; Grant et al., 2019). In Spain, different populations have developed high resistance to chlorpyrifos (Haddi et al., 2017) and moderate to chlorantraniliprole (Roditakis et al., 2017, 2018), but many others have not shown any resistance to spinosad and emamectin benzoate (Roditakis et al., 2018). The uneven distribution of resistance in field populations may have played a role in our results.
There is little information in the literature about pesticide compatibility with N. tenuis under field settings. The NE, established in the tomato nurseries was not negatively affected by two applications of the diamides flubendiamide and chlorantraniliprole, and density grew up to 49%–67% at the end of the trial (IOBC category 1). The number of mirids per plant in these plots always surpassed the economic threshold of 4.5, which is reported to result in less than 4% damaged tomatoes (Arnó et al., 2011). One application of flubendiamide was also compatible with N. tenuis, released in plants as soon as residues were dried (Wanumen et al., 2016). Two applications of emamectin benzoate impaired the population growth of the NE (IOBC 2; 9%–25% increase in density). This result is in accordance with the long duration of emamectin benzoate harmful activity to N. tenuis under semifield conditions (IOBC category C in the persistence scale; Hassan, 1994) reported by Wanumen et al. (2016). In contrast, one application of emamectin in semifield was safe for the mirid (López et al., 2011). The most deleterious pesticide was chlorpyrifos‐methyl (IOBC 3; 35%–37% reduction), followed by spinosad (IOBC 2–3), the latter compatible in extended laboratory trials after one application at a lower concentration than that used in our trials (72 instead of 110 g a.i/ha) (Arnó & Gabarra, 2011).
In sweet pepper, the biological control of the thrip F. occidentalis relies on the anthocorid O. laevigatus (very effective) and the phytoseiid mite A. swirskii (more polyphagous but with a very fast establishment in the crop) (Robledo et al., 2009). In our trial, both NE established in the crop at low population levels. Levels of F. occidentalis were very low and not homogeneous at the beginning of the trial. Thrips slightly grew under pymetrozine, spirotetramat, and sulfoxaflor (16%–57% increase), probably because these pesticides also decreased the population of O. laevigatus, and maybe because of the broad insecticide resistance in Spanish populations (Bielza, 2008, 2009).
In contrast to our results, neither spirotetramat to O. armatus nor spiromesifen (same mode of action of spirotetramat, group 23; IRAC, 2018) to O. laevigatus were toxic after one application (Bielza et al., 2009; Broughton et al., 2013). Pymetrozine, reported as harmless to O. laevigatus in semifield (van de Veire et al., 2002), can decrease fecundity and nymph hatch of O. armatus (Broughton et al., 2013), and this could have played a role in our results (IOBC 2). Also, the variability in the susceptibility of O. laevigatus to insecticides might be explained by the populations used in each study (Balanza et al., 2019).
Chlorantraniliprole, very selective to Orius species O. insidiosus (Say) and O. armatus (Gross) (Gradish et al., 2011; Broughton et al., 2013), was compatible with O. laevigatus and A. swirskii (IOBC 1). However, thrips population increase along the trail, even though there is no information in literature concerning the development of resistance to diamide compounds in Spain (Bielza & Guillén, 2014). Emamectin benzoate, slightly harmful to O. laevigatus either after one direct spray field application (Amor et al., 2012) or after two applications in our trials (IOBC 2), did not allow the pest to increase. Probably, this pesticide properly controlled F. occidentalis larvae since adults are in general resistant (Shan et al., 2012).
The predatory mite A. swirskii is rather compatible with pesticides under laboratory (flubendiamide, methoxyfenozide, spiromesifen, spirotetramat, metaflumizone) (Gradish et al., 2011; Fernández et al., 2017) or field conditions (methoxyfenozide, flonicamid, emamectin benzoate) (Colomer et al., 2011; Amor et al., 2012). Sulfoxaflor was slightly toxic (IOBC 2) but pesticide formulation is pertinent for compatibility. In contrast with our results, an experimental formulation of sulfoxaflor (60 mg a.i./L, 11.4% SC; Dow Agrosciences Ibérica S.A.) was compatible with adults of this predatory mite in the laboratory (Fernández et al., 2017). In agreement with literature, two applications of the rest of the tested pesticides were compatible with A. swirskii. Chlorantraniliprole is reported harmless to the phytoseiid mite Iphiseius degenerans (Berlese) under laboratory and greenhouse (Gradish et al., 2011; Döker et al., 2014). A short duration of the pesticide harmful activity is important for NE safety. As such, emamectin benzoate (IOBC category A in the persistence scale; Fernández et al., 2017) and spirotetramat were compatible after two applications in our trial (Koppert, 2019). However, another formulation of emamectin benzoate has been rated as slightly harmful or harmful (Koppert, 2019), probably because phytoseiid community level interactions, different in every field trial, play a role (Bakker & Jacas, 1995).
To sum up, our research results herein are suitable for employment in the rational planning of IPM programmes in vegetable greenhouses. Both the mode of action of pesticides (IRAC, 2018) and the number of applications are important for the selectivity. The diamides flubendiamide and chlorantraniliprole (IRAC group 28) can be included in tomato IPM programmes because N. tenuis coexists perfectly with them after two applications. Emamectin benzoate and metaflumizone (IRAC 6 and 22, respectively) should be handled with care because they were slightly harmful after two applications. The organophosphate chlorpyrifos‐methyl and the spynosin spinosad (IRAC 1 and 5, respectively) reduced the low populations of T. absoluta, but they compromised the activity of the NE as well. In the sweet pepper crop, two applications of most of the pesticides tested, chorantraniliprole, emamectin benzoate, spirotetramat (IRAC 23) and pymetrozine (IRAC 9) were harmless to A. swirskii and can be recommended for IPM programmes. Only the sulfoximine sulfoxaflor (IRAC 4) was slightly harmful. Orius laevigatus was less tolerant to the pesticides and only chlorantraniliprole was harmless.
Disclosure
Authors declare no conflict of interest.
Acknowledgments
This research was funded by Spanish Ministry of Science, Innovation and Universities projects AGL2013‐47603‐C2‐1‐R and AGL2017‐83498‐C2‐2‐R awarded to Elisa Viñuela and Pilar Medina, along with postdoctoral fellowships awarded to Beatriz Dáder (Spanish Ministry of Science, Innovation and Universities FJCI‐2016‐28443 and Universidad Politécnica de Madrid PINV18XFWLGK24S2US6D). We deeply appreciate the cooperation of two farmers that allowed us to perform trials in their commercial greenhouses. We are indebted to Dr. Christina Elizabeth Pease for English revision and to Dr. Ismael Sánchez from INIA for help with statistics.
References
- Abraham, C.M. , Braman, S.K. , Oetting, R.D. and Hinkle, N.C. (2013) Pesticide compatibility with natural enemies for pest management in greenhouse gerbera daisies. Journal of Economic Entomology, 106, 1590–1601. [DOI] [PubMed] [Google Scholar]
- Amor, F. , Medina, P. , Bengochea, P. , Cánovas, M. , Vega, P. , Correia, R. et al (2012) Effect of emamectin benzoate under semi‐field and field conditions on key predatory biological control agents used in vegetable greenhouse. Biocontrol Science & Technology, 22, 219–232. [Google Scholar]
- Armer, C.A. , Wiedenmann, R.N. and Bush, D.R. (1998) Plant feeding site selection on soybean by the facultatively phytophagous predator Orius insidiosus . Entomologia Experimentalis et Applicata, 86, 109–118. [Google Scholar]
- Arnó, J. and Gabarra, R. (2011) Side effects of selected insecticides on the Tuta absoluta (Lepidoptera: Gelechiidae) predators Macrolophus pygmaeus and Nesidiocoris tenuis (Hemiptera: Miridae). Journal of Pest Science, 84, 513–520. [Google Scholar]
- Bakker, F.M. and Jacas, J.A. (1995) Pesticides and phytoseiid mites: strategies for risk assessment. Ecotoxicology and Environmental Safety, 32, 58–67. [DOI] [PubMed] [Google Scholar]
- Balanza, V. , Mendoza, J.E. and Bielza, P. (2019) Variation in susceptibility and selection for resistance to imidacloprid and thiamethoxam in Mediterranean populations of Orius laevigatus . Entomologia Experimentalis et Applicata, 167, 626–635. [Google Scholar]
- Bengochea, P. , Medina, P. , Amor, F. , Cánovas, M. , Vega, P. , Correia, R. et al (2012) The effect of emamectin benzoate on two parasitoids, Aphidius colemani Viereck (Hymenoptera: Braconidae) and Eretmocerus mundus Mercet (Hymenoptera: Aphelinidae), used in pepper greenhouses. Spanish Journal of Agricultural Research, 10, 806–814. [Google Scholar]
- Bielza, P. (2008) Insecticide resistance management strategies against the western flower thrips, Frankliniella occidentalis . Pest Management Science, 64, 1131–1138. [DOI] [PubMed] [Google Scholar]
- Bielza, P. , Fernández, E. , Grávalos, C. and Izquierdo, J. (2009) Testing for nontarget effects of spiromesifen on Eretmocerus mundus and Orius laevigatus under greenhouse conditions. BioControl, 54, 229–236. [Google Scholar]
- Bielza, P. and Gillén, J. (2014) Cyantraniliprole: a valuable tool for Frankliniella occidentalius (Pergande) management. Pest Management Science, 71, 1068–1074. [DOI] [PubMed] [Google Scholar]
- Bielza, P. , Quinto, V. , Contreras, J. , Torné, M. , Martín, A. and Espinosa, P.J. (2007) Resistance to spinosad in the western flower thrips Frankliniella occidentalis (Pergande) in greenhouses of South‐eastern Spain. Pest Management Science, 63, 682–687. [DOI] [PubMed] [Google Scholar]
- Biobest (2019) Side‐effect manual. https://www.biobestgroup.com/en/side-effect-manual. Accessed 15 Jul 2019.
- Biocolor (2018) Number of hectares under biological control in Almería (Spain). http://www.biocolor.es/control-biologico/estado-control-biologico-2017/. Accessed 15 Mar 2019, in Spanish.
- Biondi, A. , Guedes, R.N.C. , Wan, F.H. and Desneux, N. (2018) Ecology, worldwide spread, and management of the invasive South American tomato pinworm, Tuta absoluta: past, present, and future. Annual Review of Entomology, 63, 239–258. [DOI] [PubMed] [Google Scholar]
- Bouagga, S. , Urbaneja, A. and Pérez‐Hedo, M. (2018) Comparative biocontrol potential of three predatory mirids when preying on sweet pepper key pests. Biological Control, 121, 168–174. [Google Scholar]
- Brodsgaard, H.F. (2004) Biological control of thrips on ornamental crops Biocontrol in Protected Culture (eds. Heinz K.M., Van Driesche R.G. & Parrella M.P.), pp. 253–264. Ball Publishing, Batavia, USA. [Google Scholar]
- Broughton, S. , Harrison, J. , Rahman, T. (2013) Effect of new and old pesticides on Orius armatus (Gross)—an Australian predator of western flower thrips, Fankliniella occidentalis (Pergande). Pest Management Science, 70, 389–397. [DOI] [PubMed] [Google Scholar]
- CABI (2018) Invasive species compendium. Frankliniella occidentalis datasheet. https://www.cabi.org/isc/datasheet/24426/. Accessed 10 Mar 2019.
- Calvo, F.J. , Bolckmans, C. and Belda, J.E. (2012a) Release rate for a pre‐plant application of Nesidiocoris tenuis for Bemisia tabaci control in tomato. BioControl, 57, 809–817. [Google Scholar]
- Calvo, F.J. , Bolckmans, K. and Belda, J.E. (2012b) Biological control‐based IPM in sweet pepper greenhouses using Amblyseius swirskii (Acari: Phytoseiidae). Biocontrol Science & Technology, 12, 1398–1416. [Google Scholar]
- Chow, A. , Chau, A. and Heinz, K.M. (2008) Control of Frankliniella occidentalis on greenhouse roses with Amblyseius (Typhlodromips) swirskii and Orius insidiosus . IOBC/WPRS Bulletin, 32, 45–48. [Google Scholar]
- Colomer, I. , Aguado, P. , Medina, P. , Heredia, R.M. , Fereres, A. , Belda, J.E. et al (2011) Field trial measuring the compatibility of methoxyfenozide and flonicamid with Orius laevigatus Fieber (Hemiptera: Anthocoridae) and Amblyseius swirskii (Athias‐Henriot) (Acari: Phytoseiidae) in commercial pepper greenhouse. Pest Management Science, 67, 1237–1244. [DOI] [PubMed] [Google Scholar]
- Crawley, M.J. (2013) The R Book. 2nd edn John Wiley & Sons, New Delhi, India. [Google Scholar]
- De Barro, P.J. , Liu, S.S. , Boykin, L.M. and Dinsdale, A.B. (2011) Bemisia tabaci: a statement of species status. Annual Review of Entomology, 56, 1–19. [DOI] [PubMed] [Google Scholar]
- De Bortoli, S.A. , Vacari, A.M. , Polanczyk, R.A. , Pires Veiga, A.C. and Goulart, R.M. (2017) Effect of Bacillus thuringiensis on parasitoids and predators Bacillus thuringiensis and Lysinibacillus sphaericus (eds. Fiuza L.M., Polanczyk R.A. & Crickmore N.), pp. 67–77. Springer Nature, Switzerland. [Google Scholar]
- Desneux, N. , Wajnberg, E. , Wyckhuys, K.A.G. , Burgio, G. , Arpaia, S. , Narváez‐Vasquez, C.A. et al (2010) Biological invasion of European tomato crops by Tuta absoluta: ecology, geographic expansion and prospects for biological control. Journal of Pest Science, 83, 197–215. [Google Scholar]
- Döker, I. , Pappas, M.L. , Samaras, K. , Triantafyllous, A. , Kazak, C. and Broufas, G.D. (2014) Compatibility of reduced‐risk insecticides with the non‐target predatory mite Iphiseius degenerans (Acari: Phytoseiidae). Pest Management Science, 71, 1267–1273. [DOI] [PubMed] [Google Scholar]
- Ehler, L.E. (2006) Perspective. Integrated pest management (IPM): definition, historical development and implementation, and the other IPM. Pest Management Science, 62, 787–789. [DOI] [PubMed] [Google Scholar]
- Elbert, A. and Nauen, R. (2000) Resistance of Bemisia tabaci to insecticides in south‐eastern Spain with special reference to neonicotinoids. Pest Management Science, 56, 60–64. [Google Scholar]
- Espinosa, P.J. , Bielza, P. , Contreras, J. and Lacasa, A. (2002) Insecticide resistance in field populations of Frankliniella occidentalis (Pergande) in Murcia (South‐east Spain). Pest Management Science, 58, 967–971. [DOI] [PubMed] [Google Scholar]
- Fernández, E. , Grávalos, C. , Haro, P.J. Cifuentes, D. and Bielza, P. (2009) Insecticide resistance status of Bemisia tabaci Q‐biotype in south‐eastern Spain. Pest Management Science, 65(8), 885–891. [DOI] [PubMed] [Google Scholar]
- Fernández, M.M. , Medina, P. , Wanumen, A. , Del Estal, P. , Smagghe, G. and Viñuela, E. (2017) Compatibility of sulfoxaflor and other modern pesticides with adults of the predatory mite Amblyseius swirskii. Residual contact and persistence studies. BioControl, 62, 197–208. [Google Scholar]
- FRAC (Fungicide resistance action committee) (2018) FRAC Code List: Fungicides sorted by mode of action (including FRAC Code numbering). http://www.phi-base.org/images/fracCodeList.pdf. Accessed 15 Mar 2019.
- Fountain, M.T.Y. and Med, N. (2015) Integrating pesticides and predatory mites in soft fruit crops. Phytoparasitica, 43, 657–667. [Google Scholar]
- Garzón, A. , Medina, P. , Amor, F. , Viñuela, E. and Budia, F. (2015) Toxicity and sublethal effects of six insecticides to last instar larvae and adults of the biocontrol agents Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae) and Adalia bipunctata (L.) (Coleoptera: Coccinellidae). Chemosphere, 132, 87–93. [DOI] [PubMed] [Google Scholar]
- Gázquez, J.C. , López, J.C. , Baeza, E.J. , Pérez‐Parra, J.J. , Pérez, C. , Meca, D.E. et al (2011) Influence of the sulphur application method on pests, diseases and natural enemies in a greenhouse pepper crop. Acta Horticulturae, 893, 1309–1316. [Google Scholar]
- Glass, R. and Egea González, F.J. (2012) Biological control in the greenhouses of Almería and challenges for a sustainable intensive production. Outlooks on Pest Management, 23, 276–279. [Google Scholar]
- Gradish, A.E. , Scott‐Dupree, C.D. , Shipp, L. , Harris, C.R. and Ferguson, G. (2011) Effect of reduced risk pesticides on greenhouse vegetable arthropod biological control agents. Pest Management Science, 67, 82–86. [DOI] [PubMed] [Google Scholar]
- Grant, C. , Jacobson, R. , Ilias, A. , Berger, M. , Vasakis, E. , Bielza, P. et al (2019) The evolution of multiple‐insecticide resistance in UK populations of tomato leafminer, Tuta absoluta . Pest Management Science, 75, 2079–2085. [DOI] [PubMed] [Google Scholar]
- Grávalos, C. , Fernández, E. , Belando, A. , Moreno, I. , Ros, C. and Bielza, P. (2014) Cross‐resistance and baseline susceptibility of Mediterranean strains of Bemisia tabaci to cyantraniliprole. Pest Management Science, 71, 1030–1036. [DOI] [PubMed] [Google Scholar]
- Han, P. , Bayram, Y. , Shaltiel‐Harpaz, L. , Sohrabi, F. , Saji, A. , Esenali, U.T. et al (2019) Tuta absoluta continues to disperse in Asia: damage, ongoing management and future challenges. Journal of Pest Science, 92, 1317–1327. [Google Scholar]
- Haddi, K. , Berger, M. , Bielza, P. , Rapisarda, C. , Williamson, M.S. , Moores, G. et al (2017) Mutation in the ace‐1 gene of the tomato leaf miner (Tuta absoluta) associated with organophosphates resistance. Journal of Applied Entomology, 141, 612–619. [Google Scholar]
- Hassan, S.A. (1994) Activities of the IOBC/WPRS‐working group “Pesticides and beneficial organisms”. IOBC/WPRS Bulletin, 17(10), 1–5. [Google Scholar]
- IBM Corp . (2015) IBM SPSS Statistics for Windows, Version 23.0. Armonk, New York, USA.
- IRAC (Insecticide Resistance Action Committee) (2018) Mode of action classification. http://www.irac-online.org/modes-of-action/. Accessed 15 Mar 2019.
- IOBC (International Organization for Biological and Integrated Control) (2018) Pesticides and beneficial organisms. https://www.iobc-wprs.org/expert_groups/01_wg_beneficial_organisms.html. Accessed 15 Mar 2019.
- IOBC (International Organization for Biological and Integrated Control) (2019) IOBC‐WPRS Pesticide Side Effect Database. http://www.iobc-wprs.org/ip_ipm/IOBC_Pesticide_Side_Effect_Database.html. Accessed 15 Jun 2019.
- Koppert (2018) Control pests products. https://www.koppert.com/products-solutions/. Accessed 15 Mar 2019.
- Koppert (2019) Side‐effect manual. https://efectos-secundarios.koppert.es/. Accessed 15 Jul 2019.
- López, J.A. , Amor, F. , Bengochea, P. , Medina, P. , Budia, F. and Viñuela, E. (2011) Toxicity of emamectin benzoate to adults of Nesidiocoris tenuis Reuter, Macrolophus pygmaeus (Rambur) (Heteroptera, Miridae) and Diglyphus isaea Walker (Hymenoptera, Eulophidae) on tomato plants. Semi‐field studies. Spanish Journal of Agricultural Research, 9, 617–622. [Google Scholar]
- Madbouni, M.A.Z. , Samih, M.A. , Qureshi, J.A. , Biondi, A. and Namvar, P. (2017) Compatibility of insecticides and fungicides with the zoophytophagous mirid predator Nesidiocoris tenuis . PLoS ONE, 12(11), e0187439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia, J.B. , Carvalho, G.A. , Medina, P. , Garzón, A. , Gontijo, P.C. and Viñuela, E. (2016) Lethal and sublethal effects of pesticides on Chrysoperla carnea larvae (Neuroptera: Chrysopidae) and the influence of rainfastness in their degradation pattern over time. Ecotoxicology, 25, 845–855. [DOI] [PubMed] [Google Scholar]
- Mansour, R. , Brévault, T. , Chailleux, A. , Cherif, A. , Grissa‐Lebdi, K. , Haddi, K. et al (2018) Occurrence, biology, natural enemies and management of Tuta absoluta in Africa. Entomologia Generalis, 38, 83–112. [Google Scholar]
- MAPAMA (Ministry of Agriculture, Food and Environment) (2018a) Survey of surface areas and crop yields of Spain: national and autonomic results (ESYRCE). https://www.mapama.gob.es/es/estadistica/temas/estadisticas-agrarias/boletin2017sm_tcm30-455983.pdf/. Accessed 15 Mar 2019, in Spanish.
- MAPAMA (Ministry of Agriculture, Food and Environment) (2018b) European community list of active substances included, excluded and under evaluation. http://www.magrama.gob.es/agricultura/pags/fitos/registro/fichas/pdf/Lista_Sustancias_activas_aaceptada_excluidas.pdf/. Accessed 15 Mar 2019, in Spanish.
- Meier, U. (2001) BBCH monography. Federal center of biological research for agriculture and silviculture. Germany (in Spanish).
- Milliken, G.A. and Johnson D.E. (1984) Analysis of messy data. Vol. 1 Designed experiments. Van Nostrand Reinhold. New York.
- OJEU (Official Journal of the European Union) (2009) Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009, establishing a framework for Community action to achieve the sustainable use of pesticides 309, 71–86. [Google Scholar]
- Panizzi, A. , Suciu, N.A. and Trevisan, M. (2017) Combined ecotoxicological risk assessment in the frame of European authorization of pesticides. Science of the Total Environment, 580, 136–146. [DOI] [PubMed] [Google Scholar]
- Parrella, M.P. and Lewis, E. (2018) Biological control in greenhouse and nursery production: present status and future directions. American Entomologist, 63, 237–250. [Google Scholar]
- Perdikis, D. and Arvaniti, K. (2016) Nymphal development on plant vs. leaf with and without prey for two omnivorous predators: Nesidiocoris tenuis (Reuter, 1895) (Hemiptera: Miridae) and Dicyphus errans (Wolff, 1804) (Hemiptera: Miridae). Entomologia Generalis, 35, 297–306. [Google Scholar]
- Pérez‐Hedo, M. and Urbaneja, A. (2015) Prospects for predatory mirid bugs as biocontrol agents of aphids in sweet peppers. Journal of Pest Science, 88, 65–73. [Google Scholar]
- Put, K. , Bollens, T. , Wäckers, F. and Pekas, A. (2016) Non‐target effects of commonly used plant protection products in roses on the predatory mite Euseius gallicus Kreiter & Tixier (Acari: Phytoseiidae). Pest Management Science, 72, 1373–1380. [DOI] [PubMed] [Google Scholar]
- Robledo Camacho, A. , Van der Bloom, J. , Sánchez Martínez, J.A. and Torres Giménez, S. (2009) Biological Control in Horticultural Greenhouses. Coexphal, FAECA, Almería, Spain. [Google Scholar]
- Roditakis, E. , Steinbach, D. , Moritz, G. , Vasakis, E. , Stavrakaki, M. , Ilias, A. et al (2017) Ryanodine receptor point mutations confer diamide insecticide resistance in tomato leafminer, Tuta absoluta (Lepidoptera: Gelechiidae). Insect Biochemistry and Molecular Biology, 80, 11–20. [DOI] [PubMed] [Google Scholar]
- Roditakis, E. , Vasakis, E. , García‐Vidal, L. , Martínez‐Aguirre, M.R. , Rison J.L., Haxaire‐Lutun, M.O. et al (2018) A four‐year survey on insecticide resistance and likelihood of chemical control failure for tomato leaf miner Tuta absoluta in the European/Asian region. Journal of Pest Science, 91, 421–435. [Google Scholar]
- Sánchez, J.A. , Alcázar, A. , Lacasa, A. , Llamas, A. and Bielza, P. (2000) Integrated pest management strategies in sweet pepper plastic houses in the southeast of Spain. IOBC/WPRS Bulletin, 23, 21–30. [Google Scholar]
- Sánchez, C. , Gallego, J.R. , Gámez, M. and Cabello, T. (2014) Intensive biological control in Spanish greenhouses: problems of the success. International Science Index, 8 (10 Part XII), 10156–1019. [Google Scholar]
- Sánchez, J.A. , Lacasa, A. , Arno, J. , Castane, C. and Alomar, O. (2009) Life history parameters for Nesidiocoris tenuis (Reuter) (Het. Miridae) under different temperature regimes. Journal of Applied Entomology, 133, 125–132. [Google Scholar]
- Shan, C. , Ma, S. , Wang, M. and Gao, G. (2012) Evaluation of insecticides against the werstern flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae) in the laboratory. Florida Entomologist, 95, 454–460. [Google Scholar]
- Sterk, G. , Hassan, S.A. , Baillod, M. , Bakker, F. , Bigler, F. , Blümel, S. et al (1999) Results of the seventh joint pesticide testing programme carried out by the IOBC/WPRS‐Working Group “Pesticides and Beneficial Organisms”. BioControl, 44, 99–117. [Google Scholar]
- Tropea Garzia, G. , Siscaro, G. , Biondi, A. and Zappala, L. (2010) Tuta absoluta, a South American pest of tomato now in the EPPO region: biology, distribution and damage. EPPO Bulletin, 42, 205–210. [Google Scholar]
- Urbaneja, A. , González‐Cabrera, J. , Arnó, J. and Gabarra, R. (2012) Prospects for the biological control of Tuta absoluta in tomatoes of the Mediterranean basin. Pest Management Science, 68, 1215–1222. [DOI] [PubMed] [Google Scholar]
- van de Veire, M. , Sterk, G. , van der Staaij, M. , Ramakers, P.M.J. and Tirry, L. (2002) Sequential testing scheme for the assessment of the side‐effects of plant protection products on the predatory bug Orius laevigatus . BioControl, 47, 101–113. [Google Scholar]
- van Lenteren, J.C. (2011) The state of commercial augmentative biological control: plenty of natural enemies, but a frustrating lack of uptake. BioControl, 57, 1–20. [Google Scholar]
- Wang, Z. and Goonewardene, L.A. (2004) The use of MIXED models in the analysis of animal experiments with repeated measures data. Canadian Journal of Animal Science, 84, 1–11. [Google Scholar]
- Wanumen, A.C. , Carvalho, G.A. , Medina, P. , Viñuela, E. and Adán, A. (2016) Residual acute toxicity of some modern insecticides toward two mirid predators of tomato pests. Journal of Economic Entomology, 109, 1079–1085. [DOI] [PubMed] [Google Scholar]
- Willow, J. , Silva, A. , Veromann, E. and Smagghe, G. (2019) Acute effect of low‐dose thiacloprid exposure synergized by tebuconazole in a parasitic wasp. PLoS ONE, 14(2), e0212456. [DOI] [PMC free article] [PubMed] [Google Scholar]


