Abstract
Even though conventionally prepared octacalcium phosphate and collagen composite (OCP/Col) has exhibited excellent bone regeneration and has recently been commercialized for treating bone defects, reproducible appositional bone formation with OCP/Col has never been achieved. The present study investigated whether appositional bone formation could be achieved by altering the density of OCP/Col and applying liquid nitrogen during the preparation of OCP/Col. The prepared OCP/Col disks had eight variations and were divided into categories according to four different type of densities (1.0, 1.3, 1.7, and 2.0) of OCP/Col and two different pre‐freezing conditions of gas phase (G group: −80°C) and liquid phase (L group: −196°C). These disks were implanted into subperiosteal pockets in rodent calvaria, five samples per each eight variations. Radiomorphometric analysis was conducted at 4 and 12 weeks after implantation, and histological analysis was conducted at 12 weeks after implantation. OCP/Col samples in the L group tended to retain their height and shape and had enhanced appositional bone formation, whereas OCP/Col samples in the G group tended to lose their height and shape and had limited appositional bone formation. The appositional bone formation increased along with growing density of OCP/Col, and L2.0 demonstrated higher appositional bone formation than other samples. These results suggest that the pre‐freezing conditions and densities of OCP/Col affect the appositional bone formation.
Keywords: bone augmentation, collagen, liquid nitrogen, multinucleated giant cells, octacalcium phosphate
1. INTRODUCTION
It is hard to place dental implants for atrophic alveolar ridges to obtain an acceptable aesthetic profile of the final prosthesis (Buser, Martin, & Belser, 2004). Also, it is often difficult to secure sufficient bone volume for edentulous patients because of severely resorbed alveolar ridges (Tallgren, 2003). To conquer these difficulties of alveolar ridges, autologous bone grafts have been used for the reconstruction and augmentation to facilitate implantation (Clavero & Lundgren, 2003). However, there are problems associated with the use of autologous bone in terms of donor site pain, morbidity, limited availability, and the need to include additional surgical sites (Jensen & Terheyden, 2009). And these drawbacks related to autografts have redoubled the search for suitable alternatives. Although hydroxyapatite (HA) and β‐tricalcium phosphate (β‐TCP) have been commonly used as bone graft substitutes (Pinholt, Ruyter, Haanaes, & Bang, 1992; Zerbo et al., 2004), autologous bone grafting is still the gold standard procedure, because these bone substitutes have low bone regenerative properties to autologous bone grafting (Hjorting‐Hansen, 2002).
Octacalcium phosphate (OCP: Ca8H2(PO4)6 5H2O) is a calcium phosphate and an advocated precursor of the initial mineral crystals of biological apatite in bone (Brown, Mathew, & Tung, 1981; Brown, Smith, Frazier, & Lehr, 1962; Crane, Popescu, Morris, Steenhuis, & Ignelzi Jr., 2006). OCP has bone regenerative properties superior to those of HA or β‐TCP (Kamakura et al., 2002), and the implanted OCP can be resorbed by multinucleated giant cells (MNGCs) which share some ultrastructural characteristics with osteoclasts (Kamakura et al., 1997). While OCP has many suitable properties for bone substitute, its innate brittleness makes it hard to sustain its shape and impedes its use in clinical applications. Thus, OCP combined with atelocollagen (OCP/Col) has been developed to overcome the limitations of OCP in terms of moldability and handling performance (Kamakura, Sasaki, Honda, Anada, & Suzuki, 2006). OCP/Col demonstrated excellent bone regeneration, better than β‐TCP and collagen composite (β‐TCP/Col) or HA and collagen composite (HA/Col) (Kamakura et al., 2007). OCP/Col has also promoted osteogenic differentiation and angiogenesis (Kouketsu et al., 2020). After the confirmation of its safety and efficacy in preclinical studies with bone defects (Kawai et al., 2011; Matsui et al., 2010; Matsui et al., 2014; Tanuma et al., 2013), doctor‐initiated and sponsor‐initiated clinical trials were conducted (Kawai et al., 2014, 2016, 2017; Miura et al., 2020), and the commercialization of OCP/Col in Japan was recently approved for the bone defects in the field of oral surgery (Kamakura, 2020; Kawai et al., 2020).
As suggested in a previous study, conventionally prepared OCP/Col achieved limited appositional bone formation because of compression stress under the periosteum (Suzuki et al., 2009). If the OCP/Col disks were supported with a polytetrafluoroethylene (PTFE) ring, which has a higher modulus than OCP/Col, the appositional bone formation was clearly accomplished by alleviating mechanical stress (Matsui et al., 2010). However, PTFE is a nonresorbable material which remains in the body and needs to be removed. The present study investigated whether appositional bone formation could be achieved by altering the density of OCP/Col to maintain its shape from compression stress.
In addition to studying the effects of altered density of OCP/Col on bone formation, this study also investigated the effects of pre‐freezing conditions. It has previously been reported that preparing meniscus with liquid nitrogen before reimplantation into the knee joint decreased inflammatory reaction and that ingrowth of repopulating cells could be observed after implantation (Arnoczky, DiCarlo, O'Brien, & Warren, 1992; Wada, Amiel, Harwood, Moriya, & Amiel, 1998). Therefore, this present study simultaneously investigated the effects of pre‐freezing conditions during the preparation of OCP/Col for appositional bone formation.
2. MATERIALS AND METHODS
2.1. Preparations of OCP/Col
The preparation of OCP/Col was done as previously described (Kajii et al., 2018). The OCP was prepared by direct precipitation to produce sieved granules with particle size 300–500 μm. Collagen was prepared from NMP collagen PS (Nippon Meat Packers, Tsukuba, Ibaraki, Japan), a lyophilized powder of pepsin‐digested atelocollagen isolated from porcine dermis. Sieved granules of OCP were added to the concentrated collagen and mixed. To produce different densities of OCP/Col, four collagen solutions with different densities (3%, 4%, 5%, and 6%) were used. OCP/Col 1.0 was prepared by mixing 3% collagen with granules of OCP to an OCP/Col ratio of 77:23. OCP/Col 1.3, 1.7, and 2.0 were prepared by 4%, 5%, and 6% collagen with the same weight ratio (77%) of OCP, respectively.
To study the effect of pre‐freezing temperature on the bone regenerative properties of the OCP/Col, two different pre‐freezing conditions were used. Half of the OCP/Col mixtures were prefrozen with gas phase (−80°C) and the other half of the mixtures were prefrozen with liquid phase (−196°C). In this study the OCP/Col mixtures prefrozen with gas phase will be referred to as group G and the mixtures prefrozen with liquid phase as group L. Therefore, the research sample included eight variations of mixtures (G1.0, G1.3, G1.7, G2.0, L1.0, L1.3, L1.7, L2.0). The resulting OCP/Col mixtures were then lyophilized and molded into disks (diameter 9 mm, thickness 2 mm). The OCP/Col disks were then prepared with dehydrothermal treatment (150°C, 24 h) in a vacuum drying oven and then sterilized using electron beam irradiation.
2.2. Implantation procedure
Twelve‐week‐old male Wistar rats (SLC Corp., Hamamatsu, Shizuoka, Japan) were used. The principles of laboratory animal care and national laws were followed, and all procedures were approved by the Animal Research Committee of Tohoku University (2016BeA‐001). Experimental rats were anesthetized with intraperitoneal dexmedetomidine hydrochloride (0.05 mg/kg), midazolam (0.12 mg/kg), and butorphanol tartrate (0.15 mg/kg). The skin and the periosteum of the calvaria were dissected and then raised from the bone surface, thus producing a periosteal pocket for disk implantation. After the sample was implanted, the ablated periosteum and skin were repositioned and sutured, respectively. Total of 40 experimental rats (5 rats per each eight variations of the implant [G1.0, G1.3, G1.7, G2.0, L1.0, L1.3, L1.7, L2.0]) were fixed at 12 weeks after implantation.
2.3. Micro‐computed tomography examination and radiomorphometry
In vivo micro‐computed tomography (CT) analysis of rat calvaria was performed 4 and 12 weeks after implantation, using an X‐ray CT system (Latheta LCT‐200; Hitachi Aloka Medical, Tokyo, Japan) after intraperitoneal injection of sodium pentobarbital (50 mg/kg), as described previously (Kajii et al., 2018). The calvaria were scanned continuously in increments of 120 μm, with pixel size of 60 μm. CT images were acquired using the following parameters: 50 kVp tube voltage, 500 μA tube current. After the CT analysis at 12 weeks, the rats were euthanized with intraperitoneal injection of overdose of sodium pentobarbital. After sacrifice, the implants were resected together with the surrounding bone and tissues and fixed in 4% paraformaldehyde in 0.1 M phosphate‐buffered saline, pH 7.4.
The height (H) between the bottom surface of the original bone and the upper surface of the sample was quantified 4 and 12 weeks after implantation by using micro‐CT image (Fig. 1). Next, the angles (θ, θ') of the upper margin were similarly quantified to reflect shape retention as it was demonstrated that this indicates bone augmentation. The apex of the implant at 4 or 12 weeks was plotted on the micro‐CT image and two inflection points on both side of the apex were plotted on a ridgeline. After that the angle centered around the apex was measured (Fig. 1).
FIGURE 1.
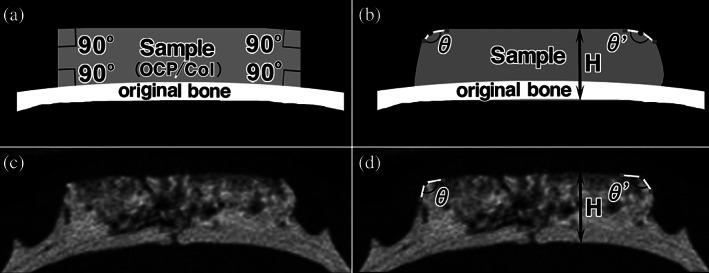
Radiomorphometric measurement of the height and the angles of the upper margin of the implanted octacalcium phosphate and collagen composite (OCP/Col). The height (H) between the bottom surface of the original bone and the angles of upper margin (θ and θ′) of the implanted OCP/Col was measured 4 and 12 weeks after implantation by using micro‐computed tomography (micro‐CT) image. To measure the angles, the apex of the implant at 4 or 12 weeks was plotted on the micro‐CT image, and two inflection points on both side of the apex were plotted on a ridgeline. After that the angle centered around the apex was measured. (a) Schema of immediately after implantation. (b) Schema of 12 weeks after implantation. (c) Micro‐CT image of 12 weeks after implantation. (d) Micro‐CT image with description of the height and upper angles
2.4. Tissue preparations and quantitative micrograph analysis
The samples were decalcified in 10% ethylenediaminetetraacetic acid in 0.01 M phosphate buffer, pH 7.4 at 4°C for 4–6 weeks after radiographs had been taken. Specimens were embedded in paraffin, and the center was extracted and sectioned coronally. Sections were stained with hematoxylin and eosin, and photographs were taken using a photomicroscope (Leica DM2500, Leica Microsystems Japan, Tokyo, Japan). Histomorphometrical analysis of the histological sections of implanted OCP/Col was performed. The percentage of newly formed bone (n‐Bone%) at 12 weeks after implantation was analyzed by using histological sections stained with hematoxylin and eosin. n‐Bone% was calculated by dividing the area of newly formed bone which was identified by the histological sections with the whole area of the implant immediately after implantation (9 mm width × 2 mm height) × 100 (Fig. 2). It was quantified two‐dimensionally using the ImageJ version 1.43 (National Institutes of Health, Bethesda, Maryland) public domain software. For detailed histological observation, whole area of the implanted OCP/Col were divided into four areas (lower marginal [LM], upper marginal [UM], lower central [LC], and upper central [UC]; Fig. 2).
FIGURE 2.
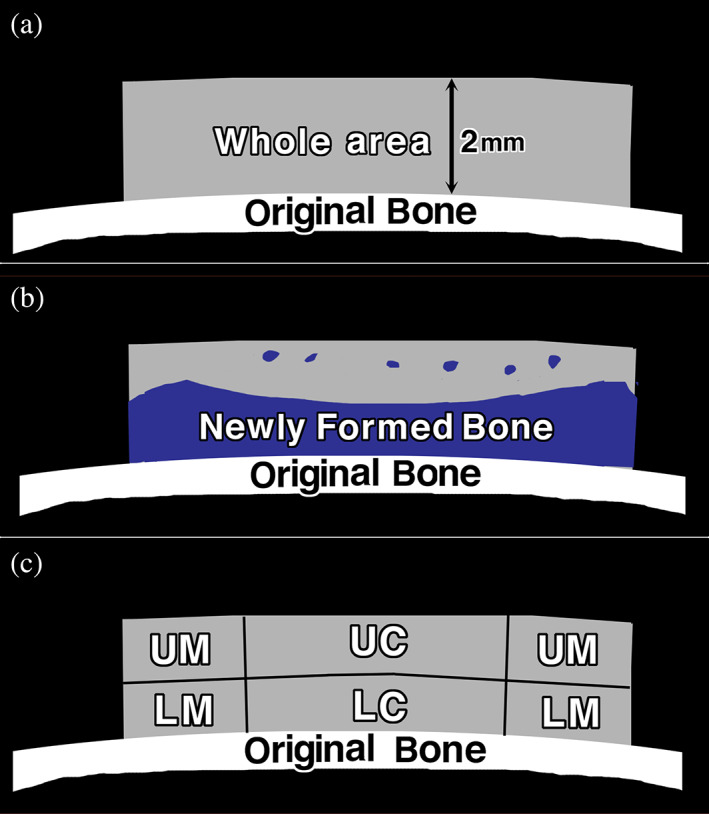
Histomorphometric measurement of the percentage of newly formed bone (n‐Bone%) and histological subsection of the implanted octacalcium phosphate and collagen composite (OCP/Col). The n‐Bone% at 12 weeks after implantation was calculated by dividing the area of newly formed bone (b) with the whole area of the implant immediately after implantation (9 mm width × 2 mm height) (a) ×100. (c) For detailed histological observation, whole area of the implanted OCP/Col were divided into four areas (lower marginal [LM], upper marginal [UM], lower central [LC], and upper central [UC])
2.5. Statistical analysis
At 12 weeks after implantation, n‐Bone% was analyzed statistically using Excel v. X (Microsoft Co., Redmond, Washington). All values are reported as mean ± standard deviation. The chi‐squared test was used to investigate normal distribution of each group, and Barlett's test was used to examine the homogeneity of variance between samples. One‐way analysis of variance and the Kruskal–Wallis test were used to compare mean values of each group. Statistical significance was determined at p < .05. In cases where significant differences were detected between the mean values, Tukey–Kramer or Scheffe's multiple comparison analysis was used as a post hoc test.
3. RESULTS
3.1. Micro‐CT analysis and radiomorphometric analysis
In G group samples, mixed granulous radiopaque and radiolucent figures were observed at 4 weeks after implantation. Additionally, radiopaque mass was observed in G2.0 at 12 weeks after implantation. Original bone in G2.0 became thinner at 4 weeks after implantation, and it became thinner in G1.7 and G2.0 at 12 weeks after implantation (Figure 3). In L group samples, granulous mixed radiopaque and radiolucent figures were observed at 4 weeks after implantation. At 12 weeks after implantation, radiopacity of samples became more dominant near the original bone and outer marginal area as the density of OCP/Col increased. In addition, the interface between OCP/Col and original bone had become unclear (Figure 3).
FIGURE 3.
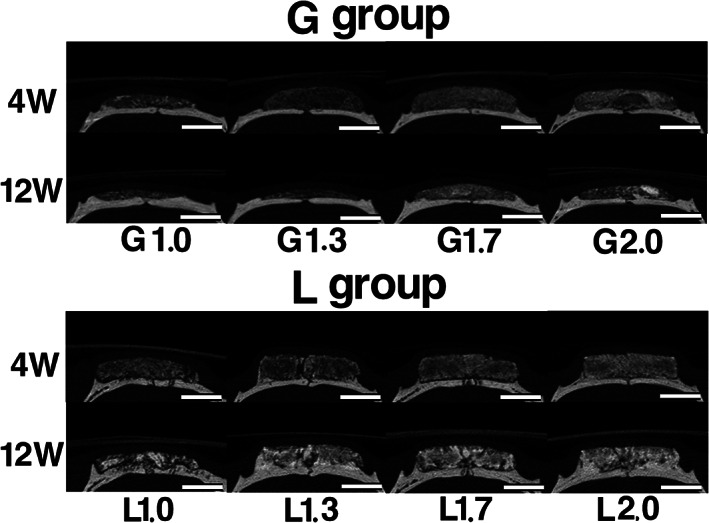
Micro‐computed tomography (micro‐CT) images at 4 and 12 weeks after implantation. The top of the images indicates the skin side, and the bottom is the original bone side. In G group samples, mixed granulous radiopaque and radiolucent figures were observed at 4 weeks after implantation. Additionally, radiopaque mass was observed in G2.0 at 12 weeks after implantation. Original bone in G2.0 became thinner at 4 weeks after implantation, and it became thinner in G1.7 and G2.0 at 12 weeks after implantation. In L group samples, mixed granulous radiopaque and radiolucent figures were observed at 4 and 12 weeks after implantation. At 12 weeks after implantation, radiopacity was more dominant near the original bone and outer marginal area, and the interface between octacalcium phosphate and collagen composite and original bone became unclear. Bars: 4 mm
The height of G1.0 was lower than those of G1.3, G1.7, and G2.0 at 4 weeks after implantation. At 12 weeks after implantation, the height of all samples was lower than their heights at 4 weeks. In the G group, the mean of the sample height at 4 weeks was 2.2 ± 0.1 mm (20 samples), and a significant difference was observed between the heights of G1.0 and G1.7. The mean of the height at 12 weeks was 1.6 ± 0.1 mm (20 samples), but no significant difference was observed among these samples. In samples G1.3, G1.7, and G2.0, there was a significant difference between the heights at 4 and 12 weeks (Figure 4a).
FIGURE 4.
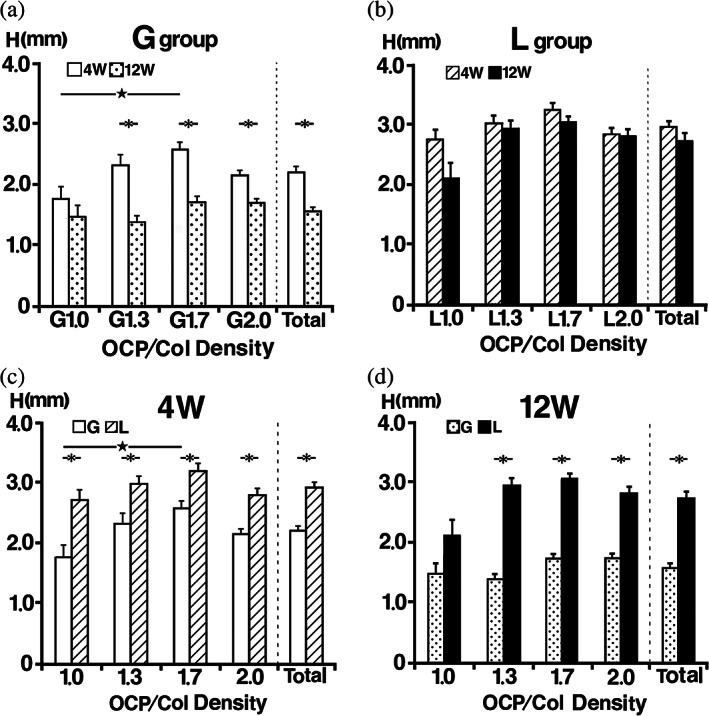
Radiomorphometric analysis of the implant height. (a) In the G group at 4 weeks, a significant difference was observed between the heights of G1.0 and G1.7. At 12 weeks, no significant difference was observed among these samples. In samples G1.3, G1.7 and G2.0, there was a significant difference between the heights at Weeks 4 and 12. (b) In the L group at 4 and 12 weeks, there was no significant difference between samples in this group at each experimental period. Although the difference was not significant, there was a noticeable change in the height of L1.0 between Weeks 4 and 12. (c) Samples with the same concentration of octacalcium phosphate and collagen composite were compared across the groups, the height of samples in the L group at Week 4 was significantly higher than those in the G group. (d) The height of samples in the L group at Week 12 was higher than those in the G group
The height of L1.0 decreased slightly at 4 weeks after implantation, but the heights of L1.3, L1.7, and L2.0 were retained. At 12 weeks after implantation, the height of L1.0 had decreased a bit more, whereas those of L1.3, L1.7, and L2.0 were retained. In the L group, the mean of sample heights at 4 weeks was 2.9 ± 0.1 mm (20 samples), and there was no significant difference between the samples in this group. The mean of height at 12 weeks was 2.7 ± 0.1 mm (20 sample), and there was no significant difference between the samples in this group. Although the difference was not significant, there was a noticeable change in the height of L1.0 between Weeks 4 and 12 (Figure 4b). Samples with the same density of OCP/Col were compared across the groups, and at Week 4 the height of samples in the L group was significantly higher than those in the G group (Figure 4c). Similarly, the height of samples in the L group was higher than those in the G group at Week 12 (Figure 4d).
Because the edges of G group samples became more obtuse and the upper angles were difficult to distinguish due to collapse of the implants, it was impossible to do analysis of the angles in the G group. Therefore, only the angles of L group samples were statistically analyzed. The edges of L1.0 and L1.3 had become obtuse, but the height of L1.3, L1.7, and L2.0 were similar to that of 4 weeks. The mean of angles at 4 weeks was 126 ± 2°, and there was no significant difference among these samples. The mean of angles at 12 weeks was 136 ± 3°, and there was significant difference between samples L1.3 and L2.0. Although the angle at 12 weeks became more obtuse from that of 4 weeks, there was no significant difference between the same OCP/Col density of 1.0, 1.3, 1.7, and 2.0 between 4 and 12 weeks. At 4 and 12 weeks after implantation, the angles of L1.3, L1.7, and L2.0 had decreased as the density of OCP/Col in samples increased (Figure 5).
FIGURE 5.
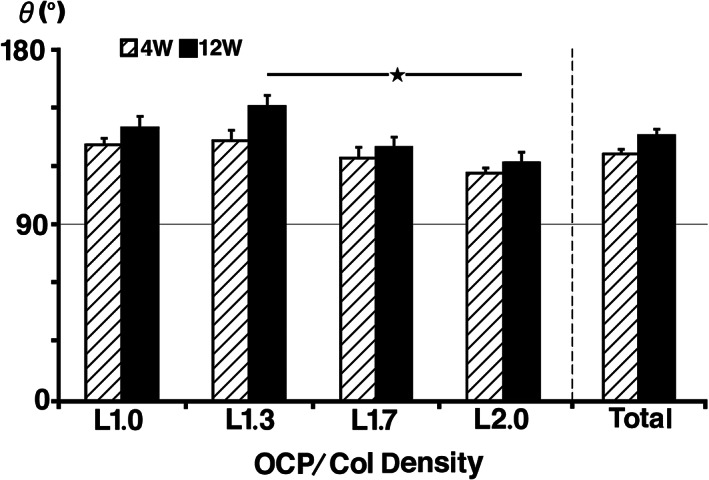
Radiomorphometric analysis of the upper angles of L group implants. At 4 weeks, there was no significant difference among the upper angles of these samples. At 12 weeks, there was significant difference between samples L1.3 and L2.0. Although the angle at 12 weeks had reduced from that of 4 weeks, there was no significant difference between octacalcium phosphate and collagen composite with the same density between 4 and 12 weeks
3.2. Histological examination of implants
In the G group, there was a little newly formed bone in the marginal area in samples. The height of G1.0 and G1.3 had decreased more than those of G1.7 and G2.0, and in cases of G1.7 and G2.0, the original bone had become thinner. In the L group, newly formed bone was dominant near the original bone and outer marginal area, and the amount of new bone increased as the density of OCP/Col in samples rose (Figure 6).
FIGURE 6.
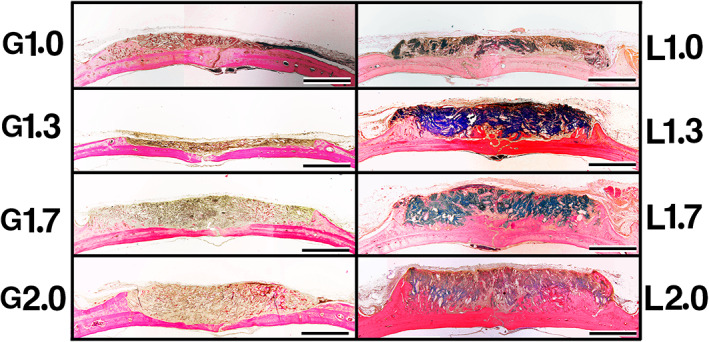
Histological overview of implants at 12 weeks postimplantation. In the G group, there was a little newly formed bone in the marginal area in all samples. The height of G1.0 and G1.3 had decreased more than that of G1.7 and G2.0, and in cases of G1.7 and G2.0, the original bone had become thinner. In the L group, newly formed bone was dominant near the original bone and outer marginal area, and the amount of new bone increased as the density of octacalcium phosphate and collagen composite in samples rose. Bars: 4 mm
In the case of G2.0, there was newly formed bone in LM area (Figure 7a), but original bone had become thinner in LC area (Figure 7b), and there was no bone near the skin side in UC area (Figure 7c). Furthermore, there were a lot of MNGCs and the implanted collagen had disintegrated in UC (Figure 7d). In the L2.0, a new bone was formed from the original bone toward the skin side in LM area (Figure 7e), and it was more dominant near the original bone in LC area (Figure 7f). Newly formed bone was observed near the skin side in UC area (Figure 7g). The implanted collagen remained its framework, and a small amount of MNGCs was observed near the skin side in UC (Figure 7h).
FIGURE 7.
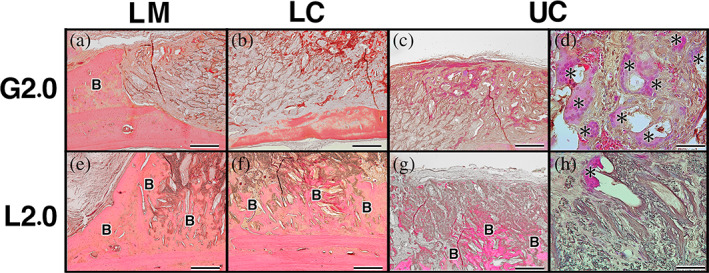
Histological examination of implants at 12 weeks postimplantation. (a) In the case of G2.0, there was newly formed bone in lower marginal area (LM). (b) Original bone had become thinner in lower central area (LC). (c) There was no bone near the skin side in upper central area (UC). (d) Additionally, there was large amount of multinucleated giant cells (MNGCs) and the implanted collagen was disintegrated in UC. (e) In the L2.0, new bone formed from the original bone toward the skin side in LM. (f) New bone was more dominant near the original bone in LC. (g) Newly formed bone was observed near the skin side in UC. (h) The implanted collagen retained its framework and a small amount of MNGCs was observed near the skin side in UC. Bars: 200 μm (a–c and e–g), Bars: 50 μm (d, h); B, newly formed bone; asterisk indicates MNGCs
3.3. Histomorphometrical examination of n‐Bone%
In the G group, n‐Bone% of the G1.0 (17.7% ± 4.8%) was higher than those of G1.7 (12.9% ± 1.8%), G2.0 (7.4% ± 1.3%), and G1.3 (5.8% ± 0.6%), and there was no significant difference among the samples of same density. In the L group, n‐Bone% of the L2.0 (40.2% ± 4.8%) was higher than those of L1.7 (24.5% ± 4.0%), L1.3 (17.1% ± 1.9%), and L1.0 (9.7% ± 3.6%), and there was no significant difference among the samples of same density. Compared with same density of OCP/Col, the n‐Bone% at 12 weeks of L group samples were higher than those of G group samples with the exception of OCP/Col density of 1.0 (Figure 8).
FIGURE 8.
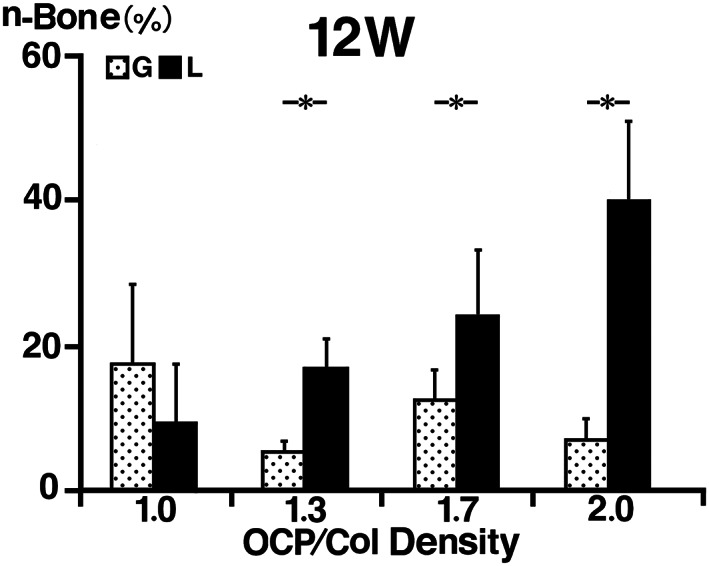
Histomorphometrical examination of the percentage of newly formed bone (n‐Bone%). In the G group, n‐Bone% of the G1.0 was higher than those of G1.3, G1.7, and G2.0, and there was no significant difference among the samples of same density. In the L group, n‐Bone% of the L2.0 was higher than those of L1.7, L1.3, and L1.0, and there was no significant difference among the samples of same density. Compared with same density of octacalcium phosphate and collagen composite (OCP/Col), the n‐Bone% at 12 weeks of L group samples was higher than those of G group samples with the exception of OCP/Col density of 1.0
4. DISCUSSION
The present study demonstrated that OCP/Col samples in the L group tended to retain their height and shape and had enhanced appositional bone formation, whereas OCP/Col samples in the G group tended to lose their height and shape and had limited appositional bone formation. This suggests that pre‐freezing temperature of the OCP/Col affects the bone regenerative properties and the shape‐retaining properties, since the L group was prefrozen with liquid phase (−196°C) and the G group was done with gas phase (−80°C).
The height of the G group progressively decreased with time, whereas the height of the L group was mostly preserved during the experimental period except for L1.0, the height of which gradually decreased. The histological examination indicated that there were a lot of MNGCs in OCP/Col of G groups, whereas a small amount of MNGCs was observed near the skin side in OCP/Col of L groups. A previous study indicated that the height of the OCP/Col decreased due to the resorption by MNGCs, possibly because of the increased mechanical load in the subperiosteal pocket, when the disks of OCP/Col were implanted into a calvarial subperiosteal pocket (Suzuki et al., 2009). As G group samples were prepared similarly to the samples used in the previous study (Kajii et al., 2018), it could be assumed that the height of G group samples decreased due to resorption by a large amount of MNGCs. It is well known that the extracellular matrix of menisci is mostly composed of collagen, and it has been reported that there was little inflammatory reaction when liquid nitrogen prepared meniscus was reimplanted into the knee joint (Arnoczky et al., 1992). Thus, it can be assumed that the heights of L group samples, which were prefrozen with liquid nitrogen, had only slight variation because the MNGCs related to inflammatory reaction were concentrated on the skin side. However, it is important to note that a previous study reported that calvarial osteocytes are more mechanosensitive than osteocytes of load bearing long bones (Vatsa et al., 2008) and that calvarial osteoblasts were demonstrated to induce more osteoclasts and in a shorter time interval than long bone osteoblasts (Wan et al., 2016). Therefore, these procedures for long bone might occur in a different manner.
The angles of the upper margin of the G group were difficult to distinguish at 4 and 12 weeks, whereas those of the L group could be observed clearly at 4 weeks after implantation and only became more obtuse at 12 weeks. Consequently, it was impossible to do analysis of the angles in the G group due to the collapse of the implants. In the L group, the angles of L1.3, L1.7, and L2.0 became less obtuse as the density of OCP/Col increased. This indicates that deformation of the disks due to compression stress from the skin side was reduced. These results might be caused by lessened resorption of OCP/Col by MNGCs, increased density of the samples, or increased hardness caused by conversion from OCP to apatite phase (Masuda, Kawai, Anada, Kamakura, & Suzuki, 2010).
In the samples with OCP/Col density of 1.3, 1.7, and 2.0, the n‐Bone% of the L group was higher than those of the G group with the same density. This indicates that pre‐freezing OCP/Col with liquid nitrogen improves new bone formation effectively. Also, the n‐Bone% of the L2.0 was higher than in other L group samples, which indicates that higher OCP/Col density in the L group enhances appositional bone formation even more. In the L group, newly formed bone was dominant near the original bone and in the outer marginal area. In the LM area, bone formation was initiated by osteoprogenitors that had invaded into the OCP/Col from the bloodstream. It has been previously reported that OCP acts together with osteoblasts, bone lining cells, and their closely committed progenitors on the original bone to enhance bone formation from the surface of the original bone (Sasano et al., 1999). Therefore, additional bone formation could be enhanced by interaction between the osteoprogenitor cells on the bone and the implanted OCP/Col. It has previously been reported that ingrowth of repopulating cells was observed when liquid nitrogen prepared meniscus was reimplanted into the knee joint of rabbit (Wada et al., 1998), and this study demonstrated that the implanted OCP/Col of the L group partly became newly formed bone, but at the site intermingled with OCP converted apatite and collagen, there was no bone formation. However, the site with no bone formation had retained its framework of OCP/Col and could still act as a scaffold for bone formation. Therefore, it can be expected that appositional bone formation occurs on the surface of the original bone or in the framework of OCP/Col. Additionally, the n‐Bone% increased as the density of OCP/Col increased. It was previously reported that collagen has promoted proliferation and attachment of host osteoblastic cells and increasing the amount of OCP has resulted in progressive enhancement of bone regeneration (Kawai et al., 2009). Consequently, it can be assumed that new bone formation is enhanced as the density of OCP/Col increases.
Original bone resorption was observed in G1.7 and G2.0. It has been previously been reported that implanted HA, which is rarely replaced by newly formed bone, has resorbed original bone when implanted into a calvarial subperiosteal pocket (Pinholt et al., 1992). The present study demonstrated that there was little bone formation with radiopacity in G1.7 and G2.0, while bone formation with radiopacity was observed in the L group. Because the radiopacity in G1.7 and G2.0 could be caused by OCP‐converted apatite, the original bone might have been resorbed by them.
These results suggest that appositional bone formation stimulated by OCP/Col was achieved when it was prefrozen with liquid phase. Additionally, the amount of newly formed bone increased, and the shape of the implant preserved when the density of OCP/Col was increased. Since this procedure needs no exogenous cell transplantation or cytokines, it could become a possible technique for appositional bone formation in the future.
CONFLICT OF INTEREST
S.K. has obtained patents on OCP/Col in Japan (#5046511). The remaining authors declare no potential conflict of interest.
ACKNOWLEDGMENTS
This study was supported in part by JSPS KAKENHI, grant number 16H03159, 16K11741, and 18K19891.
Yanagisawa T, Yasuda A, Makkonen RI, Kamakura S. Influence of pre‐freezing conditions of octacalcium phosphate and collagen composite for reproducible appositional bone formation. J Biomed Mater Res. 2020;108B:2827–2834. 10.1002/jbm.b.34613
Funding information JSPS KAKENHI, Grant/Award Numbers: 18K19891, 16K11741, 16H03159
REFERENCES
- Arnoczky, S. P. , DiCarlo, E. F. , O'Brien, S. J. , & Warren, R. F. (1992). Cellular repopulation of deep‐frozen meniscal autografts: An experimental study in the dog. Arthroscopy, 8(4), 428–436. [DOI] [PubMed] [Google Scholar]
- Brown, W. E. , Mathew, M. , & Tung, M. S. (1981). Crystal‐chemistry of octacalcium phosphate. Progress in Crystal Growth and Characterization of Materials, 4(1–2), 59–87. [Google Scholar]
- Brown, W. E. , Smith, J. P. , Frazier, A. W. , & Lehr, J. R. (1962). Crystallographic and chemical relations between octacalcium phosphate and hydroxyapatite. Nature, 196(4859), 1050–1055. [Google Scholar]
- Buser, D. , Martin, W. , & Belser, U. C. (2004). Optimizing esthetics for implant restorations in the anterior maxilla: Anatomic and surgical considerations. The International Journal of Oral & Maxillofacial Implants, 19(Suppl), 43–61. [PubMed] [Google Scholar]
- Clavero, J. , & Lundgren, S. (2003). Ramus or chin grafts for maxillary sinus inlay and local onlay augmentation: Comparison of donor site morbidity and complications. Clinical Implant Dentistry and Related Research, 5(3), 154–160. [DOI] [PubMed] [Google Scholar]
- Crane, N. J. , Popescu, V. , Morris, M. D. , Steenhuis, P. , & Ignelzi, M. A., Jr. (2006). Raman spectroscopic evidence for octacalcium phosphate and other transient mineral species deposited during intramembranous mineralization. Bone, 39(3), 434–442. [DOI] [PubMed] [Google Scholar]
- Hjorting‐Hansen, E. (2002). Bone grafting to the jaws with special reference to reconstructive preprosthetic surgery. A historical review. Mund‐, Kiefer‐ Und Gesichtschirurgie, 6(1), 6–14. [DOI] [PubMed] [Google Scholar]
- Jensen, S. S. , & Terheyden, H. (2009). Bone augmentation procedures in localized defects in the alveolar ridge: Clinical results with different bone grafts and bone‐substitute materials. The International Journal of Oral & Maxillofacial Implants, 24(Suppl), 218–236. [PubMed] [Google Scholar]
- Kajii, F. , Iwai, A. , Tanaka, H. , Matsui, K. , Kawai, T. , & Kamakura, S. (2018). Single‐dose local administration of teriparatide with a octacalcium phosphate collagen composite enhances bone regeneration in a rodent critical‐sized calvarial defect. Journal of Biomedical Materials Research. Part B, Applied Biomaterials, 106(5), 1851–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamakura, S. (2020). Development and clinical application of octacalcium phosphate/collagen composites. In Suzuki O. & Insley G. (Eds.), (pp. 289–308). Octacalcium phosphate biomaterials: Elsevier. [Google Scholar]
- Kamakura, S. , Sasaki, K. , Homma, T. , Honda, Y. , Anada, T. , Echigo, S. , & Suzuki, O. (2007). The primacy of octacalcium phosphate collagen composites in bone regeneration. Journal of Biomedical Materials Research. Part A, 83(3), 725–733. [DOI] [PubMed] [Google Scholar]
- Kamakura, S. , Sasaki, K. , Honda, Y. , Anada, T. , & Suzuki, O. (2006). Octacalcium phosphate combined with collagen orthotopically enhances bone regeneration. Journal of Biomedical Materials Research. Part B, Applied Biomaterials, 79(2), 210–217. [DOI] [PubMed] [Google Scholar]
- Kamakura, S. , Sasano, Y. , Homma‐Ohki, H. , Nakamura, M. , Suzuki, O. , Kagayama, M. , & Motegi, K. (1997). Multinucleated giant cells recruited by implantation of octacalcium phosphate (OCP) in rat bone marrow share ultrastructural characteristics with osteoclasts. Journal of Electron Microscopy, 46(5), 397–403. [DOI] [PubMed] [Google Scholar]
- Kamakura, S. , Sasano, Y. , Shimizu, T. , Hatori, K. , Suzuki, O. , Kagayama, M. , & Motegi, K. (2002). Implanted octacalcium phosphate is more resorbable than beta‐tricalcium phosphate and hydroxyapatite. Journal of Biomedical Materials Research, 59(1), 29–34. [DOI] [PubMed] [Google Scholar]
- Kawai, T. , Anada, T. , Honda, Y. , Kamakura, S. , Matsui, K. , Matsui, A. , … Suzuki, O. (2009). Synthetic octacalcium phosphate augments bone regeneration correlated with its content in collagen scaffold. Tissue Engineering. Part A, 15(1), 23–32. [DOI] [PubMed] [Google Scholar]
- Kawai, T. , Echigo, S. , Matsui, K. , Tanuma, Y. , Takahashi, T. , Suzuki, O. , & Kamakura, S. (2014). First clinical application of octacalcium phosphate collagen composite in human bone defect. Tissue Engineering. Part A, 20(7–8), 1336–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai, T. , Kamakura, S. , Matsui, K. , Fukuda, M. , Takano, H. , Iino, M. , … Takahashi, T. (2020). Clinical study of octacalcium phosphate and collagen composite in oral and maxillofacial surgery. Journal of Tissue Engineering, 11, 2041731419896449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai, T. , Matsui, K. , Iibuchi, S. , Anada, T. , Honda, Y. , Sasaki, K. , … Echigo, S. (2011). Reconstruction of critical‐sized bone defect in dog skull by octacalcium phosphate combined with collagen. Clinical Implant Dentistry and Related Research, 13(2), 112–123. [DOI] [PubMed] [Google Scholar]
- Kawai, T. , Suzuki, O. , Matsui, K. , Tanuma, Y. , Takahashi, T. , & Kamakura, S. (2017). Octacalcium phosphate collagen composite facilitates bone regeneration of large mandibular bone defect in humans. Journal of Tissue Engineering and Regenerative Medicine, 11(5), 1641–1647. [DOI] [PubMed] [Google Scholar]
- Kawai, T. , Tanuma, Y. , Matsui, K. , Suzuki, O. , Takahashi, T. , & Kamakura, S. (2016). Clinical safety and efficacy of implantation of octacalcium phosphate collagen composites in tooth extraction sockets and cyst holes. Journal of Tissue Engineering, 7, 2041731416670770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouketsu, A. , Matsui, K. , Kawai, T. , Ezoe, Y. , Yanagisawa, T. , Yasuda, A. , … Kamakura, S. (2020). Octacalcium phosphate collagen composite stimulates the expression and activity of osteogenic factors to promote bone regeneration. Journal of Tissue Engineering and Regenerative Medicine, 14, 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda, T. , Kawai, T. , Anada, T. , Kamakura, S. , & Suzuki, O. (2010). Quality of regenerated bone enhanced by implantation of octacalcium phosphate‐collagen composite. Tissue Engineering. Part C, Methods, 16(3), 471–478. [DOI] [PubMed] [Google Scholar]
- Matsui, A. , Anada, T. , Masuda, T. , Honda, Y. , Miyatake, N. , Kawai, T. , … Suzuki, O. (2010). Mechanical stress‐related calvaria bone augmentation by onlayed octacalcium phosphate‐collagen implant. Tissue Engineering. Part A, 16(1), 139–151. [DOI] [PubMed] [Google Scholar]
- Matsui, A. , Matsui, K. , Handa, T. , Tanuma, Y. , Miura, K. , Kato, Y. , … Echigo, S. (2014). The regenerated bone quality by implantation of octacalcium phosphate collagen composites in a canine alveolar cleft model. The Cleft Palate‐Craniofacial Journal, 51(4), 420–430. [DOI] [PubMed] [Google Scholar]
- Matsui, K. , Matsui, A. , Handa, T. , Kawai, T. , Suzuki, O. , Kamakura, S. , & Echigo, S. (2010). Bone regeneration by octacalcium phosphate collagen composites in a dog alveolar cleft model. International Journal of Oral and Maxillofacial Surgery, 39(12), 1218–1225. [DOI] [PubMed] [Google Scholar]
- Miura, K. I. , Sumita, Y. , Kajii, F. , Tanaka, H. , Kamakura, S. , & Asahina, I. (2020). First clinical application of octacalcium phosphate collagen composite on bone regeneration in maxillary sinus floor augmentation: A prospective, single‐arm, open‐label clinical trial. Journal of Biomedical Materials Research. Part B, Applied Biomaterials, 108(1), 243–252. [DOI] [PubMed] [Google Scholar]
- Pinholt, E. M. , Ruyter, I. E. , Haanaes, H. R. , & Bang, G. (1992). Chemical, physical, and histologic studies on four commercial apatites used for alveolar ridge augmentation. Journal of Oral and Maxillofacial Surgery, 50(8), 859–867 discussion 867‐8. [DOI] [PubMed] [Google Scholar]
- Sasano, Y. , Kamakura, S. , Homma, H. , Suzuki, O. , Mizoguchi, I. , & Kagayama, M. (1999). Implanted octacalcium phosphate (OCP) stimulates osteogenesis by osteoblastic cells and/or committed osteoprogenitors in rat calvarial periosteum. The Anatomical Record, 256(1), 1–6. [DOI] [PubMed] [Google Scholar]
- Suzuki, Y. , Kamakura, S. , Honda, Y. , Anada, T. , Hatori, K. , Sasaki, K. , & Suzuki, O. (2009). Appositional bone formation by OCP‐collagen composite. Journal of Dental Research, 88(12), 1107–1112. [DOI] [PubMed] [Google Scholar]
- Tallgren, A. (2003). The continuing reduction of the residual alveolar ridges in complete denture wearers: A mixed‐longitudinal study covering 25 years. 1972. The Journal of Prosthetic Dentistry, 89(5), 427–435. [DOI] [PubMed] [Google Scholar]
- Tanuma, Y. , Matsui, K. , Kawai, T. , Matsui, A. , Suzuki, O. , Kamakura, S. , & Echigo, S. (2013). Comparison of bone regeneration between octacalcium phosphate/collagen composite and beta‐tricalcium phosphate in canine calvarial defect. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, 115(1), 9–17. [DOI] [PubMed] [Google Scholar]
- Vatsa, A. , Breuls, R. G. , Semeins, C. M. , Salmon, P. L. , Smit, T. H. , & Klein‐Nulend, J. (2008). Osteocyte morphology in fibula and calvaria—Is there a role for mechanosensing? Bone, 43(3), 452–458. [DOI] [PubMed] [Google Scholar]
- Wada, Y. , Amiel, M. , Harwood, F. , Moriya, H. , & Amiel, D. (1998). Architectural remodeling in deep frozen meniscal allografts after total meniscectomy. Arthroscopy, 14(3), 250–257. [DOI] [PubMed] [Google Scholar]
- Wan, Q. , Schoenmaker, T. , Jansen, I. D. , Bian, Z. , de Vries, T. J. , & Everts, V. (2016). Osteoblasts of calvaria induce higher numbers of osteoclasts than osteoblasts from long bone. Bone, 86, 10–21. [DOI] [PubMed] [Google Scholar]
- Zerbo, I. R. , Zijderveld, S. A. , de Boer, A. , Bronckers, A. L. , de Lange, G. , ten Bruggenkate, C. M. , & Burger, E. H. (2004). Histomorphometry of human sinus floor augmentation using a porous beta‐tricalcium phosphate: A prospective study. Clinical Oral Implants Research, 15(6), 724–732. [DOI] [PubMed] [Google Scholar]


