ABSTRACT
Chronic neonatal lung disease (CNLD) is defined as continued need for any form of respiratory support (supplemental oxygen and/or assisted ventilation) beyond 36 weeks PMA. Low‐flow supplemental oxygen facilitates discharge from hospital of infants with CNLD who are hypoxic in air and is widely used despite lack of evidence on the most appropriate minimum mean target oxygen saturations. Furthermore, there are minimal data to guide the home monitoring, titration or weaning of supplemental oxygen in these infants. The purpose of this position statement is to provide a guide for the respiratory management of infants with CNLD, with special emphasis on role and logistics of supplemental oxygen therapy beyond the NICU stay. Reflecting a variety of clinical practices and infant comorbidities (presence of pulmonary hypertension, retinopathy of prematurity and adequacy of growth), it is recommended that the minimum mean target range for SpO2 during overnight oximetry to be 93–95% with less than 5% of total recording time to be below 90% SpO2. Safety of short‐term disconnection from supplemental oxygen should be assessed before discharge, with majority of infants with CNLD not ready for discharge until supplemental oxygen requirement is ≤0.5 L/min. Sleep‐time assessment of oxygenation with continuous overnight oximetry is recommended when weaning supplemental oxygen. Palivizumab is considered safe and effective for the reduction of hospital admissions with RSV infection in this group. This statement would be useful for paediatricians, neonatologists, respiratory and sleep physicians and general practitioners managing children with CNLD.
Keywords: bronchopulmonary dysplasia, oximetry, oxygen, palivizumab, premature infant
Short abstract
Abbreviations
- ANZNN
Australian New Zealand Neonatal Network
- AOP
apnoea of prematurity
- BOOST
Benefit Of Oxygen Saturation Targeting
- BPD
bronchopulmonary dysplasia
- CNLD
chronic neonatal lung disease
- CPAP
continuous positive airway pressure
- DI4
desaturation of 4% or greater
- EOI
expression of interest
- FiO2
fraction of inspired oxygen
- ICU
intensive care unit
- NICU
neonatal ICU
- PMA
post‐menstrual age
- RSV
Respiratory Syncytial Virus
- SIG
Special Interest Group
- SpO2
saturation of peripheral oxygen
- TSANZ
Thoracic Society of Australia and New Zealand
BACKGROUND
This position statement is developed by the working party (the authors, chaired by Nitin Kapur) of the Paediatric Special Interest Group (SIG) within the Thoracic Society of Australia and New Zealand (TSANZ). The working party was formulated by seeking expression of interest (EOI) during the Paediatric SIG meeting at the TSANZ annual scientific meeting (ASM) in April 2018. The working party was selected based on EOI received as well as consideration for geographical representation and inclusion of nursing representative. Once the working party was selected, each author was allocated one section of the position statement for literature search and drafting. The initial draft of the position statement was presented to the Australasian Paediatric Respiratory Medical Group (APRMG) at the TSANZ ASM in March 2019. This group suggested inclusion of sections on caffeine and diuretics and exclusion of section on pulmonary hypertension. The recommendations on RSV prophylaxis were also agreed upon at this meeting. A revised manuscript was circulated to the entire working group including a consumer representative, and all suggestions included. From October to December 2019, the position statement was reviewed by four reviewers allocated by the TSANZ and their recommendations were included in the revised draft. The revision was then endorsed by the Clinical care and Resources Subcommittee of the TSANZ and was given a final approval by the TSANZ board in April 2020. The document would be due for review in 2025.
INTRODUCTION
Chronic neonatal lung disease (CNLD) is the most common complication of preterm birth, estimated to affect 30–40% of very low birth weight infants. 1 Home‐based low‐flow supplemental oxygen therapy has been widely recommended 2 and accepted as a successful approach to facilitate earlier discharge of these infants and reduce healthcare costs. 3 Despite its increasing use over the last 40 years, there remains considerable variation and little objective evidence to guide the use of long‐term supplemental oxygen therapy in infants with CNLD. This position statement provides a guide for the respiratory management of preterm infants with CNLD, with special emphasis on role and logistics of supplemental oxygen therapy beyond the early neonatal period.
DEFINITIONS AND CLINICAL FEATURES
While CNLD is the most common chronic lung disease of infancy, there remains considerable controversy regarding its definition. Terms such as chronic lung disease, chronic lung disease of prematurity and most commonly bronchopulmonary dysplasia (BPD) have all been used interchangeably in literature to describe a similar spectrum of clinical state in preterm infants requiring respiratory support. For the purpose of this position paper, an infant who has a continued need for any form of respiratory support [supplemental oxygen and/or assisted ventilation which includes high‐flow oxygen, continuous positive airway pressure (CPAP) or mechanical ventilation] at 36 weeks post‐menstrual age (PMA) is defined as having CNLD. 4 Of this group, the subgroup born at ≤32 weeks gestation would classify as having BPD, based on the commonly used definition of need for treatment with supplemental oxygen for at least 28 days. 5 Severity of BPD is assessed at 36 weeks PMA: mild BPD is defined as breathing room air, moderate BPD as a fraction of inspired oxygen (FiO2) requirement of less than 0.3 and severe BPD as a FiO2 requirement of more than 0.3 and/or positive pressure. 6 CNLD and BPD are unusual in that these are diseases defined by their treatment rather than their pathophysiology. This definition is problematic as it can be significantly affected by the treating physician's preferences. In addition, the increasing use of CPAP and high‐flow nasal cannula air in nurseries means that many infants are receiving respiratory support at 36 weeks gestation, but not oxygen, further clouding the definition. Variations in definition of BPD and in oxygen treatment practice can therefore lead to marked variations in the rate of BPD diagnosis. 7 Jensen et al. recently evaluated 18 pre‐specified severity graded definitions of BPD and proposed three grades of BPD: grade 1, nasal cannula at flow rate ≤2 L/min; grade 2, nasal cannula at flow rate >2 L/min or non‐invasive positive airway pressure; and grade 3, invasive mechanical ventilation. 8
More recently, the need to develop a pathophysiological rather than treatment‐based definition of BPD has been identified. 9
PATHOPHYSIOLOGY
The vast majority of CNLD occurs secondary to BPD. BPD is caused by prolonged positive pressure ventilation (barotrauma and/or volutrauma) and supplemental oxygen in the newborn period resulting in airway and vascular remodelling leading to airflow limitation. Premature infants are particularly at risk but CNLD, as distinct from BPD, can occur in term neonates who are at risk because of other pulmonary factors including congenital diaphragmatic hernia and pulmonary hypoplasia, oligohydramnios‐associated pulmonary hypoplasia, aspiration or persistent pulmonary hypertension of the newborn. 5 Other risk factors for CNLD include presence of patent ductus arteriosus, infection/sepsis including chorioamnionitis and possible genetic predisposition (SPOCK2 gene). 10 , 11 Inflammation and oxidative stress are also thought to play a role. 12 , 13
As neonates have begun to consistently survive at increasingly younger gestational ages, the clinical syndrome and underlying aetiology of CNLD has changed. In surfactant‐treated extremely low birth weight infants (birth weight < 1000 g), the primary histopathological finding of CNLD is arrested or disrupted alveolar and microvascular development of the peripheral lung. The lungs in infants with CNLD are characterized by reduced number of simplified, larger alveoli with distortion of capillaries 14 and therefore decreased surface area for gas exchange. Pulmonary vasculature is abnormal, typified by abnormal distribution and hypertrophy of pulmonary arteriole smooth muscle which, depending upon the severity, can result in pulmonary hypertension. 5 Interstitial oedema can also occur. In comparison, neonates diagnosed with BPD in the pre‐surfactant era, managed with high‐pressure ventilation and requiring higher FiO2, had lung damage where neutrophilic inflammation and fibrosis were the key defining features. 6 Although these elements can sometimes still occur in severe cases of CNLD, they no longer represent classical features.
DEMOGRAPHY AND EPIDEMIOLOGY
CNLD is the most common form of chronic lung disease in infancy, occurring in up to 20% of infants with respiratory distress syndrome. The 2017 report from the Australian New Zealand Neonatal Network (ANZNN) 4 identified that the incidence of CNLD is highest in babies born less than 27 weeks gestation with 61% of babies born less than 24 weeks who survived to day 28 and requiring respiratory support were discharged on home oxygen. While the incidence of CNLD decreases with gestational age, at 30 weeks gestation, 12% of infants are reported subsequently to manifest chronic lung disease.
Supplemental oxygen is used in neonatal intensive care units (NICU) for over 95% of infants born before 28 weeks gestation in Australia and New Zealand.
The proportion of preterm infants being discharged on home oxygen therapy varies widely between neonatal units and is largely dependent on target oxygenation strategies. The 2017 ANZNN report describes that some 17% of infants born prior to 30 weeks gestation who survived to require respiratory support at day 28 were discharged on home oxygen.
INDICATIONS AND GOALS OF SUPPLEMENTARY OXYGEN THERAPY BEYOND THE NICU STAY
The goal of treatment of CNLD with supplemental oxygen at home is to facilitate safe early discharge and reduce the effects of long‐term hypoxia. Hypoxaemia may occur in infants with CNLD during sleep despite normal oxygen saturation when awake, 15 with potential adverse effects on many body systems. 16 , 17 Sleep‐related hypoxaemia has been associated with reduced left and right ventricular ejection fraction 18 and abnormal autonomic control of heart rate. 19 Infants with CNLD are at increased risk of pulmonary hypertension due to structural changes in the lung 20 and hypoxia‐induced pulmonary vasoconstriction may exacerbate this. 21 Supplemental oxygen is a mainstay of treatment of pulmonary hypertension associated with CNLD, 22 to reverse hypoxia‐induced vasoconstriction. 23 , 24 Time spent below 90% has also been associated with increased airway resistance, 15 a finding that is likely bidirectional, with hypoxia potentially worsening bronchial constriction, further exacerbating the abnormal lung mechanics of CNLD. 25
Infants with CNLD are also at increased risk for central sleep‐disordered breathing, with recurrent central apnoeas and periodic breathing. 26 Supplemental oxygen stabilizes the respiratory pattern, reducing central apnoeas and episodes of periodic breathing. Oxygen also promotes improved nutrition and somatic growth. 27 Despite the difficulties in gaining evidence for the benefits of avoiding hypoxia in developing infants, recommendations reasonably make the assumption that a hypoxic infant may lack the energy to learn and develop optimally, with supplemental oxygen enhancing physical activities that promote development. 28
In addition to these benefits of supplemental oxygen on physiological processes and functioning, the availability of supplemental oxygen for home use also facilitates earlier discharge of infants from hospital, thereby limiting their exposure to the risks of prolonged hospitalization and promoting parent–infant bonding and integration of the infant into family life. 29 , 30 Earlier discharge also reduces healthcare costs, 31 although this impact is difficult to measure: supplemental oxygen at discharge is associated with longer hospital stay, noting that infants with more severe lung disease and a more complicated clinical course are at risk for both a prolonged hospital stay and the use of supplemental oxygen at discharge. 32 , 33
TARGET OXYGEN SATURATION LEVELS
While arterial PaO2 measurement is considered critical to assess hypoxia in adults, oxygen saturation measured by pulse oximetry remains the most widely accepted form of oxygen assessment in infants. Median baseline saturation in healthy term infants during the first year of life is 97–98%, with less than 5% of healthy infants spending >4% of the time at SpO2 < 90%. 34 , 35 , 36 During sleep, the mean SpO2 is reported to be marginally less at 96.3% (±1.3%). 37 In contrast, preterm infants, even at term‐corrected age, tend to spend a significantly higher proportion of time <90% SpO2 (9% in one cohort of ex‐preterm infants born <29 weeks). 38 , 39
A review of the literature found no study showing a conclusive proof of the optimal target saturation post discharge in children born preterm on several outcomes. An observational study of 63 infants with BPD 40 reported a significant reduction in weight gain when oxygen therapy was ceased in a subgroup of 14 infants who had desaturations of 88–91% for more than 1 h on an overnight oximetry on room air.
There is more robust evidence available on what possibly is an appropriate SpO2 level in early neonatal period in the preterm infant. The Benefit Of Oxygen Saturation Targeting (BOOST) 1 trial randomly allocated 358 infants of <30 weeks gestation into two target SpO2 levels (91–94% vs 95–98%) from 32 weeks PMA. 41 No difference in growth or neurodevelopmental outcomes was seen at 12 months between the two groups, although those in the higher saturation target group understandably had longer median length of oxygen therapy post randomization (40 vs 17.5 days) and frequency of home oxygen therapy (30% vs 17%).
A more recent multicentre study (BOOST 2 trial) 42 included 2448 neonates of <28 weeks gestation from the UK, Australia and New Zealand and randomly allocated them within 24 h of birth into lower (85–89%) versus higher (91–95%) target SpO2 levels. The study was stopped early when the interim analysis showed significantly higher rate of death at 36 weeks PMA in the lower saturation group. Tarnow‐Mordi and Kirby 43 recently reviewed 23 publications from 2011 to 2019 discussing randomized trials of oxygen saturation targets of 85–89% versus 91–95% infants below 28 weeks gestation. Of the 18 commentaries or consensus statements, 17 recommended saturation targets above 89%. Five systematic reviews reported that the 85–89% target increased mortality but not the composite of death or disability.
Although baseline (or mean) SpO2 is routinely used as a marker of oxygenation (and hence need to initiate and/or continue supplementary oxygen), desaturation events (variably defined as 4–10% drop in SpO2 from baseline) can often result in continuation (and in some cases initiation) of supplemental oxygen in this group of infants. While data on preterm infants are limited, recent normative data 44 in term infants indicate that infants at 1 month of age have desaturations of 4% or greater (DI4) at a mean of 16 times/h, with average minimum saturation of 80%. In the same study, the DI4 improved to mean of 8.12 at 3–4 months of age. It is unclear if these intermittent desaturations are of clinical significance, and hence it is difficult to base treatment recommendations on these results, particularly in preterm infants.
Also, whilst SpO2 levels either below or above target may be associated with adverse effects, in infants of post‐term corrected age, being below target is of greater concern owing to less vulnerability to the ophthalmological and pulmonary adverse effects of hyperoxia.
Overall, while there is limited direct evidence on the most appropriate saturation levels in this group of infants, by extrapolating the data from normative term infant, 34 , 35 , 36 one observational study 40 and BOOST 1 and 2 41 , 42 studies, it may be concluded that SpO2 above 95% is probably unnecessary and those below 90% are likely associated with adverse clinical outcomes. Our recommendation is to target a minimum mean SpO2 level of 93–95% in infants with CNLD, as measured by continuous overnight oximetry with less than 5% of total recording time to be below 90% SpO2. We understand that our suggestion of using a minimum cut‐off value of 90% SpO2 is based on low‐grade evidence but is consistent with the British Thoracic Society (maintain SpO2 ≥ 93% and < 5% of time below 90%) 36 and European Respiratory Society (ERS) (maintain minimum SpO2 of 90%) guidelines. 45 Similar minimum cut‐off value of 93% suggested in the recent American Thoracic Society (ATS) guidelines (< 5% recording time to be ≤ 93% SpO2) 46 are also based on low‐grade evidence. We feel that the lower cut‐off value will require fewer infants to be discharged on home oxygen, reducing financial and emotional burden without increasing the risk of compromising patient outcomes.
PRACTICAL CONSIDERATIONS FOR HOME OXYGEN
Hospital discharge is uncommon in most parts of Australasia until the infant has an oxygen requirement of ≤0.5 L/min. 47 Supplemental oxygen is delivered via nasal cannula and at oxygen flows ≤0.5 L/min humidification is rarely required. An oxygen concentrator may be viewed as a cheaper and reliable alternative for delivering oxygen in the child's bedroom. However, they are noisy, not mobile and the direct cost to the family for the electricity required should be considered. Portable oxygen cylinders are needed in addition to a concentrator to allow outings from the house 36 , 47 and for power failures. A large cylinder (4200 L) usually lasts for 11 days of continuous use on oxygen flow of 0.25 L/min and a portable cylinder (400–470 L) lasts 24 h at the same flow. Standard tube length is 10 m plus 1.5 m on the nasal prongs. Accurate flow cannot be guaranteed if multiple lengths of tubes are connected. Low‐flow regulators for home use vary across companies and have variable starting flow rates, some as low as 0.02 L/min. All have variability listed as ±10%. Conserving devices that are used to provide intermittent flow and deliver pulsed oxygen are considered unsuitable for neonatal or paediatric use. State funded programmes generally meet the cost of supplemental oxygen therapy in Australia and New Zealand. Home caregiver education should address the risks associated with smoking and open flames near the oxygen source.
MONITORING PERI‐DISCHARGE AND AT HOME
Oximetry, measuring the ratio of oxyhaemoglobin to total haemoglobin, is the mainstay of monitoring. Newer oximeters have circumvented problems with movement artefact using adaptive filters. 48 These reduce noise and extract the arterial signal from the venous signal to more accurately report arterial oxygen saturations. 39 Spot saturations must not be used to wean oxygen as periods of hypoxia that occur with sleep, activity and feeding would be missed. 2 , 48 A continuous recording of SpO2 overnight, preferably for more than 6 hours, gives clinically useful information. 2 , 39
The use of the ‘Air Test’ prior to discharge is favoured by some units in order to establish risk in case oxygen is inadvertently discontinued at home. Infants are assessed prior to discharge to determine if they can maintain an SpO2 > 80% during disconnection from supplemental oxygen over 4 hours. 49 Shorter periods of oxygen saturation monitoring in air, typically up to 30 min, are used for monitoring readiness for discontinuation of supplemental oxygen prior to discharge or in outpatient clinic reviews. 49 , 50 Whilst there is no clear consensus on optimal oxygen profiles in air tests during outpatient reviews through infancy, targeted mean SpO2 of 93–95% with a minimum SpO2 of 88–90% in air is considered broadly acceptable. Routine monitoring with an oximeter at home is not recommended.
Overnight polysomnography provides information on carbon dioxide profile, respiratory rate, work of breathing and the presence of central and obstructive apnoea in addition to oxygen saturations. 17 This test may be considered when obstructive sleep apnoea (OSA) or significant central sleep‐disordered breathing/hypoventilation is suspected such as in syndromic children, children with craniofacial anomalies and with associated neurological problems. Follow‐up polysomnography may also be considered in an infant who does not follow the expected clinical course of progressive respiratory improvement.
WEANING OFF HOME SUPPLEMENTAL OXYGEN
There is no available evidence to guide the titration or weaning of supplemental oxygen in infants. However, the prevailing principle is to maintain the child within the SpO2 targets whilst recognizing changes in their underlying disease status (usually improving CNLD, maturity and size). The need to wean oxygen therapy over the first months of life is guided by the severity of lung disease and the presence of comorbidities, particularly retinopathy of prematurity, pulmonary hypertension and poor growth trajectory. 2 CNLD severity generally improves over time with lung growth and improved respiratory function. Respiratory control also matures resulting in fewer respiratory pauses/intermittent desaturation. Alternatively, premature infants are vulnerable and may suffer additional respiratory insults such as intercurrent respiratory infections, leading to worsening respiratory function. With growth metabolic requirements increase, potentially leading to higher supplemental oxygen requirements. The weaning recommendations are given in Box and Figure 1.
Figure 1.
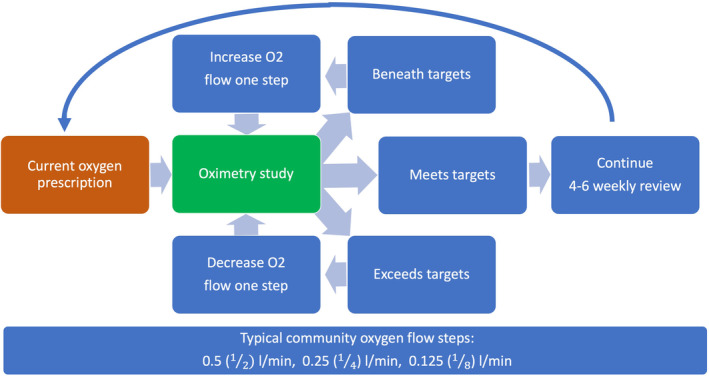
Oxygen weaning recommendation.
BOX 1. Oxygen weaning recommendations beyond the neonatal intensive care unit (NICU).
1 SpO2 is to be maintained within age appropriate targets over time.
2 SpO2 should be assessed by overnight oximetry in the days prior to discharge and have the first clinical review in a 4–6 week window.
3 Thereafter, SpO2 is recommended to be reassessed on a 4–8 weekly basis to ensure adequacy of the supplemental oxygen and allow weaning or increasing as appropriate. In the (rarer) older infant on longer term supplemental oxygen, less frequent monitoring may be appropriate.
4 Twenty‐four hour oxygen therapy is usually recommended. Some older infants may not tolerate daytime supplemental oxygen and compromising to sleep oxygen therapy may be appropriate to maximize the number of hours per day at target SpO2.
5 Supplemental oxygen for infants is usually prescribed in steps including 0.5 (1/2), 0.25 (1/4) and 0.125 (1/8) L/min. In some centres, lower flow rates may be available and appropriately utilized.
6 In general, an overnight oximetry study should be performed on the current prescription first. If a previous oximetry on this flow was recent and found to be at or above target, it may be appropriate to go straight to an assessment on a lower prescription
• If the oximetry result meets SpO2 targets (mean 93–95% and less than 5% of time below 90%), the current prescription should be continued.
• If the oximetry result is below target (mean SpO2 < 93% and/or more than 5% of time below 90%), the prescription ought to be increased a step and the oximetry repeated. If deterioration is not expected, the reason behind the deterioration should be explored.
• If the oximetry result exceeds target (mean SpO2 > 95%), then the prescription ought to be reduced a step and the oximetry repeated.
• Oximetry studies ought to be repeated as mentioned above until the infant's current appropriate prescription (or stability in room air) is identified. In some settings, this may be done as a single night's ‘titration’ study investigating multiple doses of oxygen on a single night.
7 Whilst SpO2 targets are the main guide, note also ought to be taken of the infant's overall clinical picture including work of breathing, feeding, growth and any co‐morbid cardiac disease.
8 Oxygen can be discontinued when target saturation is achieved without it.
FITNESS TO FLY
Cabins of commercial aircrafts are pressurized to an equivalent of 1500–2400 m altitude, which equates to 15–17% inspired oxygen. Infants with CNLD with normal SpO2 in air may have clinically significant desaturation at this lower FiO2 and flight assessment is recommended in infants who have required supplemental oxygen in the 6 months preceding any air travel. This can be simulated in a respiratory laboratory with the infant and parent seated in a body plethysmograph, where inspired oxygen is diluted with nitrogen to reduce the FiO2 to about 15%. A fall in SpO2 below 85% during 20 min of experimental hypoxia is an indication for either delaying the air travel or providing supplemental oxygen to the infant in flight. 51 The dose of supplemental oxygen required to normalize SpO2 at altitude can be determined during the test. If such a test is not readily accessible, supplemental oxygen should be doubled from the usual dose used at sea level or 0.25 L/min can be provided for the flight for infants who have ceased oxygen therapy in the preceding 6 months.
DIURETICS
Diuretics are the most commonly used medications in the management of established BPD. The degree of free lung water has been correlated with the severity of BPD, 52 and hence form the basis for using diuretics in this group. Long‐term use of diuretic therapy was studied in 43 oxygen‐dependent BPD infants in a randomized double‐blind, placebo‐controlled study. Those on diuretics had a significant improvement in respiratory scores, lung mechanics and FiO2 used but this did not translate into decreased duration of oxygen supplementation. 53 While a large meta‐analysis on pre‐term infants with CNLD also did show some improvement in pulmonary mechanics with chronic administration of diuretics, the authors suggested that the number of infants studied were small, with most studies from the pre‐surfactant era. Furthermore, there were no trials that demonstrated a reduction in length of stay, duration of mechanical ventilation or duration of supplemental oxygen in this group. 54 On the basis of these data, no recommendation can be given on suitability of long‐term diuretics therapy in infants with established BPD.
CAFFEINE
Apnoea of prematurity (AOP) is a common developmental disorder with an incidence that increases with decreasing gestational age. 55 Caffeine therapy potentially works in AOP via several mechanisms including enhancing sensitivity to carbon dioxide via adenosine antagonism, improving diaphragmatic contractility and increasing muscle tone. 56 , 57 While discussion on the role of caffeine in early neonatal period for prevention of BPD and treatment of AOP is outside the scope of this position statement, its use has been suggested as a treatment option for persistent apnoeas/hypopnoeas beyond term‐corrected age. A randomized trial of early (34–37 weeks PMA) versus late cessation (40 weeks PMA) of caffeine therapy in preterm infants reported that prolonged therapy resulted in fewer episodes of intermittent hypoxia and more time at goal saturation. 58 There are currently no data on the role of caffeine in reduction of length of supplemental oxygen therapy beyond term‐corrected age.
RSV PREVENTION
Ten percent of infants born <32 weeks gestation require hospital admission with RSV infection. Most with CNLD require a temporary increase in supplemental oxygen, and some require supported feeding, high‐flow nasal oxygen or admission to intensive care unit (ICU). Intubation and mechanical ventilation are rarely required.
The data on the use of palivizumab for CNLD come principally from the Impact‐RSV trial (1998), a randomized, placebo‐controlled trial of 1002 infants born <32 weeks gestation, who were <6 months old or who also had CNLD but were <2 years old. 59 Use of palivizumab resulted in 39% reduction in RSV‐related hospitalization in this group, with a 56% reduction in ICU admission.
Palivizumab is the only TGA (Australia) and Medsafe (New Zealand) licensed anti‐RSV preventative therapy. It is delivered by intramuscular injection (15 mg/kg) on a monthly basis. Each injection of palivizumab cost ~A$1750. Palivizumab is not funded by the PBS (Australia) or PHARMAC (New Zealand). Australian and New Zealand practice varies with some centres not using palivizumab at all for infants with CNLD, others using it selectively for patients with CNLD who also have other risk factors for RSV hospitalization, such as congenital heart disease or pulmonary hypertension. Recommendations for its use are outlined in Box 2.
BOX 2. Recommendations for use of palivizumab.
1 Palivizumab is safe and effective for the reduction of admissions to hospital with RSV infection in preterm infants with CNLD. Mortality from RSV infection is extremely low and there is no evidence of palivizumab reducing mortality from RSV infection in preterm infants with CNLD. As such, no firm recommendations for the use of palivizumab can be made.
2 Palivizumab is also effective at reducing hospital admissions with RSV infection in infants with congenital cardiac disease. This may justify the use of palivizumab for infants with CNLD and congenital cardiac disease or pulmonary hypertension.
3 Local epidemiology of RSV infection will determine the months to administer. Given that at least 50% of infants with CNLD will be infected by the end of the first RSV season, palivizumab is only indicated for use amongst premature infants with CNLD in their first winter.
LONG‐TERM OUTCOMES OF CNLD
The long‐term outcomes of preterm birth, both with and without BPD, have been extensively reviewed. 14 BPD has been shown to be associated with ongoing abnormalities in childhood including increased respiratory symptoms, reduced lung function and abnormal lung structure on imaging. A recent meta‐analysis of more than 50 studies has demonstrated that children with a history of BPD have a 16% lower forced expiratory volume in 1 s (FEV1) during childhood than children born at term. 60 Recent longitudinal studies suggest that lung disease during childhood and young adulthood is ongoing in survivors of preterm birth with BPD, with tracking of lung function below normal levels or even progressive decline. 61 , 62 There is no evidence that oxygen supplementation influences or improves long‐term respiratory outcomes or whether the positive effects of oxygen supplementation seen in infancy translate to better long‐term outcomes.
While there is some evidence that episodes of hypoxia during the neonatal period can be associated with poorer neurodevelopmental outcomes at 18 months, 63 a recent meta‐analysis of five trials involving almost 5000 infants, comparing higher and lower oxygen target saturations, revealed no differences in rates of major disability at 18–24 months. 64 Long‐term follow‐up of these infants would present a unique opportunity to determine whether there are neurocognitive benefits to oxygen supplementation. Currently, however, neurocognitive benefits from oxygen supplementation in infants with BPD remain putative. Moreover, neonatal care is ever changing and there is a need to evaluate newer cohorts longitudinally to truly understand outcomes in current population of preterm infants.
In conclusion, safe target SpO2 for preterm infants post discharge still remains unclear. It seems judicious to avoid lower saturations to avoid mortality and possible morbidity of chronic hypoxia. The summary of this position statement is outlined in Box 3. Future research should address the long‐term outcomes for oxygen saturation targets during early infancy and identification of best practice for weaning and discontinuation of home oxygen therapy in infants with CNLD.
BOX 3. Summary main statements.
• For infants with CNLD, supplemental oxygen therapy is recommended to maintain the minimum mean SpO2 between 93% and 95%.
• It is recommended that the safety of short‐term disconnection from supplemental oxygen is assessed before discharge.
• The majority of infants with CNLD may not be ready for discharge until their supplemental oxygen requirement is ≤0.5 L/min, which is delivered via nasal cannulae.
• Assessment of oxygenation while asleep with continuous overnight oximetry is recommended when weaning supplemental oxygen.
• There is limited objective evidence upon which to make recommendations.
Acknowledgement
Ms Sandra Addley was the consumer representative on the position paper who gave valuable feedback on the paper.
Kapur N, Nixon G, Robinson P, et al. Respiratory management of infants with chronic neonatal lung disease beyond the NICU: A position statement from the Thoracic Society of Australia and New Zealand*. Respirology. 2020;25:880–888. 10.1111/resp.13876
*This document was reviewed by the TSANZ Clinical Care and Resources Subcommittee and recommended to the TSANZ Board for endorsement. The TSANZ Board of Directors endorsed this position paper on 27 March 2020.
Received 2 April 2020; invited to revise 7 May 2020; revised 16 May 2020; accepted 21 May 2020
Handling Editors: Philip Bardin and Paul Reynolds
REFERENCES
- 1. Zysman‐Colman Z, Tremblay GM, Bandeali S, Landry JS. Bronchopulmonary dysplasia–trends over three decades. Pediatr. Child Health 2013; 18: 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fitzgerald DA, Massie RJ, Nixon GM, Jaffe A, Wilson A, Landau LI, Twiss J, Smith G, Wainwright C, Harris M. Infants with chronic neonatal lung disease: recommendations for the use of home oxygen therapy. Med. J. Aust. 2008; 189: 578–82. [DOI] [PubMed] [Google Scholar]
- 3. Balfour‐Lynn IM, Primhak RA, Shaw BNJ. Home oxygen for children: who, how and when? Thorax 2005; 60: 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow SSW, Creighton P, Chambers GM, Lui K. 2019. Report of the Australian and New Zealand Neonatal Network 2017. Sydney: ANZNN.
- 5. Baraldi E, Filippone M. Chronic lung disease after premature birth. N. Engl. J. Med. 2007; 357: 1946–55. [DOI] [PubMed] [Google Scholar]
- 6. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001; 163: 1723–1729. [DOI] [PubMed] [Google Scholar]
- 7. Hines D, Modi N, Lee SK, Isayama T, Sjörs G, Gagliardi L, Lehtonen L, Vento M, Kusuda S, Bassler D et al; International Network for Evaluating Outcomes (iNeo) of Neonates . Scoping review shows wide variation in the definitions of bronchopulmonary dysplasia in preterm infants and calls for a consensus. Acta Paediatr 2017; 106: 366–74. [DOI] [PubMed]
- 8. Jensen EA, Dysart K, Gantz MG, McDonald S, Bamat NA, Keszler M, Kirpalani H, Laughon MM, Poindexter BB, Duncan AF et al The diagnosis of bronchopulmonary dysplasia in very preterm infants. An evidence‐based approach. Am. J. Respir. Crit. Care Med. 2019; 200: 751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stoecklin B, Simpson SJ, Pillow JJ. Bronchopulmonary dysplasia: rationale for a pathophysiological rather than treatment based approach to diagnosis. Paediatr. Respir. Rev. 2019; 32: 91–7. [DOI] [PubMed] [Google Scholar]
- 10. Morty RE. Recent advances in the pathogenesis of BPD. Semin. Perinatol. 2018; 42: 404–12. [DOI] [PubMed] [Google Scholar]
- 11. Hadchouel A, Durrmeyer X, Bouzigon E, Incitti R, Huusko J, Jarreau PH, Lenclen R, Demenais F, Franco‐Montoya ML, Layouni I et al Identification of SPOCK2 as a susceptibility gene for bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2011; 184: 1164–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bonadies L, Zaramella P, Porzionato A, Muraca M, Baraldi E. Bronchopulmonary dysplasia: what's new on the horizon? Lancet Child Adolesc. Health 2018; 2: 549–51. [DOI] [PubMed] [Google Scholar]
- 13. Looi K, Evans DJ, Garratt LW, Ang S, Hillas JK, Kicic A, Simpson SJ. Preterm birth: born too soon for the developing airway epithelium? Paediatr. Respir. Rev. 2019; 31: 82–8. [DOI] [PubMed] [Google Scholar]
- 14. Simpson SJ, Hall GL, Wilson AC. Lung function following very preterm birth in the era of ‘new’ bronchopulmonary dysplasia. Respirology 2015; 20: 535–40. [DOI] [PubMed] [Google Scholar]
- 15. Garg M, Kurzner SI, Bautista DB, Keens TG. Clinically unsuspected hypoxia during sleep and feeding in infants with bronchopulmonary dysplasia. Pediatrics 1988; 81: 635–42. [PubMed] [Google Scholar]
- 16. Mclean JE, Fitzgerald D. A rational approach to home oxygen use in infants and children. Paediatr. Respir. Rev. 2006; 7: 215–22. [DOI] [PubMed] [Google Scholar]
- 17. Fitzgerald DA, Van Asperen PP, Feddema P, O'Leary P, Leslie G, Arnold J, Sullivan CS. Sleep, respiratory rate and growth hormone in chronic neonatal lung disease. Pediatr. Pulmonol. 1998; 26: 241–9. [DOI] [PubMed] [Google Scholar]
- 18. Praud JP, Cavailloles F, Boulhadour K, DeRecondo M, Guilleminault C, Gaultier C. Radionuclide evaluation of cardiac function during sleep in children with bronchopulmonary dysplasia. Chest 1991; 100: 721–5. [DOI] [PubMed] [Google Scholar]
- 19. Filtchev SI, Curzi‐Dascalova L, Spassov L, Kauffmann F, Trang HT, Gaultier C. Heart rate variability during sleep in infants with bronchopulmonary dysplasia: effects of mild decrease in oxygen saturation. Chest 1994; 106: 1711–6. [DOI] [PubMed] [Google Scholar]
- 20. Bush A, Busst CM, Knight WB, Hislop AA, Haworth SG, Shinebourne EA. Changes in pulmonary circulation in severe bronchopulmonary dysplasia. Arch. Dis. Child. 1990; 65: 739–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cerro MJ, Abman S, Diaz G, Freudenthal AH, Freudenthal F, Harikrishnan S, Haworth SG, Ivy D, Lopes AA, Raj JU et al A consensus approach to the classification of pediatric pulmonary hypertensive vascular disease: report from the PVRI Pediatric Taskforce, Panama 2011. Pulm. Circ. 2011; 1: 286–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Khemani E, McElhinney DB, Rhein L, Andrade O, Lacro RV, Thomas KC, Mullen MP. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics 2007; 120: 1260–9. [DOI] [PubMed] [Google Scholar]
- 23. Dhillon R. The management of neonatal pulmonary hypertension. Arch. Dis. Child. Fetal Neonatal Ed. 2012; 97: F223–8. [DOI] [PubMed] [Google Scholar]
- 24. Abman SH, Wolfe RR, Accurso FJ, Koops BL, Bowman CM, Wiggins JW Jr. Pulmonary vascular response to oxygen in infants with severe bronchopulmonary dysplasia. Pediatrics 1985; 75: 80–4. [PubMed] [Google Scholar]
- 25. Allen JL, Greenspan JS, Deoras KS, Keklikian E, Wolfson MR, Shaffer TH. Interaction between chest wall motion and lung mechanics in normal infants and infants with bronchopulmonary dysplasia. Pediatr. Pulmonol. 1991; 11: 37–43. [DOI] [PubMed] [Google Scholar]
- 26. McGrath‐Morrow SA, Ryan T, McGinley BM, Okelo SO, Sterni LM, Collaco JM. Polysomnography in preterm infants and children with chronic lung disease. Pediatr. Pulmonol. 2012; 47: 172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Groothius JR, Rosenberg AA. Home oxygen promotes weight gain in infants with bronchopulmonary dysplasia. Am. J. Dis. Child. 1987; 141: 992–5. [DOI] [PubMed] [Google Scholar]
- 28. Allen J, Zwerdling R, Ehrenkranz R, Gaultier C, Geggel R, Greenough A, Kleinman R, Klijanowicz A, Martinez F, Ozdemir A et al; American Thoracic Society . Statement on the care of the child with chronic lung disease of infancy and childhood. Am. J. Respir. Crit. Care Med 2003; 168: 356–96. [DOI] [PubMed]
- 29. Silva DT, Hagan R, Sly PD. Home oxygen management of neonatal chronic lung disease in Western Australia. J. Paediatr. Child Health 1995; 31: 185–8. [DOI] [PubMed] [Google Scholar]
- 30. Howard‐Glenn L. Transition to home: discharge planning for the oxygen‐dependent infant with bronchopulmonary dysplasia. J. Perinat. Neonatal Nurs. 1992; 6: 85–94. [DOI] [PubMed] [Google Scholar]
- 31. Donn S. Cost effectiveness of home management of bronchopulmonary dysplasia. Pediatrics 1982; 70: 330–1. [PubMed] [Google Scholar]
- 32. Stark AR, Adamkin DH, Batton DG, Bell EF, Bhutani VK, Denson SE, Martin GI, Watterberg KL, Barrington KJ, Hankins GD et al; American Academy of Pediatrics Committee on Fetus and Newborn . Hospital discharge of the high risk neonate. Pediatrics 2008; 122: 1119–26. [DOI] [PubMed]
- 33. Brooten D, Kumar S, Brown LP, Butts P, Finkler SA, Bakewell‐Sachs S, Gibbons A, Delivoria‐Papadopoulos M. A randomized clinical trial of early hospital discharge and home follow‐up of very‐low‐birth‐weight infants. N. Engl. J. Med. 1986; 315: 934–9. [DOI] [PubMed] [Google Scholar]
- 34. Shah PS, Hakak H, Mohamed A, Shah J, Young J, Kelly E. Oxygen saturation profile in late‐preterm and term infants: a prospective cohort study. J. Perinatol. 2014; 34: 917–20. [DOI] [PubMed] [Google Scholar]
- 35. Røsvik A, Øymar K, Kvaløy JT, Berget M. Oxygen saturation in healthy newborns; influence of birth weight and mode of delivery. J. Perinat. Med. 2009; 37: 403–6. [DOI] [PubMed] [Google Scholar]
- 36. Balfour‐Lynn IM, Field DJ, Gringras P, Hicks B, Jardine E, Jones RC, Magee AG, Primhak RA, Samuels MP, Shaw NJ et al; Paediatric Section of the Home Oxygen Guideline Development Group of the BTS Standards of Care Committee . BTS guidelines for home oxygen in children. Thorax 2009; 64(Suppl. 2): ii1–26. [DOI] [PubMed]
- 37. Horemuzova E, Katz‐Salamon M, Milerad J. Breathing patterns, oxygen and carbon dioxide levels in sleeping healthy infants during the first nine months after birth. Acta Paediatr. 2000; 89: 1284–9. [DOI] [PubMed] [Google Scholar]
- 38. Harigopal S, Satish HB, Tactac AFG, Southern KW, Shaw NJ. Oxygen saturation profile in healthy preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2011; 96: f339–42. [DOI] [PubMed] [Google Scholar]
- 39. Rhein L, Simoneau T, Davis J, Correia C, Ferrari D, Monuteaux M, Gregory M, Simoneau T, Davis J. Reference values of nocturnal oxygenations for use in outpatient oxygen weaning protocols in premature babies. Pediatr. Pulmonol. 2012; 47: 453–9. [DOI] [PubMed] [Google Scholar]
- 40. Moyer‐Mileur LJ, Nielson DW, Pfeffer KD, Witte MK, Chapman DL. Eliminating sleep‐associated hypoxemia improves growth in infants with bronchopulmonary dysplasia. Pediatrics 1996; 98: 779–83. [PubMed] [Google Scholar]
- 41. Askie LM, Henderson‐Smart DJ, Irwig L, Simpson JM. Oxygen saturation targets and outcomes in extremely preterm infants. N. Engl. J. Med. 2003; 349: 959–67. [DOI] [PubMed] [Google Scholar]
- 42. Stenson BJ, Tarnow‐Mordi WO, Darlow BA, Simes J, Juszczak E, Askie L, Battin M, Bowler U, Broadbent R, Cairns P et al Oxygen saturation and outcomes in preterm infants. N. Engl. J. Med. 2013; 368: 2094–104. [DOI] [PubMed] [Google Scholar]
- 43. Tarnow‐Mordi W, Kirby A. Current recommendations and practice of oxygen therapy in preterm infants. Clin. Perinatol. 2019; 46: 621–36. [DOI] [PubMed] [Google Scholar]
- 44. Evans HJ, Karunatilleke AS, Grantham‐Hill S, Gavlak JC. A cohort study reporting normal oximetry values in healthy infants under 4 months of age using Masimo technology. Arch. Dis. Child. 2018; 103: 868–72. [DOI] [PubMed] [Google Scholar]
- 45. Duijts L, van Meel ER, Moschino L, Baraldi E, Barnhoorn M, Bramer WM, Bolton CE, Boyd J, Buchvald F, Del Cerro MJ et al European Respiratory Society guideline on long term management of children with bronchopulmonary dysplasia. Eur. Respir. J. 2020; 55: 1900788. [DOI] [PubMed] [Google Scholar]
- 46. Hayes D Jr, Wilson KC, Krivchenia K, Hawkins SMM, Balfour‐Lynn IM, Gozal D, Panitch HB, Splaingard ML, Rhein LM, Kurland G et al Home oxygen therapy for children. An official American Thoracic Society clinical practice guideline. Am. J. Respir. Crit. Care Med. 2019; 199: e5–e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Askie LM, Henderson‐Smart DJ, Jones RA. Management of infants with chronic lung disease of prematurity in Australasia. Early Hum. Dev. 2005; 81: 135–42. [DOI] [PubMed] [Google Scholar]
- 48. Flint A, Davies MW. The use of overnight oximetry in neonates: a literature review. J. Paediatr. Child Health 2018; 54: 720–7. [DOI] [PubMed] [Google Scholar]
- 49. Saletti A, Stick S, Doherty D, Simmer K. Home oxygen therapy after preterm birth in Western Australia. J. Paediatr. Child Health 2004; 40: 519–23. [DOI] [PubMed] [Google Scholar]
- 50. Walsh MC, Wilson‐Costello D, Zadell A, Newman N, Fanaroff A. Safety, reliability, and validity of a physiologic definition of bronchopulmonary dysplasia. J. Perinatol. 2003; 23: 451–6. [DOI] [PubMed] [Google Scholar]
- 51. Ahmedzai S, Balfour‐Lynn IM, Bewick T, Buchdahl R, Coker RK, Cummin AR, Gradwell DP, Howard L, Innes JA, Johnson AO et al; British Thoracic Society Standards of Care Committee . Managing passengers with stable respiratory disease planning air travel: British Thoracic Society recommendations. Thorax 2011; 66(Suppl. 1): i1–30. [DOI] [PubMed]
- 52. Adams EW, Harrison MC. Increased lung water and tissue damage in BPD. J. Pediatr. 2004; 145: 505–7. [DOI] [PubMed] [Google Scholar]
- 53. Kao LC, Durand DJ, McCrea RC, Birch M, Powers RJ, Nickerson BG. Randomized trial of long term diuretic therapy for infants with oxygen dependent bronchopulmonary dysplasia. J. Pediatr. 1994; 124: 772–81. [DOI] [PubMed] [Google Scholar]
- 54. Stewart A, Brion LP, Ambrosiop‐Perez I. Diuretics acting on the distal renal tubule for preterm infants with (or developing) chronic lung disease. Cochrane Database Syst. Rev. 2011; 9: CD001817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Henderson‐Smart DJ, De Paoli AG. Methylxanthine treatment for apnoea in preterm infants. Cochrane Database Syst. Rev. 2010; 12: CD000140. [DOI] [PubMed] [Google Scholar]
- 56. Kraaijenga JV, Hutten GJ, de Jongh FH, van Kaam AH. The effect of caffeine on diaphragmatic activity and tidal volume in preterm infants. J. Pediatr. 2015; 167: 70–5. [DOI] [PubMed] [Google Scholar]
- 57. Atik A, Harding R, De Matteo R, Kondos‐Devcic D, Cheong J, Doyle LW, Tolcos M. Caffeine for apnea of prematurity: effects on the developing brain. Neurotoxicology 2017; 58: 94–102. [DOI] [PubMed] [Google Scholar]
- 58. Rhein LM, Dobson NR, Darnall RA, Corwin MJ, Heeren TC, Poets CF, McEntire BL, Hunt CE; Caffeine Pilot Study Group . Effects of caffeine on intermittent hypoxia in infants born prematurely: a randomized clinical trial. JAMA Pediatr. 2014; 168: 250–7. [DOI] [PubMed] [Google Scholar]
- 59. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high‐risk infants. The IMpact‐RSV Study Group. Pediatrics 1998; 102: 531–7. [PubMed] [Google Scholar]
- 60. Kotecha SJ, Edwards MO, Watkins WJ, Henderson AJ, Paranjothy S, Dunstan FD, Kotecha S. Effect of preterm birth on later FEV1: a systematic review and meta‐analysis. Thorax 2013; 68: 760–6. [DOI] [PubMed] [Google Scholar]
- 61. Simpson SJ, Turkovic L, Wilson AC, Verheggen M, Logie KM, Pillow JJ, Hall GL. Lung function trajectories throughout childhood in survivors of very preterm birth: a longitudinal cohort study. Lancet Child Adolesc. Health. 2018; 2: 350–9. [DOI] [PubMed] [Google Scholar]
- 62. Moschino L, Stocchero M, Filippone M, Carraro S, Baraldi E. Longitudinal assessment of lung function in survivors of bronchopulmonary dysplasia from birth to adulthood. The Padova BPD study. Am. J. Respir. Crit. Care Med. 2018; 198: 134–7. [DOI] [PubMed] [Google Scholar]
- 63. Poets CF, Roberts RS, Schmidt B, Whyte RK, Asztalos EV, Bader D, Bairam A, Moddemann D, Peliowski A, Rabi Y et al; Canadian Oxygen Trial Investigators . Association between intermittent hypoxemia or bradycardia and late death or disability in extremely preterm infants. JAMA 2015; 314: 595–603. [DOI] [PubMed]
- 64. Askie LM, Darlow BA, Finer N, Schmidt B, Stenson B, Tarnow‐Mordi W, Davis PG, Carlo WA, Brocklehurst P, Davies LC et al; Neonatal Oxygenation Prospective Meta‐Analysis (NeOProM) Collaboration . Association between oxygen saturation targeting and death or disability in extremely preterm infants in the Neonatal Oxygenation Prospective Meta‐Analysis Collaboration. JAMA 2018; 319: 2190–201. [DOI] [PMC free article] [PubMed]


