Abstract
Domain wall motion is detected for the first time during the transition to a ferroelastic and spin state ordered phase of a spin crossover complex. Single‐crystal X‐ray diffraction and resonant ultrasound spectroscopy (RUS) revealed two distinct symmetry‐breaking phase transitions in the mononuclear Mn3+ compound [Mn(3,5‐diBr‐sal2(323))]BPh4, 1. The first at 250 K, involves the space group change Cc→Pc and is thermodynamically continuous, while the second, Pc→P1 at 85 K, is discontinuous and related to spin crossover and spin state ordering. Stress‐induced domain wall mobility was interpreted on the basis of a steep increase in acoustic loss immediately below the the Pc‐P1 transition
Keywords: domain wall, ferroelastic materials, manganese(III), spin crossover, structural phase transition
Stresses and strains: Domain wall motion is detected for the first time in a spin crossover crystal. Mobility of ferroelastic domain walls was interpreted on the basis of a steep increase in acoustic loss below a first order transition. Spin state ordering and domain formation is associated with collapse of the Jahn–Teller distortion on switching from the spin quintet to spin triplet form of a mononuclear Mn3+ complex.
Introduction
Domain walls (DWs) in ferroic materials—ferromagnets, ferroelectrics, ferroelastics—represent the regions where there is a change in order parameter.1 The dimensions, mobility, and internal structure of domain walls continue to yield useful functionality such as magnetic racetrack memory,2 in which the supersonic motion3 of magnetic DWs is driven by spin‐polarized currents. In the last decade, work on ferroelectric oxides has unexpectedly revealed that electrical conductivity,4 or even superconductivity,5 is possible within ferroelectric DWs, despite the fact that ferroelectrics should be good insulators. Thus, far from being an inert barrier between functional ordered regions, the DW in both ferromagnets and ferroelectrics is instead recognized as a functional entity in itself, and is being investigated for applications in which “the wall is the device”.1 In this context, it is of interest to examine other types of ordered materials to probe the nature of DW structure and to look for new functionality.
Whilst most reports on ferroic properties focus on inorganic oxides, molecular systems also offer a rich playground for structural and electronic ordering. For example, in molecular crystals both intramolecular and intermolecular degrees of freedom can be modulated to induce changes in either local point‐group and/or global translational symmetry, as has been demonstrated in organic ferroelectric materials.6 The vibronic phenomenon of thermal spin state switching7 is also well known to cause significant structural reorganization in both small‐molecule transition‐metal complexes8 and solid‐state oxides.9 In spin crossover (SCO) materials, the switching is usually strongly coupled to structural degrees of freedom, with local bond‐length changes of up to 0.2 Å in each metal–donor distance due to depopulation/population of anti‐bonding orbitals during the electron pairing/unpairing process. These local distortions at the molecular scale propagate macroscopically through elastic coupling, resulting in macroscopic changes in lattice parameters.10 The variety of SCO phenomena can be understood in terms of the evolution of the totally symmetric HS fraction order parameter, γ, which may couple to a symmetry‐breaking order parameter driving spin state ordering, η, or to volume and shear strains.11 Such coupling, in turn, can give rise to large anomalies in elastic properties.12 In some SCO crystals, this drives cooperative phase transitions to produce multiple structural phases with spin state ordering over a temperature gradient.13 Such ordering phenomena have been the focus of sustained experimental14 and theoretical11, 15 investigations over the last decade but little is known about the DW architecture in the ordered phases, as in most systems studied so far, spin state ordering results in antiphase boundaries. Herein, we report magnetic, structural, and elastic properties of a new Mn3+ SCO complex, [Mn(3,5‐diBr‐sal2(323))]BPh4, 1, and show that the ferroelastic DWs in one of two spin state ordered phases are mobile in response to shear stress. The DWs detected in complex 1 are distinct from the high‐spin/low‐spin (HS/LS) phase boundary, which develops in crystalline SCO materials across a thermal gradient, and in which spatiotemporal effects can be very effectively followed by optical microscopy.16 Such examples of an isostructural phase transition between LS and HS phases do not correspond to DW formation, rather to a phase boundary. In the isostructural case both HS and LS phases have the same symmetry, so the symmetry‐breaking order parameter is 0 and the HS/LS interface is not a DW. In contrast, in the case of complex 1, the spin state and ferroelastic order parameters are both coupled with strain, making it inevitable that the DWs will contain local variations in spin state, thus realizing a new class of DW architecture.
Results and Discussion
[Mn(3,5‐diBr‐sal2(323))]BPh4, 1, belongs to the [Mn(R‐sal2(323))]+ series of Schiff base complexes, many of which exhibit thermal SCO or stabilization of the rare S=1 state.14f, 17 Dark red crystals of complex 1 were prepared in a one‐pot synthesis, Scheme 1, and magnetic susceptibility in heating and cooling modes over the temperature range 4–300 K was recorded on a SQUID magnetometer in an applied field of 0.1 T, Figure 1 a and Figure S1.
Scheme 1.
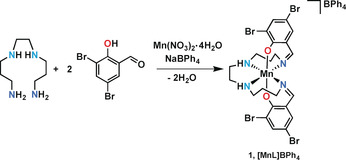
Synthesis of complex 1, [Mn(3,5‐diBr‐sal)2323]BPh4.
Figure 1.
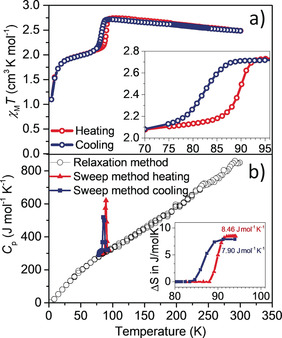
a) Plot of χ M T versus T for complex 1 in cooling (blue curve) and heating (red curve) modes between 4 and 300 K measured at 0.1 T. Inset: 8 K wide hysteretic transition. b) Heat capacity, Cp, versus T of a single crystal of complex 1 measured by two methods, the relaxation method (black circles) and the temperature sweep method (red line, warming; blue line, cooling). The temperature sweep method is sensitive to sharp changes such as first order phase transitions, whereas the relaxation method more accurately determines the magnitude of the heat capacity where it is smoothly varying with temperature. Inset: Entropy change ΔS determined from integration of the peak in the heat capacity: 7.90 J mol−1 K−1 on cooling and 8.46 J mol−1 K−1 on heating.
Plots of χ M T versus T, Figure 1 a, indicate that complex 1 is in its spin quintet form at room temperature. A 9.3 % increase in χ M T was observed on cooling from 300 K (2.49 cm3 mol−1 K) to 95 K (2.72 cm3 mol−1 K), whereupon an abrupt drop to a χ M T value of 2.1 cm3 mol−1 K was observed with a T ↓ value of 82 K, Figure S2. This represents a 50:50 ratio of spin quintet and triplet forms. A further decrease on cooling below circa 20 K is observed, which is attributed to zero‐field splitting. On heating, an abrupt and hysteretic transition was observed with T ↑=90 K.
Thus, we identify a first‐order phase transition related to spin state switching centred at 86 K with a thermal hysteresis window of 8 K. The width of the hysteresis is of the same order of magnitude as reported for other Mn3+ SCO complexes with an N4O2 2− ligand donor set.14f, 17c The transition at circa 86 K was accompanied by a change in entropy of circa 8 J mol−1 K−1, obtained by integration of the peak from heat capacity measurements, Figure 1 b. Such a large entropy change suggests that a significant component of the thermodynamic driving force is configurational, which involves both structural and electronic reorganizations accompanying the SCO behaviour. No influence of magnetic field on the heating branch was observed and only a slight upward shift in the cooling branch by 1.5 K was observed when applying fields of 1 T and 5 T, Figure S3.
Resonant ultrasound spectroscopy (RUS) revealed that three structural phases emerge over the temperature interval of the SCO, including a structural state that contains ferroelastic twin domains (see below). Single‐crystal diffraction was used to elucidate the structure in each phase and the full transition sequence is Cc→Pc→P1.
At room temperature, complex 1 crystallises in the monoclinic polar space group Cc and data in this high temperature (HT) phase was collected at 293 K and at 250 K, Table S4. The asymmetric unit comprises one full occupancy [MnIIIL]+ cation, which is chelated by a hexadentate trans‐N4O2‐ligand, Figure 2 a and Figure S5. By symmetry, the global polarization in the Cc space group lies on the (a,c) plane. The geometry around the Mn3+ centre can be described as a distorted octahedron even though the bonds involve different atoms, Mn−Namine/imine and Mn−Ophen, with bond lengths in the equatorial plane showing significant elongation, Figure S4 and Table S5. This is consistent with population of the orbital of the anti‐bonding eg* orbitals in the Jahn–Teller distorted S=2 state. The asymmetric unit also contains one disordered BPh4 − counteranion, Figure 2 a and Figures S5 and S6.
Figure 2.
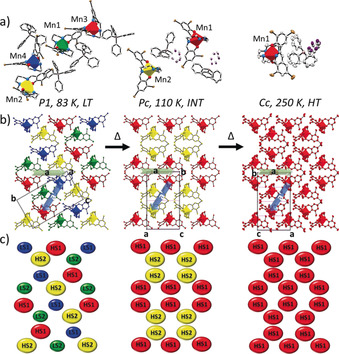
Perspective view of the LT P1 (83 K), INT Pc (110 K) and HT Cc (250 K) structures of complex 1.34 a) Representation of the asymmetric unit with distorted [MnN4O2]+ units shown as polyhedra with colour coding: HS1 (red), HS2 (yellow), LS1 (blue) and LS2 (green). b) The same structures displayed in a layered crystal packing arrangement, showing the relationships between the conventional unit cells of each. c) Simplified representation of the three structures in terms of the Mn atoms alone, with the same colour coding as in (a,b). Atoms are shown at 50 % atomic probability distributions for ellipsoids. BPh4 − anion in (b), (c) and hydrogen atoms are omitted for clarity.
Single‐crystal X‐ray diffraction data collected at different temperatures revealed a slight change in the slope of lattice parameter variations below circa 250 K and an abrupt change below circa 90 K, Figures S30 and S31. Collection of a full data set at intermediate temperatures (INT), 150 K and 110 K, confirmed a change in symmetry by the appearance of reflections not obeying the reflection conditions for the Cc cell, Figures S32 and S33. The structure was refined under monoclinic polar space group Pc and the asymmetric unit in this INT phase comprises two unique [MnIIIL]+ cations, both in the HS S=2 state. There are increases in Mn−Nimine and Mn−Namine bond lengths in both sites compared with the single S=2 site in the structures at 293 K and 250 K. The asymmetric unit in the INT phase also contains two unique disordered BPh4 − counteranions, Figure 2 a and Figures S7 and S8.
The lattice parameters show a steep decrease in a and increases in b and c below the transition at circa 90 K, Figures S30 and S31. A different set of superstructure reflections, characteristic of the loss of the c glide plane, was observed in a full data set collected at 83 K and 25 K, the low‐temperature (LT) phase, indicating that it has a different symmetry from the higher temperature phases. The symmetry decrease requires refining the structure in the space group P1, which is chiral and polar. The unit cell contains four independent [MnIIIL]+ cations and four BPh4 − counteranions, the latter now in a fully ordered configuration, Figure 2 a and Figures S9 and S10. Two of the four Mn3+ cations are in the S=1 state and two are in the S=2 state. There is no indication of a geometric Jahn–Teller effect in the S=1 Mn3+ cations. The Mn−Nimine and Mn−Namine bond lengths in all the measured structures are similar to those of other related Mn3+ S=1 and S=2 complexes, Table S5.14f, 17
Values of ΣMn, Φ, and ζ,18 which define the degree of octahedral distortion in relation to spin state changes, are also higher, as expected, for the S=2 Mn3+ cations in the HT and INT phases (293 K, 250 K, 150 K, 110 K) than for the LT S=1 Mn3+ cations observed at 83 K and 25 K, Table S6.
Only weak hydrogen‐bonding interactions between the Mn3+ complex cation(s) and the BPh4 − counteranion(s) were found in HT (250 K), INT (110 K) and LT (83 K) phases, Figures S20–S25. A full Hirshfeld surface analysis, mapped over d norm, of complex 1, Figures S26–S28, shows that the three main contributions to the intermolecular interactions are H⋅⋅⋅H, H⋅⋅⋅Br and H⋅⋅⋅C with an increase in H⋅⋅⋅Br and a decrease in H⋅⋅⋅H interactions in the LT phase compared to the INT and HT phase, Figure S29. The slight changes in intermolecular interactions may generate a critical elastic energy, which directly affects the spin state causing the hysteretic response between the INT and LT phases. The spin state distribution across the three phases is summarized in Tables S5 and S6 and in the structure diagrams in Figure 2 b,c and Figures S12–S19 in which the striped spin state order of the Mn3+ complex cations in the low‐temperature regime is apparent.
The thermal evolution of complex 1 is unambiguously associated with the two phase transitions in the sequence Cc→Pc→P1. Given the group–subgroup relationship between the Cc and Pc space groups, which have the same translation symmetry (a,b,c),19 the Cc→Pc transition is allowed to be second order. The related order parameter, q, describing the associated structural order, has the symmetry of irreducible representation (irrep) at the border of the Brillouin zone, Y1. This is not the case for the transition between the Pc and P1 phases because there is no group–subgroup relationship; some translation symmetry operator exists in the Pc phase and not in the P1 phase and vice versa, Figure 2. Such a reconstructive phase transition must be first order.
Lattice distortions associated with structural, magnetic, electronic or any other phase transition between structures that have a group–subgroup relationship depend formally on coupling between a thermodynamic order parameter, q, and components of a second rank strain tensor, [e i].20 The lowest order coupling terms permitted by symmetry are λi ei q 2, i=1,2,3,5, and λi ei 2 q 2, i=4,6, for Cc→Pc. Coupling of the form λi ei q 2 gives ei∝q 2.20 Strain variations calculated from lattice parameter data in Figure S36 reveal unambiguously that q 2 for the Cc→Pc transition varies continuously through the transition temperature, T c, and has a non‐linear dependence on temperature in the stability field of the Pc structure, Figure 3 a.
Figure 3.
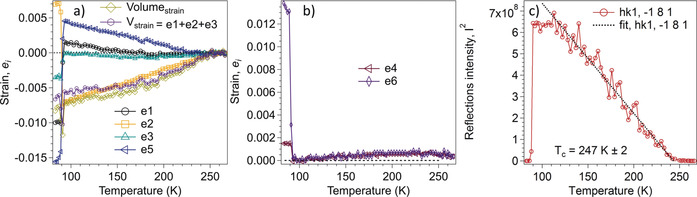
a, b) Temperature dependence of strain components for the Pc and P1 structures, as defined with respect to the parent Cc structure. All the strains vary continuously through the Cc→Pc transition at circa 247 K and discontinuously through the Pc→P1 transition at circa 90 K. c) Variations of the square of the intensity, I k, of the superstructure reflection with hk1=−181, which appears in diffraction patterns from the Pc phase. The data show I k 2∝(T c−T), within experimental uncertainty, and T c=247±2 K.
The precise form of the non‐linearity is highly sensitive to the choice of baseline for the reference states. An alternative approach is to follow by X‐ray diffraction the temperature dependence of the intensity Ik of superlattice reflections associated with the Cc→Pc transition, corresponding to Bragg peaks (hkl) with h+k odd. Figure 3 c shows that below Tc it appears that the fit of I k 2 has linear dependence so I k 2∝(T c−T) and, hence the order parameter scales as, q 4∝(T c−T), indicating that the transition is close to being Landau tricritical in character with T c=247±2 K. By way of contrast, the Pc→P1 transition is very obviously first order, with a large discontinuity at circa 90 K, Figure 3 a–c.
The two phase transitions are also evident in variations in elastic properties obtained by RUS from a small single crystal. This technique is commonly used to investigate phase transitions,21 and has been used once previously for a non‐symmetry‐breaking SCO material.22 The square of the frequencies, f, of mechanical resonance peaks of a single crystal scales with different combinations of elastic moduli. The peak widths at half maximum height provide a measure of acoustic dissipation in the form of the inverse mechanical quality factor, Q −1. A stack of spectra collected from a single crystal during cooling from 295 to 4 K, Figure 4 a, revealed a small shift in the frequency trends of all resonance peaks below circa 250 K. There was then a more marked shift in resonance frequencies below circa 85 K. The widths of individual peaks also increased abruptly below circa 85 K. On heating back up to room temperature from 7 K, Figure 4 d, the resonance peaks returned to the same positions as in the cooling sequence, confirming that the crystal survived the two phase transitions without cracking.
Figure 4.
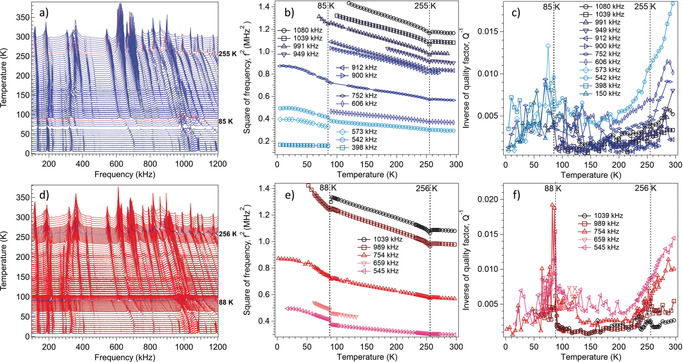
a,d) RUS spectra as a function of frequency for a single crystal of complex 1, stacked up the y‐axis in proportion to the temperature at which they were collected. The y‐axis is really amplitude in volts but has been relabelled as temperature. Spectra were collected during (a) cooling and (d) heating between circa 295 K and circa 4 K. The highlighted red lines (cooling) and blue lines (heating) indicate the expected location of the transitions at circa 255 K and circa 85 K. b,e) f 2 and c,f) Q −1 data from fitting of selected resonances with an asymmetric Lorentzian function, showing the continuous structural phase transition at circa 255 K and the discontinuous transition at circa 85 K.
It is well understood that changes in the elastic constants of single crystals at phase transitions depend primarily on the form and strength of the coupling between the driving order parameter and strain.23 Coupling of the form λi ei q 2 is expected to give rise to discontinuous softening as the crystal is cooled through the transition temperature of a classical displacive transition, with the magnitude of the effect depending sensitively on λi 2. However, the Cc→Pc transition is marked by a slight minimum in f 2 followed by an increase in the slope of the stiffening trend with falling temperature. The Pc→P1 transition is marked by a small discontinuity and a larger increase in slope of the stiffening trend, Figure 4 b,e. Such stiffening occurs either when the values of λi are negligibly small, which is not the case here, or when the time required for relaxation of the order parameter in response to a strain induced by some external stress is short in comparison with the timescale of the applied stress. Changes in the elastic constants during the Pc→P1 transition can be attributed to the partial spin state conversion due to the stronger bonding nature of the LS state.
As shown in Figure 5, variations of f 2 for the Pc phase with respect to values extrapolated linearly from the stability field of the Cc structure, expressed as Δf 2, have nonlinear form similar to variations of the strains, that is, ei∝Δf 2∝q 2. This confirms that relaxation of the order parameter requires times greater than circa 10−6 s, given that the observed resonance frequencies are circa 105–106 Hz. The pattern of acoustic loss also adds insights into the nature of the phase transitions. A normal expectation for displacive transitions is that Q −1 values, Figure 4 c,f, are low in the high‐symmetry phase, with the possibility of a peak at the transition point marking a critical slowing down of the atomic motions responsible for the transitions, and high in the low‐symmetry phase if there is a loss mechanism involving a transformation microstructure such as ferroelastic twin walls.21
Figure 5.
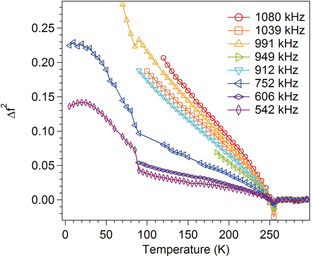
Variations of changes in f 2 with respect to values for the Cc structure obtained by extrapolation of linear fits to data collected above 255 K.
Instead, the steep reduction in values of Q −1 as the Cc→Pc transition point is approached from above is more reminiscent of the magnetic ordering transition in YMnO3,24 as well as structural transitions involving hydrogen bonding in a metal–organic framework25 and in the mineral lawsonite.26 In each of these cases, the transitions were interpreted as having a significant component of order–disorder character, and this was confirmed by calorimetric measurements in the case of lawsonite.27 Dynamical clustering of ordered regions ahead of the transition contributes to relatively high acoustic losses by coupling with local strains and this disappears below the transition when the ordering is static.
By way of contrast with the Cc→Pc transition, there is a steep increase in Q −1 immediately below the Pc→P1 transition, as would be typical of a ferroelastic transition in which the loss is due to mobility of ferroelastic twin walls in response to an applied stress. Typical examples are (Ca,Sr)TiO3 and Sr(Zr,Ti)O3 perovskites at temperatures below the cubic–tetragonal transition.28 The RUS evidence of acoustic loss is thus that crystals with P1 symmetry contain ferroelastic twins even when they developed by a first‐order transition from the Pc structure, Figure S16, and that the twin walls are at least partially mobile under the influence of external stress. Given that there is coupling between the ferroelastic and spin state order parameters, it is inevitable that these domain walls will contain local variations in the degree of spin state order that also must respond to the external stress.
As described in the introduction, the discovery of mobile ferroelastic DWs in complex 1 is quite distinct from the motion of the HS/LS boundary in isostructural single‐crystal SCO samples16 and instead represents a new phenomenon. It will now be important to establish a method to determine the velocity of DW motion in ferroelastic SCO systems, which will enable meaningful comparison with their ferromagnetic and ferroelectric counterparts. In these latter materials there are marked differences between the magnetic‐field induced supersonic speeds of 750–1000 m s−1 achievable in ferromagnetic thin films29 and nanowires,30 and the much slower motion of ferroelectric DWs, in which velocities are also much more sensitive to sample preparation and orientation.31 Internal DW structure is also typically complex in ferroic materials; in ferromagnets, in which the magnitude of the quantized spins cannot change across the wall, the magnetization is inverted by chiral out‐of‐plane (Néel) or in‐plane (Bloch) rotation of the spins.1 In contrast, ferroelectric DWs are Ising‐like as the non‐quantized polarization axis can vary in size and gradually reverse its sign.1 It will therefore be of interest to further probe the internal structure of ferroelastic DWs in SCO crystals, nanomaterials7a and films32 using advanced imaging techniques suitable for different physical scales.8
Conclusion
In summary, we have demonstrated formation of mobile ferroelastic twin walls in a Mn3+ SCO crystal with strong coupling between spin state and elastic order parameters. The spin quintet form of Mn3+ SCO compounds exhibits a pronounced Jahn–Teller effect, which can be easily injected into or removed from the lattice by changing the spin state via thermal or other perturbations. This large change in structural distortion is likely to have contributed to the considerable elastic strain in the Pc→P1 transition. As spin state switching results in a change in both magnetization, through a change in the overall spin state of the transition metal ion, and large atomic displacements, such compounds are ideal for magnetoelectric applications. These include, for example, data storage devices with an electrical input and magnetic read‐out, which would avoid the problems of reading ferroelectric random access memory (FeRAM).33 In the case of complex 1, all three structural phases are polar and therefore potentially ferroelectric. Thus, it is of interest to explore these aspects and to investigate the potential for ferroelectric ordering and DW conductivity in our ongoing studies on this and related materials.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Supplementary
Supplementary
Supplementary
Acknowledgements
We thank Science Foundation Ireland (SFI) for generous support via an Investigator Project Award (12/IP/1703 to G.G.M.). This research was also supported by the Irish Research Council GOIPG/2016/73 fellowship (V.B.J.). Travel grants for research visits to LANL, Cambridge and Rennes for V.B.J. were funded by Augustinus Fonden (grant no. 18‐0338), Oticon Fonden (grant no. 17‐3813), Reinholdt W. Jorck og Hustrus Fond (grant no. 18‐JI‐0573), P.A. Fiskers Fond, A.P. Møller og Hustru Chastine Mc‐Kinney Møllers Fond til almene Formaal and Christian og Ottilia Brorsons Rejselegat for yngre videnskabsmænd og kvinder. RUS facilities in Cambridge were funded by grants to M.A.C. from the Natural Environment Research Council of Great Britain (grant nos. NE/B505738/1 and NE/F17081/1) and from the Engineering and Physical Sciences Research Council (grant no. EP/I036079/1). The NHMFL facility at LANL is funded by the U.S. National Science Foundation through Cooperative Grant No. DMR‐1157490, the State of Florida, and the U.S. Department of Energy. Scientific work at LANL was supported by the Laboratory‐Directed Research and Development program (LDRD) followed by the Center for Molecular Magnetic Quantum Materials (M2QM), an Energy Frontier Research Center funded by the US Department of Energy, Office of Science, Basic Energy Sciences under Award DE SC0019330.
V. B. Jakobsen, E. Trzop, L. C. Gavin, E. Dobbelaar, S. Chikara, X. Ding, K. Esien, H. Müller-Bunz, S. Felton, V. S. Zapf, E. Collet, M. A. Carpenter, G. G. Morgan, Angew. Chem. Int. Ed. 2020, 59, 13305.
Contributor Information
Dr. Vibe B. Jakobsen, https://people.ucd.ie/grace.morgan.
Prof. Eric Collet, Email: eric.collet@univ-rennes1.fr.
Prof. Michael A. Carpenter, Email: mc43@cam.ac.uk.
Prof. Grace G. Morgan, Email: grace.morgan@ucd.ie.
References
- 1. Catalan G., Seidel J., Ramesh R., Scott J. F., Rev. Mod. Phys. 2012, 84, 119–156. [Google Scholar]
- 2. Parkin S. S., Hayashi M., Thomas L., Science 2008, 320, 190–194. [DOI] [PubMed] [Google Scholar]
- 3.
- 3a. Demokritov S. O., Kirilyuk A. I., Kreines N. M., Kudinov V. I., Smirnov V. B., Chetkin M. V., JETP Lett. 1988, 48, 294; [Google Scholar]
- 3b. Demokritov S. O., Kirilyuk A. I., Kreines N. M., Kudinov V. I., Smirnov V. B., Chetkin M. V., J. Magn. Magn. Mater. 1991, 102, 339–353. [Google Scholar]
- 4. Seidel J., Martin L. W., He Q., Zhan Q., Chu Y. H., Rother A., Hawkridge M. E., Maksymovych P., Yu P., Gajek M., Balke N., Kalinin S. V., Gemming S., Wang F., Catalan G., Scott J. F., Spaldin N. A., Orenstein J., Ramesh R., Nat. Mater. 2009, 8, 229–234. [DOI] [PubMed] [Google Scholar]
- 5. Mostovoy M., Phys. Rev. Lett. 2006, 96, 067601. [DOI] [PubMed] [Google Scholar]
- 6.
- 6a. Horiuchi S., Tokura Y., Nat. Mater. 2008, 7, 357–366; [DOI] [PubMed] [Google Scholar]
- 6b. Zhang W., Xiong R.-G., Chem. Rev. 2012, 112, 1163–1195; [DOI] [PubMed] [Google Scholar]
- 6c. Horiuchi S., Tokunaga Y., Giovannetti G., Picozzi S., Itoh H., Shimano R., Kumai R., Tokura Y., Nature 2010, 463, 789–792; [DOI] [PubMed] [Google Scholar]
- 6d. Naber R. C. G., Asadi K., Blom P. W. M., de Leeuw D. M., de Boer B., Adv. Mater. 2010, 22, 933–945. [DOI] [PubMed] [Google Scholar]
- 7.
- 7a. Bousseksou A., Molnár G., Salmon L., Nicolazzi W., Chem. Soc. Rev. 2011, 40, 3313–3335; [DOI] [PubMed] [Google Scholar]
- 7b. Halcrow M. A., Spin crossover Materials, Wiley, Hoboken, 2013; [Google Scholar]
- 7c. Lefter C., Davesne V., Salmon L., Molnár G., Demont P., Rotaru A., Bousseksou A., Magnetochemistry 2016, 2, 18; [Google Scholar]
- 7d. Gütlich P., Garcia Y., Goodwin H. A., Chem. Soc. Rev. 2000, 29, 419–427; [Google Scholar]
- 7e. Gütlich P., Gaspar A. B., Garcia Y., Beilstein J. Org. Chem. 2013, 9, 342–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Collet E., Guionneau P., C. R. Chim. 2018, 21, 1133–1151. [Google Scholar]
- 9.
- 9a. Rao C. N. R., Seikh M. M., Narayana C., Top. Curr. Chem. 2004, 234, 1–21; [Google Scholar]
- 9b. Zhou J.-S., Yan J.-Q., Goodenough J. B., Phys. Rev. B 2005, 71, 220103; [Google Scholar]
- 9c. Guo E.-J., Desautels R., Keavney D., Roldan M. A., Kirby B. J., Lee D., Liao Z., Charlton T., Herklotz A., Zac Ward T., Fitzsimmons M. R., Lee H. N., Sci. Adv. 2019, 5, eaav5050; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9d. Vogt T., Hriljac J. A., Hyatt N. C., Woodward P., Phys. Rev. B 2003, 67, 140401; [Google Scholar]
- 9e. Khalifah P., Osborn R., Huang Q., Zandbergen H. W., Jin R., Liu Y., Mandrus D., Cava R. J., Science 2002, 297, 2237–2240. [DOI] [PubMed] [Google Scholar]
- 10.
- 10a. Nishino M., Boukheddaden K., Konishi Y., Miyashita S., Phys. Rev. Lett. 2007, 98, 247203; [DOI] [PubMed] [Google Scholar]
- 10b. Enachescu C., Nishino M., Miyashita S., Boukheddaden K., Varret F., Rikvold P. A., Phys. Rev. B 2015, 91, 104102; [Google Scholar]
- 10c. Traiche R., Sy M., Boukheddaden K., J. Phys. Chem. C 2018, 122, 4083–4096; [Google Scholar]
- 10d. Miyashita S., Konishi Y., Nishino M., Tokoro H., Rikvold P. A., Phys. Rev. B 2008, 77, 014105; [DOI] [PubMed] [Google Scholar]
- 10e. Nakada T., Mori T., Miyashita S., Nishino M., Todo S., Nicolazzi W., Rikvold P. A., Phys. Rev. B 2012, 85, 054408; [Google Scholar]
- 10f. Nishino M., Miyashita S., Phys. Rev. B 2013, 88, 014108; [Google Scholar]
- 10g. Enachescu C., Stoleriu L., Nishino M., Miyashita S., Stancu A., Lorenc M., Bertoni R., Cailleau H., Collet E., Phys. Rev. B 2017, 95, 224107; [Google Scholar]
- 10h. Rikvold P. A., Brown G., Miyashita S., Omand C., Nishino M., Phys. Rev. B 2016, 93, 064109. [Google Scholar]
- 11. Watanabe H., Tanaka K., Bréfuel N., Cailleau H., Létard J.-F., Ravy S., Fertey P., Nishino M., Miyashita S., Collet E., Phys. Rev. B 2016, 93, 014419. [Google Scholar]
- 12.
- 12a. Singh Y., Oubouchou H., Nishino M., Miyashita S., Boukheddaden K., Phys. Rev. B 2020, 101, 054105; [Google Scholar]
- 12b. Affes K., Fourati H., Slimani A., Boukheddaden K., J. Phys. Soc. Jpn. 2019, 88, 124701; [Google Scholar]
- 12c. Cruddas J., Powell B. J., J. Am. Chem. Soc. 2019, 141, 19790–19799. [DOI] [PubMed] [Google Scholar]
- 13.
- 13a. Chernyshov D., Hostettler M., Tornroos K. W., Burgi H. B., Angew. Chem. Int. Ed. 2003, 42, 3825–3830; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2003, 115, 3955–3960; [Google Scholar]
- 13b. Shatruk M., Phan H., Chrisostomo B. A., Suleimenova A., Coord. Chem. Rev. 2015, 289–290, 62–73. [Google Scholar]
- 14.
- 14a. Trzop E., Zhang D., Piñeiro-Lopez L., Valverde-Muñoz F. J., Muñoz M. C., Palatinus L., Guerin L., Cailleau H., Real J. A., Collet E., Angew. Chem. Int. Ed. 2016, 55, 8675–8679; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 8817–8821; [Google Scholar]
- 14b. Bonnet S., Siegler M. A., Costa J. S., Molnár G., Bousseksou A., Spek A. L., Gamez P., Reedijk J., Chem. Commun. 2008, 5619–5621; [DOI] [PubMed] [Google Scholar]
- 14c. Griffin M., Shakespeare S., Shepherd H. J., Harding C. J., Létard J.-F., Desplanches C., Goeta A. E., Howard J. A. K., Powell A. K., Mereacre V., Garcia Y., Naik A. D., Müller-Bunz H., Morgan G. G., Angew. Chem. Int. Ed. 2011, 50, 896–900; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 926–930; [Google Scholar]
- 14d. Murnaghan K. D., Carbonera C., Toupet L., Griffin M., Dîrtu M. M., Desplanches C., Garcia Y., Collet E., Létard J.-F., Morgan G. G., Chem. Eur. J. 2014, 20, 5613–5618; [DOI] [PubMed] [Google Scholar]
- 14e. Vieira B. J. C., Coutinho J. T., Santos I. C., Pereira L. C. J., Waerenborgh J. C., da Gama V., Inorg. Chem. 2013, 52, 3845–3850; [DOI] [PubMed] [Google Scholar]
- 14f. Fitzpatrick A. J., Trzop E., Müller-Bunz H., Dîrtu M. M., Garcia Y., Collet E., Morgan G. G., Chem. Commun. 2015, 51, 17540–17543; [DOI] [PubMed] [Google Scholar]
- 14g. Boonprab T., Lee S. J., Telfer S. G., Murray K. S., Phonsri W., Chastanet G., Collet E., Trzop E., Jameson G. N. L., Harding P., Harding D. J., Angew. Chem. Int. Ed. 2019, 58, 11811–11815; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 11937–11941. [Google Scholar]
- 15.
- 15a. Paez-Espejo M., Sy M., Boukheddaden K., J. Am. Chem. Soc. 2016, 138, 3202–3210; [DOI] [PubMed] [Google Scholar]
- 15b. Ndiaye M., Boukheddaden K., J. Phys. Soc. Jpn. 2020, 89, 014004; [Google Scholar]
- 15c. Nishino M., Miyashita S., Phys. Rev. B 2015, 92, 184404; [Google Scholar]
- 15d. Nishino M., Enachescu C., Miyashita S., Phys. Rev. B 2019, 100, 134414; [Google Scholar]
- 15e. Nakada T., Rikvold P. A., Mori T., Nishino M., Miyashita S., Phys. Rev. B 2011, 84, 054433; [Google Scholar]
- 15f. Miyashita S., Konishi Y., Tokoro H., Nishino M., Boukheddaden K., Varret F., Prog. Theor. Exp. Phys. 2005, 114, 719–735. [Google Scholar]
- 16.
- 16a. Sy M., Traiche R., Fourati H., Singh Y., Varret F., Boukheddaden K., J. Phys. Chem. C 2018, 122, 20952–20962; [Google Scholar]
- 16b. Fourati H., Milin E., Slimani A., Chastanet G., Abid Y., Triki S., Boukheddaden K., Phys. Chem. Chem. Phys. 2018, 20, 10142–10154; [DOI] [PubMed] [Google Scholar]
- 16c. Slimani A., Varret F., Boukheddaden K., Chong C., Mishra H., Haasnoot J., Pillet S., Phys. Rev. B 2011, 84, 094442; [Google Scholar]
- 16d. Chong C., Slimani A., Varret F., Boukheddaden K., Collet E., Ameline J.-C., Bronisz R., Hauser A., Chem. Phys. Lett. 2011, 504, 29–33; [Google Scholar]
- 16e. Slimani A., Varret F., Boukheddaden K., Garrot D., Oubouchou H., Kaizaki S., Phys. Rev. Lett. 2013, 110, 087208; [DOI] [PubMed] [Google Scholar]
- 16f. Sy M., Varret F., Boukheddaden K., Bouchez G., Marrot J., Kawata S., Kaizaki S., Angew. Chem. Int. Ed. 2014, 53, 7539–7542; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 7669–7672; [Google Scholar]
- 16g. Varret F., Slimani A., Boukheddaden K., Chong C., Mishra H., Collet E., Haasnoot J., Pillet S., New J. Chem. 2011, 35, 2333–2340; [Google Scholar]
- 16h. Boukheddaden K., Loutete-Dangui E. D., Codjovi E., Castro M., Rodriguéz-Velamazán J. A., Ohkoshi S., Tokoro H., Koubaa M., Abid Y., Varret F., J. Appl. Phys. 2011, 109, 013520; [Google Scholar]
- 16i. Paez-Espejo M., Sy M., Varret F., Boukheddaden K., Phys. Rev. B 2014, 89, 024306; [Google Scholar]
- 16j. Traiche R., Sy M., Oubouchou H., Bouchez G., Varret F., Boukheddaden K., J. Phys. Chem. C 2017, 121, 11700–11708; [Google Scholar]
- 16k. Slimani A., Boukheddaden K., Varret F., Oubouchou H., Nishino M., Miyashita S., Phys. Rev. B 2013, 87, 014111. [DOI] [PubMed] [Google Scholar]
- 17.
- 17a. Morgan G. G., Murnaghan K. D., Müller-Bunz H., McKee V., Harding C. J., Angew. Chem. Int. Ed. 2006, 45, 7192–7195; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2006, 118, 7350–7353; [Google Scholar]
- 17b. Pandurangan K., Gildea B., Murray C., Harding C. J., Müller-Bunz H., Morgan G. G., Chem. Eur. J. 2012, 18, 2021–2029; [DOI] [PubMed] [Google Scholar]
- 17c. Martinho P. N., Gildea B., Harris M. M., Lemma T., Naik A. D., Müller-Bunz H., Keyes T. E., Garcia Y., Morgan G. G., Angew. Chem. Int. Ed. 2012, 51, 12597–12601; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 12765–12769; [Google Scholar]
- 17d. Gildea B., Harris M. M., Gavin L. C., Murray C. A., Ortin Y., Müller-Bunz H., Harding C. J., Lan Y., Powell A. K., Morgan G. G., Inorg. Chem. 2014, 53, 6022–6033; [DOI] [PubMed] [Google Scholar]
- 17e. Barker A., Kelly C. T., Kühne I. A., Hill S., Krzystek J., Wix P., Esien K., Felton S., Müller-Bunz H., Morgan G. G., Dalton Trans. 2019, 48, 15560–15566; [DOI] [PubMed] [Google Scholar]
- 17f. Jakobsen V. B., O'Brien L., Novitchi G., Müller-Bunz H., Barra A.-L., Morgan G. G., Eur. J. Inorg. Chem. 2019, 4405–4411. [Google Scholar]
- 18.
- 18a. Drew M. G. B., Harding C. J., McKee V., Morgan G. G., Nelson J., J. Chem. Soc. Chem. Commun. 1995, 1035–1038; [Google Scholar]
- 18b.R. Ketkaew, Y. Tantirungrotechai, D. J. Harding, P. Harding, M. Marchivie, OctaDist: A tool for calculating distortion parameters in coordination complexes. https://octadist.github.io. [DOI] [PubMed]
- 19. Hatch D. M., Stokes H. T., Isotropy Subgroups Of The 230 Crystallographic Space Groups, World Scientific, Singapore, 1989. [Google Scholar]
- 20. Carpenter M. A., Salje E. K. H., Graeme-Barber A., Eur. J. Mineral. 1998, 10, 621–691. [Google Scholar]
- 21. Carpenter M. A., J. Phys. Condens. Matter 2015, 27, 263201. [DOI] [PubMed] [Google Scholar]
- 22. Mason H. E., Li W., Carpenter M. A., Hamilton M. L., Howard J. A. K., Sparkes H. A., New J. Chem. 2016, 40, 2466–2478. [Google Scholar]
- 23. Carpenter M. A., Salje E. K. H., Eur. J. Mineral. 1998, 10, 693–812. [Google Scholar]
- 24. Thomson R. I., Chatterji T., Howard C. J., Palstra T. T., Carpenter M. A., J. Phys. Condens. Matter 2014, 26, 045901. [DOI] [PubMed] [Google Scholar]
- 25. Zhang Z., Li W., Carpenter M. A., Howard C. J., Cheetham A. K., CrystEngComm 2015, 17, 370–374. [Google Scholar]
- 26. McKnight R. E. A., Carpenter M. A., Darling T. W., Buckley A., Taylor P. A., Am. Mineral. 2007, 92, 1665–1672. [Google Scholar]
- 27. Martín-Olalla J.-M., Hayward S. A., Meyer H.-W., Ramos S., Cerro J. D., Carpenter M. A., Eur. J. Mineral. 2001, 13, 5–14. [Google Scholar]
- 28.
- 28a. McKnight R. E. A., Howard C. J., Carpenter M. A., J. Phys. Condens. Matter 2009, 21, 015901; [DOI] [PubMed] [Google Scholar]
- 28b. McKnight R. E. A., Kennedy B. J., Zhou Q., Carpenter M. A., J. Phys. Condens. Matter 2009, 21, 015902; [DOI] [PubMed] [Google Scholar]
- 28c. Perks N. J., Zhang Z., Harrison R. J., Carpenter M. A., J. Phys. Condens. Matter 2014, 26, 505402. [DOI] [PubMed] [Google Scholar]
- 29. Yang S.-H., Ryu K.-S., Parkin S., Nat. Nanotechnol. 2015, 10, 221–226. [DOI] [PubMed] [Google Scholar]
- 30. Hertel R., Andreas C., Magnetic Nano- and Microwires (Ed.: M. Vázquez), Woodhead Publishing, Sawston, 2015, pp. 653–677. [Google Scholar]
- 31.
- 31a. McGilly L. J., Feigl L., Sluka T., Yudin P., Tagantsev A. K., Setter N., Nano Lett. 2016, 16, 68–73; [DOI] [PubMed] [Google Scholar]
- 31b. Bakaul S. R., Kim J., Hong S., Cherukara M. J., Zhou T., Stan L., Serrao C. R., Salahuddin S., Petford-Long A. K., Fong D. D., Holt M. V., Adv. Mater. 2020, 32, 1907036. [DOI] [PubMed] [Google Scholar]
- 32. Ruben M., Kumar K. S., Angew. Chem. Int. Ed. 2020, 10.1002/anie.201911256; [DOI] [Google Scholar]; Angew. Chem. 2020, 10.1002/ange.201911256. [DOI] [Google Scholar]
- 33. Eerenstein W., Mathur N. D., Scott J. F., Nature 2006, 442, 759–765. [DOI] [PubMed] [Google Scholar]
- 34. Deposition Number(s) 1971455 (293 K), 1971456 (250 K), 1971457 (150 K), 1971458 (110 K), 1971459 (83 K), and 1971460 (25 K) contain(s) the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Supplementary
Supplementary
Supplementary


