Abstract
Objective
To clarify the detailed pharmacokinetics (PK) of orally administered voriconazole in tear fluid (TF) of horses for evaluating the efficacy of voriconazole secreted into TF against equine keratomycosis.
Animals studied
Five healthy Thoroughbred horses.
Procedures
Voriconazole was administrated through a nasogastric tube to each horse at a single dose of 4.0 mg/kg. TF and blood samples were collected before and periodically throughout the 24 hours after administration. Voriconazole concentrations in plasma and TF samples were analyzed using liquid chromatography‐electrospray tandem‐mass spectrometry. The predicted voriconazole concentration in both samples following multiple dosing every 24 hours was simulated by the superposition principle.
Results
The mean maximum voriconazole concentrations in plasma and TF were 3.3 μg/mL at 1.5 h and 1.9 μg/mL at 1.6 h, respectively. Mean half‐life in both samples were 16.4 and 25.2 h, respectively. The ratio of predicted AUC0–24 at steady state in TF (51.3 μg∙h/mL) to previously published minimum inhibitory concentration (MIC) of Aspergillus and Fusarium species was >100 and 25.7, respectively.
Conclusions
This study demonstrated the detailed single‐dose PK of voriconazole in TF after oral administration and simulated the predicted concentration curves in a multiple oral dosing. Based on the analyses of PK‐PD, the simulation results indicated that repeated oral administration of voriconazole at 4.0 mg/kg/d achieves the ratio of AUC to MIC associated with treatment efficacy against Aspergillus species. The detailed PK‐PD analyses against pathogenic fungi in TF can be used to provide evidence‐based medicine for equine keratomycosis.
Keywords: Aspergillus, horse, keratomycosis, pharmacodynamics, pharmacokinetics, tear fluid
1. INTRODUCTION
Equine keratomycosis is common in warm and humid climates, 1 , 2 , 3 and Aspergillus species are the most common cause in North America, Spain, and Japan. 1 , 2 , 4 , 5 , 6 , 7 , 8 , 9 Equine keratomycosis can lead to vision and/or globe loss; therefore, aggressive medical treatments are required to retain the visual function of affected horses. 9 , 10 , 11 Voriconazole, a broad‐spectrum triazole anti‐fungal drug, is effective against various pathogenic fungi species 12 , 13 , 14 , 15 and is used in the treatment of equine keratomycosis. Voriconazole 1.0% solution effectively penetrates healthy equine cornea after topical administration, 16 and multiple doses at 1 to 4 hours intervals have been suggested as a treatment option for some cases of equine keratomycosis. 17 Although topical administration of voriconazole solution may be performed easily by using a subpalpebral lavage system, continuous rate infusion (CRI) of voriconazole solution using a CRI pumps is not recommended because voriconazole becomes unstable owing to the high temperature in CRI pumps. 18 Furthermore, during long treatment periods for equine keratomycosis, use of a CRI system or frequent topical administration may become difficult because of CRI device‐related issues and/or horses being fractious. 17 , 19 Oral administration of voriconazole achieves excellent systemic bioavailability, 20 and, based on published minimum inhibitory concentration (MIC) results, dosing at 4.0 mg/kg once daily achieves adequate plasma concentrations for the treatment of systemic aspergillosis in horses. 16 , 21 , 22 Oral administration of voriconazole would be one of the treatment options for keratomycosis because it has been reported that voriconazole was secreted into the tear fluid (TF) after oral administration. 20 , 21
Several orally administered anti‐microbial drugs are secreted at sufficient concentrations into the TF to effectively treat corneal and conjunctival diseases. 23 , 24 , 25 For these drugs, the relationship between pharmacokinetics (PK) and pharmacodynamic (PD) parameters in both the TF and plasma permits determination of the optimal dosing schedule. 26 , 27 Although orally administered voriconazole reaches detectable TF concentrations, 20 , 21 the PK‐PD parameters of oral administration as related to TF and plasma concentrations are unclear. Knowledge of the PK and PD of this anti‐fungal agent in TF would enable a more effective treatment of equine keratomycosis.
We aimed to clarify the detailed PK of voriconazole in TF after a single oral administration in healthy adult Thoroughbred horses and to infer the PK following multiple doses. An additional objective was to evaluate the efficacy of voriconazole against equine keratomycosis based on PK‐PD analyses in oral administration.
2. MATERIALS AND METHODS
2.1. Horses
Five healthy adult Thoroughbred horses with normal eyes —one female, three males, and one gelding—were used, with ages ranging from 3 to 5 years and bodyweights, which were weighed with a horse weight scale, ranging from 395 to 475 kg and all horses were examined with a physical examination, a complete blood cell count (Nihon Kohden) and serum biochemical analysis (Fujifilm). No horses included in this study had signs of ocular disease as determined by ophthalmic examinations with slit‐lamp biomicroscopy (Welch Allyn Coaxial ophthalmoscopes, Welch Allyn)and fluorescein staining (AYUMI Pharmaceutical Corporation). All examinations were performed by two veterinarians (A.O and N.T). The same examinations including a physical examination, a complete blood cell count, serum biochemical analysis, and the ophthalmic examinations were also performed immediately after drug administration and 3 hours after final sample collection.
Horses were housed in separate stalls and fed typical rations of grass hay. Food was withheld 12 hours before and 4 hours after drug administration. All horses had access to freshwater ad libitum throughout the study. This study adhered to the Association for Research in Vision and Ophthalmology statement for the Use of Animals in Ophthalmic and Vision Research. All study protocols were approved by the Animal Care Committee of the Equine Research Institute of the Japan Racing Association (approval number 18 301T3 0 200 075).
2.2. Drug administration
Voriconazole tablets (Vfend; Pfizer Inc) were crushed into a powder. The drug was accurately weighed at a dose of 4 mg/kg for each horse with a calibrated electronic scale and administered through a nasogastric tube with 500 mL of distilled water. The tube was further rinsed with 500 mL of distilled water to ensure that the drug reached the stomach in the horse.
2.3. Sample collection
Blood samples (8 mL) were collected into lithium heparin tubes via a 14‐G catheter inserted in the jugular vein at 0 min (before drug administration), 5, 10, 20, 30, 40, and 50 min and 1, 1.25, 1.5, 2, 3, 4, 8, 12, and 24 hours after administration. Blood samples were centrifuged for 10 minutes at 2000 g to harvest the plasma, and all plasma samples were stored at −80°C until further analysis.
Tear fluid was collected by placing a filter paper strip cut with sterile scissors (20 × 8 mm) on the conjunctival sac in the right and left eyes at 0, 1, 2, 4, 8, 12, and 24 hours after administration. These sampling procedures were performed on both eyes within 5 minutes of each other. The filter paper strip was removed and transferred to a collection tube in which a pipette tip was placed. The filter paper was placed above the pipette tip in the collection tube. The pipette tip worked as a base for supporting the filter paper in centrifugal extraction. TF samples were harvested from the filter paper by centrifuging the collection tubes for 15 minutes at 3000 g at 4°C. Tear film samples harvested from both eyes of the same horse at the same time point were combined at all sampling times, resulting in seven total samples from each horse. Each TF sample was weighted using an electronic scale and stored at −80°C until further analysis. The stability of voriconazole against freezing has been reported in the previous study. 28 In our preliminary experiment, the voriconazole absorbed on the filter paper was sufficiently recovered by this procedure.
2.4. Sample preparation
All samples were assayed within 1 month from collection. To analyze concentrations of voriconazole, 0.1 mL each of the plasma or TF samples were placed into glass tubes and mixed with 0.3 mL of internal standard solution comprising 1.0 μg/mL of diazepam(Sigma‐Aldrich Co.) in pure water. The extraction of voriconazole from both samples was performed by liquid‐liquid extraction with tert‐butyl methyl ether. Tert‐butyl methyl ether (3 mL) was added to each tube. After agitation for 5 minutes using a vortex mixer, tubes were centrifuged for 5 min at 2200 g. Supernatants were transferred into new glass tubes, dried at 40°C under nitrogen gas, and then resuspended with 1.0 mL of 50% acetonitrile solution. Each sample was transferred into an autosampler vial, and 5.0 μL of each sample was injected into the liquid chromatography‐electrospray tandem‐mass spectrometer (LC‐ MS/MS).
2.5. Liquid chromatography‐electrospray tandem‐mass spectrometer
Concentrations of voriconazole in the plasma and TF samples were quantified using LC‐MS/MS that was performed with an AB Sciex QTRAP 4000 LC‐MS/MS system (AB Sciex) equipped with a Shimadzu Prominence HPLC system (Shimadzu Corp.). The mobile phase comprised 0.1% formic acid (A) and acetonitrile (B). The analytical column used was the Atlantis T3 (3.0 μm × 50 mm). Chromatographic separation of analytes was performed with gradient elution with increasing mobile phase B (20%‐95% from 0 to 5 min) at a flow rate of 0.2 mL/min. Selected reaction monitoring in the positive ion mode was used to monitor transitions at m/z 350 → 281 for voriconazole and m/z 285 → 193 for the diazepam standard.
2.6. Calibration and validation
Since it was difficult to collect TF sufficiently, physiological saline was applied to the blank solution of calibration and validation in the TF analysis. Standard calibration curves for the plasma and TF samples were prepared by adding voriconazole standard (Sigma‐Aldrich Co.) to blank horse plasma and physiological saline, respectively. The quantification linear range was 0.1‐5.0 μg/mL. The calibration curve was constructed by linear regression analysis using peak area ratios (peak area of voriconazole divided by the internal standard) against concentrations of standards, and the coefficient of correlation for the curve was >0.999. All standard and unknown sample analyses were conducted in duplicate.
2.7. Pharmacokinetics and pharmacodynamics analysis
Pharmacokinetics parameters of voriconazole in plasma and TF samples for each horse were calculated with a noncompartment analysis using Phoenix WinNonlin Version 6.3 (Pharsight). The maximum concentration (C max) and the time to C max (T max) of voriconazole in the plasma and TF samples were directly obtained from the concentration curves. AUC from time 0 to 24 hours (AUC0–24), AUC from time 0 to infinity (AUC0–inf), and the area under the first moment curve from time 0 to infinity (AUMC) for the plasma and TF samples were calculated using the trapezoidal method. The mean residence time (MRT) was calculated by AUMC/AUC. The elimination rate constant in TF (kel) was estimated from the terminal phase of the concentration‐time curve using log‐linear regression, and the elimination half‐life (t1/2β) was calculated as loge2/kel.
Predicted concentration curves of voriconazole in plasma and TF following multiple dosing at an oral dose of 4.0 mg/kg every 24 hours were simulated by the superposition principle because the time vs. concentration of voriconazole in plasma and TF showed a high correlation and the disposition processes of drug were considered a linear regression.
Voriconazole at 0.5 μg/mL was used as a target therapeutic concentration for Aspergillus species 16 , 20 , 22 as previous susceptibility studies reported that, at or below that concentration, voriconazole inhibited more than 90% of the 376 isolates tested. 29 In contrast, a voriconazole concentration of 2.0 μg/mL was used as a target therapeutic concentration for Fusarium species based on MIC50 and MIC90 data from previous susceptibility studies utilizing isolates from human keratitis patients. 12 , 30
3. RESULTS
No adverse effects such as a loss of appetite, gastrointestinal dysfunctions, or ocular dysfunctions were observed in the horses. The systemic findings including a complete blood cell count and serum biochemical analysis remained within the reference intervals.
Mean (±SD) recoveries of voriconazole from plasma and TF samples were 92.6 ± 4.48% and 93.5 ± 2.20% for 0.2 μg/mL, 98.3 ± 1.81% and 93.3 ± 1.19% for 2.0 μg/mL, respectively. The intra‐, inter‐day precisions and accuracy for the plasma sample were 4.83%, 5.25% and 92.6%, 82.6% at 0.2 µg/mL and 1.85%, 7.84% and 98.3%, 93.3% at 2.0 µg/mL, respectively. The intra‐, inter‐day precisions and accuracy for the TF sample were 2.90%, 3.70% and 113%, 113% at 0.2 µg/mL and 1.53%, 1.85% and 106%, 107% at 2.0 µg/mL, respectively.
Concentrations of voriconazole measured in the plasma and TF after the single oral administration are shown in Figure 1. The total volume of TF per sample ranged from 0.3 to 1.6 mL (mean, 1.16 mL). The drug was quantifiable in the plasma and TF of all horses at 20 min and 1 h after administration, respectively. The voriconazole concentrations in both samples peaked within 1‐2 hours and then progressively decreased, but the concentrations remained above 1.0 µg/mL until the final sampling point. The TF to plasma concentration ratio (TF/P) was approximately 0.7 during the first 2 hours and increased thereafter. PK parameters of voriconazole in the plasma and TF are summarized in Table 1. The PK parameters of TF had longer t 1/2β, lower C max, and similar AUC0–inf compared with that of plasma.
Figure 1.
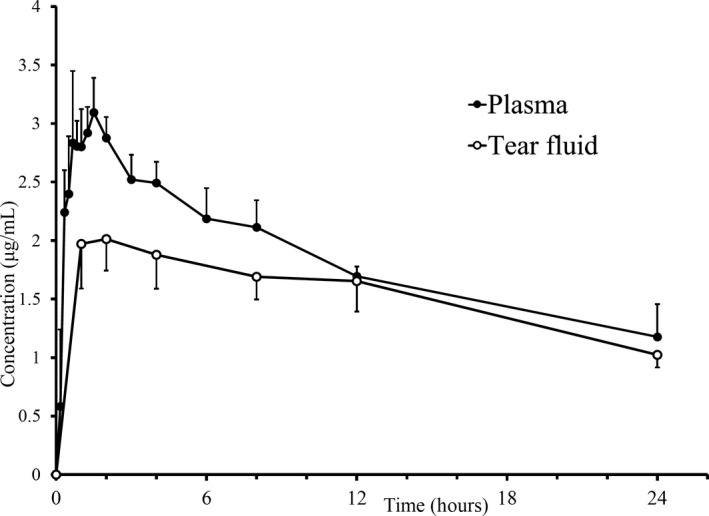
Voriconazole concentration (mean ± SD) in plasma (solid circles) and tear fluid (open circles) samples after single oral administration of 4 mg/kg bodyweight in five healthy Thoroughbred horses. Concentrations were lower in tear fluid than in plasma during the first 4 h after administration (*P < .05)
Table 1.
Pharmacokinetic parameters (mean ± SD) of voriconazole in plasma and tear fluid samples
| Parameters | Plasma | Tear fluid |
|---|---|---|
| C max (μg/mL) | 3.29 ± 0.10 | 1.85 ± 0.26 |
| T max (h) | 1.45 ± 0.37 | 1.60 ± 0.55 |
| t 1/2α (h) | 0.25 ± 0.08 | NA |
| t 1/2β (h) | 16.4 ± 4.37 | 25.2 ± 9.19 |
| AUC0–24 (μg∙h/mL) | 44.0 ± 3.89 | 36.8 ± 4.54 |
| AUC0–inf (μg∙h/mL) | 73.3 ± 19.6 | 74.8 ± 17.0 |
| AUMC (μg∙h2/mL) | 1916 ± 1074 | 2868 ± 1827 |
| MRT (h) | 24.7 ± 6.75 | 36.2 ± 13.3 |
C max and T max, maximum concentration and time to C max, respectively; t 1/2α and t 1/2β, absorption and elimination half‐lives, respectively; AUC (AUC0‐24 and AUC0‐inf), area under the concentration vs time curve (AUC from time 0 to 24 h and AUC from time 0 h to infinity, respectively).
Abbreviations: AUMC, area under the first moment curve from time 0 to infinity; MRT, mean residence time; NA, not applicable.
The predicted concentration curves of voriconazole in plasma and TF by multiple oral dosing simulations are shown in Figure 2. Since the predicted trough concentration did not change from 3 days after the start of the experiment, concentrations of voriconazole in the plasma and TF were considered to achieve the steady state after the third administration. The mean (±SD) peak and trough concentrations in the steady state were 4.28 ± 0.58 μg/mL and 2.2 ± 0.4 μg/mL in the plasma and 3.10 ± 0.38 μg/mL and 1.2 ± 0.4 μg/mL in the TF, respectively. The predicted AUC0–24 in the steady state was calculated to be 71.1 μg∙h/mL in the plasma and 51.3 μg∙h/mL in the TF. Using 0.5 μg/mL as the target MIC of Aspergillus species, the AUC to MIC ratio (AUC/MIC) for voriconazole in TF after a single administration and after 3 days administration was calculated to be 73.6 and 102.6, respectively. Also, using 2.0 μg/mL as the target MIC of Fusarium species, the AUC/MIC for voriconazole in TF after a single administration and after 3 days administration was calculated to be 18.4 and 25.7, respectively.
Figure 2.
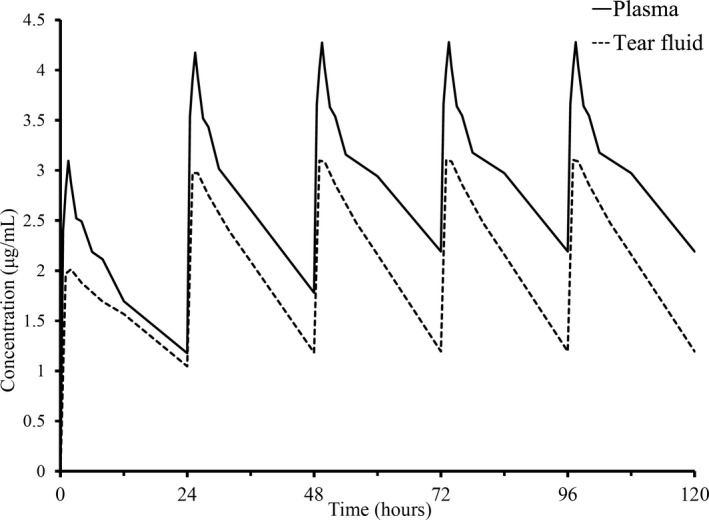
Predicted voriconazole concentrations in plasma (solid line) and tear fluid (dotted line) based on simulated multiple oral administrations of 4 mg/kg bodyweight at 24‐h intervals. Theoretical concentrations of voriconazole in the plasma and TF achieved a steady state after the third administration because the predicted trough concentration from the simulation did not change after day 3 of the first administration
4. DISCUSSION
This is the first detailed study to assess the PK of voriconazole in the TF of healthy Thoroughbred horses after a single oral administration. Further, the predicted drug concentrations in the plasma and TF induced by multiple dosing with administration at 24 hours intervals were demonstrated. PK parameters in the TF have been reported for several drugs 25 , 26 , 27 , 31 and relationships between PK and PD parameters were used to optimize dosing regimens and increase the efficacy of anti‐fungals. 32 Thus, it is important to investigate the PK–PD relationship to effectively treat corneal and conjunctival infections through drug secretion into the TF after oral administration.
Although no adverse effects were observed during this study, visual disturbances are one of the major adverse effects of oral voriconazole administration in humans, 33 and anti‐microbial‐associated enterocolitis is one of the commonest adverse drug effects of anti‐microbials in equine practice. 34 Previous studies describing voriconazole pharmacokinetics in horses reported the absence of any serious adverse effect after oral administration, even at a single dose of 10 or 3 mg/kg twice daily for 10 days. 16 , 20 , 21 , 22 Although longitudinal studies evaluating large populations will be needed to clarify the safety of oral administration of voriconazole, the voriconazole dose used in this study was considered safe.
Voriconazole was detected in the plasma within 20 minutes of its oral administration in all horses. Previous studies have similarly reported rapid increases in plasma concentrations of this drug after oral administration in horses, human beings, dogs, and cats. 20 , 33 , 35 , 36 This has been attributed to the high solubility and permeability of voriconazole. 33 It was confirmed that voriconazole was absorbed rapidly in healthy horses after a single oral administration in this study.
Here, there were differences in concentrations of voriconazole between the TF and plasma samples up to 4 hours after oral administration, and the TF/P was approximately 0.7 during the first 2 hours. Drugs secreted in the TF are considered to arise from unbound drug in the plasma, and the plasma protein binding rate of voriconazole is reportedly 32%. 22 Therefore, voriconazole concentrations in TF appear to represent free drug in the plasma during early stages after administration. At time points from 8 hours after administration, the voriconazole concentration in the TF was not different from that in plasma, with TF/P ratios >0.8. The values of t1/2β and MRT in this study indicated that the elimination of voriconazole in TF was slower than that in plasma. Since secreted tears are temporarily stored and excreted from lacrimal puncta anatomically, the clearance of the drug in TF may be low.
The plasma PK parameters, including the C max, AUC0–24 and t 1/2β, in the present study were approximately 30% higher than those in a previous study with the same fasting conditions. 22 This may be due to the difference in drug properties and administration route. Voriconazole was administered for each horse via dose syringe with the drug suspended in 60 mL corn syrup in the previous study, 22 whereas we administered voriconazole powder through a nasogastric tube with 500 mL of distilled water. These different dosing methods may have affected the results of PK profiles of voriconazole. In human medicine, the mean plasma voriconazole concentration in elderly patients (>65 years) is reportedly 80% higher than that in younger patients after oral administration. 33 Similarly, the voriconazole concentration in body fluids is reportedly higher in geriatric horses than in younger horses. 21 However, age differences cannot be compared because this is not described in the previous study. 22 Additionally, CYP2C19, the major metabolizing enzyme for voriconazole, exhibits genetic polymorphisms; therefore, the rate of voriconazole metabolism varies between human beings and between different races. 33 , 37 Hence, a study using several horse breeds to evaluate PK parameters for voriconazole observed large inter‐subject variations. 21 Although the amounts, activities, and genetic polymorphisms of the metabolizing enzymes for voriconazole in horses remain unclear, the metabolic rate of voriconazole is likely to differ depending on the breed.
The PD target studies of triazole drugs, such as voriconazole, have revealed strong relationships between efficacy and the PK‐PD index of AUC/MIC. 38 , 39 One of the most common pathogenic causes of equine keratomycosis is the Aspergillus species, 2 , 4 , 5 , 6 , 7 , 8 and voriconazole shows marked fungicidal activity towards those mycosis. 40 , 41 In a previous PK‐PD study of voriconazole, it was shown that a AUC/MIC >36.4 is associated with treatment efficacy for 90% of mice infected with Aspergillus species for that experiments. 39 Using 0.5 μg/mL as the target MIC of Aspergillus species, the AUC/MIC for voriconazole in the TF after a single administration was >70 in this study. Thus, the results suggested that a single oral administration of voriconazole 4 mg/kg could achieve an adequate concentration in the TF against Aspergillus species. Fusarium species, the second most common group of causative pathogens of equine keratomycosis, 17 exhibit higher MIC than Aspergillus species. In this study, the Cmax of voriconazole in TF did not achieve therapeutic concentrations for Fusarium species (2.0 μg/mL as the target MIC) after a single oral administration; however, the multiple dosing simulation suggested that the effective concentrations were maintained in TF more than 12 hours after the second administration. Unfortunately, there was no available study on an AUC/MIC associated with treatment efficacy for Fusarium species and no available susceptibility studies on equine Fusarium species in detail, further PK‐PD and susceptibility studies for this fungus will be needed to clarify the therapeutic effect against equine keratomycosis.
In human medicine, the administration of voriconazole with a loading dose higher (generally 1.5‐2 times higher) than the maintenance dose is recommended to rapidly achieve the steady state. 33 A previous report showed that oral administration of voriconazole at 10 mg/kg for horses achieved a maximum plasma concentration of 4.7‐8.5 μg/mL without clinical adverse effects. 20 Although further studies will be needed to ascertain the safety and dose of a high loading dose, a multiple oral dosing regimen included a high loading dose of voriconazole on the first day may be more effective for treating equine keratomycosis. Our results suggested the clinical relevance of oral administration of voriconazole, which is much simpler than other approaches, will likely help maintain an effective concentration for treating equine keratomycosis based on the PK‐PD in TF.
This study evaluated voriconazole PK profiles in the plasma and TF of healthy horses without ocular dysfunctions. The extent of lacrimation and corneal permeability are probably different for healthy horses and horses suffering from keratomycosis. Additional studies are required to determine PK profiles for voriconazole in such cases and clarify factors that influence the relationship between voriconazole concentrations in the plasma and TF. We also set a fasting time similar to the previous equine study 22 to eliminate the effects of feed that could affect the PK profiles. Naturally, this condition will be different from clinical situation of keratomycosis cases that were not treated under the fasting. In the previous human study, it has been reported that the bioavailability of voriconazole is reduced when taken with food compared with fasting. 42 Further studies will be needed to clarify the effect on PK profiles of voriconazole under various feedings.
In our study, PK profiles of voriconazole for the plasma and TF in the healthy Thoroughbred horses after a single oral administration of 4 mg/kg were evaluated. The drug was rapidly distributed into the TF and maintained at a concentration of >1.0 μg/mL for at least 24 hours. The voriconazole concentration in TF maintained above the target MIC of Aspergillus species, and the results of PK‐PD analysis indicated that the oral dosing regimen once a day was effective in treatment of ocular diseases caused by this fungus. The results of simulation in the multiple dosing showed that this drug could also be effective against lower susceptibility fungi by the continuous dosing for more than 2 days. These detailed PK‐PD analyses for treatment of pathogenic fungi infections can be helpful to provide evidence‐based medicine for equine keratomycosis. Although further studies are needed to determine the factors that affect voriconazole bioavailability, oral administrations of voriconazole for equine keratomycosis would be an effective treatment option in some situations.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Yoshiro Sato for his research preparation. All funding source of this research was internal funding of Japan Racing Association. The authors have declared no conflicts of interest.
Tamura N, Okano A, Kuroda T, et al. Utility of systemic voriconazole in equine keratomycosis based on pharmacokinetic‐pharmacodynamic analysis of tear fluid following oral administration. Vet Ophthalmol. 2020;23:640–647. 10.1111/vop.12764
REFERENCES
- 1. GalÁn A, MartÍn‐SuÁrez EM, Gallardo JM, Molleda JM. Clinical findings and progression of 10 cases of equine ulcerative keratomycosis (2004–2007). Equine Vet Educ. 2009;21:236‐242. [Google Scholar]
- 2. Coad CT, Robinson NM, Wilhelmus KR. Antifungal sensitivity testing for equine keratomycosis. Am J Vet Res. 1985;46:676‐678. [PubMed] [Google Scholar]
- 3. Brooks DE. Equine keratomycosis: an international problem. Equine Vet Educ. 2009;21:243‐246. [Google Scholar]
- 4. Nasisse MP, Nelms S. Equine ulcerative keratitis. Vet Clin North Am Equine Pract. 1992;8:537‐555. [DOI] [PubMed] [Google Scholar]
- 5. Hamor RE, Whelan NC. Equine infectious keratitis. Vet Clin North Am Equine Pract. 1999;15:623‐646. [DOI] [PubMed] [Google Scholar]
- 6. Brooks DE, Andrew SE, Dillavou CL, Ellis G, Kubilis PS. Antimicrobial susceptibility patterns of fungi isolated from horses with ulcerative keratomycosis. Am J Vet Res. 1998;59:138‐142. [PubMed] [Google Scholar]
- 7. Ledbetter EC, Patten VH, Scarlett JM, Vermeylen FM. In vitro susceptibility patterns of fungi associated with keratomycosis in horses of the northeastern United States: 68 cases (1987–2006). J Am Vet Med Assoc. 2007;231:1086‐1091. [DOI] [PubMed] [Google Scholar]
- 8. Wada S, Hobo S, Ode H, Niwa H, Moriyama H. Equine keratomycosis in Japan. Vet Ophthalmol. 2013;16:1‐9. [DOI] [PubMed] [Google Scholar]
- 9. Sansom J, Featherstone H, Barnett KC. Keratomycosis in six horses in the United Kingdom. Vet Rec. 2005;156:13‐17. [DOI] [PubMed] [Google Scholar]
- 10. Gaarder JE, Rebhun WC, Ball MA, Patten V, Shin S, Erb H. Clinical appearances, healing patterns, risk factors, and outcomes of horses with fungal keratitis: 53 cases (1978–1996). J Am Vet Med Assoc. 1998;213:105‐112. [PubMed] [Google Scholar]
- 11. Andrew SE, Brooks DE, Smith PJ, Gelatt KN, Chmielewski NT, Whittaker CJ. Equine ulcerative keratomycosis: visual outcome and ocular survival in 39 cases (1987–1996). Equine Vet J. 1998;30:109‐116. [DOI] [PubMed] [Google Scholar]
- 12. Marangon FB, Miller D, Giaconi JA, Alfonso EC. In vitro investigation of voriconazole susceptibility for keratitis and endophthalmitis fungal pathogens. Am J Ophthalmol. 2004;137:820‐825. [DOI] [PubMed] [Google Scholar]
- 13. Johnson EM, Szekely A, Warnock DW. In‐vitro activity of voriconazole, itraconazole and amphotericin B against filamentous fungi. J Antimicrob Chemother. 1998;42:741‐745. [DOI] [PubMed] [Google Scholar]
- 14. Espinel‐Ingroff A. In vitro activity of the new triazole voriconazole (UK‐109,496) against opportunistic filamentous and dimorphic fungi and common and emerging yeast pathogens. J Clin Microbiol. 1998;36:198‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Radford SA, Johnson EM, Warnock DW. In vitro studies of activity of voriconazole (UK‐109,496), a new triazole antifungal agent, against emerging and less‐common mold pathogens. Antimicrob Agents Chemother. 1997;41:841‐843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clode AB, Davis JL, Salmon J, Michau TM, Gilger BC. Evaluation of concentration of voriconazole in aqueous humor after topical and oral administration in horses. Am J Vet Res. 2006;67:296‐301. [DOI] [PubMed] [Google Scholar]
- 17. Brooks DE, Matthews A, Clode AB. Diseases of the cornea In: Gilger BC, eds. Equine Ophthalmology, 3rd ed Hoboken, NJ: John Wiley & Sons, Inc; 2016:252‐368. [Google Scholar]
- 18. Smith KM, Maxwell L, Gull T, Payton ME, Gilmour MA. Stability of 1% voriconazole solution in a constant‐rate infusion pump for topical ocular delivery to horses. Vet Ophthalmol. 2014;17(Suppl 1):82‐89. [DOI] [PubMed] [Google Scholar]
- 19. Matthews AG. Ophthalmic antimicrobial therapy in the horse. Equine Vet Educ. 2009;21:271‐280. [Google Scholar]
- 20. Colitz CM, Latimer FG, Cheng H, Chan KK, Reed SM, Pennick GJ. Pharmacokinetics of voriconazole following intravenous and oral administration and body fluid concentrations of voriconazole following repeated oral administration in horses. Am J Vet Res. 2007;68:1115‐1121. [DOI] [PubMed] [Google Scholar]
- 21. Passler NH, Chan HM, Stewart AJ, et al. Distribution of voriconazole in seven body fluids of adult horses after repeated oral dosing. J Vet Pharmacol Ther. 2010;33:35‐41. [DOI] [PubMed] [Google Scholar]
- 22. Davis JL, Salmon JH, Papich MG. Pharmacokinetics of voriconazole after oral and intravenous administration to horses. Am J Vet Res. 2006;67:1070‐1075. [DOI] [PubMed] [Google Scholar]
- 23. Collum LM, McGettrick P, Akhtar J, Lavin J, Rees PJ. Oral acyclovir (Zovirax) in herpes simplex dendritic corneal ulceration. Br J Ophthalmol. 1986;70:435‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gerhardt N, Schulz BS, Werckenthin C, Hartmann K. Pharmacokinetics of enrofloxacin and its efficacy in comparison with doxycycline in the treatment of Chlamydophila felis infection in cats with conjunctivitis. Vet Rec. 2006;159:591‐594. [DOI] [PubMed] [Google Scholar]
- 25. Baker A, Plummer CE, Szabo NJ, Barrie KP, Brooks DE. Doxycycline levels in preocular tear film of horses following oral administration. Vet Ophthalmol. 2008;11:381‐385. [DOI] [PubMed] [Google Scholar]
- 26. Thomasy SM, Covert JC, Stanley SD, Maggs DJ. Pharmacokinetics of famciclovir and penciclovir in tears following oral administration of famciclovir to cats: a pilot study. Vet Ophthalmol. 2012;15:299‐306. [DOI] [PubMed] [Google Scholar]
- 27. Hartmann A, Krebber R, Daube G, Hartmann K. Pharmacokinetics of pradofloxacin and doxycycline in serum, saliva, and tear fluid of cats after oral administration. J Vet Pharmacol Ther. 2008;31:87‐94. [DOI] [PubMed] [Google Scholar]
- 28. Amoros‐Reboredo P, Bastida‐Fernandez C, Guerrero‐Molina L, Soy‐Muner D, Lopez‐Cabezas C. Stability of frozen 1% voriconazole ophthalmic solution. Am J Health Syst Pharm. 2015;72:479‐482. [DOI] [PubMed] [Google Scholar]
- 29. Pfaller JB, Messer SA, Hollis RJ, Diekema DJ, Pfaller MA. In vitro susceptibility testing of Aspergillus spp.: comparison of Etest and reference microdilution methods for determining voriconazole and itraconazole MICs. J Clin Microbiol. 2003;41:1126‐1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lalitha P, Shapiro BL, Srinivasan M, et al. Antimicrobial susceptibility of Fusarium, Aspergillus, and other filamentous fungi isolated from keratitis. Arch Ophthalmol. 2007;125:789‐793. [DOI] [PubMed] [Google Scholar]
- 31. Fukuda M, Murano H, Sasaki K. Dynamics of fluoroquinolone agents in tear fluid–a comparison of the dynamic in tears of human and rabbit eyes. Nihon Ganka Gakkai Zasshi. 1994;98:721‐726. [PubMed] [Google Scholar]
- 32. Mouton JW, Dudley MN, Cars O, Derendorf H, Drusano GL. Standardization of pharmacokinetic/pharmacodynamic (PK/PD) terminology for anti‐infective drugs: an update. J Antimicrob Chemother. 2005;55:601‐607. [DOI] [PubMed] [Google Scholar]
- 33. Theuretzbacher U, Ihle F, Derendorf H. Pharmacokinetic/pharmacodynamic profile of voriconazole. Clin Pharmacokinet. 2006;45:649‐663. [DOI] [PubMed] [Google Scholar]
- 34. Hollis AR, Wilkins PA. Current controversies in equine antimicrobial therapy. Equine Vet Educ. 2009;21:216‐224. [Google Scholar]
- 35. Vishkautsan P, Papich MG, Thompson GR 3rd, Sykes JE. Pharmacokinetics of voriconazole after intravenous and oral administration to healthy cats. Am J Vet Res. 2016;77:931‐939. [DOI] [PubMed] [Google Scholar]
- 36. Lemetayer JD, Dowling PM, Taylor SM, Papich MG. Pharmacokinetics and distribution of voriconazole in body fluids of dogs after repeated oral dosing. J Vet Pharmacol Ther. 2015;38:451‐456. [DOI] [PubMed] [Google Scholar]
- 37. Hyland R, Jones BC, Smith DA. Identification of the cytochrome P450 enzymes involved in the N‐oxidation of voriconazole. Drug Metab Dispos. 2003;31:540‐547. [DOI] [PubMed] [Google Scholar]
- 38. Lepak AJ, Andes DR. Antifungal pharmacokinetics and pharmacodynamics. Cold Spring Harb Perspect Med. 2015;5:a019653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mavridou E, Bruggemann RJ, Melchers WJ, Verweij PE, Mouton JW. Impact of cyp51A mutations on the pharmacokinetic and pharmacodynamic properties of voriconazole in a murine model of disseminated aspergillosis. Antimicrob Agents Chemother. 2010;54:4758‐4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Manavathu EK, Cutright JL, Chandrasekar PH. Organism‐dependent fungicidal activities of azoles. Antimicrob Agents Chemother. 1998;42:3018‐3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Krishnan S, Manavathu EK, Chandrasekar PH. A comparative study of fungicidal activities of voriconazole and amphotericin B against hyphae of Aspergillus fumigatus. J Antimicrob Chemother. 2005;55:914‐920. [DOI] [PubMed] [Google Scholar]
- 42. Purkins L, Wood N, Kleinermans D, Greenhalgh K, Nichols D. Effect of food on the pharmacokinetics of multiple‐dose oral voriconazole. Br J Clin Pharmacol. 2003;56(Suppl 1):17‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]


