Abstract
Hypertensive retinopathy refers to the retinal vascular changes associated with systemic arterial hypertension. Hypertensive retinopathy can be divided into chronic and acute phases. A cross‐sectional study was performed to explore a method of measurement in the diameters of retinal vessels for diagnosis of chronic hypertensive retinopathy based on spectral domain optical coherence tomography (SD‐OCT). The central retinal artery diameter (CRAD), the central retinal vein diameter (CRVD), and the artery‐to‐vein ratio (AVR) were measured. A total of 119 subjects with 119 eyes were included in this study, in which 56 subjects with 56 eyes were included in hypertensive group and 63 subjects with 63 eyes were included in normotensive group. There were significant differences between the two groups in the CRAD (t = −2.14, P = .04) and the AVR (t = −2.59, P = .01). The cutoff point of 0.75 was determined by receiver operating characteristic (ROC) curve (area under the curve, AUC 0.786; 95% confidence interval, 95% CI 0.70‐0.87). Multivariate logistic regression analysis showed the probability of AVR below to 0.75 was more in patients with high systolic blood pressure (odds ratio OR 4.39; P = .048), more in male (OR 4.15; P = .004) and more in smokers (OR 5.80; P = .01). Bland‐Altman plots showed small mean bias between the measurements of the two technicians in the CRAD, the CRVD, and the AVR. In summary, application of SD‐OCT is an accurate, reproducible, convenient method for measuring the diameters of retinal vessels. It is valuable for the diagnosis of chronic stage of hypertensive retinopathy.
Keywords: chronic stage, hypertensive retinopathy, spectral domain optical coherence tomography
1. INTRODUCTION
The global burden of hypertension is expected to affect one third of the world's population by 2025. 1 Hypertension has an unfavorable impact on target organs. 2 , 3 Hypertensive retinopathy refers to the retinal vascular changes associated with systemic arterial hypertension. 4 The blood vessels of the retina are the only parts of the circulation system that can be viewed directly and noninvasively in vivo. 5 Hypertensive retinopathy can be divided into chronic and acute phases. 6 There are several classifications for hypertensive retinopathy. The most commonly used classification was that of Keith‐Wagener‐Barker 7 which showed four grades of hypertensive retinopathy as follows: Grade I, retinal arterial narrowing; Grade II, retinal arteriovenous nicking; Grade III, retinal hemorrhages, cotton‐wool spots, hard exudates; and Grade IV, Grade III changes plus optic disc swelling. 8 Grades I and II are commonly seen in practice and reflect chronic changes. Grades III and IV changes are less frequently seen and are reflective of an acute increase in blood pressure.
Vasospasm and generalized arteriolar narrowing of the retina were presented in the initial response of the retinal circulation to raised blood pressure. 4 Previous study showed limitations in the diagnosis of chronic hypertensive retinopathy in clinic. 6 Previous classic methods for measuring retinal vascular caliber included traditional direct and indirect ophthalmoscopes and fundus color photography. 9 Limitations such as a time‐consuming process, mydriasis, and close contact with patients were presented in previous methods for measurements. In the situation of the coronavirus disease 2019 (COVID‐19) pandemic, reduction of opportunities in close contact with patients for protecting medical workers was very necessary. 10
A cross‐sectional study was performed for exploring an accurate, reproducible, convenient method of measurement in the diameters of retinal vessels for diagnosis of chronic hypertensive retinopathy based on SD‐OCT.
2. METHODS
2.1. Study design and participants
This cross‐sectional study was conducted from January 2019 to December 2019 at Beijing Friendship Hospital. A total of 119 subjects with 119 eyes without any other ocular diseases were included in this study, in which 56 subjects with 56 eyes were included in hypertensive group and 63 subjects with 63 eyes were included in normotensive group. This study was approved by the institutional review board and was conducted in conformance with the Declaration of Helsinki. All participants signed informed consent.
Inclusion criteria for subjects with hypertension were based on histories of antihypertensive agents use or the diagnosis of physicians. The diagnosis of essential hypertension was defined as seated systolic blood pressure (SBP) >140 mm Hg and/or diastolic blood pressure (DBP) >90 mm Hg during at least 3 measurements. 11
Exclusion criteria were subjects who that had intraocular surgery before, high myopia (more than −6.00 diopters), glaucoma, senile cataract that diminished image quality, and vascular retinopathy (eg, diabetic retinopathy, retinal artery occlusion, retinal vein occlusion, and age‐related macular degeneration).
2.2. Examinations
All participants were examined by two experienced technicians. Results of measurements were retrieved from the two technicians and were calculated the average value. Circular scans of the optic disc were performed by SD‐OCT (NIDEK RS‐3000, Japan). Cross‐sections of the retinal vessels were presented in a group of reflections of vascular walls which were ellipse in SD‐OCT, and the diameters of the retinal vessels were presented by the distance between the reflections of vascular walls (Figure 1). Data on a single eye were chosen randomly for inclusion in the study. If the quality of image was poor, the image of the other eye was accepted. Color fundus photography combined with IVAN software (Version 1.3; Nicola Ferrier, Madison, WI, USA) was performed in the measurements of retinal vascular caliber after mydriasis. In our study, fundus color photography was taken as standard.
Figure 1.
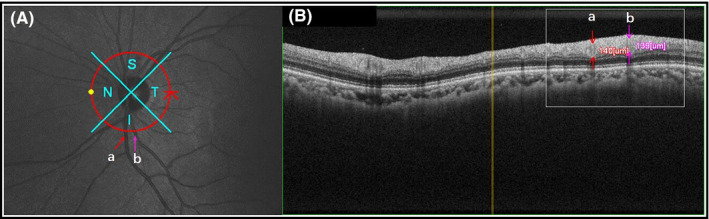
Circular scan of the optic disc was shown in the infrared image (A). Cross‐sections of the retinal vessel (a, vein; b, artery) were presented in a group of heterogeneous reflectivity which were ellipse (B). S, superior; I, inferior; N, nasal; T, temporal
2.3. Statistical analysis
Statistical analyses were performed using Stata 13, Texas, 77845 USA. Vessel diameter values and the artery‐to‐vein ratios were presented as mean ± standard deviation. Student's t test was used to compare quantitative data with normal distribution. Categorical variables were analyzed using chi‐square tests. ROC curve analysis was performed to evaluate the accuracy of the method of measurement. Multivariate logistic regression analysis was performed to identify associations between dependent and independent variables. The results are expressed as adjusted odds ratios (ORs) with 95% CIs. Bland‐Altman plots with mean bias and limits of agreement at 95% CIs were performed to evaluate the reproducibility of the method of measurement. Statistical significance was accepted as a two‐sided test with an alpha level of 0.05. A P value of < .05 was considered statistically significant.
3. RESULTS
One hundred and nineteen subjects with 119 eyes were included in this study. There were 40 males and 79 females, with an average age of 63.4 ± 12.1 years. Table 1 showed that 56 subjects in hypertensive group (22 males, 39.3%; 34 females, 60.7%) and 63 subjects (18 males, 28.6%; 45 females, 71.4%) in normotensive group were enrolled. The mean age of subjects in these two groups (hypertensive group and normotensive group) was 66.4 ± 10.9 years and 60.7 ± 12.7 years, respectively. There were no significant differences in age (t = 2.64, P = .070), male rated (χ2 = 1.53, P = .217) between the two groups. The details of the antihypertensive medication of 56 subjects in hypertensive group were as follows: calcium channel blocker (CCB), 21 subjects (37.5%); angiotensin‐converting enzyme inhibitor/angiotensin receptor blockers (ACEI/ARBs), 8 subjects (14.3%); CCB combined with beta‐blockers (BBs), 13 subjects (23.2%); CCB combined with diuretics, 4 subjects (7.1%); CCB combined with ACEI/ARBs, 3 subjects (5.4%); CCB, ACEI/ARBs combined with diuretics, 3 subjects (5.4%); and 4 subjects (7.1%) did not use any antihypertensive agents.
Table 1.
Clinical characteristics of the study population
| Variable | Hypertension (n = 56) | Normotension (n = 63) | P value |
|---|---|---|---|
| Age, y | 66.4 ± 10.9 | 60.7 ± 12.7 | .070 |
| Male, n (%) | 22 (39.3) | 18(28.6) | .217 |
| Diabetes history, n (%) | 24 (42.9) | 6 (9.5) | <.001** |
| Coronary heart disease history, n (%) | 9 (16.1) | 4 (6.3) | .090 |
| Cerebral infarction history, n (%) | 7 (12.5) | 2 (3.2) | .055 |
| Hyperlipidemia history, n (%) | 13 (23.2) | 7 (11.1) | .078 |
| CRAD, μm | 123.29 ± 12.55 | 128.21 ± 12.53 | .035* |
| CRVD, μm | 162.59 ± 20.74 | 157.86 ± 16.21 | .166 |
| AVR | 0.77 ± 0.11 | 0.82 ± 0.10 | .011* |
| Superior RNFL, μm | 120.93 ± 17.99 | 126.73 ± 16.63 | .070 |
| Inferior RNFL, μm | 128.09 ± 24.82 | 132.00 ± 21.41 | .358 |
| Nasal RNFL, μm | 69.93 ± 15.67 | 71.95 ± 18.87 | .529 |
| Temporal RNFL, μm | 73.71 ± 21.50 | 73.27 ± 16.05 | .898 |
Abbreviations: AVR, artery‐to‐vein ratio; CRAD, central retinal artery diameter; CRVD, central retinal vein diameter; DBP, diastolic blood pressure; RNFL, retinal nerve fiber layer; SBP, systolic blood pressure.
P < .05
P < .01
In the hypertensive group, CRAD, CRVD, and AVR were 123.29 ± 12.55 μm, 162.59 ± 20.74 μm, and 0.77 ± 0.11, while in the normotensive group which were 128.21 ± 12.53 μm, 157.86 ± 16.21 μm, and 0.82 ± 0.10, respectively. There were significant differences between the two groups in CRAD (t = −2.14, P = .035) and AVR (t = −2.59, P = .011). There were no significant differences between the two groups in CRVD (t = 1.39, P = .166; Figure 2).
Figure 2.
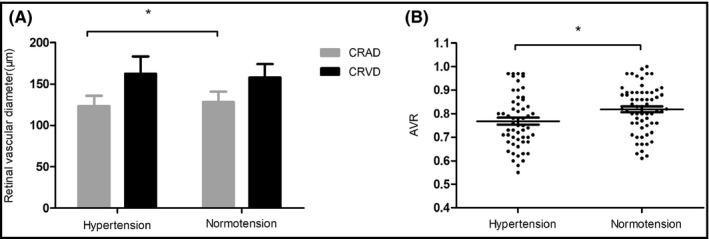
Comparison of retinal vascular diameter (A) and AVR (B) between hypertensive group and normotensive group. CRAD, central retinal artery diameter; CRVD, central retinal vein diameter; AVR, artery‐to‐vein ratio; *P < .05
The accuracy of the method of measurement was evaluated by ROC curve analysis, and the maximum value of Youden index was determined as cutoff point of the AVR. Fundus color photography was taken as standard. The cutoff point of 0.75 showed a sensitivity of 68.6%, a specificity of 79.4%. (AUC 0.786; Standard error 0.04; 95% CI 0.70‐0.87; Figure 3).
Figure 3.
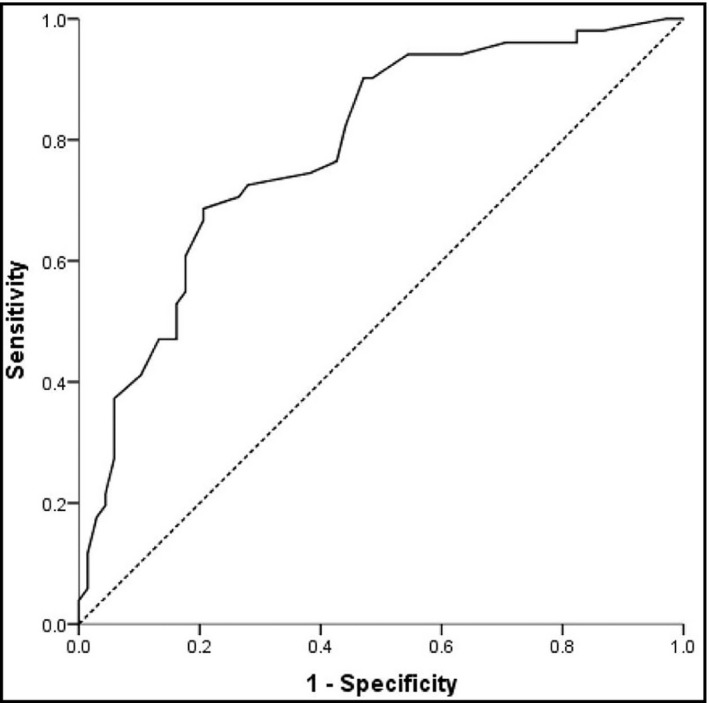
The cutoff value of AVR for retinal arteriosclerosis was determined by receiver operating characteristic (ROC) curve. The cutoff point of 0.75 showed a sensitivity of 68.6% and a specificity of 79.4%. Area under the curve (AUC) 0.786; standard error 0.04; 95% CI 0.70‐0.87. AVR, artery‐to‐vein ratio
Table 2 showed that multivariate logistic regression analysis was performed to analyze the correlations between the AVR and sex, age, smoking history, SBP, DBP, pulse pressure (PP), thickness of RNFL, previous history of diabetes, hyperlipidemia, coronary heart disease, and cerebral infarction. The probability of AVR below to 0.75 was more in patients with high systolic blood pressure (OR, 4.39; 95% CI, 1.01‐19.05; P = .048), more in male (OR, 4.15; 95% CI, 1.58‐10.93; P = .004) and more in smokers (OR, 5.80; 95% CI, 1.43‐23.57; P = .014).
Table 2.
Multivariable logistic regression of AVR in patients
| Variable | Odds Ratio | Std. Err | 95% Cl | P value |
|---|---|---|---|---|
| Sex (male vs female) | 4.15 | 0.49 | 1.58‐10.93 | .004** |
| Age, y | 0.73 | 0.54 | 0.25‐2.10 | .560 |
| Smoking (yes vs no) | 5.80 | 0.72 | 1.43‐23.57 | .014* |
| Clinic SBP, mm Hg | 4.39 | 0.75 | 1.01‐19.05 | .048* |
| Clinic DBP, mm Hg | 0.34 | 0.91 | 0.06‐2.03 | .238 |
| PP, mm Hg | 2.40 | 0.55 | 0.82‐7.06 | .111 |
| Superior RNFL, μm | 1.63 | 0.78 | 0.36‐7.48 | .528 |
| Inferior RNFL, μm | 0.63 | 0.51 | 0.24‐1.71 | .368 |
| Nasal RNFL, μm | 1.73 | 0.49 | 0.66‐4.51 | .262 |
| Temporal RNFL, μm | 0.78 | 0.51 | 0.28‐2.12 | .622 |
| Hypertension history (yes vs no) | 0.93 | 0.60 | 0.29‐2.97 | .897 |
| Diabetes history (yes vs no) | 0.45 | 0.67 | 0.12‐1.67 | .234 |
| Hyperlipidemia history (yes vs no) | 1.77 | 0.66 | 0.49‐6.47 | .386 |
| Coronary heart disease history (yes vs no) | 1.80 | 0.80 | 0.37‐8.68 | .467 |
| Cerebral infarction history (yes vs no) | 0.98 | 0.93 | 0.16‐6.10 | .984 |
Cutoff point of AVR was 0.75 (<0.75, ≥0.75). Adjusted variables were sex (male vs female), age (>65, ≤65), smoking (yes vs no), SBP (>140 mm Hg, ≤140 mm Hg), DBP (>90 mm Hg, ≤90 mm Hg), PP (>50 mm Hg, ≤50 mm Hg), superior RNFL (≤140 μm, >140 μm), inferior RNFL (≤140 μm, >140 μm), nasal RNFL (≤70 μm, >70 μm), temporal RNFL (≤70 μm, >70 μm), hypertension history (yes vs no), diabetes history (yes vs no), hyperlipidemia history (yes vs no), coronary heart disease history (yes vs no), and cerebral infarction history (yes vs no). Data are expressed as hazard ratios (95% confidence intervals) followed by P value.
Abbreviations: AVR, artery‐to‐vein ratio; DBP, diastolic blood pressure; PP, pulse pressure; RNFL, retinal nerve fiber layer; SBP, systolic blood pressure.
P < .05,
P < .01.
Bland‐Altman analysis was performed to evaluate the reproducibility of the method of measurement in the comparison of the two experienced technicians. Mean bias and limits of agreement at 95% CIs were presented graphically in Bland‐Altman plots. Comparison of the measurements between the two technicians in CRAD (mean 0.68 μm; 95% limits of agreement, 95% LoA − 5.87, 7.23 μm), CRVD (mean 0.28 μm; 95% LoA − 3.15, 3.71 μm), and AVR (mean 0.003; 95% LoA − 0.04, 0.04) was presented in Bland‐Altman plots (Figure 4).
Figure 4.
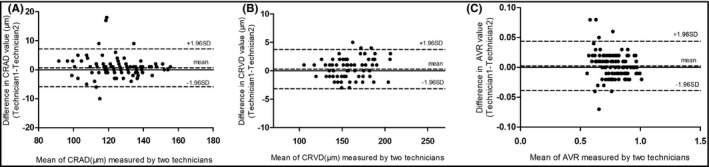
Comparison of the measurements between technician 1 and technician 2 in CRAD (A), CRVD (B), and AVR (C) by Bland‐Altman plots. (A) mean 0.68 μm; 95% limits of agreement, 95% LoA − 5.87, 7.23 μm; (B) mean 0.28 μm; 95% LoA − 3.15, 3.71 μm; (C) mean 0.003; 95% LoA − 0.04, 0.04. AVR, artery‐to‐vein ratio; CRAD, central retinal artery diameter; CRVD, central retinal vein diameter; SD, standard deviation
4. DISCUSSION
Previous classic methods for measuring retinal vascular caliber included traditional direct and indirect ophthalmoscopes and fundus color photography. 9 Fundus fluorescence angiography (FFA) 9 and optical coherence tomography angiography (OCTA) 12 can be used to evaluate the hemodynamics and vascular density of retinal blood flow, but cannot measure retinal vascular diameter. Traditional direct and indirect ophthalmoscopes cannot quantify the data which is affected by the refractive medium opacity. Color fundus photography combined with IVAN software (Version 1.3; Nicola Ferrier, Madison, WI, USA) was performed in the measurements of retinal vascular caliber after mydriasis. 13 SD‐OCT 14 has been widely used and highly sensitive imaging modality in various ophthalmologic disciplines for evaluating the macula and the optic nerve. 15 In our study, fundus color photography was taken as standard. The accuracy of measurement of the SD‐OCT was evaluated by ROC curve analysis. The AUC value of 0.786 (95% CI 0.70‐0.87) showed that SD‐OCT had diagnostic value in measuring the diameters of blood vessels. Zhu's study 16 showed that fifty‐five healthy individuals were recruited for observation by SD‐OCT, but their study did not evaluate the accuracy of measurement in terms of ROC curve. Muraoka's research 17 focused more on the measurement of the thickness of retinal vessel wall; however, our point of view was that the resolution of the retinal vessel wall measured by SD‐OCT cannot be so accurate in actual clinical practice.
The reproducibility of SD‐OCT measurements is important for assessing the interobserver variability. The application of ophthalmoscope in the measurement of retinal vascular diameter has poor reproducibility. The diameters of retinal vessels were observed by direct or indirect ophthalmoscope, and the results vary greatly in different examiners. In our study, Bland‐Altman plots showed good agreements in CRAD (mean 0.68 μm; 95% LoA − 5.87, 7.23 μm), CRVD (mean 0.28 μm; 95% LoA − 3.15, 3.71 μm), and AVR (mean 0.003; 95% LoA − 0.04, 0.04). There were 5 points (4.2%), 3 points (2.5%), and 7 points (5.9%) outside the 95%LoA in the CRAD, the CRVD, and the AVR, respectively. Goldenberg's study 18 once demonstrated the noninvasive method for measuring the diameters of retinal blood vessels by means of an SD‐OCT imaging modality, but their study did not evaluate the reproducibility of the measurement in terms of reproducibility by presenting Bland‐Altman plots or other reliability index.
The convenience of clinical application of SD‐OCT in the measurements of retinal vascular diameters needs to be fully considered. In the situation of the COVID‐19 pandemic, reduction of opportunities in close contact with patients for protecting medical workers was very necessary. Limitations such as a time‐consuming process, mydriasis, and close contact with patients were presented in direct and indirect ophthalmoscopes for the measurements of retinal vascular diameters. Additional software such as IVAN software was required to combine with color fundus photography to measure the diameters of retinal vessels after mydriasis, which required higher technology of data processing. 19 In our study, the diameters of retinal vessels were measured by SD‐OCT which was a noninvasive method, non‐mydriatic, fast, non–close contact with the subject, and easy to be operated.
Previous study 20 showed limitations in the diagnosis of chronic hypertensive retinopathy in clinic. Pache's study 6 suggested that a differentiated division into four stages was not possible. According to the classification of Neubauer, 21 a modification of the classification of Keith and Wagener, that distinguishes between fundus hypertonic (stages I–II = mild) and hypertensive retinopathy (stages III–IV = severe). However, it was difficult to distinguish between stages I and II. Previous study 22 indicated that the normal value of AVR was 2:3. If the artery caliber was narrow, the AVR could be 1:2 or 1:3. Goldenberg's study 18 indicated that the AVR was 0.9 at all points of measurement based on SD‐OCT in healthy subjects. The previous gold standards of AVR were not uniform. There was no gold standard of AVR based on SD‐OCT. In our study, according to the maximum value of Youden index, the cutoff point of 0.75 which showed a sensitivity of 68.6% and a specificity of 79.4% was determined by ROC curve. In the future, a large sample of research could be needed to further confirm the gold standard of AVR based on SD‐OCT.
Our study showed that the CRAD and the AVR in the hypertensive group were smaller than that in the normotensive group. High systolic blood pressure, male, and smoking are independent factors associated with lower AVR. A multi‐ethnic study 23 confirms that a narrower retinal arteriolar diameter and wider venular diameter are associated with the development of hypertension. Previous studies 24 , 25 , 26 reported that systolic and diastolic blood pressure were associated with retinal arteriolar narrowing. Our findings further confirmed the conclusions of our predecessors. Female may be the protective factor of retinal vascular caliber due to the effect of estrogen. 27 Previous studies 28 have shown that smoking is a risk factor for hypertension. Furthermore, our findings further found that smoking is also a risk factor associated with smaller retinal vascular diameter. Tapp's study 29 suggested that the large curvature of retinal arterioles was related to the higher systolic blood pressure, mean arterial pressure, and pulse pressure. In our study, after adjustment for the model, the results of pulse pressure were negative.
In future research, SD‐OCT will be used for the quantitative evaluation of retinal hemorrhages, cotton‐wool spots, hard exudates, and optic disc swelling, then to distinguish the acute phases of hypertensive retinopathy from other diseases. We will consider for standardly grouping based on antihypertensive medication to further explore the effects of different antihypertensive medication combinations on retinal vascular changes by SD‐OCT.
In summary, application of SD‐OCT is an accurate, reproducible, convenient method for measuring the diameters of retinal vessels. It is valuable for the diagnosis of chronic stage of hypertensive retinopathy.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
Xue Feng performed the analysis, drafted the manuscript, and designed the figures. Haiwei Wang performed the analysis and measurements. Yuanyuan Kong and Hong Qi participated in the correction and the statistical analysis of the revision. Junyan Zhang, Jingfang He, and Bozheng Zhang were involved in data analysis. Jianqiang Zhang performed the measurements and was involved in planning. Yanling Wang aided in interpreting the results, worked on the manuscript, was involved in planning, and supervised the work. All authors discussed the results and contributed to the final manuscript.
ACKNOWLEDGMENTS
We thank all the staff of Beijing Friendship Hospital, Beijing Moslem People's Hospital, Fuxing Hospital, Bothwin Clinical Study Consultant, and Peking University Third Hospital for their contributions to this work.
Feng X, Wang H, Kong Y, et al. Diagnosis of chronic stage of hypertensive retinopathy based on spectral domain optical coherence tomography. J Clin Hypertens. 2020;22:1247–1252. 10.1111/jch.13935
Xue Feng and Haiwei Wang contribute equally to this work.
REFERENCES
- 1. Haeusler KG, Huttner HB, Kuramatsu JB. Comment on 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2019;40:2092. [DOI] [PubMed] [Google Scholar]
- 2. Stroke O. Neuroimaging of Intracranial Atherosclerosis Trail I. Stroke outcome and neuroimaging of intracranial atherosclerosis (SONIA): design of a prospective, multicenter trial of diagnostic tests. Neuroepidemiology. 2004;23:23‐32. [DOI] [PubMed] [Google Scholar]
- 3. Omboni S, Posokhov I, Parati G, et al. Variable association of 24‐h peripheral and central hemodynamics and stiffness with hypertension‐mediated organ damage: the VASOTENS Registry. J Hypertens. 2020;38(4):701‐715. [DOI] [PubMed] [Google Scholar]
- 4. Wong TY, Mitchell P. The eye in hypertension. Lancet. 2007;369:425‐435. [DOI] [PubMed] [Google Scholar]
- 5. Dumitrescu AG, Voinea L, Badarau IA, Paun VA, Schowe M, Ciuluvica R. Update on retinal vascular caliber. Rom J Ophthalmol. 2017;61:171‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pache M, Kube T, Wolf S, Kutschbach P. Do angiographic data support a detailed classification of hypertensive fundus changes? J Hum Hypertens. 2002;16:405‐410. [DOI] [PubMed] [Google Scholar]
- 7. Aissopou EK, Papathanassiou M, Nasothimiou EG, et al. The Keith‐Wagener‐Barker and Mitchell‐Wong grading systems for hypertensive retinopathy: association with target organ damage in individuals below 55 years. J Hypertens. 2015;33:2303‐2309. [DOI] [PubMed] [Google Scholar]
- 8. Chida T. Comparative study of essential hypertension based on age and the Keith‐Wagener‐Barker fundus classification, with reference to X‐ray kymography and electrocardiography. Igaku Kenkyu. 1964;34:46‐75. [PubMed] [Google Scholar]
- 9. Littlewood R, Mollan SP, Pepper IM, Hickman SJ. The utility of fundus fluorescein angiography in neuro‐ophthalmology. Neuroophthalmology. 2019;43:217‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu Z, Sun CB. Conjunctiva is not a preferred gateway of entry for SARS‐CoV‐2 to infect respiratory tract. J Med Virol. 2020. 10.1002/jmv.25859. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ikonomidis I, Voumvourakis A, Makavos G, et al. Association of impaired endothelial glycocalyx with arterial stiffness, coronary microcirculatory dysfunction, and abnormal myocardial deformation in untreated hypertensives. J Clin Hypertens. 2018;20(4):672‐679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tey KY, Teo K, Tan ACS, et al. Optical coherence tomography angiography in diabetic retinopathy: a review of current applications. Eye Vis. 2019;6(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wei FF, Thijs L, Yu CG, et al. Retinal microvasculature in relation to central hemodynamics in a Flemish population. Hypertension. 2019;74:606‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pilch M, Wenner Y, Strohmayr E, et al. Automated segmentation of retinal blood vessels in spectral domain optical coherence tomography scans. Biomed Opt Express. 2012;3:1478‐1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dysli M, Ruckert R, Munk MR. Differentiation of underlying pathologies of macular edema using spectral domain optical coherence tomography (SD‐OCT). Ocul Immunol Inflamm. 2019;27:474‐483. [DOI] [PubMed] [Google Scholar]
- 16. Zhu TP, Tong YH, Zhan HJ, Ma J. Update on retinal vessel structure measurement with spectral‐domain optical coherence tomography. Microvasc Res. 2014;95:7‐14. [DOI] [PubMed] [Google Scholar]
- 17. Muraoka Y, Tsujikawa A, Kumagai K, et al. Age‐ and hypertension‐dependent changes in retinal vessel diameter and wall thickness: an optical coherence tomography study. Am J Ophthalmol. 2013;156:706‐714. [DOI] [PubMed] [Google Scholar]
- 18. Goldenberg D, Shahar J, Loewenstein A, Goldstein M. Diameters of retinal blood vessels in a healthy cohort as measured by spectral domain optical coherence tomography. Retina. 2013;33:1888‐1894. [DOI] [PubMed] [Google Scholar]
- 19. Ajaz A, Aliahmad B, Kumar H, Sarossy M, Kumar DK. Agreement study between color and IR retinal images based on retinal vasculature morphological parameters. BMC Ophthalmol. 2019;19:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schrier RW. Treating high‐risk diabetic hypertensive patients with comorbid conditions. Am J Kidney Dis. 2000;36:S10‐S17. [DOI] [PubMed] [Google Scholar]
- 21. Neubauer H. Fundus oculi findings in arterial hypertension? Der Internist. 1974;15:485‐496. [PubMed] [Google Scholar]
- 22. Kralev S, Zimmerer E, Buchholz P, et al. Microvascular retinal changes in patients presenting with acute coronary syndromes. Microvasc Res. 2010;79:150‐153. [DOI] [PubMed] [Google Scholar]
- 23. Kawasaki R, Cheung N, Wang JJ, et al. Retinal vessel diameters and risk of hypertension: the multiethnic study of atherosclerosis. J Hypertens. 2009;27:2386‐2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kochli S, Endes K, Infanger D, Zahner L, Hanssen H. Obesity, blood pressure, and retinal vessels: a meta‐analysis. Pediatrics. 2018;141:e20174090. [DOI] [PubMed] [Google Scholar]
- 25. Chew SK, Xie J, Wang JJ. Retinal arteriolar diameter and the prevalence and incidence of hypertension: a systematic review and meta‐analysis of their association. Curr Hypertens Rep. 2012;14:144‐151. [DOI] [PubMed] [Google Scholar]
- 26. Lin JM, Hsu KL, Chiang FT, Tseng CD, Tseng YZ. Influence of isolated diastolic hypertension identified by ambulatory blood pressure on target organ damage. Int J Cardiol. 1995;48:311‐316. [DOI] [PubMed] [Google Scholar]
- 27. He Y, Li SM, Kang MT, et al. Association between blood pressure and retinal arteriolar and venular diameters in Chinese early adolescent children, and whether the association has gender difference: a cross‐sectional study. BMC Ophthalmol. 2018;18:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Song H, Feng D, Wang R, et al. The urban‐rural disparity in the prevalence and risk factors of hypertension among the elderly in China—a cross‐sectional study. PeerJ. 2019;7:e8015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tapp RJ, Owen CG, Barman SA, et al. Associations of retinal microvascular diameters and tortuosity with blood pressure and arterial stiffness: United Kingdom Biobank. Hypertension. 2019;74:1383‐1390. [DOI] [PMC free article] [PubMed] [Google Scholar]


