Abstract
Background/Objectives
The prevalence of atopic dermatitis (AD) has increased significantly in industrialised countries in recent decades but data about the incidence or prevalence of AD in Australia are sparse. We aimed to determine the prevalence and incidence of AD among patients seen in Australian general practice and the use of specified medicines.
Methods
This was a cross‐sectional study of 2.1 million patients attending 494 general practices in the MedicineInsight program from 1 January 2017 to 31 December 2018. We assessed the prevalence (lifetime and current), incidence, management and severity of AD.
Results
The lifetime (ever diagnosed) prevalence of AD in this general practice population was 16.4% and was greater in females (17.3%) than males (15.3%). One in five patients with AD were classified as having moderate‐to‐severe disease. Prevalence over the last two years was 6.3%. The incidence of AD in 2018 was 2.0% and was greater in females (2.2%) and for patients aged 0–4 years (3.9%). Patients with AD had an increased risk of insomnia, anxiety and depression, compared to those with no recorded AD. For AD patients, topical corticosteroids were the most commonly prescribed AD medication (36.5%) and topical calcineurin inhibitors the least (0.1%), with systemic corticosteroids (15.6%) more commonly prescribed than other immunosuppressants (0.9%).
Conclusions
Our findings provide important insights into the epidemiology of AD and its management in Australian general practice. This information is likely to be useful in planning effective interventions to support GPs in the optimal management of patients with AD.
Keywords: atopic dermatitis, Australia, eczema, general practice, incidence, management, MedicineInsight, prevalence, therapies
Introduction
Atopic dermatitis, also called eczema, is a chronic, relapsing, pruritic, inflammatory skin disease that occurs more frequently in children than adults.1 The prevalence of eczema varies widely between populations and countries2, 3 and is said to have doubled or tripled in industrialised countries in recent decades.4, 5 The population prevalence of eczema in Australia was estimated to be 16% in 4‐year‐olds (2017)6 and 20.3% in 1‐year‐olds (2013).7
Atopic dermatitis has substantial effects on the psychosocial well‐being and quality of life of patients,8, 9 who often have comorbidities such as asthma, allergic rhinoconjunctivitis, food allergies and mental illnesses. Evidence shows an increased prevalence of depression, anxiety and conduct disorder in children severely affected by atopic dermatitis.10 Children and adolescents with atopic dermatitis have approximately 50% greater risk of attention deficit hyperactivity disorder, which is likely a result of sleep disturbance.11 Adults with atopic dermatitis are three times as likely to have depression compared with healthy individuals,12 and the prevalence of suicidal ideation exceeds 20%.13
Atopic dermatitis also places a tremendous financial burden on patients, their families, and society, through direct medical costs and decreased productivity.14, 15 Severe atopic dermatitis costs over AUS$ 6000 annually per child in direct medical, hospital and treatment costs, as well as time off work and distress for caregivers and the family.16
Mild atopic dermatitis is typically managed in primary care by avoiding irritants and disease triggers and by using emollients (moisturisers) and standard topical anti‐inflammatory therapies such as corticosteroids, calcineurin inhibitors and phosphodiesterase type 4 inhibitors.17 Other therapies used to manage atopic dermatitis include systemic agents such as corticosteroids, biologics and other immunosuppressants; phototherapy; and other agents (such as antihistamines and antibacterial medicines). The Australian management consensus recommends referral to a dermatologist or immunologist if atopic dermatitis is not responsive to standard treatment, causes significant distress and interferes with sleep, school or work, an allergy is suspected, or there are recurrent infections.17
Although the burden of atopic dermatitis is said to be increasing in Australia,3, 18 national information about the prevalence and particularly the incidence of atopic dermatitis is limited. Using routinely collected general practice data from the MedicineInsight program, this study aimed to 1) determine the prevalence and incidence of atopic dermatitis among patients seen in Australian general practice; 2) describe the use of medicines for the management of patients with atopic dermatitis; and 3) classify patients with atopic dermatitis by disease severity and assess relevant comorbidities.
Methods
Study design and data source
We conducted a descriptive study using MedicineInsight, a national general practice data program developed and managed by NPS MedicineWise with funding support from the Australian Government Department of Health.19 MedicineInsight extracts and collates longitudinal, de‐identified patient health records, including demographics, encounters (excluding progress notes), diagnoses, prescriptions and pathology tests from MedicalDirector and Best Practice clinical information systems. On 1 July 2019, MedicineInsight included records for about 3.5 million regular patients (approximately 15% of the Australian population) from more than 5000 GPs in 715 general practices across Australia. When compared with Medicare Benefits Schedule data, the characteristics of current regular MedicineInsight patients are broadly comparable to those of patients who visited a GP in 2016–17.19
Study participants
We included patients who had valid information for age and gender and were regular attenders (at least three consultations between 1 January 2017 and 31 December 2018 in accordance with the Royal Australian College of General Practitioners’ (RACGP) definition of an active patient) at a practice that met data quality requirements (described elsewhere).19
A sub‐cohort was used to estimate the incidence of atopic dermatitis and included patients from the main study population (Figure S1) who met additional inclusion criteria: did not have a diagnosis of atopic dermatitis recorded prior to 1 January 2018 (historical diagnosis) and had at least 12 months of follow‐up (attendance) at the practice prior to 1 January 2018.
Atopic dermatitis diagnoses
We developed condition coding algorithms using information from the diagnosis (medical history), reason for encounter and reason for prescription fields, and included both coded and free‐text data. Patients were defined as having atopic dermatitis if they had a relevant term recorded at any time (ever) from their earliest record until the end of the study: ‘atopic dermatitis’; or ‘eczema’ (except seborrhoeic); or ‘other dermatitis (not seborrhoeic) and a personal/family history of atopy (ever)’ (Fig. 1). ‘Atopic conditions’ included atopy, atopic dermatitis, eczema, asthma, allergic rhinoconjunctivitis and food allergies including milk (dairy), eggs, nuts (tree and peanuts), sesame, fish and seafood. A full list of included terms is in Table S1.
Figure 1.
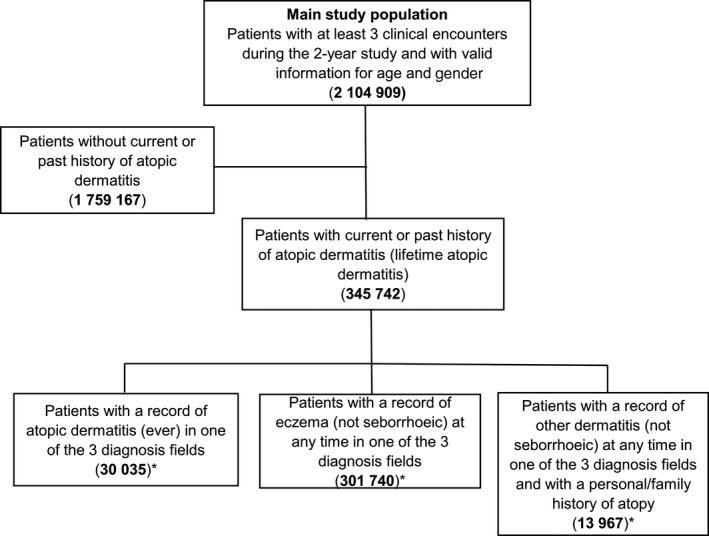
Flow chart for selection of the main study population and atopic dermatitis prevalent population. *Patients in these groups are mutually exclusive, with the order of priority being ‘atopic dermatitis’, then ‘eczema’ and then ‘dermatitis (other)’ plus personal/family history of atopy. That is, patients with recorded ‘atopic dermatitis’ are counted first, then those with recorded ‘eczema’ (and no recorded ‘atopic dermatitis’) and then the rest.
Management of atopic dermatitis
We described the type of specified atopic dermatitis medications prescribed and eosinophil count tests, among patients with atopic dermatitis. While not a diagnostic test, an elevated eosinophil count can support the diagnosis of atopy.20
Medicine groups assessed included topical corticosteroids (TCS), topical calcineurin inhibitors (TCI), systemic corticosteroids, systemic immunosuppressants (ciclosporin, methotrexate, azathioprine or mycophenolate) and antibacterial medicines (mupirocin, dicloxacillin, flucloxacillin, cefalexin, clindamycin [non‐topical form], trimethoprim/sulfamethoxazole or erythromycin). The classification of topical corticosteroids by potency is described in Table S2.
MedicineInsight prescribing information includes whether the prescription is marked as eligible for Australian Government subsidy (under the Pharmaceutical Benefits Scheme [PBS] or the Repatriation Pharmaceutical Benefits Scheme [RPBS], which is available to specified war veterans and their families) or not eligible, namely ‘private’. Medicines were identified using the Anatomical Therapeutic Chemical Classification System code, ‘medicine active ingredient’, ‘medicine name’ and, where required, the route of administration and strength. A patient was defined as having one of the assessed medicine groups if they had at least one recorded relevant prescription in the 2‐year study period. Since it is not mandatory for prescribers to record a reason for prescription, this field is often incomplete. Therefore, some of the specified atopic dermatitis medicine prescriptions counted for the patients with atopic dermatitis may have been for indications other than atopic dermatitis.
A patient was defined as having an eosinophil count test result if they had at least one properly recorded test result in the 2‐year study period – identified by the relevant Logical Observation Identifiers Names and Codes. Eosinophilia was defined as at least one eosinophil count test result where the upper limit value exceeded the laboratory’s normal reference range for that patient.
Classification of atopic dermatitis severity
There is no validated measure of atopic dermatitis severity for routinely collected clinical data. Data, such as objective signs and subjective symptoms on which validated measures such as the SCORing Atopic Dermatitis index are based, are not available in MedicineInsight, and this presents challenges in classifying patients according to severity. For this analysis, patients were classified into two categories – ‘mild’ or ‘moderate‐to‐severe' – in accordance with the Australian consensus recommendations for management of atopic dermatitis.17 A record of systemic therapies for atopic dermatitis or a referral to a specialist were used as indicators of disease severity in this study.
Patients with atopic dermatitis were classified as having ‘moderate‐to‐severe’ atopic dermatitis if they had either a prescription for systemic immunosuppressants or corticosteroids or if they had a referral to a dermatologist/immunologist/allergist, recorded during the study period. Otherwise, they were classified as having ‘mild’ atopic dermatitis.
Outcomes
Prevalence
Atopic dermatitis is a chronic relapsing condition where some patients outgrow the disease. Because it is difficult to define current atopic dermatitis in routinely collected data, both lifetime and current prevalence estimates were assessed.
Lifetime prevalence was estimated as the proportion of the main study population who had current, or a past history of, atopic dermatitis (ever‐recorded diagnosis).
Current prevalence was estimated as the proportion of the main study population who had a diagnosis of atopic dermatitis recorded during the 2‐year study period.
Incidence
The incidence of atopic dermatitis was estimated as the proportion of the sub‐analysis cohort (Figure S1) who had a new diagnosis of atopic dermatitis recorded for the first time during 2018.
Covariates
Socio‐demographic characteristics included age (based on year of birth), gender, state/territory, Socio‐Economic Indexes for Areas (SEIFA) and remoteness. State/territory, remoteness and SEIFA were based on patients’ residential postcodes. Remoteness was determined in accordance with the ABS geographical framework ‘Remoteness Areas’.21 SEIFA was determined according to the Australian Bureau of Statistics Index of Relative Socio‐Economic Advantage and Disadvantage (IRSAD).22 IRSAD is an indicator of relative economic and social advantage/disadvantage position within an area compared with the rest of the country.
We assessed the presence of relevant comorbidities including insomnia, anxiety and depression (see Table S1 for detailed clinical definitions).
Statistical analysis
We used descriptive statistics including frequencies, proportions and relative risks adjusted for confounding factors, age and gender. 95% confidence intervals (CIs) were adjusted for clustering by practice site. Non‐overlap of 95% CIs was used to determine significant differences between groups.23 Severity of atopic dermatitis and medications were assessed among patients with an ever‐recorded diagnosis of atopic dermatitis (‘lifetime atopic dermatitis’). Data management and analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Ethics
Approval to conduct this study was granted by the MedicineInsight independent Data Governance Committee (reference number: 2018‐014). NPS MedicineWise has RACGP NREEC ethics approval (NREEC 17‐017) for the standard operation and the use of the MedicineInsight program by NPS MedicineWise.
RESULTS
Characteristics of the main study population
The main study population comprised 2 104 909 regular patients from 494 general practices (Fig. 1). Of this population, 56.6% were female, 63.7% resided in major cities, and the median age was 43.0 years.
Characteristics of patients with atopic dermatitis
There were 345 742 patients with a current or historical (lifetime) diagnosis of atopic dermatitis recorded, of whom 21.4% were classified as having ‘moderate‐to‐severe' disease (data not shown). Just over one in seven patients (14.9%) with atopic dermatitis were aged below 10 years (Table 1).
Table 1.
Socio‐demographic characteristics of patients with and without atopic dermatitis (ever) (2017–2018)
| Characteristic |
Patients with atopic dermatitis (ever) (N = 345 742) |
Patients without atopic dermatitis (N = 1 759 167) |
||
|---|---|---|---|---|
| Number | % (95% CI) | Number | % (95% CI) | |
| Gender | ||||
| Male | 140 182 | 40.5 (40.0, 41.1) | 773 875 | 44.0 (43.5, 44.4) |
| Female | 205 560 | 59.5 (58.9, 60.0) | 985 292 | 56.0 (55.6, 56.5) |
| Age group (years) | ||||
| 0–4 | 27 111 | 7.8 (7.2, 8.5) | 117 268 | 6.7 (6.4, 7.0) |
| 5–9 | 24 285 | 7.0 (6.7, 7.3) | 85 558 | 4.9 (4.7, 5.0) |
| 10–14 | 18 382 | 5.3 (5.1, 5.5) | 71 351 | 4.1 (3.9, 4.2) |
| 15–19 | 17 159 | 5.0 (4.8, 5.2) | 82 615 | 4.7 (4.5, 4.8) |
| 20–24 | 16 648 | 4.8 (4.6, 5.0) | 101 685 | 5.8 (5.4, 6.2) |
| 25–29 | 15 766 | 4.6 (4.3, 4.8) | 113 691 | 6.5 (6.1, 6.8) |
| 30–34 | 16 375 | 4.7 (4.5, 5.0) | 125 141 | 7.1 (6.8, 7.5) |
| 35–39 | 17 131 | 4.9 (4.7, 5.2) | 124 109 | 7.1 (6.8, 7.3) |
| 40–44 | 16 859 | 4.9 (4.7, 5.0) | 113 550 | 6.4 (6.3, 6.6) |
| 45–49 | 19 206 | 5.6 (5.4, 5.7) | 122 939 | 7.0 (6.8, 7.1) |
| 50–54 | 19 106 | 5.5 (5.4, 5.7) | 114 209 | 6.5 (6.4, 6.6) |
| 55–59 | 21 664 | 6.3 (6.1, 6.5) | 118 907 | 6.8 (6.6, 6.9) |
| 60–64 | 21 297 | 6.2 (6.0, 6.4) | 110 821 | 6.3 (6.1, 6.5) |
| 65–69 | 22 402 | 6.5 (6.2, 6.7) | 104 068 | 5.9 (5.7, 6.2) |
| 70–74 | 22 531 | 6.5 (6.2, 6.8) | 91 724 | 5.2 (4.9, 5.5) |
| 75–79 | 18 123 | 5.2 (4.9, 5.5) | 64 032 | 3.6 (3.4, 3.9) |
| 80–84 | 14 492 | 4.2 (3.9, 4.4) | 45 253 | 2.6 (2.4, 2.8) |
| 85–89 | 9988 | 2.9 (2.7, 3.1) | 30 266 | 1.7 (1.6, 1.8) |
| 90+ | 7217 | 2.1 (1.9, 2.3) | 21 980 | 1.3 (1.1, 1.4) |
| State/territory | ||||
| ACT | 6084 | 1.8 (0.4, 3.1) | 34 764 | 2.0 (0.4, 3.6) |
| NSW | 133 168 | 38.5 (32.7, 44.3) | 690 161 | 39.2 (33.7, 44.7) |
| NT | 3132 | 0.9 (0.2, 1.6) | 30 316 | 1.7 (0.5, 3.0) |
| QLD | 45 073 | 13.0 (9.7, 16.4) | 295 278 | 16.8 (12.9, 20.6) |
| SA | 12 340 | 3.6 (1.6, 5.6) | 45 387 | 2.6 (1.1, 4.1) |
| TAS | 27 360 | 7.9 (4.5, 11.3) | 103 194 | 5.9 (3.3, 8.4) |
| VIC | 87 262 | 25.2 (19.4, 31.0) | 359 929 | 20.5 (15.6, 25.3) |
| WA | 31 323 | 9.1 (5.8, 12.3) | 200 138 | 11.4 (7.4, 15.3) |
| Remoteness | ||||
| Major city | 222 913 | 64.5 (59.1, 69.9) | 1 118 700 | 63.6 (58.7, 68.5) |
| Inner regional | 78 799 | 22.8 (18.5, 27.1) | 417 392 | 23.7 (19.6, 27.8) |
| Outer regional | 40 004 | 11.6 (7.7, 15.4) | 196 000 | 11.1 (8.1, 14.2) |
| Remote/very remote | 4026 | 1.2 (0.5, 1.8) | 27 075 | 1.5 (0.7, 2.4) |
| Socio‐economic status (SEIFA IRSAD quintiles) | ||||
| 1 (least advantaged) | 59 038 | 17.1 (13.4, 20.7) | 265 479 | 15.1 (12.2, 18.0) |
| 2 | 59 526 | 17.2 (14.3, 20.1) | 315 878 | 18.0 (15.2, 20.7) |
| 3 | 75 964 | 22.0 (18.9, 25.1) | 403 309 | 22.9 (19.8, 26.0) |
| 4 | 72 125 | 20.9 (18.2, 23.5) | 378 739 | 21.5 (18.8, 24.2) |
| 5 (most advantaged) | 78 951 | 22.8 (18.9, 26.8) | 394 743 | 22.4 (18.9, 25.9) |
| Data missing | 138 | 0.0 (0.0, 0.1) | 1019 | 0.1 (0.0, 0.1) |
| Comorbid conditions | ||||
| Insomnia | 46 957 | 13.6 (12.9, 14.3) | 128 587 | 7.3 (7.0, 7.6) |
| Anxiety | 70 751 | 20.5 (19.6, 21.3) | 263 427 | 15.0 (14.4, 15.5) |
| Depression | 78 083 | 22.6 (21.7, 23.5) | 296 267 | 16.8 (16.2, 17.4) |
CI, confidence interval; IRSAD, Index of Relative Socio‐economic Advantage and Disadvantage; SEIFA, Socio‐economic Indexes for Areas.
Lifetime and current prevalence of atopic dermatitis
The estimated lifetime prevalence of atopic dermatitis was 16.4% (Table 2). Recorded lifetime prevalence for patients aged below 15 and those ≥65 years, and for patients resident in South Australia, Tasmania and Victoria, was greater than the overall population prevalence. It was greater for females (17.3%; 95% CI 16.4–18.1) than males (15.3%; 95% CI 14.6–16.0).
Table 2.
Lifetime prevalence and current prevalence of atopic dermatitis (2017–2018)
| Characteristic |
Lifetime prevalence of atopic dermatitis (N = 2 104 909) |
Current prevalence of atopic dermatitis (N = 2 104 909) |
|---|---|---|
| % (95% CI) | % (95% CI) | |
| Overall | 16.4 (15.7, 17.2) | 6.3 (6.0, 6.6) |
| Gender | ||
| Male | 15.3 (14.6, 16.0) | 6.1 (5.8, 6.4) |
| Female | 17.3 (16.5, 18.1) | 6.5 (6.2, 6.8) |
| Age group (years) | ||
| 0–4 | 18.8 (17.9, 19.7) | 13.8 (13.1, 14.5) |
| 5–9 | 22.1 (21.1, 23.1) | 8.3 (7.9, 8.7) |
| 10–14 | 20.5 (19.4, 21.6) | 6.5 (6.2, 6.8) |
| 15–19 | 17.2 (16.2, 18.2) | 5.8 (5.5, 6.1) |
| 20–24 | 14.1 (13.1, 15.0) | 5.6 (5.3, 5.9) |
| 25–29 | 12.2 (11.5, 12.9) | 5.2 (4.9, 5.5) |
| 30–34 | 11.6 (10.9, 12.2) | 5.0 (4.8, 5.3) |
| 35–39 | 12.1 (11.5, 12.7) | 4.9 (4.7, 5.2) |
| 40–44 | 12.9 (12.2, 13.6) | 4.8 (4.5, 5.1) |
| 45–49 | 13.5 (12.8, 14.2) | 4.6 (4.3, 4.9) |
| 50–54 | 14.3 (13.6, 15.1) | 4.9 (4.6, 5.2) |
| 55–59 | 15.4 (14.6, 16.2) | 5.3 (4.9, 5.6) |
| 60–64 | 16.1 (15.3, 16.9) | 5.4 (5.1, 5.8) |
| 65–69 | 17.7 (16.8, 18.7) | 5.9 (5.5, 6.3) |
| 70–74 | 19.7 (18.7, 20.8) | 6.5 (6.1, 7.0) |
| 75–79 | 22.1 (20.9, 23.2) | 7.5 (6.9, 8.0) |
| 80–84 | 24.3 (23.0, 25.6) | 8.0 (7.4, 8.6) |
| 85–89 | 24.8 (23.5, 26.2) | 7.6 (7.0, 8.2) |
| 90+ | 24.7 (23.3, 26.1) | 7.1 (6.5, 7.7) |
| State/territory | ||
| ACT | 14.9 (11.3, 18.5) | 7.0 (4.9, 9.0) |
| NSW | 16.2 (15.0, 17.4) | 6.2 (5.7, 6.7) |
| NT | 9.4 (7.9, 10.8) | 4.0 (2.9, 5.1) |
| QLD | 13.2 (11.9, 14.5) | 5.3 (4.7, 5.8) |
| SA | 21.4 (17.6, 25.2) | 6.5 (5.6, 7.5) |
| TAS | 21.0 (19.0, 22.9) | 7.0 (5.9, 8.0) |
| VIC | 19.5 (17.8, 21.2) | 7.6 (7.0, 8.3) |
| WA | 13.5 (11.7, 15.3) | 5.6 (5.0, 6.3) |
| Remoteness | ||
| Major city | 16.6 (15.6, 17.6) | 6.6 (6.3, 7.0) |
| Inner regional | 15.9 (14.7, 17.1) | 5.7 (5.2, 6.1) |
| Outer regional | 16.9 (14.9, 19.0) | 6.0 (5.1, 6.8) |
| Remote/very remote | 12.9 (9.8, 16.1) | 5.2 (4.1, 6.3) |
| Socio‐economic status (SEIFA IRSAD quintiles) | ||
| 1 (least advantaged) | 18.2 (16.6, 19.8) | 6.6 (5.8, 7.3) |
| 2 | 15.9 (14.8, 16.9) | 6.1 (5.6, 6.6) |
| 3 | 15.8 (14.7, 17.0) | 6.2 (5.8, 6.6) |
| 4 | 16.0 (14.9, 17.1) | 6.4 (5.9, 6.9) |
| 5 (most advantaged) | 16.7 (15.4, 17.9) | 6.3 (5.9, 6.8) |
CI, confidence interval; IRSAD, Index of Relative Socio‐economic Advantage and Disadvantage; SEIFA, Socio‐economic Indexes for Areas.
The estimated current prevalence of atopic dermatitis was 6.3% (Table 2). Recorded current prevalence was greater for patients aged 0–4 years (13.8%) than for other age groups; greater for residents of major cities than inner regional and remote areas; and greater for residents of Victoria than Northern Territory, Queensland, Western Australia and New South Wales. The socio‐demographic characteristics of patients with ‘current atopic dermatitis’ were similar to those with ‘lifetime atopic dermatitis’ (Table S3).
Incidence of atopic dermatitis
Of the 1.35 million patients in the sub‐analysis cohort, 27 414 were diagnosed with atopic dermatitis during 2018, giving an estimated incidence of 2.0% (Table 3). Incidence was greater for patients aged 0–4 years (3.9%) than for other age groups, and greater for females (2.2%; 95% CI 2.1–2.3) than males (1.9%; 95% CI 1.8–2.0) (Table 3). The incidence of atopic dermatitis was similar between sexes for those aged 0–4 years, but higher in female than male patients aged 5–39 and 45–54 years (Fig. 2). The socio‐demographic characteristics of the sub‐analysis cohort and patients with incident atopic dermatitis are presented in Table S4.
Table 3.
Incidence of atopic dermatitis in 2018
| Characteristic |
Sub‐analysis cohort (N = 1 349 224) |
Patients with incident atopic dermatitis | |
|---|---|---|---|
| Number | % (95% CI) | ||
| Overall | 1 349 224 | 27 414 | 2.0 (1.9, 2.1) |
| Gender | |||
| Male | 592 491 | 11 079 | 1.9 (1.8, 2.0) |
| Female | 756 733 | 16 335 | 2.2 (2.1, 2.3) |
| Age group (years) | |||
| 0–4 | 51 828 | 2017 | 3.9 (3.6, 4.2) |
| 5–9 | 67 623 | 1727 | 2.5 (2.4, 2.7) |
| 10–14 | 58 001 | 1250 | 2.2 (2.0, 2.3) |
| 15–19 | 64 222 | 1311 | 2.0 (1.9, 2.2) |
| 20–24 | 68 658 | 1309 | 1.9 (1.8, 2.0) |
| 25–29 | 72 959 | 1288 | 1.8 (1.6, 1.9) |
| 30–34 | 84 554 | 1535 | 1.8 (1.7, 2.0) |
| 35–39 | 90 546 | 1642 | 1.8 (1.7, 1.9) |
| 40–44 | 88 823 | 1560 | 1.8 (1.6, 1.9) |
| 45–49 | 100 092 | 1628 | 1.6 (1.5, 1.7) |
| 50–54 | 95 017 | 1581 | 1.7 (1.5, 1.8) |
| 55–59 | 100 594 | 1842 | 1.8 (1.7, 2.0) |
| 60–64 | 94 523 | 1765 | 1.9 (1.7, 2.0) |
| 65–69 | 89 736 | 1837 | 2.1 (1.9, 2.2) |
| 70–74 | 80 413 | 1744 | 2.2 (2.0, 2.3) |
| 75–79 | 57 053 | 1388 | 2.4 (2.2, 2.6) |
| 80–84 | 40 061 | 1048 | 2.6 (2.4, 2.8) |
| 85–89 | 26 156 | 600 | 2.3 (2.1, 2.5) |
| 90+ | 18 365 | 342 | 1.9 (1.6, 2.1) |
CI, confidence interval.
Figure 2.
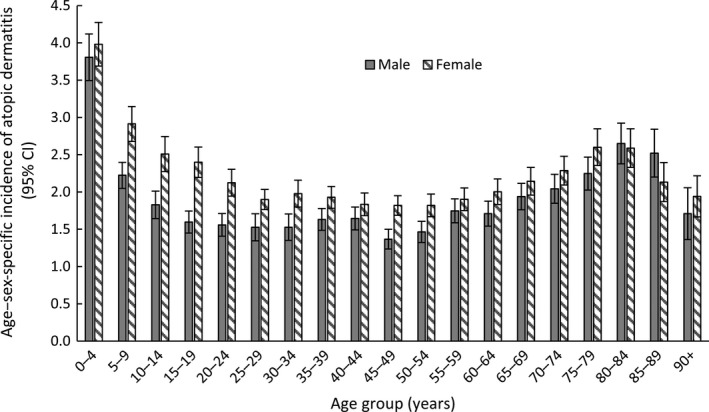
Age–sex‐specific incidence of atopic dermatitis in 2018.
Comorbid conditions
Among 345 742 patients who had ever had atopic dermatitis, 46 957 (13.6%) had a record of insomnia, 70 751 (20.5%) anxiety and 78 083 (22.6%) depression (Table 1). After adjusting for age and gender, having atopic dermatitis increased the risk of insomnia, anxiety and depression by 79%, 44% and 41%, respectively, compared with the general patient population without atopic dermatitis (data not shown).
Management of atopic dermatitis
Topical corticosteroids were the most commonly prescribed group of medicines with one in three patients with atopic dermatitis having at least one prescription for a topical corticosteroid during the study period (Table 4). The most commonly prescribed class of topical corticosteroids was potent TCS (22.5%), followed by moderate (11.5%), mild (7.5%) and very potent TCS (0.8%). The least prescribed group of medicines were topical calcineurin inhibitors (0.1%). Among patients with ‘lifetime atopic dermatitis’, 15.6% had at least one prescription for systemic corticosteroids, 0.9% had at least one prescription for other systemic immunosuppressants; 40.3% did not have a recorded prescription for any of the specified medicine groups (topical corticosteroids, topical calcineurin inhibitors, systemic agents and antibacterial medicines) during the 2‐year study period.
Table 4.
Number and proportion of patients with at least one recorded prescription in any of the atopic dermatitis medicine groups (2017–2018)
| Medicine group |
Patients with atopic dermatitis (ever) † (N = 345 742) |
% of patients with atopic dermatitis (ever) | 95% CI |
|---|---|---|---|
| Topical corticosteroids | 126 096 | 36.5 | 35.6, 37.3 |
| Mild | 25 888 | 7.5 | 7.1, 7.9 |
| Moderate | 39 608 | 11.5 | 10.9, 12.0 |
| Potent | 77 806 | 22.5 | 21.8, 23.2 |
| Very potent | 2767 | 0.8 | 0.7, 0.9 |
| Topical calcineurin inhibitors | 250 | 0.1 | 0.0, 0.1 |
| Systemic corticosteroids | 53 944 | 15.6 | 14.9, 16.4 |
| Systemic immunosuppressants | 3001 | 0.9 | 0.8, 0.9 |
| Antibacterial medicines ‡ | 112 092 | 32.4 | 31.6, 33.2 |
Note that the number of patients in each medicine group and in each class of topical corticosteroids is not mutually exclusive as some patients may be prescribed medicines from more than one group and/or from more than one class of topical corticosteroids.
Antibacterial medicines included in this analysis are mupirocin, dicloxacillin, flucloxacillin, cefalexin, clindamycin (non‐topical form), trimethoprim/sulfamethoxazole and erythromycin.
Patients with atopic dermatitis were more likely to have an eosinophil count recorded (59.5%; 95% CI 58.2–60.8) than patients without atopic dermatitis (54.2%; 95% CI 53.0–55.4). Patients with atopic dermatitis were 60% more likely to have eosinophilia compared to those without atopic dermatitis (age–sex‐adjusted relative risk 1.6; 95% CI 1.5–1.6) (data not shown).
Discussion
This is the first large‐scale Australian study to estimate the prevalence, incidence and management of atopic dermatitis using routinely collected general practice data. Over 2.1 million regular patients, including 345 742 identified as having atopic dermatitis, were included. As atopic dermatitis is a chronic relapsing condition with some patients outgrowing the disease, we estimated both the lifetime prevalence (current or past history of atopic dermatitis) and current prevalence (atopic dermatitis recorded during the 2‐year study period), in order to provide a more comprehensive picture.
The estimated lifetime prevalence of atopic dermatitis in the Australian general practice setting was 16.4%, and the current prevalence was 6.3%. As expected, the prevalence of current atopic dermatitis was highest for younger patients but was more common for older patients than previously recognised.24 The high prevalence of eczema among older MedicineInsight patients could represent a true finding or an overestimate resulting from the relative incompleteness of records among younger, healthier patients compared to older patients; mortality attrition bias arising if deceased patients are less likely to have eczema recorded; and misclassification bias from the inclusion of patients with ‘other dermatitis and a family or personal history of atopy’ and historical eczema diagnoses that resolved.
A population‐based study in Melbourne estimated current prevalence (eczema symptoms in the past year) of eczema in 4‐year‐olds as 16% in 2017 and lifetime prevalence (ever‐recorded diagnosis) as 27.6%.6 Our study found current and lifetime prevalence of AD in patients aged 0–4 years of 13.8% and 18.8%, respectively. Two other population‐based studies in Victoria also reported estimated current prevalence of atopic dermatitis, one in school‐aged children as 16.3%25 and the other in adults aged 20 and over as 6.9%26. Both studies used a survey followed by physical examination for identifying atopic dermatitis and reported higher prevalence of mild (and minimal) atopic dermatitis, compared to our patient cohort, which may explain the higher overall prevalence in these studies compared to ours. Evidence from the International Study of Asthma and Allergies in Childhood demonstrated a wide variation in the current prevalence of eczema globally – from 0.9% in India to 22.5% in Ecuador for 6‐ to 7‐year‐olds and from 0.2% in China to 24.6% in Colombia for 13‐ to 14‐year‐olds.2 This variation may reflect true differences, or disparate study designs, sampling methodologies, definitions of eczema and reported age groups.
Incidence of atopic dermatitis in the general practice setting was 2.0% in 2018. The highest incidence of atopic dermatitis was in the youngest age group (0–4 years: 3.9%). Gender‐related differences were observed for some age groups, namely a higher incidence among females than males aged 5–39 and 45–54 years. This is one of the few studies to report the incidence of atopic dermatitis in Australia.
Topical corticosteroids are first‐line treatment and very effective for most cases of atopic dermatitis.27 Just over a third of patients with atopic dermatitis had a prescription for topical corticosteroids during the study period. Potent topical corticosteroids were the most common class of TCS and included methylprednisolone, mometasone furoate and betamethasone dipropionate. As topical calcineurin inhibitors are second‐line atopic dermatitis therapy for patients where topical corticosteroid therapy is contraindicated or has failed,27, 28, 29 and may be more expensive than their corresponding class of topical corticosteroids,30 this may explain the very small proportion of patients prescribed them during the 2‐year study. Non‐corticosteroid immunosuppressants, generally prescribed by specialists, were not surprisingly, sparsely recorded.
Guidelines recommend oral corticosteroids be used for atopic dermatitis only with careful consideration.17, 28, 29 Our finding that one in six patients with atopic dermatitis were prescribed oral corticosteroids is potentially concerning. Several factors might explain the relatively high use of oral corticosteroids in this cohort of atopic dermatitis patients including potential gaps in consumer and GP knowledge about optimising the use of topical therapies before moving to oral corticosteroids, and use for conditions other than atopic dermatitis.
About 40% of patients with a history of atopic dermatitis did not have a prescription for any of the specified medicine groups (topical corticosteroids, topical calcineurin inhibitors, systemic agents and antibacterial medicines) over the 2 years of the study. It is possible that some patients may have outgrown the disease; obtained their prescriptions from a non‐MedicineInsight GP or a specialist; and been managed only with over‐the‐counter therapies (such as mild topical corticosteroids or emollients) or with other therapies not assessed in this study.
We found that patients with atopic dermatitis were more likely to have eosinophilia than those without atopic dermatitis, which is similar to findings reported elsewhere.31
Consistent with other studies,10, 11, 12 our findings demonstrate that patients with atopic dermatitis were at increased risk of comorbidities, including insomnia, anxiety and depression, compared to patients with no record of atopic dermatitis. This reinforces the importance of health professionals addressing comorbidities which decrease the quality of life for patients with atopic dermatitis.17
The strengths of this analysis include the size and national coverage of the MedicineInsight data. As MedicineInsight is an open cohort and patients in Australia can visit multiple general practices, we used a cohort of regularly attending patients, likely to be receiving most of their care at a MedicineInsight practice, to help improve data quality. The use of clinical records reduces subjective biases found in self‐reported health surveys, because these data comprise GP‐identified diagnoses and medicines prescribed for patients. MedicineInsight has comprehensive medicines information including both PBS/RPBS‐subsidised and private (non‐subsidised) medicines.
These data have limitations, in addition to those inherent in routinely collected data described elsewhere.19 For privacy reasons, MedicineInsight does not include data from progress notes, which may contain further clinical information. Depending on individual GP recording practices, a diagnosis for a current condition may have only been recorded historically, and not routinely, thus underestimating current prevalence. Utilisation of medicines, particularly mild TCS (including 0.5% and 1% hydrocortisone preparations which are available over the counter), may have been underestimated. Some people may have been prescribed a relevant medication (such as oral prednisolone) for a different condition – because the reason for prescription was not commonly recorded, we could not directly link relevant prescriptions with the diagnosis of atopic dermatitis. Lastly, while our definition of ‘moderate‐to‐severe' atopic dermatitis would have identified most patients with severe atopic dermatitis, patients with moderate disease may have been underestimated if they did not use systemic therapies or have a record for referral to a specialist.
Our findings provide important insights about the epidemiology of atopic dermatitis and its management in Australian general practice. This information is useful for planning effective interventions to support GPs, and other primary care providers, in the optimal management of their patients with atopic dermatitis, particularly those experiencing uncontrolled atopic dermatitis and acute flares.
Supporting information
Figure S1 Flowchart for the sub‐analysis cohort and incident atopic dermatitis population.
Table S1 Clinical definitions used to identify atopic and co‐morbid conditions.
Table S2 Classification of topical corticosteroids by potency.
Table S3 Socio‐demographic characteristics for patients with ‘current’ atopic dermatitis (2017–2018).
Table S4 Socio‐demographic characteristics for the sub‐analysis cohort and patients with incident atopic dermatitis (2018).
Acknowledgements
We are grateful to the general practices, general practitioners and patients who allow the use of de‐identified information for MedicineInsight. We thank the Research Advisory Group, particularly Judy Wettenhall, and NPS MedicineWise staff Margaret Wall, Lisa Quick, Emma Grahame, Sarah Norman and Isla Hains for their valuable input. This study was supported by an unrestricted grant from Pfizer Pty Ltd (Australia) to VentureWise Pty Ltd, who commissioned NPS MedicineWise to design and develop this study. The study was conducted with complete independence from Pfizer Pty Ltd, who did not have any input into the design or content of the study or into this publication.
Conflict of interest: DR is an Advisory Board member, consultant or principal investigator for the following: Abbvie, Eli Lilly, MSD, Pfizer and Sanofi Genzyme. SS received speaking fees and research support from AbbVie, BMS, Ego, Eli Lilly and Company, Janssen‐Cilag, Leo Pharma, and Novartis, and has been an advisory board member for AbbVie, Ego, Eli Lilly and Company, Janssen‐Cilag, Johnson and Johnson Pacific, Leo Pharma, Novartis, Pfizer and Sanofi Genzyme. The other co‐authors are employees of NPS MedicineWise.
Kendal Chidwick, MSc Epidemiology. Doreen Busingye, PhD Epidemiology. Allan Pollack, FRACS (Orth). Rawa Osman, MPharm. Jeannie Yoo, FRACGP. Suzanne Blogg, MPH. Diana Rubel, FACD. Saxon Smith, FACD.
REFERENCES
- 1. Australasian Society of Clinical Immunology and Allergy . Eczema (Atopic Dermatitis). 2019 27 June 2019 (Accessed 1 July 2019); Available from: https://www.allergy.org.au/patients/skin‐allergy/eczema.
- 2. Odhiambo JA, Williams HC, Clayton TO et al Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J. Allergy Clin. Immunol. 2009; 124: 1251–8.e23. [DOI] [PubMed] [Google Scholar]
- 3. Deckers IA, McLean S, Linssen S et al Investigating international time trends in the incidence and prevalence of atopic eczema 1990–2010: a systematic review of epidemiological studies. PLoS ONE 2012; 7: e39803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann. Nutr. Metab. 2015; 66(Suppl 1): 8–16. [DOI] [PubMed] [Google Scholar]
- 5. Bieber T. Atopic dermatitis. N. Engl. J. Med. 2008; 358: 1483–94. [DOI] [PubMed] [Google Scholar]
- 6. Peters RL, Koplin JJ, Gurrin LC et al The prevalence of food allergy and other allergic diseases in early childhood in a population‐based study: HealthNuts age 4‐year follow‐up. J. Allergy Clin. Immunol. 2017; 140: 145–53.e8. [DOI] [PubMed] [Google Scholar]
- 7. Martin PE, Koplin JJ, Eckert JK et al The prevalence and socio‐demographic risk factors of clinical eczema in infancy: a population‐based observational study. Clin. Exp. Allergy 2013; 43: 642–51. [DOI] [PubMed] [Google Scholar]
- 8. Harris VR, Cooper AJ. Atopic dermatitis: the new frontier. Med. J. Aust. 2017; 207: 351–6. [DOI] [PubMed] [Google Scholar]
- 9. Weidinger S, Novak N. Atopic dermatitis. Lancet 2016; 387: 1109–22. [DOI] [PubMed] [Google Scholar]
- 10. Yaghmaie P, Koudelka CW, Simpson EL. Mental health comorbidity in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2013; 131: 428–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schmitt J, Buske‐Kirschbaum A, Roessner V. Is atopic disease a risk factor for attention‐deficit/hyperactivity disorder? A systematic review. Allergy 2010; 65: 1506–24. [DOI] [PubMed] [Google Scholar]
- 12. Dalgard FJ, Gieler U, Tomas‐Aragones L et al The psychological burden of skin diseases: a cross‐sectional multicenter study among dermatological out‐patients in 13 European countries. J. Invest. Dermatol. 2015; 135: 984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dieris‐Hirche J, Gieler U, Petrak F et al Suicidal ideation in adult patients with atopic dermatitis: a german cross‐sectional study. Acta Derm. Venereol. 2017; 97: 1189–95. [DOI] [PubMed] [Google Scholar]
- 14. Drucker AM, Wang AR, Li WQ et al The burden of atopic dermatitis: summary of a report for the national eczema association. J. Invest. Dermatol. 2017; 137: 26–30. [DOI] [PubMed] [Google Scholar]
- 15. Adamson AS. The economics burden of atopic dermatitis. Adv. Exp. Med. Biol. 2017; 1027: 79–92. [DOI] [PubMed] [Google Scholar]
- 16. Mooney E, Rademaker M, Dailey R et al Adverse effects of topical corticosteroids in paediatric eczema: Australasian consensus statement. Australas. J. Dermatol. 2015; 56: 241–51. [DOI] [PubMed] [Google Scholar]
- 17. Smith S, Baker C, Gebauer K et al Atopic dermatitis in adults: An Australian management consensus. Australas. J. Dermatol. 2019; 61: 23–32. [DOI] [PubMed] [Google Scholar]
- 18. Williams H, Stewart A, von Mutius E et al Is eczema really on the increase worldwide? J Allergy Clin Immunol 2008; 121: 947–54.e15. [DOI] [PubMed] [Google Scholar]
- 19. Busingye D, Gianacas C, Pollack A et al Data Resource Profile: MedicineInsight, an Australian national primary health care database. Int J Epidemiol 2019. 10.1093/ije/dyz147. [DOI] [PubMed] [Google Scholar]
- 20. Australasian Society of Clinical Immunology and Allergy . Laboratory Tests in the Diagnosis of Allergic Diseases. 2019 January 2010 (Accessed 17 October 2019); Available from: https://www.allergy.org.au/hp/papers/tests‐in‐the‐diagnosis‐of‐allergic‐diseases.
- 21. Australian Bureau of Statistics . Australian Statistical Geography Standard (ASGS): Volume 5 ‐ Remoteness Structure, July 2016. 2018 (Accessed 7 May 2018); Available from: http://www.abs.gov.au/ausstats/abs@.nsf/Latestproducts/1270.0.55.005Main%20Features1July%202016?opendocument&tabname=Summary&prodno=1270.0.55.005&issue=July%202016&num=&view=.
- 22. Australian Bureau of Statistics . Socio‐Economic Indexes for Areas. 2018. (Accessed 7 May 2018); Available from: http://www.abs.gov.au/websitedbs/censushome.nsf/home/seifa.
- 23. Krzywinski M, Altman N. Points of significance: error bars. Nat. Methods 2013; 10: 921–2. [DOI] [PubMed] [Google Scholar]
- 24. Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatol. Clin. 2017; 35: 283–9. [DOI] [PubMed] [Google Scholar]
- 25. Marks R, Kilkenny M, Plunkett A et al The prevalence of common skin conditions in Australian school students: 2. Atopic dermatitis. Br J Dermatol 1999; 140: 468–73. [DOI] [PubMed] [Google Scholar]
- 26. Plunkett A, Merlin K, Gill D et al The frequency of common nonmalignant skin conditions in adults in central Victoria, Australia. Int. J. Dermatol. 1999; 38: 901–8. [DOI] [PubMed] [Google Scholar]
- 27. Eichenfield LF, Tom WL, Berger TG et al Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J. Am. Acad. Dermatol. 2014; 71: 116–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rubel D, Thirumoorthy T, Soebaryo RW et al Consensus guidelines for the management of atopic dermatitis: an Asia‐Pacific perspective. J. Dermatol. 2013; 40: 160–71. [DOI] [PubMed] [Google Scholar]
- 29. Wollenberg A, Barbarot S, Bieber T et al Consensus‐based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J. Eur. Acad. Dermatol. Venereol. 2018; 32: 657–82. [DOI] [PubMed] [Google Scholar]
- 30. Strathie Page S, Weston S, Loh R. Atopic dermatitis in children. Aust. Fam. Physician 2016; 45: 293–6. [PubMed] [Google Scholar]
- 31. Jenerowicz D, Czarnecka‐Operacz M, Silny W. Peripheral blood eosinophilia in atopic dermatitis. Acta Dermatovenerol. Alp. Pannonica Adriat. 2007; 16: 47–52. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Flowchart for the sub‐analysis cohort and incident atopic dermatitis population.
Table S1 Clinical definitions used to identify atopic and co‐morbid conditions.
Table S2 Classification of topical corticosteroids by potency.
Table S3 Socio‐demographic characteristics for patients with ‘current’ atopic dermatitis (2017–2018).
Table S4 Socio‐demographic characteristics for the sub‐analysis cohort and patients with incident atopic dermatitis (2018).


