Abstract
Learning that certain cues or environments predict threat enhances survival by promoting appropriate fear and the resulting defensive responses. Adapting to changing stimulus contingencies by learning that such cues no longer predict threat, or distinguishing between these threat‐related and other innocuous stimuli, also enhances survival by limiting fear responding in an appropriate manner to conserve resources. Importantly, a failure to inhibit fear in response to harmless stimuli is a feature of certain anxiety and trauma‐related disorders, which are also associated with dysfunction of the neural circuitry underlying learned fear and its inhibition. Interestingly, these disorders are up to twice as common in women, compared to men. Despite this striking sex difference in disease prevalence, the neurobiological factors involved remain poorly understood. This is due in part to the majority of relevant preclinical studies having neglected to include female subjects alongside males, which has greatly hindered progress in this field. However, more recent studies have begun to redress this imbalance and emerging evidence indicates that there are significant sex differences in the inhibition of learned fear and associated neural circuit function. This paper provides a narrative review on sex differences in learned fear and its inhibition through extinction and discrimination, along with the key gonadal hormone and brain mechanisms involved. Understanding the endocrine and neural basis of sex differences in learned fear inhibition may lead to novel insights on the neurobiological mechanisms underlying the enhanced vulnerability to develop anxiety‐related disorders that are observed in women.
Keywords: discrimination, extinction, fear conditioning, female, generalization, male
We review studies on sex differences in learned fear inhibition. Overall, females show less fear extinction and discrimination than males, involving sex differences in the underlying brain areas. Estrogen regulates fear extinction and discrimination in females. Understanding the neurobiological basis of sex differences in learned fear inhibition may shed light on why women are more prone to anxiety, which is linked to deficits in learned fear inhibition and relevant neural circuit function.
Abbreviations
- ACC
anterior cingulate cortex
- BDNF
brain‐derived neurotrophic factor
- BLA
basolateral amygdala
- CeA
central amygdala
- COMT
catechol‐O‐methyltransferase
- CS
conditioned stimulus
- DH
dorsal hippocampus
- ERα
estrogen receptor α
- ERβ
estrogen receptor β
- IL
infralimbic cortex
- La
lateral amygdala
- LTP
long‐term potentiation
- mPFC
medial prefrontal cortex
- pERK
phosphorylated extracellular signal‐regulated kinase
- PFC
prefrontal cortex
- PL
prelimbic cortex
- PTSD
post‐traumatic stress disorder
- SNP
single nucleotide polymorphism
- TrkB
tropomyosin‐related kinase B
- US
unconditioned stimulus
- vmPFC
ventromedial prefrontal cortex
1. INTRODUCTION
Anxiety and trauma‐related disorders can be serious forms of psychiatric disease associated with a huge socioeconomic burden, given their significant lifetime prevalence and inadequate treatment options with currently available psychological therapies and medications. The etiology of anxiety‐related disorders is complex and involves interactions between various genetic and environmental factors (Craske et al., 2017). Epidemiological evidence indicates a clear role for biological sex in determining the vulnerability to develop anxiety‐related disorders, such that their prevalence is up to twice as high in women compared to men (Kornfield, Hantsoo, & Epperson, 2018; Lebron‐Milad & Milad, 2012; McLean, Asnaani, Litz, & Hofmann, 2011; Perrin et al., 2014). Yet even though this risk is widely acknowledged to be markedly greater in women than in men, there is still relatively little known about the neurobiological mechanisms involved.
Certain anxiety‐related disorders are characterized by abnormally persistent emotional memories of fear‐related stimuli, and impaired inhibition of learned fear is thought to be an endophenotype of such diseases, including post‐traumatic stress disorder (PTSD) (Jovanovic, Kazama, Bachevalier, & Davis, 2012; Singewald & Holmes, 2019; Zuj, Palmer, Lommen, & Felmingham, 2016). Investigating the neural circuit basis of learned fear and its inhibition from a translational perspective has been possible using broadly similar experimental paradigms in rodent and human studies. This research has identified several homologous brain areas (e.g., amygdala, hippocampus, prefrontal cortex (PFC); Figure 1) across species that play crucial roles in various Pavlovian fear learning and memory processes, including the inhibition of learned fear. Importantly, this neural circuitry is also implicated in the cognitive and emotional regulation deficits that are key features of anxiety and PTSD (Asok, Kandel, & Rayman, 2019a; Dunsmoor & Paz, 2015; Sevenster, Visser, & D'Hooge, 2018; Tovote, Fadok, & Lüthi, 2015). Preclinical studies determining the neural mechanisms underpinning learned fear inhibition have therefore been pivotal for gaining a better understanding of the pathophysiology underlying these disorders. However, until recently, most of these animal studies have only examined the inhibition of learned fear and its neural circuit basis in males (Lebron‐Milad & Milad, 2012). Investigating the neurobiological underpinnings of sex differences in learned fear inhibition may thus shed light on the mechanisms that mediate the greatly increased prevalence of anxiety‐related disorders that are observed in women in comparison with men.
Figure 1.
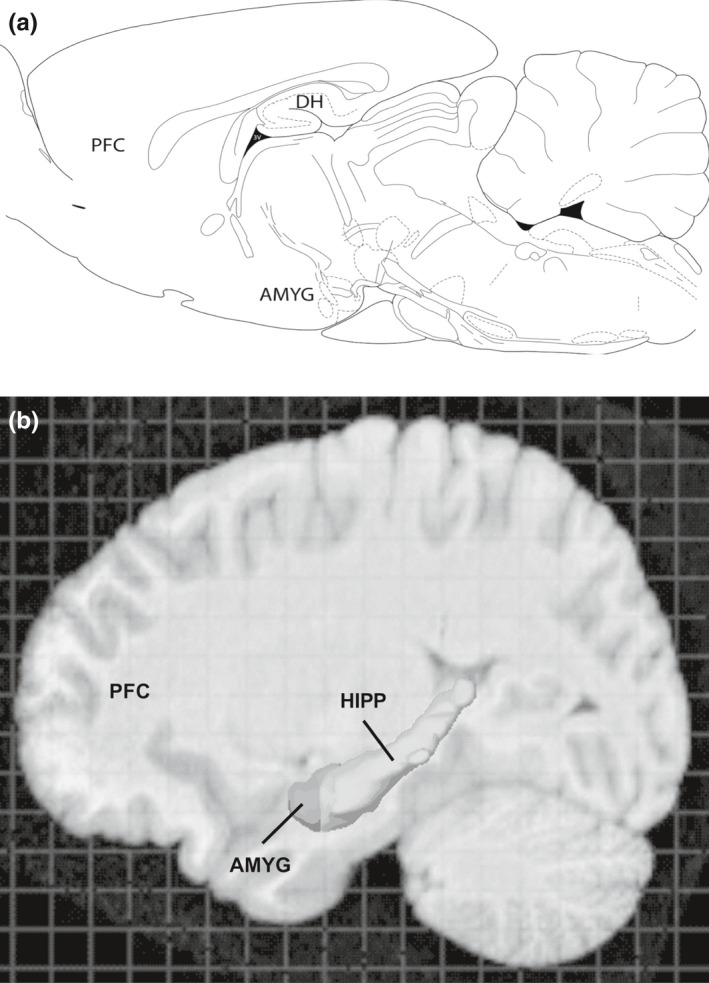
Brain areas involved in learned fear and its inhibition in (a) rats (adapted from Paxinos & Watson, 2007) and (b) humans (adapted from Allen Human Brain Atlas; Ball, Gilbert, & Overly, 2012). AMYG: amygdala; DH; dorsal hippocampus; HIPP: hippocampus; PFC: prefrontal cortex
In this narrative review, we begin by providing a brief overview of the various factors involved in sex differences in anxiety‐related disorders before reviewing the literature on sex differences in Pavlovian fear learning and memory. We then focus on the growing body of evidence from rodent and human studies indicating important sex differences in two types of learned fear inhibition: extinction and discrimination. Our coverage of both fear extinction and fear discrimination distinguishes this review from other recent reviews on the topic (Garcia, Walker, & Zoellner, 2018; Jasnow, Lynch, Gilman, & Riccio, 2017; Li & Graham, 2017; Ramikie & Ressler, 2018; Velasco, Florido, Milad, & Andero, 2019). Emerging evidence indicates that these sex differences in learned fear inhibition involve gonadal hormone signaling and are accompanied by altered function of the underlying neural circuitry. We then conclude with future directions to take this important area of research forward. While there are also key sex differences in the effects of stress on the encoding and inhibition of learned fear (Maeng & Milad, 2015; Merz & Wolf, 2017), this topic is beyond the scope of the present review.
2. OVERVIEW OF SEX DIFFERENCES IN ANXIETY‐RELATED DISORDERS
It is now well established that there are marked sex differences in certain fear‐ and stress‐related psychiatric diseases, such as anxiety‐related disorders. For example, women are up to 60% more likely to suffer from an anxiety disorder and up to twice as likely to suffer from PTSD, compared to men. Women are more likely to be diagnosed with co‐morbid anxiety, eating and mood disorders, and experience a greater burden with illness than men (Lebron‐Milad & Milad, 2012; McLean et al., 2011). Women are also more likely to report greater severity and persistence of PTSD symptoms, with these sex differences remaining when the exposure frequency to traumatic events is equivalent between women and men (Breslau, 2002; Seedat, Stein, & Carey, 2005). The reasons for these discrepancies in disease course between the sexes remain poorly understood but are thought to be multifactorial in nature. These factors include hormonal status, stress reactivity, temperament, cognition, environmental effects and societal influences (Catuzzi & Beck, 2014; McLean & Anderson, 2009). Different trauma types and co‐morbid disorders are associated with different levels of PTSD risk. At the same time, there are differences in the types of trauma to which women and men are more likely to be exposed and in the likelihood of having co‐morbidities that are linked to increased PTSD risk. For example, sexual abuse, which is more commonly and frequently experienced by women than men, is associated with a higher PTSD risk compared to other types of trauma. Such factors might contribute to sex differences in PTSD prevalence, although they are unlikely to account for them fully (Kornfield et al., 2018; Perrin et al., 2014).
Recent research has identified genetic factors involved in moderating anxiety and PTSD risk in a sex‐dependent manner. Several studies have examined the link between gene variants of catechol‐O‐methyltransferase (COMT), an enzyme important for metabolizing the neurotransmitter dopamine in certain brain areas (e.g., PFC), and anxiety‐related traits or disorders in men and women (Harrison & Tunbridge, 2008). Studies have shown an association between COMT genotype and symptoms or traits related to panic and anxiety that was specific to women (Domschke et al., 2004; Olsson et al., 2005; Stein, Fallin, Schork, & Gelernter, 2005), while other studies have reported a link between COMT genotype and anxiety endophenotypes or panic disorder in males only (Konishi, Tanii, Otowa, Sasaki, Motomura, et al., 2014; Konishi, Tanii, Otowa, Sasaki, Tochigi, et al., 2014; Lee & Prescott, 2014). However, some studies have also found an association that was independent of sex, or found no such association in either sex (Hoth et al., 2006; Howe at al., 2016; Wray et al., 2008). Differences in the specific gene variants involved, population demographics (e.g., ethnicity), and the diagnostic or trait criteria used between the studies might help to explain these discrepancies. More recent studies have discovered a role for a variant of the gene encoding the PAC1 receptor in influencing PTSD risk and symptoms specifically in women. The PAC1 receptor mediates signaling by pituitary adenylate cyclase‐activating polypeptide, an important regulator of cellular responses to stress (Hammack & May, 2015). A single nucleotide polymorphism (SNP) in the PAC1 receptor has been linked to PTSD diagnosis and symptom severity selectively in women (Ressler et al., 2011; Almli et al., 2013; Lind et al., 2017; but see also Chang et al., 2012). PAC1 receptor genotype has also been associated with emotional numbing symptoms in traumatized mothers (Wang et al., 2013). Interestingly, this SNP is located in an estrogen response element and a recent study has shown interactions between PAC1 receptor genotype, circulating estradiol levels and symptom severity in women with PTSD (Mercer et al., 2016).
3. SEX DIFFERENCES IN FEAR LEARNING AND MEMORY ENCODING
A feature of phobias and PTSD in particular is the abnormal persistence of emotional memory for fear‐related cues or contexts. Preclinical studies examining various (typically Pavlovian) fear learning and memory processes have proved useful as animal models of relevance to this feature of such anxiety‐related disorders. During Pavlovian fear conditioning, a neutral conditioned stimulus (CS), such as a discrete cue (e.g., sound) or a context (e.g., testing chamber), is associated with an aversive unconditioned stimulus (US; e.g., mild electric shock). Following conditioning, the association between the CS and US consolidates into long‐term memory. Later presentation of the conditioned cue or re‐exposure to the conditioned context initially results in the expression of conditioned fear responding, which has typically been inferred by quantifying freezing behavior elicited by the CS (Tovote et al., 2015).
Rodent studies have investigated sex differences in Pavlovian fear conditioning and memory retrieval. Many studies have shown reduced contextual fear conditioning in females, compared to males, as indicated by decreased freezing during the later retrieval of contextual fear memory (Maren, Oca, & Fanselow, 1994; Gupta, Sen, Diepenhorst, Rudick, & Maren, 2001; Wiltgen, Sanders, Behne, & Fanselow, 2001; Gresack, Schafe, Orr, & Frick, 2009; Chang et al., 2009; Ribeiro et al., 2010; Barker & Galea, 2010; Bethancourt, Vásquez, & Britton, 2011; Daviu, Andero, Armario, & Nadal, 2014; Ujjainwala, Courtney, Wojnowski, Rhodes, & Christian, 2019; Clark, Drummond, Hoyer, & Jacobson, 2019; but see Blume et al., 2017). Sex differences in nociception during conditioning are unlikely to explain this finding given the evidence for enhanced shock sensitivity in females (Dalla & Shors, 2009). Studies have found that females show more locomotor activity than males (Aguilar et al., 2003; Bethancourt et al., 2011; Daviu et al., 2014; Day, Reed, & Stevenson, 2016), raising the possibility that the reduction in contextual fear shown by females simply reflects that they are more active. However, several studies have reported no sex differences in cued fear conditioning (Baker‐Andresen, Flavell, Li, & Bredy, 2013; Baran, Armstrong, Niren, Hanna, & Conrad, 2009; Chen et al., 2014; Clark et al., 2019; Fenton, Halliday, Mason, Bredy, & Stevenson, 2016; Fenton et al., 2014; Maes, 2002; Maren et al., 1994; Markus & Zecevic, 1997). This suggests that greater locomotion in females is unlikely to account for sex differences in contextual fear, although some studies have also found reduced cued fear conditioning in females (Baran, Armstrong, Niren, & Conrad, 2010; Clark et al., 2019; Kosten, Miserendino, Bombace, Lee, & Kim, 2005; Pryce, Lehmann, & Feldon, 1999). However, this reduction in contextual fear conditioning in females agrees with other evidence indicating reduced spatial‐related learning and memory performance more generally in comparison with males (Yagi & Galea, 2019).
Studies have also examined the involvement of gonadal hormones in mediating these sex differences in Pavlovian fear learning and memory encoding. Ovariectomy was shown to enhance contextual fear conditioning in females, such that it abolished sex differences in contextual fear, and estrogen replacement in ovariectomized females reversed this effect (Gupta et al., 2001; Matsuda et al., 2015). Gonadectomy has been found to reduce contextual but not cued fear conditioning in males (McDermott, Liu, & Schrader, 2012). Other studies have found that cued fear expression did not differ across the estrous cycle or after ovariectomy in females, while gonadectomy enhanced cued fear expression in males, and this effect was mitigated by testosterone in an androgen receptor‐dependent manner (Chen et al., 2014; Milad, Igoe, Lebron‐Milad, & Novales, 2009). Moreover, androgen receptor overexpression was found to reduce contextual fear conditioning, which was reversed by gonadectomy or androgen receptor antagonism (Ramzan, Azam, Monks, & Zovkic, 2018). These different roles for gonadal hormones in regulating contextual and cued fear may involve differences in their sites of action in the neural circuitry involved, which is discussed below. Studies on sex differences in fear conditioning in humans are also covered below.
4. SEX DIFFERENCES IN FEAR EXTINCTION
Fear extinction is the reduction in learned fear responding that occurs with repeated presentations of the conditioned cue or prolonged re‐exposure to the conditioned context in the absence of the US. There has been intense interest in delineating the psychological and neurobiological mechanisms underlying this type of learned fear inhibition, given that it forms the theoretical basis for exposure therapy and is deficient in anxiety‐related disorders such as PTSD (Sevenster et al., 2018; Singewald & Holmes, 2019; Tovote et al., 2015; Zuj et al., 2016). Recent studies have shown sex differences in the extinction of Pavlovian fear memory.
4.1. Rodent studies
In studies investigating cued fear extinction, females have been shown to express more learned fear during extinction training and/or subsequent extinction memory testing, suggesting that females show resistance to extinction (Baker‐Andresen et al., 2013; Baran et al., 2009; Clark et al., 2019; Fenton et al., 2014, 2016; Greiner, Müller, Norris, Ng, & Sangha, 2019; Petrovich & Lougee, 2011). In contrast, there has been less research conducted on sex differences in contextual fear extinction and the findings have been mixed. One study found reduced extinction of contextual fear in females, compared to males (Matsuda et al., 2015). Another study reported enhanced contextual fear extinction in females, although the interpretation of this finding is complicated by the decrease in contextual fear shown by females at the start of extinction training (Daviu et al., 2014). Females have been shown to exhibit more spontaneous fear recovery over time after cued or contextual fear extinction, compared to males (Fenton et al., 2014, 2016; Matsuda et al., 2015). Contextual fear expression during cued fear extinction was also found to be enhanced in females relative to males (Baran et al., 2009; Fenton et al., 2014, 2016), raising the possibility that there are sex differences in the contextual regulation of fear extinction. However, another study found no sex differences in fear renewal, which is the return of fear after extinction when the cue is presented in a different context (Maes, 2002). Other studies have found sex differences in fear extinction but only in combination with certain genetic or environmental factors. Ter Horst, Carobrez, Mark, Kloet, and Oitzl (2012) showed that forebrain‐specific deletion of the mineralocorticoid receptor gene reduced fear extinction in females but not in males. Exercise has been shown to improve extinction memory and reduce later fear renewal in males but not females (Bouch et al., 2017).
Whether females show reduced fear extinction in relation to males can be influenced by gonadal hormones. Milad et al. (2009) found no sex differences in cued extinction recall unless variations in estrous cycle phase were accounted for in the females. Extinction during metestrus, when estrogen and progesterone levels are lower, resulted in reduced extinction recall in comparison with males and to females extinguished during proestrus, when estrogen and progesterone levels are higher (Figure 2a). Other studies have found enhanced extinction recall when extinction was conducted during proestrus, compared to other estrous cycle phases (Gruene, Roberts, Thomas, Ronzio, & Shansky, 2015a; Rey, Lipps, & Shansky, 2014). Reduced cued extinction learning has been shown during diestrus, when gonadal hormone levels are also lower, compared to proestrus (Blume et al., 2017). Moreover, estradiol or progesterone given to females undergoing cued extinction during metestrus resulted in enhanced extinction recall, whereas blocking estrogen or progesterone signaling in females extinguished during proestrus reduced extinction recall (Maeng, Cover, et al., 2017a; Milad et al., 2009). Estrogen receptor beta (ERβ), but not estrogen receptor alpha (ERα), agonist treatment before cued fear extinction during metestrus was found to enhance extinction recall, while estradiol given immediately, but not 4 hr, after extinction also enhanced later extinction recall (Zeidan et al., 2011). Ovariectomy or hormonal contraceptive treatment, both of which reduce circulating gonadal hormone levels, has been shown to reduce cued fear extinction in females (Graham & Milad, 2013; Parrish, Bertholomey, Pang, Speth, & Torregrossa, 2019). This effect of hormonal contraceptives can be reversed by ERα or ERβ agonist treatment (Graham & Milad, 2013). Another study found that estradiol given during cued fear extinction enhanced subsequent extinction recall, whereas progesterone had different effects depending on when it was administered relative to extinction. Progesterone facilitated the effect of estradiol on extinction recall when given 6 hr before extinction training but blocked the facilitatory effect of estradiol on extinction recall when given 24 hr before extinction training (Graham & Daher, 2016). Estrogen regulation of cued fear extinction was found to be dose‐dependent and modulated by NMDA receptor signaling (Graham & Scott, 2018a, 2018b). Both testosterone and estrogen can also regulate cued fear extinction in males. Gonadotropin‐releasing hormone agonist treatment before extinction increased circulating testosterone levels and enhanced extinction recall in one study (Maeng, Taha, et al., 2017b), while another study showed that inhibiting estradiol synthesis during extinction impaired later extinction recall in males (Graham & Milad, 2014). These findings indicate that estrogen and testosterone enhance the encoding of cued extinction memory, while progesterone may regulate extinction in a more complex manner.
Figure 2.
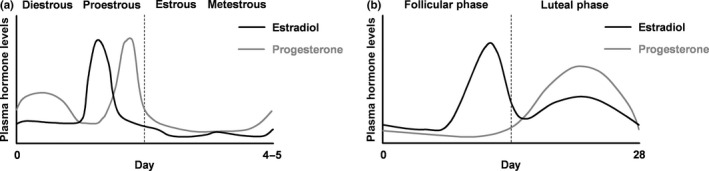
Schematic representation of fluctuations in the levels of estrogen and progesterone over the course of the (a) estrous cycle in female rats and (b) menstrual cycle in women. Vertical dotted lines represent the approximate time of ovulation (adapted from Maeng & Milad, 2015)
The role of gonadal hormones in regulating contextual fear extinction is less clear. One study in naturally cycling females showed reduced extinction learning during proestrus, compared to diestrus (Blume et al., 2017). Another study showed that females conditioned during proestrus and estrus displayed enhanced extinction, compared to males and to females conditioned during diestrus. Estrogen, but not progesterone, also enhanced contextual fear extinction in ovariectomized females. Moreover, ERβ, but not ERα, agonism enhanced contextual fear extinction in ovariectomized females (Chang et al., 2009). However, McDermott, Liu, Ade, and Schrader (2015) showed that chronic treatment with high levels of estradiol reduced contextual fear extinction. These discrepancies might involve methodological differences between the studies (e.g., estrous cycle phase during conditioning vs. extinction, naturally cycling vs. ovariectomized females, acute vs. chronic drug treatment). The potential roles of progesterone and testosterone in modulating contextual fear extinction remain unclear.
Sex differences in the return of learned fear after extinction have also been shown in juveniles, where fear renewal, reinstatement (the return of fear after non‐reinforced US exposure) and spontaneous fear recovery were observed in prepubescent females but not males (Park, Ganella, & Kim, 2017). This suggests that such sex differences in fear relapse after extinction can occur independently of the postpubertal activational effects of gonadal hormones and may instead involve their organizational effects during early development (Donner & Lowry, 2013). Alternatively, these sex differences may occur independently of gonadal hormone signaling and involve genetic and epigenetic effects, or possibly interactions between these different factors (Ratnu, Emami, & Bredy, 2017).
4.2. Human studies
Compared to the rodent literature, there have been far fewer comparable human studies conducted in healthy volunteers that have investigated this issue and demonstrated sex differences in fear extinction, unless variations in the gonadal hormone status of the women were taken into account (see below). One of the first such studies to be conducted found no sex differences in cued fear extinction (Fredrikson, Hugdahl, & Ohman, 1976). A recent study used a cued fear conditioning paradigm with a highly aversive film clip as the US to investigate sex differences in re‐experiencing intrusive memories related to the film clip, which were later elicited by the cue or occurred spontaneously after conditioning (Rattel et al., 2019). Cue‐triggered intrusive memories in this paradigm are thought to model conditioned responding to trauma reminders, which is a feature of PTSD, and may thus resemble real‐life traumatic experiences more than the aversive stimuli that are typically used in such studies (Wegerer, Blechert, Kerschbaum, & Wilhelm, 2013). Women were found to experience more of these intrusive memories and associated distress, compared to men. Moreover, this sex difference in intrusive memories was linked to reduced fear extinction in women, compared to men (Rattel et al., 2019).
As has been reported in rodent studies, sex differences in fear extinction in healthy volunteers emerge when women are stratified according to their gonadal hormone status. Milad et al. (2006) investigated cued fear conditioning and extinction in men and in women in the early or late follicular phases of the menstrual cycle, when estrogen is at lower or higher levels, respectively (Figure 2b). Men showed more fear conditioning than women, who did not differ based on their menstrual cycle phase. During extinction, there were no sex or menstrual cycle phase differences. However, women in the late follicular phase showed reduced extinction recall, compared to men and to women in the early follicular phase, suggesting an inverse relationship between estrogen levels and extinction memory. Another study replicated this sex difference in fear conditioning but found instead that higher levels of estradiol during extinction learning were associated with enhanced extinction recall in women. Furthermore, women with low levels of estradiol during extinction showed reduced extinction recall, compared to men and to women with high levels of estradiol (Milad et al., 2010). This relationship between estradiol levels during extinction and fear during later extinction recall in healthy women has been replicated in subsequent studies (Li & Graham, 2016; White & Graham, 2016; Zeidan et al., 2011). Lower levels of estradiol have also been associated with higher fear during extinction and intrusive memory strength in healthy women (Wegerer, Kerschbaum, Blechert, & Wilhelm, 2014). The contradictory findings of Milad et al. (2006) in comparison with these more recent studies might have resulted from relating extinction recall to the phase of the estrous cycle instead of directly to gonadal hormone levels. It is possible that variability in cycle length, hormone levels or accuracy of cycle phase determination may contribute to such discrepancies (Graham, Li, Black, & Öst, 2018; Pineles et al., 2016). The potential involvement of progesterone and testosterone in contributing to sex differences in fear extinction in humans is still incompletely understood (Milad et al., 2010; Pace‐Schott et al., 2013; Wegerer et al., 2014; Zeidan et al., 2011).
There is evidence for sex differences in fear conditioning and extinction in PTSD. Women with PTSD were found to show enhanced fear conditioning, compared to men with PTSD (Inslicht et al., 2013). Shvil et al. (2014) found that men with PTSD had reduced extinction recall, compared to women with PTSD, whereas there were no sex differences in fear conditioning or extinction in healthy trauma‐exposed controls. Gonadal hormone status can also influence fear extinction in anxiety‐related disorders. One study investigated associations between estradiol levels and fear conditioning and extinction in women with or without PTSD (Glover et al., 2012). There was no link between estradiol and fear conditioning in either group. However, low estradiol levels were associated with higher fear expression during extinction but only in women with PTSD. Another study found that women with PTSD showed reduced extinction recall, compared to women without PTSD. Moreover, while low levels of estradiol and progesterone predicted reduced extinction recall in women without PTSD, reduced extinction recall in women with PTSD was predicted by low estradiol and high progesterone levels (Pineles et al., 2016). Li and Graham (2016) found that, compared to high estradiol levels, low levels of estradiol during extinction in women with spider phobia resulted in greater fear during later extinction recall. Similarly, in spider phobic women undergoing a single session of exposure therapy, hormonal contraceptive use and lower estradiol levels during the session were associated with poorer outcomes post‐treatment (Graham et al., 2018). Taken together, these findings suggest that the relationship between estrogen and extinction is similar in women with or without PTSD, while the role of progesterone is, again, less clear.
5. SEX DIFFERENCES IN FEAR DISCRIMINATION
In addition to aberrant fear memory persistence and impaired fear extinction, certain anxiety‐related disorders are characterized by the overgeneralization of fear in response to harmless cues or contexts. This feature of such disorders has been investigated by examining fear discrimination and generalization using analogous behavioral paradigms across animal and human studies. In these paradigms, one cue or context (CS+) typically becomes associated with the US, while another cue or context (CS−) does not, resulting in the CS+ and CS‐ predicting threat and safety, respectively. Similarly, in conditioned inhibition (e.g., A+/AB−) paradigms, one cue typically becomes associated with the US to predict threat (A+) unless it is presented in conjunction with another cue (B), in which case presentation of the two cues together results in no US presentation (AB−) and instead signals safety (Christianson et al., 2012). Fear discrimination is thought to be a form of learned fear inhibition by the safety signal. Moreover, fear generalization in anxiety‐related disorders has been conceptualized as a deficit in fear inhibition resulting from deficient safety signaling and may represent a biomarker for these disorders (Asok, Kandel, et al., 2019a; Dunsmoor & Paz, 2015; Jovanovic et al., 2012). Emerging evidence indicates that there are sex differences in fear discrimination and safety signaling.
5.1. Rodent studies
Studies have shown less contextual fear discrimination (i.e., more generalization) in females, compared to males (Asok, Hijazi, et al., 2019b; Keiser et al., 2017; Lynch, Cullen, Jasnow, & Riccio, 2013; Reppucci, Kuthyar, & Petrovich, 2013). One study also reported reduced contextual fear discrimination in females, compared to males, in response to predator odor stress (Homiack, O'Cinneide, Hajmurad, Dohanich, & Schrader, 2018). This sex difference in contextual fear discrimination has been shown to depend on the time between conditioning and retrieval memory testing, such that generalization in females only emerged over several days after conditioning (Lynch et al., 2013). However, other studies have found no sex differences in contextual fear discrimination or in the incubation of generalized fear over time (Germer, Kahl, & Fendt, 2019; Keeley, Bye, Trow, & McDonald, 2015). Methodological differences (e.g., conditioned responses used to index fear) between the studies may help to explain these discrepancies. It is also worth noting that studies on sex differences in contextual fear discrimination using naturally cycling females have not systematically investigated the potential contributions of estrous cycle phase or gonadal hormone levels (e.g., compared to the fear extinction literature), which may provide another explanation for these discrepant findings.
Estrogen can influence contextual fear discrimination, but, in contrast to its facilitatory effect on fear extinction, estrogen has been found to reduce fear discrimination in females. Ovariectomy was shown to prevent the emergence of contextual fear generalization over time, whereas ovariectomy combined with estradiol replacement resulted in similar generalized fear over time as in naturally cycling females (Lynch et al., 2013). This effect of estradiol was found to be mediated by regulating memory retrieval in an ERβ‐dependent manner (Lynch et al., 2014). Interestingly, testosterone has been shown to reduce contextual fear generalization in males through its aromatization to estrogen (Lynch, Vanderhoof, et al., 2016a). Gonadectomized males were found to generalize their fear, which was prevented by testosterone or estradiol treatment; the latter effect involved both ERα and ERβ signaling. However, gonadectomized males treated with a non‐aromatizable androgen or an aromatase inhibitor showed fear generalization. Taken together, these findings suggest that estrogen has opposing effects on contextual fear discrimination in females and males, with estrogen reducing discrimination in females and enhancing discrimination in males. A role for progesterone in modulating contextual fear discrimination has yet to be established.
More recent studies have investigated sex differences in cued fear discrimination, but their findings have also been mixed. Males and females have been shown to discriminate and generalize, respectively, between cues predicting threat or safety (Aranda‐Fernandez, Gaztañaga, Arias, & Chotro, 2016; Day et al., 2016; Greiner et al., 2019). However, other studies have shown successful cued fear discrimination in both males and females (Clark et al., 2019; Gilman, DaMert, Meduri, & Jasnow, 2015), or even greater fear discrimination in females, compared to males (Foilb, Bals, Sarlitto, & Christianson, 2017). Another study showed that adversity in adolescence resulted in a later reduction in discrimination between cues predicting threat, uncertainty and safety in females but not males, although the female controls did successfully distinguish between these different cues (Walker, Andreansky, Ray, & McDannald, 2018). Day et al. (2016) found that the nature of sex differences in fear discrimination during retrieval testing depended on the extent of training received. Males showed successful discrimination after extended, but not limited training, whereas females discriminated after limited training but generalized after extended training. Discrimination in males involved safety signaling by the CS−, and generalization in females was due to reduced safety signaling by the CS− as shown by a retardation test, where the CS− was used as the cue during subsequent fear conditioning (Christianson et al., 2012). If the CS− signaled safety during discrimination, then later conditioning to the CS‐ was expected to be reduced (or retarded), which was the case for males but not females (Day et al., 2016). A summation test can also demonstrate safety signaling by the CS− during fear discrimination, where presenting the CS+ and CS− together would be expected to reduce fear in comparison with CS+ presentation alone. In conditioned inhibition paradigms, summation testing can demonstrate the inhibitory properties of B, where presenting B together with A+ or another conditioned cue would be expected to reduce conditioned responding relative to presentation of either conditioned cue in the absence of B (Christianson et al., 2012). Some studies have shown conditioned inhibition in males but not females (Aranda‐Fernandez et al., 2016; Greiner et al., 2019), while another study found no sex differences in conditioned inhibition (Foilb et al., 2017). However, estrous cycle phase was not determined in the studies that used naturally cycling females to examine sex differences in cued fear discrimination or conditioned inhibition. Again, this raises the possibility that variations in gonadal hormone levels between studies may account for these contradictory findings.
In contrast to contextual fear discrimination, little research has investigated gonadal hormone regulation of cued fear discrimination and safety signaling. Toufexis, Myers, Bowser, and Davis (2007) found that gonadectomized males and females both showed conditioned inhibition, which was disrupted by estradiol replacement but only in females. This suggests a sex‐specific role for estrogen in regulating safety signaling. Whether progesterone or testosterone play roles in sex differences in cued fear discrimination or safety signaling remains unknown. A sex difference in conditioned inhibition has also been shown in juveniles, where prepubescent males but not females showed conditioned inhibition (Aranda‐Fernandez et al., 2016). This suggests that such sex differences may also involve the organizational effects of gonadal hormones, genetic and epigenetic effects, or interactions between some or all of these factors.
5.2. Human studies
Compared to fear extinction, less research has been conducted on sex differences in fear discrimination in humans. In one study in healthy volunteers, women showed reduced contextual fear discrimination, compared to men. Moreover, women taking hormonal contraceptives discriminated less than free‐cycling women, although there were no differences in discrimination when comparing between the follicular and luteal phases of the menstrual cycle (Lonsdorf et al., 2015). Glover et al. (2013) found that cued fear discrimination and safety signaling were reduced with low, compared to high, estradiol levels in both non‐traumatized and traumatized women. Similarly, safety signaling was shown to be reduced with low, compared to high, estradiol levels in women with or without spider phobia (Li & Graham, 2016). However, a study investigating cued fear discrimination during pregnancy, when gonadal hormone levels are high, found that while non‐pregnant women showed discrimination, pregnant women showed generalization that was associated with PTSD symptoms (Michopoulous et al., 2015). It is worth noting that the hormonal milieu during pregnancy is very different from the menstrual cycle, suggesting a possible explanation for this discrepant finding. Another study in traumatized children found reduced cued fear discrimination in girls, compared to boys (Gamwell et al., 2015), suggesting that such sex differences may also occur independently of any activational effects of gonadal hormones.
6. NEURAL MECHANISMS IMPLICATED IN SEX DIFFERENCES IN LEARNED FEAR AND ITS INHIBITION
The neural circuitry underlying different Pavlovian fear learning and memory processes is distributed across various interconnected brain areas, including the amygdala, hippocampus and PFC. During contextual fear conditioning, the hippocampus and basolateral amygdala (BLA) are important for encoding the contextual representation and context–US association, respectively. In contrast, the lateral amygdala (La) is vital for associating the CS and US during cued fear conditioning, while the central amygdala (CeA) mediates learned fear expression. The medial PFC (mPFC) plays a key role in both contextual and cued fear memory processing. This neural circuit is also important for learned fear inhibition through extinction and discrimination (Anagnostaras, Gale, & Fanselow, 2001; Asok, Kandel, et al., 2019a; Dunsmoor & Paz, 2015; Sevenster et al., 2018; Tovote et al., 2015). Evidence indicates that sex differences in fear conditioning, extinction and discrimination involve each of these brain areas.
6.1. Rodent studies
Reduced contextual fear conditioning in females, compared to males, was found to be associated with diminished long‐term potentiation (LTP), a model of learning‐induced synaptic plasticity, in the dorsal hippocampus (DH) (Maren et al., 1994). This sex difference in contextual fear conditioning has also been related to decreased levels of phosphorylated extracellular signal‐regulated kinase (pERK), an important molecular mediator of memory encoding, in the ventral hippocampus in females (Gresack et al., 2009). In terms of the inhibitory effect of estrogen on contextual fear conditioning, Gupta et al. (2001) found that estradiol treatment after ovariectomy decreased LTP in DH, compared to ovariectomized controls treated with vehicle. Ovariectomy has also been shown to reduce LTP in La, which was reversed by estradiol. The enhancement of cued fear conditioning in males resulting from gonadectomy was found to be associated with enhanced LTP in La, and this was reversed by testosterone (Chen et al., 2014). Reduced contextual fear conditioning caused by androgen receptor overexpression in males was shown to be related to alterations in the expression of various plasticity‐related genes in DH (Ramzan et al., 2018). Enhanced learned fear expression in females, compared to males, was found to be associated with greater neuronal activity and excitability in both La and BLA (Blume et al., 2017). In a study examining the brain mechanisms involved in the cessation of feeding by learned fear cues, it was shown that different neural circuits were activated by feeding and fear, except that mPFC was recruited under both conditions in females but not males (Reppucci & Petrovich, 2018). Another study investigating the effects of mPFC lesions on cued fear learning found that such lesions enhanced the rate of conditioning in females but not males (Baran et al., 2010). Compared to males, PAC1 receptor expression in mPFC was found to be higher in females and blocking PAC1 receptor signaling in mPFC reduced trace fear conditioning, a paradigm in which a brief interval occurs between CS and US presentation, in females but not males (Kirry et al., 2018).
Sex differences in fear extinction have been linked to mPFC function. Lesions to mPFC have been shown to reduce cued fear extinction learning in females but not males, whereas mPFC lesions reduced the later recall of extinction in males but not females (Baran et al., 2010). Extinction encoding is regulated by brain‐derived neurotrophic factor (BDNF), an important mediator of synaptic plasticity, acting in mPFC in males (Peters, Dieppa‐Perea, Melendez, & Quirk, 2010; Rosas‐Vidal, Do‐Monte, Sotres‐Bayon, & Quirk, 2014). Resistance to cued fear extinction in females, compared to males, was found to be related to reduced BDNF expression in mPFC involving increased methylation of the BDNF gene in this area. Furthermore, agonism of the tropomyosin‐related kinase B (TrkB) receptor, which mediates BDNF signaling, in mPFC reduced cued fear expression during extinction and later fear renewal in females (Baker‐Andresen et al., 2013). Studies in males have shown that the prelimbic (PL) and infralimbic (IL) subregions of mPFC mediate learned fear expression and extinction, respectively (Sierra‐Mercado, Padilla‐Coreano, & Quirk, 2011; Vidal‐Gonzalez, Vidal‐Gonzalez, Rauch, & Quirk, 2006). Enhanced cued fear expression and spontaneous fear recovery in females, compared to males, were found to be associated with persistent theta and gamma oscillatory activity in PL and a failure of gamma activation in IL (Fenton et al., 2014, 2016). In a study investigating the neural correlates of individual differences in fear extinction, differences in the morphology of IL neurons receiving projections from BLA were found between subpopulations of males displaying high or low levels of extinction recall. However, there were no morphological differences in these neurons between females who displayed high or low extinction recall, suggesting that individual differences in extinction recall were mediated by distinct neural processes in females and males (Gruene, Roberts, et al., 2015a). Another study found that reduced contextual fear extinction in females, compared to males, was associated with decreased pERK expression in DH but not mPFC (Matsuda et al., 2015).
Gonadal hormone regulation of fear extinction also involves this neural circuitry. A study found differences in cued and contextual fear extinction during diestrus and proestrus that were associated with differences in neuronal excitability in La and BLA during these estrous cycle phases. Cued fear extinction and La inhibition were enhanced during proestrus, compared to diestrus, while contextual fear extinction and BLA inhibition were enhanced during diestrus, compared to proestrus (Blume et al., 2017). Compared to vehicle, females treated with estradiol after cued fear extinction were shown to have lower amygdala and higher IL activation during later extinction recall (Zeidan et al., 2011). Another study found that females given estradiol before cued fear extinction showed enhanced extinction recall and a corresponding decrease in CeA activation and an increase in the ratio of activation between IL‐PL and IL‐CeA (Maeng, Taha, et al., 2017b). A study investigating dopamine–estrogen interactions underlying cued fear extinction in females found opposing effects of D1 receptor agonism during proestrus versus other estrous cycle phases on later extinction recall and accompanying activation of IL neurons receiving BLA projections. Rey et al. (2014) found that D1 receptor agonist treatment before extinction during proestrus impaired extinction recall and this was associated with reduced IL activation. Conversely, D1 receptor agonism before extinction during other phases of the estrous cycle enhanced extinction recall, which was related to augmented IL activation. Estradiol has been shown to potentiate synaptic plasticity in an ERβ‐ and NMDA receptor‐dependent manner in IL (Galvin & Ninan, 2014), suggesting a mechanism by which estrogen enhances extinction memory encoding. Estradiol or ERβ agonism has been shown to enhance contextual fear extinction in ovariectomized females by acting in DH (Chang et al., 2009). In contrast, reduced contextual fear extinction resulting from chronic treatment with high levels of estradiol has been associated with reduced COMT levels in DH but not mPFC (McDermott et al., 2015).
Compared to fear conditioning and its extinction, few studies have examined the neural mechanisms involved in sex differences in fear discrimination. One study showed that contextual fear discrimination in males was associated with DH activation, whereas reduced discrimination in females was linked more to BLA recruitment (Keiser et al., 2017). Another study showed that reduced contextual fear discrimination with predator odor stress in females, compared to males, was associated with sex differences in signaling by cAMP response element binding protein, a crucial molecular mediator of learning and memory, in DH (Homiack et al., 2018). Contextual fear generalization induced by estradiol treatment was found to depend on ERβ activation in DH (Lynch, Winiecki, Vanderhoof, Riccio, & Jasnow, 2016b). More recently, the anterior cingulate cortex (ACC) was found to be a site of action for the effects of estradiol on contextual fear generalization. Glutamate receptor signaling in DH and ACC was also recently shown to be involved in mediating this effect of estradiol, given that AMPA or NMDA receptor antagonism in these areas blocked contextual fear generalization induced by estradiol (Adkins, Lynch, Hagerdorn, Esterhuizen, & Jasnow, 2019). In contrast to contextual fear discrimination, the neural underpinnings of sex differences in cued fear discrimination and safety signaling remain poorly understood.
6.2. Human studies
Recent studies investigating brain function in very large cohorts of healthy volunteers have shown clear sex differences in the resting‐state connectivity of neural circuits comprising the hippocampus, amygdala and mPFC (Conrin et al., 2018; Zhan et al., 2017). Evidence also indicates that there are sex differences in the activity of these areas in relation to fear conditioning and extinction. One study found that, compared to men, women showed more activation of the amygdala and ACC during cued fear conditioning. No sex differences in amygdala or ACC activation were found during extinction. However, during extinction recall, men showed higher activity in ACC, compared to women (Lebron‐Milad et al., 2012). Gonadal hormone status modulates brain activation in relation to fear extinction in healthy women. Higher estradiol levels during extinction have been associated with increased activation of ventromedial PFC (vmPFC), which is thought to be the human homolog of the rodent IL. These higher estradiol levels during extinction were also found to be related to enhanced extinction recall concomitant with increased activation of vmPFC and amygdala (Zeidan et al., 2011). Another study found that women taking oral contraceptives showed more activation in amygdala, ACC and vmPFC during cued fear extinction, compared to women in the luteal phase of the menstrual cycle (Merz et al., 2012). The brain areas involved in sex differences in fear discrimination in humans remain to be elucidated.
Genetic factors that have been identified to moderate risk for anxiety‐related disorders depending on sex can influence amygdala and hippocampus function. COMT genotype was found to regulate amygdala activation in response to threat‐related stimuli in healthy women but not men (Domschke et al., 2012). PAC1 receptor genotype has been shown to modulate activity in the hippocampus during contextual fear conditioning in healthy women (Pohlack et al., 2015). PAC1 receptor genotype in traumatized women was also found to regulate activity in and connectivity between the amygdala and hippocampus in response to threatening stimuli (Stevens et al., 2014). Studies have shown sex differences in PFC function in anxiety‐related disorders. In dental phobia, men were found to have greater PFC activation in response to fear‐related stimuli, compared to women (Schienle, Köchel, & Leutgeb, 2011; Schienle, Scharmüller, Leutgeb, Schäfer, & Stark, 2013). In a study investigating brain function in relation to cued fear extinction in PTSD, no sex differences were found in healthy trauma‐exposed controls, but men with PTSD showed reduced extinction recall linked to enhanced ACC activation, compared to women with PTSD (Shvil et al., 2014).
7. FUTURE DIRECTIONS
The evidence reviewed above indicates that there are important sex differences in learned fear and its inhibition, which are influenced by gonadal hormone signaling and accompanied by differences in the function of the relevant neural circuitry (summarized in Tables 1, 2, 3, 4). On balance, studies across species have shown that both fear extinction and discrimination are reduced in females, compared to males. Rodent and human studies are also in general agreement with respect to estrogen regulation of fear extinction in females. However, less research has investigated the role of gonadal hormones in regulating fear discrimination and the findings from the rodent and human studies conducted to date seem contradictory. Given that impaired inhibition of learned fear and associated neural circuit dysfunction are characteristic of certain anxiety‐related disorders, further investigation of the neurobiological basis of sex differences in learned fear inhibition is a potentially fruitful area of research to better understand why women are so much more prone to developing these disorders than men. Below we suggest various lines of enquiry for future work in this area.
Table 1.
Summary of studies on sex differences in and gonadal hormone regulation of fear extinction in rodents
| Subjects | Measures/manipulations | Differences/effects | Neural mechanism(s) | Reference |
|---|---|---|---|---|
| M and F rats | Cued extinction learning | M > F | Not examined | Baran et al. (2009) |
| M and F rats | mPFC lesion effects on cued extinction learning and recall | Lesions ↓ extinction learning in F and ↓ recall in M | mPFC lesions | Baran et al. (2010) |
| M and F rats | Cued extinction learning | M > F | Not examined | Petrovich and Lougee (2011) |
| M and F mice | Cued extinction recall | M > F | BDNF DNA methylation and mRNA expression in mPFC | Baker‐Andresen et al. (2013) |
| F mice | TrkB‐R agonist effects on cued extinction and fear renewal | ↓ cued fear expression during extinction and ↓ fear renewal | TrkB‐R signaling in mPFC | Baker‐Andresen et al. (2013) |
| M and F rats |
Cued fear expression Spontaneous fear recovery |
F > M F > M |
PL theta activity PL theta activity |
Fenton et al. (2014) |
| M and F rats |
Cued fear expression Spontaneous fear recovery |
F > M F > M |
PL gamma activity IL gamma activity |
Fenton et al. (2016) |
| M and F rats | Cued extinction recall | M > F | Not examined | Voulo & Parsons (2017) |
| M and F mice | Cued extinction learning | M > F | Not examined | Clark et al. (2019) |
| M and F rats | Cued extinction learning and recall | M > F | Not examined | Greiner et al. (2019) |
| M and F rats | Fear renewal after cued extinction | M = F | Not examined | Maes (2002) |
| M and F mice | Cued extinction learning | M > F | Forebrain‐specific deletion of MR gene | Ter Horst et al. (2012) |
| M and F rats | Exercise effects on cued extinction recall and fear renewal | ↑ recall and ↓ fear renewal in M but not F | Not examined | Bouchet et al. (2017) |
| M and F rats | Cued extinction, during different estrous cycle phases in F | Extinction during Met ↓ recall versus extinction during Pro and M | Not examined | Milad et al. (2009) |
| F rats | Cued extinction during different estrous cycle phases | Extinction during Est/Met/Di ↓ recall versus extinction during Pro | Not examined | Rey et al. (2014) |
| F rats | D1‐R agonist effects on cued extinction during different estrous cycle phases | Cycle phase‐dependent effects on recall | IL activation | Rey et al. (2014) |
| F rats | Cued extinction during different estrous cycle phases | Extinction during Met/Di/Est ↓ extinction learning and recall versus extinction during Pro | Not examined | Gruene, Roberts, et al. (2015a) |
| M and F rats | Individual differences in cued extinction | Differences in recall linked to differences in IL neuron morphology in M but not FExtinction during | IL neuron morphology | Gruene, Roberts, et al. (2015a) |
| F rats | Cued extinction during different estrous cycle phases | Extinction during Di ↓ extinction learning versus extinction during Pro | La activity | Blume et al. (2017) |
| F rats | E or P effects during cued extinction in Met | E or P ↑ recall | Not examined | Milad et al. (2009) |
| F rats | ER or PR blockade effects during cued extinction in Pro | ER or PR blockade ↓ recall | Not examined | Milad et al. (2009) |
| F rats | ERα or ERβ agonist effects during cued extinction in Met | ERβ but not ERα agonism ↑ recall | Not examined | Zeidan et al. (2011) |
| F rats | E effects after cued extinction in Met | E given immediately but not 4 hr after extinction ↑ recall | Amygdala and IL activation | Zeidan et al. (2011) |
| F rats | E effects during cued extinction in Met | E ↑ recall | CeA activation and ratios of IL‐PL and IL‐CeA activation | Maeng, Cover, et al. (2017a) |
| F rats | HC effects on cued extinction | HC ↓ extinction learning and recall via ERα and ERβ | Not examined | Graham and Milad (2013) |
| F rats | OVX or HC effects on cued extinction | OVX/HC ↓ extinction learning and recall via E | Not examined | Parrish et al. (2019) |
| OVX F rats | E or E + P effects on cued extinction | E ↑ recall, time‐dependent modulation of E effects by P | Not examined | Graham &Daher (2016) |
| F rats | E effects on cued extinction during different estrous cycle phases | Dose‐ and cycle phase‐dependent effects on recall | Not examined | Graham and Scott (2018a) |
| Naturally cycling and OVX F rats | NMDA‐R modulation of E effects on cued extinction | E effects on extinction NMDA‐R‐dependent | Not examined | Graham and Scott (2018b) |
| M rats | GnRH agonist effects during cued extinction | GnRH agonism ↑ recall via ↑ T | Not examined | Maeng, Taha, et al. (2017b) |
| M rats | Aromatase inhibitor effects during cued extinction | Aromatase inhibition ↓ recall via ↑ E | Not examined | Graham and Milad (2014) |
| F rats | Contextual extinction during different estrous cycle phases | Extinction during Pro ↓ extinction learning versus extinction during Di | BLA activity | Blume et al. (2017) |
| Juvenile M and F rats | Fear relapse after cued extinction | Renewal, reinstatement and spontaneous recovery shown in F but not M | Not examined | Park et al. (2017) |
| M and F rats | Contextual fear conditioning, during different estrous cycle phases in F, and extinction | F conditioned during Pro or Est ↑ extinction versus F conditioned during Di and M | Not examined | Chang et al. (2009) |
| OVX F rats | E or P effects on contextual extinction | E but not P enhanced extinction | Not examined | Chang et al. (2009) |
| OVX F rats | ERα or ERβ agonist effects on contextual extinction | ERβ but not ERα agonism ↑ extinction | Not examined | Chang et al. (2009) |
| OVX F mice | Chronic E effects on contextual extinction | High dose of chronic E ↓ extinction | COMT expression in DH | McDermott et al. (2015) |
| M and F mice |
Contextual extinction learning Spontaneous fear recovery |
M > F F > M |
pERK expression in DH | Matsuda et al. (2015) |
Abbreviations: BDNF, brain‐derived neurotrophic factor; BLA, basolateral amygdala; CeA, central amygdala; COMT, catechol‐O‐methyltransferase; D1‐R, dopamine D1 receptor; DH, dorsal hippocampus; Di, diestrus; E, estradiol; ER, estrogen receptor; ERα, estrogen receptor α; ERβ, estrogen receptor β; Est, estrus; F, female; GnRH, gonadotropin‐releasing hormone; HC, hormonal contraceptive; IL, infralimbic cortex; La, lateral amygdala; M, male; Met, metestrus; mPFC, medial prefrontal cortex; MR, mineralocorticoid receptor; NMDA‐R, NMDA receptor; OVX, ovariectomized; P, progesterone; pERK, phosphorylated extracellular signal‐regulated kinase; PL, prelimbic cortex; PR, progesterone receptor; Pro, proestrus; T, testosterone; TrkB‐R, tropomyosin‐related kinase B receptor.
Table 2.
Summary of studies on sex differences in and gonadal hormone regulation of fear extinction in humans
| Subjects | Measures/ manipulations | Differences/ effects | Neural mechanism(s) | References |
|---|---|---|---|---|
| M and W | Extinction | M = W | Not examined | Fredrikson et al. (1976) |
| M and W | Extinction | M > W | Not examined | Rattel et al. (2019) |
| M and W | Extinction, during different menstrual cycle phases in W | Extinction in LF ↓ recall versus extinction in EF and M | Not examined | Milad et al. (2006) |
| M and W | Extinction, in W with low or high E | Low E during extinction ↓ recall versus high E during extinction and M | Not examined | Milad et al. (2010) |
| W | Low or high E during extinction | Low E during extinction ↓ recall versus high E during extinction | vmPFC and amygdala activity | Zeidan et al. (2011) |
| W | Low or high E during extinction | Low E during extinction ↓ recall versus high E during extinction | Not examined | White and Graham (2016) |
| W | Low or high E during extinction | Low E during extinction ↓ recall versus high E during extinction | Not examined | Wegerer et al. (2014) |
| M and W with PTSD | Extinction recall | W > M | ACC activity | Shvil et al. (2014) |
| W with PTSD | Low or high E during extinction | Low E ↓ extinction learning versus high E | Not examined | Glover et al. (2012) |
| W with PTSD | Extinction | Low E and high P during extinction predicted ↓ recall | Not examined | Pineles et al. (2016) |
| W with phobia | Low or high E during extinction | Low E during extinction ↓ recall versus high E during extinction | Not examined | Li and Graham (2016) |
| W with phobia | Low or high E during exposure therapy | Low E during exposure therapy predicted ↓ post‐treatment outcomes | Not examined | Graham et al. (2018) |
Abbreviations: ACC, anterior cingulate cortex; E, estradiol; EF, early follicular; LF, late follicular; M, men; PTSD, post‐traumatic stress disorder; vmPFC, ventromedial prefrontal cortex; W, women.
Table 3.
Summary of studies on sex differences in and gonadal hormone regulation of fear discrimination in rodents
| Subjects | Measures/ manipulations | Differences/ effects | Neural mechanism(s) | References |
|---|---|---|---|---|
| M and F rats | Context discrimination | M > F | Not examined | Reppucci et al. (2013) |
| M and F rats | Context discrimination | M > F | Not examined | Lynch et al. (2013) |
| OVX F rats | E effects on context discrimination | OVX ↑ and OVX + E ↓ discrimination | Not examined | Lynch et al. (2013) |
| M and F mice | Context discrimination | M > F | DH and BLA activation | Keiser et al. (2017) |
| M and F mice | Context discrimination | M > F | Not examined | Asok, Hijazi, et al. (2019b) |
| M and F rats | Context discrimination with predator odor | M > F | CREB signaling in DH | Homiack et al. (2018) |
| M and F rats | Context discrimination | M = F | Not examined | Keely et al. (2015) |
| M and F mice | Context discrimination | M = F | Not examined | Germer et al. (2019) |
| OVX F rats | E, ERα agonist or ERβ agonist effects on context discrimination |
E ↓ discrimination retrieval ERβ but not ERα agonism ↓ retrieval |
Not examined | Lynch et al. (2014) |
| OVX F rats | ER antagonist effects on context discrimination | Blocked effects of E or ERβ agonism on discrimination | DH | Lynch, Winiecki, et al. (2016b) |
| OVX F rats | Glu‐R modulation of E effects on context discrimination | E effects on discrimination Glu‐R‐dependent | ACC and DH | Adkins et al. (2019) |
| GDX M rats | T, E, ERα agonist or ERβ agonist effects on context discrimination |
T or E ↑ discrimination ERα or ERβ agonism ↑ discrimination |
Not examined | Lynch, Winiecki, et al. (2016b) |
| M and F rats | Cued discrimination |
F > M with limited training M > F with extended training |
Not examined | Day et al. (2016) |
| M and F rats | Cued discrimination | Early adversity ↓ discrimination in F but not M | Not examined | Walker et al. (2018) |
| M and F mice | Cued discrimination | M = F | Not examined | Gilman et al. (2015) |
| M and F mice | Cued discrimination | M = F | Not examined | Clark et al. (2019) |
| M and F rats |
Cued discrimination Conditioned inhibition |
F > M F = M |
Not examined | Foilb et al. (2017) |
| Juvenile M and F rats | Conditioned inhibition | M > F | Not examined | Aranda‐Fernandez et al. (2016) |
| M and F rats | Conditioned inhibition | M > F | Not examined | Greiner et al. (2019) |
| GDX M and OVX F rats | E effects on conditioned inhibition | E ↓ conditioned inhibition in F but not M | Not examined | Toufexis et al. (2007) |
Abbreviations: ACC, anterior cingulate cortex; BLA, basolateral amygdala; CREB, cAMP response element binding protein; DH, dorsal hippocampus; E, estradiol; ER, estrogen receptor; ERα, estrogen receptor α; ERβ, estrogen receptor β; F, female; GDX, gonadectomized; Glu‐R, glutamate receptor; M, male; OVX, ovariectomized; T, testosterone.
Table 4.
Summary of studies on sex differences in and gonadal hormone regulation of fear discrimination in humans
| Subjects | Measures/ manipulations | Differences/ effects | Neural mechanism(s) | References |
|---|---|---|---|---|
| M and W | Context discrimination | M and free‐cycling W > W on HC | Not examined | Lonsdorf et al. (2015) |
| Healthy and traumatized W | E effects on cued discrimination and conditioned inhibition | Low E ↓ discrimination and conditioned inhibition versus high E | Not examined | Glover et al. (2013) |
| Healthy and spider phobic W | E effects on cued discrimination | Low E ↓ discrimination versus high E | Not examined | Li and Graham (2016) |
| Non‐pregnant and pregnant W | Cued discrimination | Non‐pregnant > Pregnant | Not examined | Michopoulous et al. (2015) |
| Traumatized boys and girls | Cued discrimination | Boys > Girls | Not examined | Gamwell et al. (2015) |
Abbreviations: E, estradiol; HC, hormonal contraceptive; M, men; W, women.
Compared to rodent studies, the evidence for sex differences in learned fear inhibition in humans is less clear without accounting for the gonadal hormone status of women. Genetic and environmental factors are more easily controlled for in rodent studies, which might contribute to this disparity. Future studies examining learned fear inhibition in men and women could take certain genetic or environmental factors into account to determine whether this results in more robust sex differences emerging for this anxiety‐related endophenotype. For example, it would be interesting to stratify women in such studies based on gene (e.g., COMT, PAC1 receptor) variants that moderate the function of relevant brain areas and confer risk for developing anxiety‐related disorders in a sex‐dependent manner.
Both rodent and human studies show that sex differences in fear extinction do emerge when accounting for variations in the estrogen status of females, such that estradiol enhances extinction. Similarly, human studies on fear discrimination broadly agree with these fear extinction studies, with estrogen enhancing discrimination in women. In contrast, estrogen reduces fear discrimination and safety signaling in ovariectomized rodents, although whether fluctuating estrogen levels in naturally cycling females can also affect fear discrimination and safety signaling remains to be examined in a systematic manner. Further studies should investigate this important issue given the potential implications for using estrogen‐based medicines (e.g., as adjuncts to exposure therapy) to treat anxiety‐related disorders in the future.
Rodent studies indicate a role for progesterone in regulating fear extinction, but whether this is also the case for humans is less clear, while progesterone regulation of fear discrimination remains unknown. Progesterone has anxiolytic effects through its metabolite allopregnanolone, a neurosteroid that potentiates inhibitory transmission by facilitating GABA‐A receptor signaling. Moreover, progesterone metabolism has been implicated in anxiety‐related disorders in both men and women (Pineles et al., 2018; Rasmusson et al., 2019; Schule, Nothdurfter, & Rupprecht, 2014). However, facilitating inhibitory transmission may also interfere with the efficacy of psychological treatments such as exposure therapy by impairing fear extinction (Singewald, Schmuckermair, Whittle, Holmes, & Ressler, 2015). Therefore, further research on the modulation of learned fear inhibition and associated neural circuit function by progesterone‐related signaling is warranted.
Studies investigating sex differences in fear reconsolidation, along with their potential endocrine and neural underpinnings, have only recently emerged (Franzen, Giachero, & Bertoglio, 2019; Meir Drexler, Merz, Hamacher‐Dang, & Wolf, 2016; da Silva, Takahashi, Bertoglio, Andreatini, & Stern, 2016). Learned fear retrieval can destabilize the memory engram, which requires its reconsolidation to maintain, strengthen or update fear memory. Disrupting reconsolidation to weaken fear memory is a potential therapeutic strategy for treating anxiety‐related disorders, but most of the relevant studies to date have been conducted in males. Various boundary conditions, including memory strength, are important for determining whether memories undergo reconsolidation after retrieval, such that strong fear memory can be more resistant to reconsolidation disruption (Lee, Nader, & Schiller, 2017). Future studies could thus examine whether there are sex differences in the mechanisms underlying fear reconsolidation.
It is worth noting that the conclusions drawn above on sex differences in learned fear and its inhibition in rodents are based largely on studies that were conducted using Pavlovian conditioning paradigms that inferred learned fear from freezing behavior. Studies conducted using other paradigms (e.g., eye‐blink conditioning, active avoidance) or behavioral measures of learned fear (e.g., fear‐potentiated startle) have found enhanced learning in females, compared to males, which involves the organizational and activational effects of gonadal hormones (Dalla & Shors, 2009). A recent study found sex differences in cued fear extinction that were response‐specific, such that extinction was reduced in females, compared to males, based on fear‐potentiated startle but not freezing (Voulo & Parsons, 2017). Interpreting the results of studies investigating sex differences in learned fear and extinction based solely on freezing can also be challenging because not only are females more active generally but they can also adopt more active fear responding, compared to males (Arakawa, 2019). Recent studies have found that some females exhibited active “darting” behavior in addition to freezing during cued fear conditioning and extinction, whereas males were much more likely to express freezing instead of darting (Colom‐Lapetina, Li, Pelegrina‐Perez, & Shansky, 2019; Gruene, Flick, Stefano, Shea, & Shansky, 2015b). Another study found that females showed fear generalization based on freezing but discriminated based on darting (Greiner et al., 2019); however, the levels of darting were very low overall and other studies have reported negligible darting in females during fear conditioning, extinction or discrimination (Blume et al., 2017; Foilb et al., 2017). Therefore, more research is needed to further characterize this predominantly sex‐specific conditioned fear response. Determining the mechanisms underpinning active fear responding and its inhibition is relevant to understanding avoidance, a symptom associated with poor long‐term outcomes in anxiety‐related disorders (Hendricks et al., 2016). A study showed that PTSD symptoms were associated with experimentally conditioned avoidance, which was more pronounced in women than in men (Sheynin et al., 2017). Rodent studies have shown that the extinction of passive (i.e., freezing) and active (i.e., avoidance) fear responding is mediated by partially overlapping neural circuits (Bravo‐River, Roman‐Ortiz, Brignoni‐Perez, Sotres‐Bayon, & Quirk, 2014; Martinez‐Rivera, Bravo‐Rivera, Velázquez‐Díaz, Montesinos‐Cartagena, & Quirk, 2019), although potential sex differences in the brain mechanisms involved remain to be determined.
While it is of real interest that females and males display more active and passive forms of learned fear responding, respectively, this also makes it more difficult to determine the nature of sex differences based on the expression of different behaviors between the sexes. Future studies in rodents examining sex differences in learned fear inhibition could also measure physiological responses (e.g., sympathetic activation as measured by changes in heart rate or blood pressure) elicited by conditioned cues or contexts alongside behavior. This is done routinely in such human studies (i.e., skin conductance response) and may complement the behavioral measures by allowing for directly comparing between males and females. Determining the neural basis of sex differences in the physiological responses associated with learned fear and its inhibition may also enhance the translational validity of rodent studies.
8. CONCLUSION
When considered from an adaptive perspective, resistance to extinction may promote learned fear responding in the face of cues or contexts that were once but are no longer predictive of threat. Similarly, generalizing across cues or contexts may promote learned fear in response to a wider range of more ambiguous stimuli or environments that can potentially predict threat. In evolutionary terms, such circumstances may actually confer a survival advantage to females and the offspring in their care. However, this phenotype related to risk aversion, which recent rodent studies have shown is enhanced in females relative to males (Orsini, Willis, Gilbert, Bizon, & Setlow, 2016; Pellman, Schuessler, Tellakat, & Kim, 2017), may also contribute to the greater vulnerability to anxiety‐related disorders that is seen in women in comparison with men.
CONFLICT OF INTEREST
The authors have no competing interests to declare.
AUTHOR CONTRIBUTIONS
HLLD and CWS drafted and revised the paper and approved the final version.
ACKNOWLEDGEMENTS
HLLD was supported by a Biotechnology and Biological Sciences Research Council Doctoral Training Partnership [grant number BB/J014508/1] and the University of Nottingham. These funders had no role in any aspect of this paper. We thank Christine Stubbendorff for her insightful comments on the draft manuscript.
Day HLL, Stevenson CW. The neurobiological basis of sex differences in learned fear and its inhibition. Eur J Neurosci.2020;52:2466–2486. 10.1111/ejn.14602
Edited by Christina Dalla.
The peer review history for this article is available at https://publons.com/publon/10.1111/EJN.14602
DATA AVAILABILITY STATEMENT
Not applicable (this review reports no primary data).
REFERENCES
- Adkins, J. M. , Lynch, J. F. 3rd , Hagerdorn, P. , Esterhuizen, M. , & Jasnow, A. M. (2019). Anterior cingulate cortex and dorsal hippocampal glutamate receptors mediate generalized fear in female rats. Psychoneuroendocrinology, 107, 109–118. 10.1016/j.psyneuen.2019.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar, R. , Gil, L. , Gray, J. A. , Driscoll, P. , Flint, J. , Dawson, G. R. , … Tobeña, A. (2003). Fearfulness and sex in F2 Roman rats: Males display more fear though both sexes share the same fearfulness traits. Physiology & Behavior, 78, 723–732. 10.1016/S0031-9384(03)00043-X [DOI] [PubMed] [Google Scholar]
- Almli, L. M. , Mercer, K. B. , Kerley, K. , Feng, H. , Bradley, B. , Conneely, K. N. , & Ressler, K. J. (2013). ADCYAP1R1 genotype associates with post‐traumatic stress symptoms in highly traumatized African‐American females. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics, 162B, 262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostaras, S. G. , Gale, G. D. , & Fanselow, M. S. (2001). Hippocampus and contextual fear conditioning: Recent controversies and advances. Hippocampus, 11, 8–17. [DOI] [PubMed] [Google Scholar]
- Arakawa, H. (2019). Age and sex differences in the innate defensive behaviors of C57BL/6 mice exhibited in a fear conditioning paradigm and upon exposure to a predatory odor. Physiology & Behavior, 204, 264–274. 10.1016/j.physbeh.2019.02.030 [DOI] [PubMed] [Google Scholar]
- Aranda‐Fernandez, P. E. , Gaztañaga, M. , Arias, C. , & Chotro, M. G. (2016). Conditioned inhibition in preweanling rats. Developmental Psychobiology, 58, 98–106. 10.1002/dev.21359 [DOI] [PubMed] [Google Scholar]
- Asok, A. , Hijazi, J. , Harvey, L. R. , Kosmidis, S. , Kandel, E. R. , & Rayman, J. B. (2019b). Frontiers in Behavioural Neurosciences, 13, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asok, A. , Kandel, E. R. , & Rayman, J. B. (2019a). The neurobiology of fear generalization. Frontiers in Behavioural Neurosciences, 12, 329 10.3389/fnbeh.2018.00329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker‐Andresen, D. , Flavell, C. R. , Li, X. , & Bredy, T. W. (2013). Activation of BDNF signaling prevents the return of fear in female mice. Learning & Memory, 20, 237–240. 10.1101/lm.029520.112 [DOI] [PubMed] [Google Scholar]
- Ball, S. , Gilbert, T. L. , & Overly, C. C. (2012). The human brain online: An open resource for advancing brain research. PLoS Biology, 10, e1001453 10.1371/journal.pbio.1001453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran, S. E. , Armstrong, C. E. , Niren, D. C. , & Conrad, C. D. (2010). Prefrontal cortex lesions and sex differences in fear extinction and perseveration. Learning Memory, 17, 267–278. 10.1101/lm.1778010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran, S. E. , Armstrong, C. E. , Niren, D. C. , Hanna, J. J. , & Conrad, C. D. (2009). Chronic stress and sex differences on the recall of fear conditioning and extinction. Neurobiology of Learning and Memory, 91, 323–332. 10.1016/j.nlm.2008.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker, J. M. , & Galea, L. A. (2010). Males show stronger contextual fear conditioning than females after context pre‐exposure. Physiology & Behavior, 99, 82–90. [DOI] [PubMed] [Google Scholar]
- Bethancourt, J. A. , Vásquez, C. E. , & Britton, G. B. (2011). Sex‐dependent effects of long‐term oral methylphenidate treatment on spontaneous and learned fear behaviors. Neuroscience Letters, 496, 30–34. [DOI] [PubMed] [Google Scholar]
- Blume, S. R. , Freedberg, M. , Vantrease, J. E. , Chan, R. , Padival, M. , Record, M. J. , … Rosenkranz, J. A. (2017). Sex‐ and estrus‐dependent differences in rat basolateral amygdala. Journal of Neuroscience, 37, 10567–10586. 10.1523/JNEUROSCI.0758-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchet, C. A. , Lloyd, B. A. , Loetz, E. C. , Farmer, C. E. , Ostrovskyy, M. , Haddad, N. , … Greenwood, B. N. (2017). Acute exercise enhances the consolidation of fear extinction memory and reduces conditioned fear relapse in a sex‐dependent manner. Learning Memory, 24, 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo‐Rivera, C. , Roman‐Ortiz, C. , Brignoni‐Perez, E. , Sotres‐Bayon, F. , & Quirk, G. J. (2014). Neural structures mediating expression and extinction of platform‐mediated avoidance. Journal of Neuroscience, 34, 9736–9742. 10.1523/JNEUROSCI.0191-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau, N. (2002). Gender differences in trauma and posttraumatic stress disorder. The Journal of Gender‐Specific Medicine, 5, 34–40. [PubMed] [Google Scholar]
- Catuzzi, J. E. , & Beck, K. D. (2014). Anxiety vulnerability in women: A two‐hit hypothesis. Experimental Neurology, 259, 75–80. 10.1016/j.expneurol.2014.01.023 [DOI] [PubMed] [Google Scholar]
- Chang, S. C. , Xie, P. , Anton, R. F. , De Vivo, I. , Farrer, L. A. , Kranzler, H. R. , … Koenen, K. C. (2012). No association between ADCYAP1R1 and post‐traumatic stress disorder in two independent samples. Molecular Psychiatry, 17, 239–241. [DOI] [PubMed] [Google Scholar]
- Chang, Y. J. , Yang, C. H. , Liang, Y. C. , Yeh, C. M. , Huang, C. C. , & Hsu, K. S. (2009). Estrogen modulates sexually dimorphic contextual fear extinction in rats through estrogen receptor beta. Hippocampus, 19, 1142–1150. [DOI] [PubMed] [Google Scholar]
- Chen, L. S. , Tzeng, W. Y. , Chuang, J. Y. , Cherng, C. G. , Gean, P. W. , & Yu, L. (2014). Roles of testosterone and amygdaloid LTP induction in determining sex differences in fear memory magnitude. Hormones and Behavior, 66, 498–508. [DOI] [PubMed] [Google Scholar]
- Christianson, J. P. , Fernando, A. B. , Kazama, A. M. , Jovanovic, T. , Ostroff, L. E. , & Sangha, S. (2012). Inhibition of fear by learned safety signals: A mini‐symposium review. Journal of Neuroscience, 32, 14118–14124. 10.1523/JNEUROSCI.3340-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, J. W. , Drummond, S. P. , Hoyer, D. , & Jacobson, L. H. (2019). Sex differences in mouse models of fear inhibition: Fear extinction, safety learning, and fear‐safety discrimination. British Journal of Pharmacology. 10.1111/bph [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colom‐Lapetina, J. , Li, A. J. , Pelegrina‐Perez, T. C. , & Shansky, R. M. (2019). Behavioral diversity across classic rodent models is sex‐dependent. Frontiers in Behavioural Neurosciences, 13, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrin, S. D. , Zhan, L. , Morrissey, Z. D. , Xing, M. , Forbes, A. , Maki, P. , … Leow, A. D. (2018). From default mode network to the basal configuration: Sex differences in the resting‐state brain connectivity as a function of age and their clinical correlates. Frontiers in Psychiatry, 9, 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske, M. G. , Stein, M. B. , Eley, T. C. , Milad, M. R. , Holmes, A. , Rapee, R. M. , & Wittchen, H. U. (2017). Anxiety disorders. Nature Reviews Disease Primers, 3, 17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva, T. R. , Takahashi, R. N. , Bertoglio, L. J. , Andreatini, R. , & Stern, C. A. (2016). Evidence for an expanded time‐window to mitigate a reactivated fear memory by tamoxifen. European Neuropsychopharmacology, 26, 1601–1609. 10.1016/j.euroneuro.2016.08.005 [DOI] [PubMed] [Google Scholar]
- Dalla, C. , & Shors, T. J. (2009). Sex differences in learning processes of classical and operant conditioning. Physiology & Behavior, 97, 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daviu, N. , Andero, R. , Armario, A. , & Nadal, R. (2014). Sex differences in the behavioural and hypothalamic‐pituitary‐adrenal response to contextual fear conditioning in rats. Hormones and Behavior, 66, 713–723. [DOI] [PubMed] [Google Scholar]
- Day, H. L. , Reed, M. M. , & Stevenson, C. W. (2016). Sex differences in discriminating between cues predicting threat and safety. Neurobiology of Learning and Memory, 133, 196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domschke, K. , Baune, B. T. , Havlik, L. , Stuhrmann, A. , Suslow, T. , Kugel, H. , … Dannlowski, U. (2012). Catechol‐O‐methyltransferase gene variation: Impact on amygdala response to aversive stimuli. NeuroImage, 60(4), 2222–2229. 10.1016/j.neuroimage.2012.02.039 [DOI] [PubMed] [Google Scholar]
- Domschke, K. , Freitag, C. M. , Kuhlenbäumer, G. , Schirmacher, A. , Sand, P. , Nyhuis, P. , … Deckert, J. (2004). Association of the functional V158M catechol‐O‐methyl‐transferase polymorphism with panic disorder in women. International Journal of Neuropsychopharmacology, 7, 183–188. 10.1017/S146114570400416X [DOI] [PubMed] [Google Scholar]
- Donner, N. C. , & Lowry, C. A. (2013). Sex differences in anxiety and emotional behavior. Pflügers Archiv ‐ European Journal of Physiology, 465, 601–626. 10.1007/s00424-013-1271-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor, J. E. , & Paz, R. (2015). Fear generalization and anxiety: Behavioral and neural mechanisms. Biological Psychiatry, 78, 336–343. [DOI] [PubMed] [Google Scholar]
- Fenton, G. E. , Halliday, D. M. , Mason, R. , Bredy, T. W. , & Stevenson, C. W. (2016). Sex differences in learned fear expression and extinction involve altered gamma oscillations in medial prefrontal cortex. Neurobiology of Learning and Memory, 135, 66–72. [DOI] [PubMed] [Google Scholar]
- Fenton, G. E. , Pollard, A. K. , Halliday, D. M. , Mason, R. , Bredy, T. W. , & Stevenson, C. W. (2014). Persistent prelimbic cortex activity contributes to enhanced learned fear expression in females. Learning Memory, 21, 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foilb, A. R. , Bals, J. , Sarlitto, M. C. , & Christianson, J. P. (2017). Sex differences in fear discrimination do not manifest as differences in conditioned inhibition. Learning Memory, 25, 49–53. 10.1101/lm.045500.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen, J. M. , Giachero, M. , & Bertoglio, L. J. (2019). Dissociating retrieval‐dependent contextual aversive memory processes in female rats: Are there cycle‐dependent differences? Neuroscience, 406, 542–553. 10.1016/j.neuroscience.2019.03.035 [DOI] [PubMed] [Google Scholar]
- Fredrikson, M. , Hugdahl, K. , & Ohman, A. (1976). Electrodermal conditioning to potentially phobic stimuli in male and female subjects. Biological Psychology, 4, 305–314. [DOI] [PubMed] [Google Scholar]
- Galvin, C. , & Ninan, I. (2014). Regulation of the mouse medial prefrontal cortical synapses by endogenous estradiol. Neuropsychopharmacology, 39, 2086–2094. 10.1038/npp.2014.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamwell, K. , Nylocks, M. , Cross, D. , Bradley, B. , Norrholm, S. D. , & Jovanovic, T. (2015). Fear conditioned responses and PTSD symptoms in children: Sex differences in fear‐related symptoms. Developmental Psychobiology, 57, 799–808. 10.1002/dev.21313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, N. M. , Walker, R. S. , & Zoellner, L. A. (2018). Estrogen, progesterone, and the menstrual cycle: A systematic review of fear learning, intrusive memories, and PTSD. Clinical Psychology Review, 66, 80–96. 10.1016/j.cpr.2018.06.005 [DOI] [PubMed] [Google Scholar]
- Germer, J. , Kahl, E. , & Fendt, M. (2019). Memory generalization after one‐trial contextual fear conditioning: Effects of sex and neuropeptide S receptor deficiency. Behavioral Brain Research, 361, 159–166. 10.1016/j.bbr.2018.12.046 [DOI] [PubMed] [Google Scholar]
- Gilman, T. L. , DaMert, J. P. , Meduri, J. D. , & Jasnow, A. M. (2015). Grin1 deletion in CRF neurons sex‐dependently enhances fear, sociability, and social stress responsivity. Psychoneuroendocrinology, 58, 33–45. 10.1016/j.psyneuen.2015.04.010 [DOI] [PubMed] [Google Scholar]
- Glover, E. M. , Jovanovic, T. , Mercer, K. B. , Kerley, K. , Bradley, B. , Ressler, K. J. , & Norrholm, S. D. (2012). Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biological Psychiatry, 72, 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover, E. M. , Mercer, K. B. , Norrholm, S. D. , Davis, M. , Duncan, E. , Bradley, B. , … Jovanovic, T. (2013). Inhibition of fear is differentially associated with cycling estrogen levels in women. Journal of Psychiatry and Neuroscience, 38, 341–348. 10.1503/jpn.120129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, B. M. , & Daher, M. (2016). Estradiol and progesterone have opposing roles in the regulation of fear extinction in female rats. Neuropsychopharmacology, 41, 774–780. 10.1038/npp.2015.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, B. M. , Li, S. H. , Black, M. J. , & Öst, L. G. (2018). The association between estradiol levels, hormonal contraceptive use, and responsiveness to one‐session‐treatment for spider phobia in women. Psychoneuroendocrinology, 90, 134–140. 10.1016/j.psyneuen.2018.02.019 [DOI] [PubMed] [Google Scholar]
- Graham, B. M. , & Milad, M. R. (2013). Blockade of estrogen by hormonal contraceptives impairs fear extinction in female rats and women. Biological Psychiatry, 73, 371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, B. M. , & Milad, M. R. (2014). Inhibition of estradiol synthesis impairs fear extinction in male rats. Learning Memory, 21, 347–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, B. M. , & Scott, E. (2018a). Effects of systemic estradiol on fear extinction in female rats are dependent on interactions between dose, estrous phase, and endogenous estradiol levels. Hormones and Behavior, 97, 67–74. 10.1016/j.yhbeh.2017.10.009 [DOI] [PubMed] [Google Scholar]
- Graham, B. M. , & Scott, E. (2018b). Estradiol‐induced enhancement of fear extinction in female rats: The role of NMDA receptor activation. Progress in Neuro‐Psychopharmacology and Biological Psychiatry, 86, 1–9. 10.1016/j.pnpbp.2018.05.003 [DOI] [PubMed] [Google Scholar]
- Greiner, E. M. , Müller, I. , Norris, M. R. , Ng, K. H. , & Sangha, S. (2019). Sex differences in fear regulation and reward‐seeking behaviors in a fear‐safety‐reward discrimination task. Behavioural Brain Research, 368, 111903 10.1016/j.bbr.2019.111903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack, J. E. , Schafe, G. E. , Orr, P. T. , & Frick, K. M. (2009). Sex differences in contextual fear conditioning are associated with differential ventral hippocampal extracellular signal‐regulated kinase activation. Neuroscience, 159, 451–467. 10.1016/j.neuroscience.2009.01.009 [DOI] [PubMed] [Google Scholar]
- Gruene, T. M. , Flick, K. , Stefano, A. , Shea, S. D. , & Shansky, R. M. (2015b). Sexually divergent expression of active and passive conditioned fear responses in rats. eLife, 4, e11352 10.7554/eLife.11352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruene, T. M. , Roberts, E. , Thomas, V. , Ronzio, A. , & Shansky, R. M. (2015a). Sex‐specific neuroanatomical correlates of fear expression in prefrontal‐amygdala circuits. Biological Psychiatry, 78, 186–193. 10.1016/j.biopsych.2014.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, R. R. , Sen, S. , Diepenhorst, L. L. , Rudick, C. N. , & Maren, S. (2001). Estrogen modulates sexually dimorphic contextual fear conditioning and hippocampal long‐term potentiation (LTP) in rats. Brain Research, 888, 356–365. [DOI] [PubMed] [Google Scholar]
- Hammack, S. E. , & May, V. (2015). Pituitary adenylate cyclase activating polypeptide in stress‐related disorders: Data convergence from animal and human studies. Biological Psychiatry, 78, 167–177. 10.1016/j.biopsych.2014.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, P. J. , & Tunbridge, E. M. (2008). Catechol‐O‐methyltransferase (COMT): A gene contributing to sex differences in brain function, and to sexual dimorphism in the predisposition to psychiatric disorders. Neuropsychopharmacology, 33(13), 3037–3045. 10.1038/sj.npp.1301543 [DOI] [PubMed] [Google Scholar]
- Hendriks, S. M. , Spijker, J. , Licht, C. M. M. , Hardeveld, F. , de Graaf, R. , Batelaan, N. M. , … Beekman, A. T. F. (2016). Long‐term disability in anxiety disorders. BMC Psychiatry, 16, 248 10.1186/s12888-016-0946-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homiack, D. , O'Cinneide, E. , Hajmurad, S. , Dohanich, G. P. , & Schrader, L. A. (2018). Effect of acute alarm odor exposure and biological sex on generalized avoidance and glutamatergic signaling in the hippocampus of Wistar rats. Stress, 21, 292–303. 10.1080/10253890.2018.1484099 [DOI] [PubMed] [Google Scholar]
- Hoth, K. F. , Paul, R. H. , Williams, L. M. , Dobson‐Stone, C. , Todd, E. , Schofield, P. R. , … Gordon, E. (2006). Associations between the COMT Val/Met polymorphism, early life stress, and personality among healthy adults. Neuropsychiatric Disease and Treatment, 2, 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe, A. S. , Buttenschøn, H. N. , Bani‐Fatemi, A. , Maron, E. , Otowa, T. , Erhardt, A. , … De Luca, V. (2016). Candidate genes in panic disorder: Meta‐analyses of 23 common variants in major anxiogenic pathways. Molecular Psychiatry, 21, 665–679. 10.1038/mp.2015.138 [DOI] [PubMed] [Google Scholar]
- Inslicht, S. S. , Metzler, T. J. , Garcia, N. M. , Pineles, S. L. , Milad, M. R. , Orr, S. P. , … Neylan, T. C. (2013). Sex differences in fear conditioning in posttraumatic stress disorder. Journal of Psychiatric Research, 47, 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasnow, A. M. , Lynch, J. F. 3rd , Gilman, T. L. , & Riccio, D. C. (2017). Perspectives on fear generalization and its implications for emotional disorders. Journal of Neuroscience Research, 95, 821–835. [DOI] [PubMed] [Google Scholar]
- Jovanovic, T. , Kazama, A. , Bachevalier, J. , & Davis, M. (2012). Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology, 62, 695–704. 10.1016/j.neuropharm.2011.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley, R. J. , Bye, C. , Trow, J. , & McDonald, R. J. (2015). Strain and sex differences in brain and behaviour of adult rats: Learning and memory, anxiety and volumetric estimates. Behavioral Brain Research, 288, 118–131. 10.1016/j.bbr.2014.10.039 [DOI] [PubMed] [Google Scholar]
- Keiser, A. A. , Turnbull, L. M. , Darian, M. A. , Feldman, D. E. , Song, I. , & Tronson, N. C. (2017). Sex differences in context fear generalization and recruitment of hippocampus and amygdala during retrieval. Neuropsychopharmacology, 42, 397–407. 10.1038/npp.2016.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirry, A. J. , Herbst, M. R. , Poirier, S. E. , Maskeri, M. M. , Rothwell, A. C. , Twining, R. C. , & Gilmartin, M. R. (2018). Pituitary adenylate cyclase‐activating polypeptide (PACAP) signaling in the prefrontal cortex modulates cued fear learning, but not spatial working memory, in female rats. Neuropharmacology, 133, 145–154. 10.1016/j.neuropharm.2018.01.010 [DOI] [PubMed] [Google Scholar]
- Konishi, Y. , Tanii, H. , Otowa, T. , Sasaki, T. , Motomura, E. , Fujita, A. , … Okada, M. (2014). Gender‐specific association between the COMT Val158Met Polymorphism and openness to experience in panic disorder patients. Neuropsychobiology, 69(3), 165–174. 10.1159/000360737 [DOI] [PubMed] [Google Scholar]
- Konishi, Y. , Tanii, H. , Otowa, T. , Sasaki, T. , Tochigi, M. , Umekage, T. , … Okada, M. (2014). Gene×gene×gender interaction of BDNF and COMT genotypes associated with panic disorder. Progress in Neuro‐Psychopharmacology and Biological Psychiatry, 51, 119–125. 10.1016/j.pnpbp.2014.01.020 [DOI] [PubMed] [Google Scholar]
- Kornfield, S. L. , Hantsoo, L. , & Epperson, C. N. (2018). What does sex have to do with it? The role of sex as a biological variable in the development of posttraumatic stress disorder. Current Psychiatry Reports, 20, 39 10.1007/s11920-018-0907-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten, T. A. , Miserendino, M. J. , Bombace, J. C. , Lee, H. J. , & Kim, J. J. (2005). Sex‐selective effects of neonatal isolation on fear conditioning and foot shock sensitivity. Behavioral Brain Research, 157, 235–244. 10.1016/j.bbr.2004.07.001 [DOI] [PubMed] [Google Scholar]
- Lebron‐Milad, K. , Abbs, B. , Milad, M. R. , Linnman, C. , Rougemount‐Bücking, A. , Zeidan, M. A. , … Goldstein, J. M. (2012). Sex differences in the neurobiology of fear conditioning and extinction: A preliminary fMRI study of shared sex differences with stress‐arousal circuitry. Biology of Mood & Anxiety Disorders, 2, 7 10.1186/2045-5380-2-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebron‐Milad, K. , & Milad, M. R. (2012). Sex differences, gonadal hormones and the fear extinction network: Implications for anxiety disorders. Biology of Mood & Anxiety Disorders, 2, 3 10.1186/2045-5380-2-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. L. C. , Nader, K. , & Schiller, D. (2017). An update on memory reconsolidation updating. Trends in Cognitive Sciences, 21, 531–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, L. O. , & Prescott, C. A. (2014). Association of the catechol‐O‐methyltransferase val158met polymorphism and anxiety‐related traits: A meta‐analysis. Psychiatric Genetics, 24, 52–69. 10.1097/YPG.0000000000000018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S. , & Graham, B. M. (2016). Estradiol is associated with altered cognitive and physiological responses during fear conditioning and extinction in healthy and spider phobic women. Behavioral Neuroscience, 130, 614–623. 10.1037/bne0000166 [DOI] [PubMed] [Google Scholar]
- Li, S. H. , & Graham, B. M. (2017). Why are women so vulnerable to anxiety, trauma‐related and stress‐related disorders? The potential role of sex hormones. Lancet Psychiatry, 4, 73–82. 10.1016/S2215-0366(16)30358-3 [DOI] [PubMed] [Google Scholar]
- Lind, M. J. , Marraccini, M. E. , Sheerin, C. M. , Bountress, K. , Bacanu, S. A. , Amstadter, A. B. , & Nugent, N. R. (2017). Association of posttraumatic stress disorder with rs2267735 in the ADCYAP1R1 gene: A meta‐analysis. Journal of Traumatic Stress, 30, 389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf, T. B. , Haaker, J. , Schümann, D. , Sommer, T. , Bayer, J. , Brassen, S. , … Kalisch, R. (2015). Sex differences in conditioned stimulus discrimination during context‐dependent fear learning and its retrieval in humans: The role of biological sex, contraceptives and menstrual cycle phases. Journal of Psychiatry and Neuroscience, 40, 368–375. 10.1503/jpn.140336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch, J. 3rd , Cullen, P. K. , Jasnow, A. M. , & Riccio, D. C. (2013). Sex differences in the generalization of fear as a function of retention intervals. Learn. Mem., 20, 628–632. [DOI] [PubMed] [Google Scholar]
- Lynch, J. F. 3rd , Dejanovic, D. , Winiecki, P. , Mulvany, J. , Ortiz, S. , Riccio, D. C. , & Jasnow, A. M. (2014). Activation of ERβ modulates fear generalization through an effect on memory retrieval. Hormones and Behavior, 66, 421–429. [DOI] [PubMed] [Google Scholar]
- Lynch, J. F. 3rd , Vanderhoof, T. , Winiecki, P. , Latsko, M. S. , Riccio, D. C. , & Jasnow, A. M. (2016a). Aromatized testosterone attenuates contextual generalization of fear in male rats. Hormones and Behavior, 84, 127–135. 10.1016/j.yhbeh.2016.06.007 [DOI] [PubMed] [Google Scholar]
- Lynch, J. F. 3rd , Winiecki, P. , Vanderhoof, T. , Riccio, D. C. , & Jasnow, A. M. (2016b). Hippocampal cytosolic estrogen receptors regulate fear generalization in females. Neurobiology of Learning and Memory, 130, 83–92. 10.1016/j.nlm.2016.01.010 [DOI] [PubMed] [Google Scholar]
- Maeng, L. Y. , Cover, K. K. , Taha, M. B. , Landau, A. J. , Milad, M. R. , & Lebrón‐Milad, K. (2017a). Estradiol shifts interactions between the infralimbic cortex and central amygdala to enhance fear extinction memory in female rats. Journal of Neuroscience Research, 95, 163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng, L. Y. , & Milad, M. R. (2015). Sex differences in anxiety disorders: Interactions between fear, stress, and gonadal hormones. Hormones and Behavior, 76, 106–117. 10.1016/j.yhbeh.2015.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng, L. Y. , Taha, M. B. , Cover, K. K. , Glynn, S. S. , Murillo, M. , Lebron‐Milad, K. , & Milad, M. R. (2017b). Acute gonadotropin‐releasing hormone agonist treatment enhances extinction memory in male rats. Psychoneuroendocrinology, 82, 164–172. 10.1016/j.psyneuen.2017.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes, J. H. (2002). No sex difference in contextual control over the expression of latent inhibition and extinction in Pavlovian fear conditioning in rats. Neurobiology of Learning and Memory, 78, 258–278. 10.1006/nlme.2002.4058 [DOI] [PubMed] [Google Scholar]
- Maren, S. , De Oca, B. , & Fanselow, M. S. (1994). Sex differences in hippocampal long‐term potentiation (LTP) and Pavlovian fear conditioning in rats: Positive correlation between LTP and contextual learning. Brain Research, 661, 25–34. 10.1016/0006-8993(94)91176-2 [DOI] [PubMed] [Google Scholar]
- Markus, E. J. , & Zecevic, M. (1997). Sex differences and estrous cycle changes in hippocampus‐dependent fear conditioning. Psychobiology, 25, 246–252. [Google Scholar]
- Martínez‐Rivera, F. J. , Bravo‐Rivera, C. , Velázquez‐Díaz, C. D. , Montesinos‐Cartagena, M. , & Quirk, G. J. (2019). Prefrontal circuits signaling active avoidance retrieval and extinction. Psychopharmacology (Berl), 236, 399–406. 10.1007/s00213-018-5012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda, S. , Matsuzawa, D. , Ishii, D. , Tomizawa, H. , Sutoh, C. , & Shimizu, E. (2015). Sex differences in fear extinction and involvements of extracellular signal‐regulated kinase (ERK). Neurobiology of Learning and Memory, 123, 117–124. [DOI] [PubMed] [Google Scholar]
- McDermott, C. M. , Liu, D. , Ade, C. , & Schrader, L. A. (2015). Estradiol replacement enhances fear memory formation, impairs extinction and reduces COMT expression levels in the hippocampus of ovariectomized female mice. Neurobiology of Learning and Memory, 118, 167–177. 10.1016/j.nlm.2014.12.009 [DOI] [PubMed] [Google Scholar]
- McDermott, C. M. , Liu, D. , & Schrader, L. A. (2012). Role of gonadal hormones in anxiety and fear memory formation and inhibition in male mice. Physiology & Behavior, 105, 1168–1174. [DOI] [PubMed] [Google Scholar]
- McLean, C. P. , & Anderson, E. R. (2009). Brave men and timid women? A review of the gender differences in fear and anxiety. Clinical Psychology Review, 29, 496–505. [DOI] [PubMed] [Google Scholar]
- McLean, C. P. , Asnaani, A. , Litz, B. T. , & Hofmann, S. G. (2011). Gender differences in anxiety disorders: Prevalence, course of illness, comorbidity and burden of illness. Journal of Psychiatric Research, 45, 1027–1035. 10.1016/j.jpsychires.2011.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meir Drexler, S. , Merz, C. J. , Hamacher‐Dang, T. C. , & Wolf, O. T. (2016). Cortisol effects on fear memory reconsolidation in women. Psychopharmacology (Berl), 233, 2687–2697. 10.1007/s00213-016-4314-x [DOI] [PubMed] [Google Scholar]
- Mercer, K. B. , Dias, B. , Shafer, D. , Maddox, S. A. , Mulle, J. G. , Hu, P. , … Ressler, K. J. (2016). Functional evaluation of a PTSD‐associated genetic variant: estradiol regulation and ADCYAP1R1. Translational Psychiatry, 6(12), e978–e978. 10.1038/tp.2016.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz, C. J. , Tabbert, K. , Schweckendiek, J. , Klucken, T. , Vaitl, D. , Stark, R. , & Wolf, O. T. (2012). Neuronal correlates of extinction learning are modulated by sex hormones. Soc. Cogn. Affect. Neurosci., 7, 819–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz, C. J. , & Wolf, O. T. (2017). Sex differences in stress effects on emotional learning. Journal of Neuroscience Research, 95, 93–105. [DOI] [PubMed] [Google Scholar]
- Michopoulos, V. , Rothbaum, A. O. , Corwin, E. , Bradley, B. , Ressler, K. J. , & Jovanovic, T. (2015). Psychophysiology and posttraumatic stress disorder symptom profile in pregnant African‐American women with trauma exposure. Arch. Womensment. Health, 18, 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad, M. R. , Goldstein, J. M. , Orr, S. P. , Wedig, M. M. , Klibanski, A. , Pitman, R. K. , & Rauch, S. L. (2006). Fear conditioning and extinction: Influence of sex and menstrual cycle in healthy humans. Behavioral Neuroscience, 120, 1196–1203. 10.1037/0735-7044.120.5.1196 [DOI] [PubMed] [Google Scholar]
- Milad, M. R. , Igoe, S. A. , Lebron‐Milad, K. , & Novales, J. E. (2009). Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience, 164, 887–895. 10.1016/j.neuroscience.2009.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad, M. R. , Zeidan, M. A. , Contero, A. , Pitman, R. K. , Klibanski, A. , Rauch, S. L. , & Goldstein, J. M. (2010). The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience, 168, 652–658. 10.1016/j.neuroscience.2010.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson, C. A. , Anney, R. J. , Lotfi‐Miri, M. , Byrnes, G. B. , Williamson, R. , & Patton, G. C. (2005). Association between the COMT Val158Met polymorphism and propensity to anxiety in an Australian population‐based longitudinal study of adolescent health. Psychiatric Genetics, 15, 109–115. 10.1097/00041444-200506000-00007 [DOI] [PubMed] [Google Scholar]
- Orsini, C. A. , Willis, M. L. , Gilbert, R. J. , Bizon, J. L. , & Setlow, B. (2016). Sex differences in a rat model of risky decision making. Behavioral Neuroscience, 130, 50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace‐Schott, E. F. , Spencer, R. M. , Vijayakumar, S. , Ahmed, N. A. , Verga, P. W. , Orr, S. P. , … Milad, M. R. (2013). Extinction of conditioned fear is better learned and recalled in the morning than in the evening. Journal of Psychiatric Research, 47, 1776–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, C. H. , Ganella, D. E. , & Kim, J. H. (2017). Juvenile female rats, but not male rats, show renewal, reinstatement, and spontaneous recovery following extinction of conditioned fear. Learning Memory, 24, 630–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish, J. N. , Bertholomey, M. L. , Pang, H. W. , Speth, R. C. , & Torregrossa, M. M. (2019). Estradiol modulation of the renin‐angiotensin system and the regulation of fear extinction. Translational Psychiatry, 9, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos, G. , & Watson, C. (2007) The rat brain in stereotaxic coordinates, 6th edn. Cambridge, UK: Academic Press. [Google Scholar]
- Pellman, B. A. , Schuessler, B. P. , Tellakat, M. , & Kim, J. J. (2017). Sexually dimorphic risk mitigation strategies in rats. Eneuro, 4(1), ENEURO.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin, M. , Vandeleur, C. L. , Castelao, E. , Rothen, S. , Glaus, J. , Vollenweider, P. , & Preisig, M. (2014). Determinants of the development of post‐traumatic stress disorder, in the general population. Social Psychiatry and Psychiatric Epidemiology, 49, 447–457. [DOI] [PubMed] [Google Scholar]
- Peters, J. , Dieppa‐Perea, L. M. , Melendez, L. M. , & Quirk, G. J. (2010). Induction of fear extinction with hippocampal‐infralimbic BDNF. Science, 328, 1288–1290. 10.1126/science.1186909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich, G. D. , & Lougee, M. A. (2011). Sex differences in fear‐induced feeding cessation: Prolonged effect in female rats. Physiology & Behavior, 104, 996–1001. 10.1016/j.physbeh.2011.06.020 [DOI] [PubMed] [Google Scholar]
- Pineles, S. L. , Nillni, Y. I. , King, M. W. , Patton, S. C. , Bauer, M. R. , Mostoufi, S. M. , … Orr, S. P. (2016). Extinction retention and the menstrual cycle: Different associations for women with posttraumatic stress disorder. Journal of Abnormal Psychology, 125, 349–355. 10.1037/abn0000138 [DOI] [PubMed] [Google Scholar]
- Pineles, S. L. , Nillni, Y. I. , Pinna, G. , Irvine, J. , Webb, A. , Arditte Hall, K. A. , … Rasmusson, A. M. (2018). PTSD in women is associated with a block in conversion of progesterone to the GABAergic neurosteroids allopregnanolone and pregnanolone measured in plasma. Psychoneuroendocrinology, 93, 133–141. 10.1016/j.psyneuen.2018.04.024 [DOI] [PubMed] [Google Scholar]
- Pohlack, S. T. , Nees, F. , Ruttorf, M. , Cacciaglia, R. , Winkelmann, T. , Schad, L. R. , … Flor, H. (2015). Neural mechanism of a sex‐specific risk variant for posttraumatic stress disorder in the type I receptor of the pituitary adenylate cyclase activating polypeptide. Biological Psychiatry, 78, 840–847. 10.1016/j.biopsych.2014.12.018 [DOI] [PubMed] [Google Scholar]
- Pryce, C. R. , Lehmann, J. , & Feldon, J. (1999). Effect of sex on fear conditioning is similar for context and discrete CS in Wistar, Lewis and Fischer rat strains. Pharmacology, Biochemistry and Behavior, 64, 753–759. 10.1016/S0091-3057(99)00147-1 [DOI] [PubMed] [Google Scholar]
- Ramikie, T. S. , & Ressler, K. J. (2018). Mechanisms of sex differences in fear and posttraumatic stress disorder. Biological Psychiatry, 83, 876–885. [DOI] [PubMed] [Google Scholar]
- Ramzan, F. , Azam, A. B. , Monks, D. A. , & Zovkic, I. B. (2018). Androgen receptor is a negative regulator of contextual fear memory in male mice. Hormones and Behavior, 106, 10–18. [DOI] [PubMed] [Google Scholar]
- Rasmusson, A. M. , King, M. W. , Valovski, I. , Gregor, K. , Scioli‐Salter, E. , Pineles, S. L. , … Pinna, G. (2019). Relationships between cerebrospinal fluid GABAergic neurosteroid levels and symptom severity in men with PTSD. Psychoneuroendocrinology, 102, 95–104. 10.1016/j.psyneuen.2018.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnu, V. S. , Emami, M. R. , & Bredy, T. W. (2017) Genetic and epigenetic factors underlying sex differences in the regulation of gene expression in the brain. Journal of Neuroscience Research, 95, 301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattel, J. A. , Wegerer, M. , Miedl, S. F. , Blechert, J. , Grünberger, L. M. , Craske, M. G. , & Wilhelm, F. H. (2019). Peritraumatic unconditioned and conditioned responding explains sex differences in intrusions after analogue trauma. Behavior Research and Therapy, 116, 19–29. [DOI] [PubMed] [Google Scholar]
- Reppucci, C. J. , Kuthyar, M. , & Petrovich, G. D. (2013). Contextual fear cues inhibit eating in food‐deprived male and female rats. Appetite, 69, 186–195. 10.1016/j.appet.2013.06.004 [DOI] [PubMed] [Google Scholar]
- Reppucci, C. J. , & Petrovich, G. D. (2018). Neural substrates of fear‐induced hypophagia in male and female rats. Brain Structure and Function, 223, 2925–2947. [DOI] [PubMed] [Google Scholar]
- Ressler, K. J. , Mercer, K. B. , Bradley, B. , Jovanovic, T. , Mahan, A. , Kerley, K. , … May, V. (2011). Post‐traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature, 470, 492–497. 10.1038/nature09856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey, C. D. , Lipps, J. , & Shansky, R. M. (2014). Dopamine D1 receptor activation rescues extinction impairments in low‐estrogen female rats and induces cortical layer‐specific activation changes in prefrontal‐amygdala circuits. Neuropsychopharmacology, 39, 1282–1289. 10.1038/npp.2013.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro, A. M. , Barbosa, F. F. , Godinho, M. R. , Fernandes, V. S. , Munguba, H. , Melo, T. G. , … Silva, R. H. (2010). Sex differences in aversive memory in rats: Possible role of extinction and reactive emotional factors. Brain and Cognition, 74, 145–151. 10.1016/j.bandc.2010.07.012 [DOI] [PubMed] [Google Scholar]
- Rosas‐Vidal, L. E. , Do‐Monte, F. H. , Sotres‐Bayon, F. , & Quirk, G. J. (2014). Hippocampal–prefrontal BDNF and memory for fear extinction. Neuropsychopharmacology, 39, 2161–2169. 10.1038/npp.2014.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schienle, A. , Köchel, A. , & Leutgeb, V. (2011). Frontal late positivity in dental phobia: A study on gender differences. Biological Psychology, 88, 263–269. 10.1016/j.biopsycho.2011.08.010 [DOI] [PubMed] [Google Scholar]
- Schienle, A. , Scharmüller, W. , Leutgeb, V. , Schäfer, A. , & Stark, R. (2013). Sex differences in the functional and structural neuroanatomy of dental phobia. Brain Structure and Function, 218, 779–787. [DOI] [PubMed] [Google Scholar]
- Schüle, C. , Nothdurfter, C. , & Rupprecht, R. (2014). The role of allopregnanolone in depression and anxiety. Progress in Neurobiology, 113, 79–87. [DOI] [PubMed] [Google Scholar]
- Seedat, S. , Stein, D. J. , & Carey, P. D. (2005). Post‐traumatic stress disorder in women. CNS Drugs, 19, 411–427. 10.2165/00023210-200519050-00004 [DOI] [PubMed] [Google Scholar]
- Sevenster, D. , Visser, R. M. , & D'Hooge, R. (2018). A translational perspective on neural circuits of fear extinction: Current promises and challenges. Neurobiology of Learning and Memory, 155, 113–126. 10.1016/j.nlm.2018.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheynin, J. , Shind, C. , Radell, M. , Ebanks‐Williams, Y. , Gilbertson, M. W. , Beck, K. D. , & Myers, C. E. (2017). Greater avoidance behavior in individuals with posttraumatic stress disorder symptoms. Stress, 20, 285–293. 10.1080/10253890.2017.1309523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvil, E. , Sullivan, G. M. , Schafer, S. , Markowitz, J. C. , Campeas, M. , Wager, T. D. , … Neria, Y. (2014). Sex differences in extinction recall in posttraumatic stress disorder: A pilot fMRI study. Neurobiology of Learning and Memory, 113, 101–108. 10.1016/j.nlm.2014.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra‐Mercado, D. , Padilla‐Coreano, N. , & Quirk, G. J. (2011). Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology, 36, 529–538. 10.1038/npp.2010.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singewald, N. , & Holmes, A. (2019). Rodent models of impaired fear extinction. Psychopharmacology (Berl), 236, 21–32. 10.1007/s00213-018-5054-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singewald, N. , Schmuckermair, C. , Whittle, N. , Holmes, A. , & Ressler, K. J. (2015). Pharmacology of cognitive enhancers for exposure‐based therapy of fear, anxiety and trauma‐related disorders. Pharmacology & Therapeutics, 149, 150–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein, M. B. , Fallin, M. D. , Schork, N. J. , & Gelernter, J. (2005). COMT polymorphisms and anxiety‐related personality traits. Neuropsychopharmacology, 30(11), 2092–2102. 10.1038/sj.npp.1300787 [DOI] [PubMed] [Google Scholar]
- Stevens, J. S. , Almli, L. M. , Fani, N. , Gutman, D. A. , Bradley, B. , Norrholm, S. D. , … Ressler, K. J. (2014). PACAP receptor gene polymorphism impacts fear responses in the amygdala and hippocampus. Proceedings of the National Academy of Sciences of the United States of America, 111, 3158–3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter Horst, J. P. , Carobrez, A. P. , van der Mark, M. H. , de Kloet, E. R. , & Oitzl, M. S. (2012). Sex differences in fear memory and extinction of mice with forebrain‐specific disruption of the mineralocorticoid receptor. European Journal of Neuroscience, 36, 3096–3102. [DOI] [PubMed] [Google Scholar]
- Toufexis, D. J. , Myers, K. M. , Bowser, M. E. , & Davis, M. (2007). Estrogen disrupts the inhibition of fear in female rats, possibly through the antagonistic effects of estrogen receptor alpha (ERalpha) and ERbeta. Journal of Neuroscience, 27, 9729–9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovote, P. , Fadok, J. P. , & Lüthi, A. (2015). Neuronal circuits for fear and anxiety. Nature Reviews Neuroscience, 16, 317–331. [DOI] [PubMed] [Google Scholar]
- Ujjainwala, A. L. , Courtney, C. D. , Wojnowski, N. M. , Rhodes, J. S. , & Christian, C. A. (2019). Differential impacts on multiple forms of spatial and contextual memory in diazepam binding inhibitor knockout mice. Journal of Neuroscience Research, 97, 683–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco, E. R. , Florido, A. , Milad, M. R. , & Andero, R. (2019). Sex differences in fear extinction. Neuroscience and Biobehavioral Reviews, 103, 81–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal‐Gonzalez, I. , Vidal‐Gonzalez, B. , Rauch, S. L. , & Quirk, G. J. (2006). Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learning Memory, 13, 728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voulo, M. E. , & Parsons, R. G. (2017). Response‐specific sex difference in the retention of fear extinction. Learning Memory, 24, 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, R. A. , Andreansky, C. , Ray, M. H. , & McDannald, M. A. (2018). Early adolescent adversity inflates threat estimation in females and promotes alcohol use initiation in both sexes. Behavioral Neuroscience, 132, 171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Cao, C. , Wang, R. , Qing, Y. , Zhang, J. , & Zhang, X. Y. (2013). PAC1 receptor (ADCYAP1R1) genotype is associated with PTSD's emotional numbing symptoms in Chinese earthquake survivors. Journal of Affective Disorders, 150, 156–159. [DOI] [PubMed] [Google Scholar]
- Wegerer, M. , Blechert, J. , Kerschbaum, H. , & Wilhelm, F. H. (2013). Relationship between fear conditionability and aversive memories: Evidence from a novel conditioned‐intrusion paradigm. PLoS ONE, 8, e79025 10.1371/journal.pone.0079025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegerer, M. , Kerschbaum, H. , Blechert, J. , & Wilhelm, F. H. (2014). Low levels of estradiol are associated with elevated conditioned responding during fear extinction and with intrusive memories in daily life. Neurobiology of Learning and Memory, 116, 145–154. 10.1016/j.nlm.2014.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, E. C. , & Graham, B. M. (2016). Estradiol levels in women predict skin conductance response but not valence and expectancy ratings in conditioned fear extinction. Neurobiology of Learning and Memory, 134, 339–348. 10.1016/j.nlm.2016.08.011 [DOI] [PubMed] [Google Scholar]
- Wiltgen, B. J. , Sanders, M. J. , Behne, N. S. , & Fanselow, M. S. (2001). Sex differences, context preexposure, and the immediate shock deficit in Pavlovian context conditioning with mice. Behavioral Neuroscience, 115, 26–32. 10.1037/0735-7044.115.1.26 [DOI] [PubMed] [Google Scholar]
- Wray, N. R. , James, M. R. , Dumenil, T. , Handoko, H. Y. , Lind, P. A. , Montgomery, G. W. , & Martin, N. G. (2008). Association study of candidate variants of COMT with neuroticism, anxiety and depression. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics, 147B, 1314–1318. 10.1002/ajmg.b.30744 [DOI] [PubMed] [Google Scholar]
- Yagi, S. , & Galea, L. A. (2019). Sex differences in hippocampal cognition and neurogenesis. Neuropsychopharmacology, 44, 200–213. 10.1038/s41386-018-0208-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan, M. A. , Igoe, S. A. , Linnman, C. , Vitalo, A. , Levine, J. B. , Klibanski, A. , … Milad, M. R. (2011). Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biological Psychiatry, 70, 920–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan, L. , Jenkins, L. M. , Wolfson, O. E. , GadElkarim, J. J. , Nocito, K. , Thompson, P. M. , … Leow, A. D. (2017). The significance of negative correlations in brain connectivity. The Journal of Comparative Neurology, 525, 3251–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuj, D. V. , Palmer, M. A. , Lommen, M. J. , & Felmingham, K. L. (2016). The centrality of fear extinction in linking risk factors to PTSD: A narrative review. Neuroscience and Biobehavioral Reviews, 69, 15–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable (this review reports no primary data).


