Abstract
BACKGROUND
Whole blood donors are at risk of becoming iron deficient. To monitor iron stores, Sanquin implemented a new deferral policy based on ferritin levels, in addition to the traditional hemoglobin measurements.
METHODS
Ferritin levels are determined in every fifth donation, as well as in all first‐time donors. Donors with ferritin levels <15 ng/mL (WHO threshold) are deferred for 12 months; those ≥15 and ≤30 ng/mL for 6 months. The first results were analyzed and are presented here.
RESULTS
The results show that 25% of women (N = 20151, 95% CI 24%‐25%) and 1.6% of men (N = 10391, 95% CI 1.4%‐1.8%) have ferritin levels ≤30 ng/mL at their first blood center visit. For repeat (non‐first‐time) donors, these proportions are higher: 53% of women (N = 28329, 95% CI 52%‐54%) and 42% of men (N = 31089, 95% CI 41%‐43%). After a 6‐month deferral, in 88% of returning women (N = 3059, 95% CI 87%‐89%) and 99% of returning men (N = 3736, 95% CI 98%‐99%) ferritin levels were ≥15 ng/mL. After a 12‐month deferral, in 74% of returning women (N = 486, 95% CI 70%‐78%) and 95% of returning men (N = 479, 95% CI 94%‐97%) ferritin levels increased to ≥15 ng/mL.
CONCLUSION
Deferral of donors whose pre‐donation ferritin levels were ≤30 ng/mL might prevent donors from returning with ferritin levels <15 ng/mL. This policy is promising to mitigate effects of repeated donations on iron stores.
Sanquin is the national blood service in The Netherlands. In addition to securing safe blood products for patients, it has a responsibility to its voluntary non‐remunerated donors to diminish the risk of developing health problems related to whole blood donation. One of these risks is iron deficiency anemia or iron deficient erythropoiesis. During whole‐blood donation, a donor gives half a liter of blood, containing 210 to 240 mg iron bound to hemoglobin (Hb). 1 This iron is first replaced from iron stores (of which ferritin level is an indicator), which are then slowly replenished by an increased iron uptake from food. These stores are on average 411 mg in women under 50, 591 mg in women over 50, and 880 mg in men. 1 , 2 , 3 Thus, the amount of iron lost during donation is relatively large in comparison to the total iron stores, especially in premenopausal women.
To monitor donorsʼ iron statuses, donorsʼ hemoglobin levels are measured before each donation using a photometer (HemoCue) after finger prick sampling. Donors are eligible for donation if their hemoglobin level is at least 7.8 mmol/L (12.6 g/dL) for women, or 8.4 mmol/L (13.5 g/dL) for men. A hemoglobin level below this threshold may indicate iron deficiency anemia, which needs to be prevented. Yet, donors with normal hemoglobin levels can already be iron deficient without anemia. 4 This happens when the body is not given enough time to replenish its iron stores between donations, using only hemoglobin measurements as an iron marker.
Several studies have analyzed iron recovery after donation with similar results. 5 , 6 In a study of 50 male donors, followed after whole blood donation, blood volume is restored first. About 4 days post‐donation, hemoglobin levels are at the lowest point and start to increase as stored iron is released to replenish hemoglobin. At the same time, ferritin levels decline and reach their lowest point about 29 days post‐donation. After 56 days (the minimum interval between two whole blood donations for men in The Netherlands), average measured ferritin levels are 27 ng/mL in repeat male donors, compared to an average of 49 ng/mL directly prior to donation. At that time point, the average hemoglobin level is 9.1 mmol/L, almost back to the average starting value of 9.2 mmol/L. 5 Donors in this study did not take iron supplements.
Several strategies to better monitor iron status in donors have been proposed, such as hemoglobin‐guided donation intervals, ferritin‐guided donation intervals, and iron supplementation. 7 Sanquin has chosen to implement a ferritin‐based deferral policy for its donors. The policy started in November 2017; donors are deferred for 6 or 12 months in case their ferritin levels are ≤30 or <15 ng/mL respectively, even though their hemoglobin was above the threshold and they were eligible to donate otherwise. These thresholds were based on WHO standards, which state that ferritin levels <15 ng/mL indicate iron deficiency, while higher levels reflect the size of the iron stores. 8 However, one should be aware that ferritin is also an acute‐phase reactant. 9
The main aim of the policy is to prevent donorsʼ ferritin levels from dropping below 15 ng/mL. Without regular ferritin testing, donors with low ferritin levels (≤30 ng/mL) but hemoglobin levels above the threshold will keep donating every few months, with the risk that their iron reserves decline until hemoglobin levels fall below the threshold. By measuring ferritin levels every fifth donation, Sanquin tries to prevent donors from future deferral, thus preventing them from becoming overt iron deficient (with or without anemia). The choice to measure ferritin every fifth donation rather than at a different frequency is arbitrary and not based on extensive research.
Data on hemoglobin and ferritin levels collected during the first 18 months since the implementation of this ferritin deferral protocol were analyzed to determine: 1) the distribution of ferritin levels in new donors, providing a reference distribution of ferritin levels in healthy individuals that have never donated blood before; 2) the difference in ferritin distribution between new and repeat donors; and 3) the difference in donor ferritin levels before and after deferral, which provides information on the effectiveness of donor deferral to prevent donors from returning to donate with iron deficiency.
In evaluating the deferral policy based on ferritin levels, there are three important aspects to consider. The first is the effectiveness of the policy in preventing donors returning with ferritin levels below 15 ng/mL. The second and third are the effects of the policy on the blood supply and on donor health, respectively. This article analyses the first aspect in depth; an exhaustive analysis of all three aspects is outside the scope of the current study and will become possible in due time.
METHODS
At Sanquin, the national blood establishment in The Netherlands, every person who signs up to become a blood donor is first invited for a donor intake. This initial visit is meant to screen for infectious diseases and assessment of blood type and potential antibodies, without donation. Prospect donors that meet all the criteria of the donor health questionnaire and have a negative infectious disease and antibody screen become a blood donor and are invited for their first donation a few weeks later.
The ferritin‐based deferral policy prescribes that ferritin is measured at the intake visit for all first‐time donors, and at every fifth donation in repeat donors. Donors are considered “first‐time” donors only for their first donation and are considered “repeat” donors after that. Unlike hemoglobin, which is measured by point‐of‐care testing and gives the result directly, ferritin is measured in serum samples which are analyzed within a few days after the donation has taken place. At the intake, this makes no difference, because no donation takes place during this visit. However, for repeat donors, the ferritin level is assessed after the donation has taken place, from a sample pouch that is collected along with the donated blood. This means that donors are deferred after donation (they are notified of their deferral by letter), and that ferritin measurements are available from repeat donors that have hemoglobin levels above the donation threshold only. There is currently no evidence that donating with low ferritin levels is dangerous or unhealthy, as long as hemoglobin levels are adequate. Therefore, this donation is considered to be safe even if the ferritin measurement comes back below the threshold.
Ferritin levels are assessed with the Architect i2000 by Abbott Diagnostics. Ferritin levels are divided into three categories with different consequences for the donor:
– Ferritin <15 ng/mL: the donor is deferred from donation for 12 months.
– 15 ≤ Ferritin ≤30 ng/mL: the donor is deferred from donation for 6 months.
– Ferritin >30 ng/mL: no deferral, the donor can return for the next donation after the regular minimum donation interval (56 days for men, 122 days for women).
Sanquin does not have a policy to advise donors to take iron supplements for low ferritin or hemoglobin levels, although they are free to take over‐the‐counter iron supplements on their own initiative. The deferral periods are meant to give the donors a “break” from blood donation, allowing iron stores to recover solely by iron intake from donorsʼ regular diets.
Sanquin collects approximately 400,000 whole blood donations annually, from over 270,000 donors. 10 Data for this study were collected between November 2017 and April 2019 on donors who gave consent for the use of their data for scientific research (>99% of all donors give this consent).
To compare the ferritin distributions in first‐time and repeat donors, for each donor only the first ferritin measurement is considered. For first‐time donors, this is the ferritin measurement taken at the pre‐donation screening. For repeat donors, this is the ferritin measurement taken at the fifth donation since the implementation of the protocol. If the same donor has a consecutive ferritin measurement, five donations later, that measurement is not used in this analysis, so that every donor only occurs once in the data set.
To assess the effectiveness of the deferral for preventing donors from returning to donate while iron deficient, we compare pre‐deferral ferritin levels to post‐deferral ferritin levels, of all deferred donors of whom post‐deferral ferritin measurements were available. We compared pre‐deferral ferritin levels in donors with and without a post‐deferral measurement to check for selection bias. In donors without post‐deferral measurement, we selected only those who were eligible for donation again (i.e., their deferral period has ended).
For donors who do have a post‐deferral ferritin measurement, we calculated the average daily increase in ferritin levels for each donor. Note that since ferritin recovery does not progress linearly, the averages do not represent the actual increase on any given day, 6 but this method can be used to compare recovery rates between women and men.
All analyses are performed in the R programming language and environment for statistical computing. 11 Plots are produced with the ggplot2 package. 12 Distributions are asymmetric and are therefore characterized by the median value and the interquartile range (IQR). Density plots presented are kernel density estimates; the bandwidth is selected by Silvermanʼs rule of thumb. 13
RESULTS
Ferritin levels were measured at least once in 30,542 first‐time donors (20,151 women) and 59,418 repeat donors (28,329 women). Figure 1 shows the distribution of ferritin levels for various combinations of sex and age categories. In first‐time donors, men had substantially higher ferritin levels than women, and ferritin levels increased with age: median ferritin levels ranged from 96 to 173 ng/mL in men and from 43 to 81 ng/mL in women by age group. In repeat donors, the median values were more similar for both sexes, ranging from 22‐35 ng/mL in men and from 28 to 36 ng/mL in women. Table 1 shows the median ferritin level and IQR for all age groups.
Fig .1.
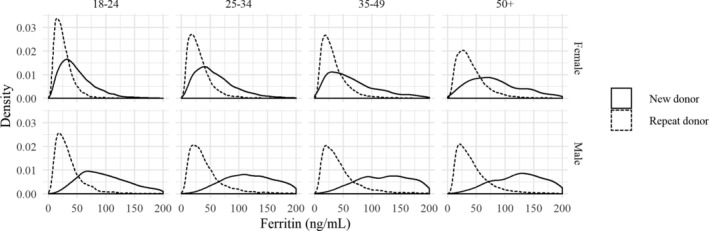
Distributions of ferritin levels in new (solid line) and repeat donors (dashed line) for various combinations of sex and age category. Sample sizes range from 1037 (male new donors age 50+) to 14,848 (male repeat donors age 50+).
TABLE 1.
Median ferritin levels and interquartile range (IQR) in first‐time and repeat donors, by sex and age category
| Sex | Age category | First‐time donors | Repeat donors | ||
|---|---|---|---|---|---|
| N | Median ferritin level (IQR) | N | Median ferritin level (IQR) | ||
| Female | 18‐24 | 9713 | 43 (27‐65) | 4537 | 22 (15‐33) |
| Female | 25‐34 | 5071 | 52 (33‐79) | 5045 | 26 (17‐39) |
| Female | 35‐49 | 3801 | 58 (33‐98) | 7411 | 28 (18‐43) |
| Female | 50+ | 1566 | 81 (50‐128) | 11,336 | 35 (23‐53) |
| Male | 18‐24 | 3896 | 96 (66‐135) | 2048 | 28 (18‐43) |
| Male | 25‐34 | 3424 | 136 (95‐191) | 3928 | 34 (21‐53) |
| Male | 35‐49 | 2167 | 154 (102‐224) | 7063 | 35 (22‐56) |
| Male | 50+ | 904 | 173 (120‐256) | 18,050 | 36 (23‐56) |
Overall, 25% of female first‐time donors (95% CI 24%‐25%) and 1.6% of male first‐time donors (95% CI 1.4%‐1.8%) had ferritin levels below the threshold of 30 ng/mL at the intake visit. These proportions were considerably higher in repeat donors: 53% of women (95% CI 52%‐54%) and 42% of men (95% CI 41%‐43%) had a ferritin level ≤30 ng/mL. These outcomes again show that men have significantly higher ferritin levels than women (as witnessed by the confidence intervals), and that repeat donors are much more likely to have low ferritin levels than first‐time donors, although this difference is much more pronounced in men (25‐fold increase) than in women (two‐fold increase). This leads to substantially smaller differences in ferritin levels between men and women for repeat donors than in first‐time donors.
Most donors with low ferritin levels had a ferritin level between 15 and 30 ng/mL, but 5.3% of female first‐time donors and 0.1% of male first‐time donors already had ferritin levels <15 ng/mL. In repeat donors, these low levels were observed in 15% of female and 9.4% of male donors.
We calculated the moving average (window size of 1000 observations) of the proportion of donors that were deferred due to low ferritin levels as a function of age. We did this separately for sex, donor type (new/repeat donor), and ferritin deferral category (<15 ng/mL and between 15‐30 ng/mL). In Fig. 2, the proportion of deferrals as a function of donor age is shown for each combination of deferral type, donor type, and sex. Confidence intervals are not shown due to the proximity of the lines, but they are all extremely narrow. The difference in deferral probability between female and male donors was substantially larger in new donors than in repeat donors. In male repeat donors, the association between age and deferral rate was negative and almost linear. In female repeat donors, there was a clear non‐linear dependency on age: after an initial decrease until the age of 25, there was an increase until the age of 40, after which it started to decrease again.
Fig .2.
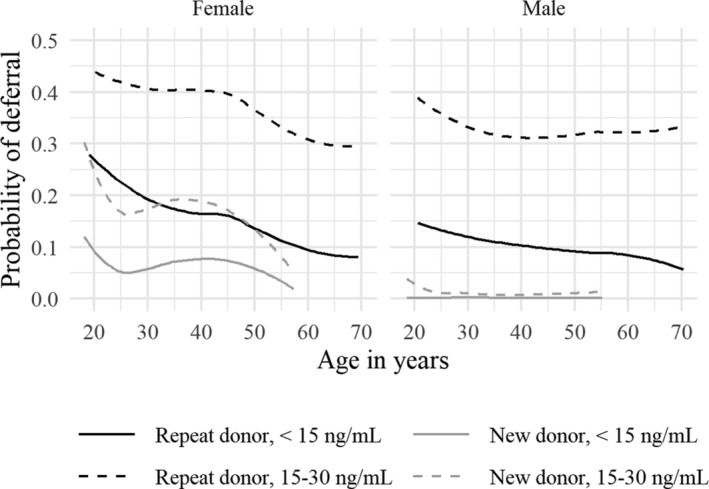
Probability of deferral due to low ferritin as a function of age in repeat (black) and new (gray) donors. Both deferral for 6 months (ferritin between 15 and 30 ng/mL, dashed line) and deferral for 12 months (ferritin under 15, solid line) are shown. The difference in age ranges is due to the fact that new donors are only accepted until the age of 65, whereas repeat donors can keep donating for several more years.
We also analyzed the difference between pre‐ and post‐deferral ferritin levels for both 6‐ and 12‐month deferral. To check for selection bias, we compared the pre‐deferral ferritin levels of donors with and without a post‐deferral measurement. Table 2 shows the number of deferred donors, the number of donors whose deferral period has ended, and those who have already returned. It shows that pre‐deferral ferritin levels in donors who returned after deferral do not differ from those in the complete group. This indicates that the group of donors with a post‐deferral ferritin measurement are likely to be a representative sample of all deferred donors with respect to ferritin. However, there is a difference in return rate between the sexes: approximately 80% of men versus only 60% of women have returned out of those whose deferral period has ended. The return rates include donors who have returned after deferral but did not have a repeat ferritin measurement due to a low hemoglobin level.
TABLE 2.
The total number of donors deferred, those that are eligible to return for donation at the time of analysis for this study (deferred at least 7 months ago for 6‐month deferral, and at least 13 months ago for 12‐month deferral), and those that have already returned for donation. For each group the median ferritin level and interquartile range (IQR) at deferral are given. Percentages behind the number of donors returned after deferral are with respect to the number of donors whose deferral period has ended and therefore could have returned after deferral
| Six‐month deferral | Twelve‐month deferral | |||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| Number of donors deferred | 15,008 | 10,296 | 5974 | 2952 |
| Median ferritin at deferral (IQR) | 22 ng/mL (19‐26) | 22 ng/mL (18‐26) | 11 ng/mL (9‐13) | 12 ng/mL (10‐13) |
| Number of donors whose deferral period has ended | 6181 | 4576 | 906 | 596 |
| Median ferritin at deferral (IQR) | 23 ng/mL (19‐26) | 22 ng/mL (18‐26) | 10 ng/mL (8‐12) | 12 ng/mL (10‐13) |
| Number of donors returned after deferral | 3258 (53%) | 3883 (85%) | 540 (60%) | 490 (82%) |
| Median ferritin at deferral (IQR) | 22 ng/mL (18‐26) | 22 ng/mL (18‐26) | 11 ng/mL (9‐13) | 12 ng/mL (10‐13) |
After a ferritin deferral period, the deferral rate due to low hemoglobin levels is considerably lower than it is in general. The overall hemoglobin deferral rate is 8.4% for women and 4.6% for men. After a 12‐month deferral, 6.1% of women and 1.6% of men are immediately deferred again because their hemoglobin levels are below the threshold. After a six‐month deferral, these percentages are 4.4% for women and 2.8% for men.
The changes in ferritin levels after 6‐ and 12‐month deferrals are summarized in Table 3. After either deferral period, the majority of donors who returned had an increased ferritin level, men more so than women. More than 95% of returning male donors had a ferritin level of 15 ng/mL or higher after either deferral type. In female donors, this proportion was 88% after six‐month deferral and 73% after 12‐month deferral. The difference in ferritin recovery rate between men and women makes sense considering the differences in ferritin levels observed in first‐time donors. These differences can likely be attributed to the same physiological cause(s).
TABLE 3.
Ferritin levels of donors who return after 6‐month deferral (ferritin level between 15 and 30 ng/mL) or 12‐month deferral (ferritin level <15 ng/mL). Symbols in the bottom three rows indicate whether the ferritin level has dropped (↓), has gone up one (↑) or two (↑↑) categories, or has stayed in the same category (=)
| Six‐month deferral | Twelve‐month deferral | |||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| Number of donors returned | 3059 | 3736 | 486 | 479 |
| Donors with increased ferritin | 61% | 91% | 91% | 99% |
| Median total increase (IQR) | 4 ng/mL (−3‐11) | 15 ng/mL (7‐25) | 12 ng/mL (5‐22) | 27 ng/mL (16‐39) |
| Median increase per day | 0.016 ng/mL | 0.071 ng/mL | 0.030 ng/mL | 0.068 ng/mL |
| Ferritin after deferral <15 ng/mL | 12% (↓) | 1.5% (↓) | 26% (=) | 4.6% (=) |
| Ferritin after deferral 15‐30 ng/mL | 54% (=) | 30% (=) | 43% (↑) | 27% (↑) |
| Ferritin after deferral >30 ng/mL | 34% (↑) | 68% (↑) | 30% (↑↑) | 68% (↑↑) |
The rate of ferritin recovery differed between female and male donors. The median of the average daily increase in women was higher for 12‐month deferral than for six‐month deferral: 0.030 ng/mL/day versus 0.016 ng/mL/day. In men, they were more similar: 0.068 ng/mL/day for 12‐month deferral and 0.071 ng/mL/day for six‐month deferral. After either period of deferral, ferritin recovery rates were substantially higher in men than in women.
DISCUSSION
This study shows that in first‐time donors who have never donated blood, womenʼs ferritin levels are lower than menʼs, and they increase with age. Ferritin levels in repeat donors are substantially lower and therefore the deferral rate is higher, for both sexes. The difference in ferritin levels between male and female donors is considerably smaller in repeat than in first‐time donors, regardless of age. Finally, after having a measured ferritin level below 30 ng/mL and being deferred for 6 or 12 months, the vast majority of returning female and almost all returning male donors have ferritin levels of 15 ng/mL or higher.
The differences we have observed between male and female first‐time donors can partly be explained by the effect of the menstrual cycle on iron stores. After menopause, this additional iron loss is no longer present and womenʼs ferritin levels increase. 14 The fact that sex differences are much smaller among repeat donors suggests that regular blood donation leads to a lower ferritin level, which impacts men more than women as their natural ferritin stores are generally higher. Multiple studies have found that an increase in the number of donations results in decreased iron stores, even though hemoglobin levels remain above the threshold for donation. 15 , 16 Our results suggest that this relationship is less strong in women. This can be explained by the shorter minimum donation interval for men (56 days vs. 122 days for women), which allows them to donate five times a year, compared to three times a year for women. Also, donation frequency is the best predictor for decreased iron stores. 17 Further research into the precise relationship between donation frequency, total number of donations and trends in ferritin levels is ongoing, for instance in the INTERVAL study. 16 Another explanation is that women are more easily deferred than men; the hemoglobin threshold for donation is much closer to the average hemoglobin value in women than in men. Women with low iron stores are already deferred by the hemoglobin test alone, so their (likely low) ferritin levels have not been measured in this study.
Sex differences can also be seen in the percentage of donors that return after donation: men are more likely to return than women. Before we try to explain this difference, we should keep in mind that donors only come back after they are actively invited by Sanquin by means of an invitation algorithm (based on daily demand for blood and blood types). The effect of this procedure may hinder the outcome of the current analysis. However, studies on donor return rates after deferral are consistent in finding a higher return rate for men than for women, although the magnitude of the difference varies. 18 , 19 , 20
Regarding the increase in ferritin after deferral, we assume that this is larger than what would have occurred in case the donors had not been deferred according to the policy. This assumption is based on studies mentioned in the introduction, which show that donors need at least 168 days for ferritin levels to recover. 5 , 6 This indicates that a longer deferral period gives donors more time to restore their ferritin stores by taking a break from their regular donation schedule. Nonetheless, a considerable number of donors is deferred again based on their ferritin level upon their return, especially women.
In male donors, the average daily ferritin increase is higher in donors who were deferred for 6 months than in those deferred for 12 months. This is interesting, because during the first 29 days after donation, ferritin levels are still decreasing. 6 It might indicate that after the initial decrease, ferritin recovery starts off at a high rate which then tapers off. We see different results in women: the average daily ferritin increase is higher in women deferred for 12 months than those deferred for 6 months. Even though recovery rates of donors deferred for 12 versus 6 months cannot be compared because of their different ferritin levels before deferral (<15 vs. ≤30 ng/mL), it is remarkable that the ratio between these rates differs between men and women. One explanation might be that ferritin recovery takes longer for women, so the increase starts later in the process. However, no differences between men and women were found in ferritin recovery speed in control groups of oral iron supplementation studies, although sample sizes were relatively low (about 20 people per group). 5 , 21 A larger‐scale study that measures donorsʼ ferritin levels in the weeks following a donation could provide more insight.
Some blood services supply blood donors with iron supplements in order to prevent iron deficiency, which can lead to restless‐leg syndrome and pica, especially pagophagia, the inclination to chew ice. 22 , 23 There is no solid evidence for an association between low iron stores and fatigue and cognition. 23 Some studies did find that iron supplements improve cognition in adolescents and women, but most of these have small sample sizes and are methodologically weak, with evidence of publication bias. 21 The INTERVAL study did not find any effect of shortening the donation interval on cognitive function in an analysis of health survey questionnaires given to more than 45,000 donors. 16 An analysis on more than 16,000 donors participating in the Danish Blood Donor Study did not find an association between low ferritin levels and self‐reported mental and physical health either. 24
Regardless of its possible health effects, several studies have shown that iron supplementation increases the speed of recovery of iron stores and hemoglobin levels after blood donation. 5 , 25 However, iron supplementation can also have unintended and unwanted side effects, which may impact compliance of iron supplementation and can deter donors. For this reason, as well as the lack of scientific consensus on how iron supplementation in blood donors should be installed, Sanquin chose to introduce ferritin‐guided donation intervals rather than iron supplementation to mitigate effects of repeated donation on iron stores.
Although the ferritin‐guided deferral policy seems to help donors maintain appropriate ferritin levels, it also raises some concerns. In the past few years, the proportion of new female donors under 25 years of age has been increasing rapidly in The Netherlands. 26 Ferritin levels below 30 ng/mL are very common among young women who have never donated blood. If this trend continues, the proportion of first‐time donors that immediately gets deferred from donation based on ferritin levels will continue to increase. Deferral of these potential donors may lead to a lower availability of blood products and has a larger effect on the blood supply than hemoglobin‐based deferral. One to three donations are lost for every 6‐month deferral, and three to five for every 12‐month deferral, depending on sex. Additionally, by deferring donors not only for low hemoglobin, but also for low ferritin levels, the chances of a donor being deferred are increased. However, for the long‐term this increased chance may decrease again, as deferral due to low ferritin can lower hemoglobin deferral rates. In our data set, we found that the hemoglobin deferral rate decreases by half after ferritin deferral as compared to the overall hemoglobin deferral rate. Deferral can also cause donors to become unmotivated and not return to the blood center, especially first‐time donors. 18 , 19 Compensating for lost donations by recruiting new donors could therefore be a less desirable consequence. Therefore, it is important to carefully monitor donor availability when introducing ferritin‐guided donation intervals. One should also note that the frequency of measuring ferritin levels (every fifth donation) is mostly arbitrary and loosely based on a trade‐off between cost and benefit. Measuring more often would identify donors at risk of iron deficiency earlier, but also increases cost and loss of potential donations due to deferral.
From our results, we conclude that repeat donors have considerably lower ferritin levels and smaller differences between sexes in comparison to first‐time donors. Deferral of donors with ferritin levels ≤30 ng/mL seems to prevent the majority of donors, male donors in particular, from returning to donate with iron deficiency.
Comparisons to a control group are needed to establish whether ferritin levels are indeed higher in groups of donors than they would have been without ferritin‐guided donation intervals. Furthermore, longer‐term research is needed to assess whether this policy can maintain donorsʼ ferritin levels within the appropriate range.
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
AUTHOR CONTRIBUTIONS
Designed research (MV, MPJ, KvdH), statistical analyses (MV, MPJ, MvL), data interpretation (MV, MPJ, KvdH, MvL), literature research (MV, KvdH, MvK), writing and checking of final manuscript (MV, MPJ, KvdH, MvK).
ACKNOWLEDGMENTS
This research was carried out under the BloodStArt project, funded by the Sanquin Blood Supply Foundation (PPO 18‐14/L2337).
REFERENCES
- 1. Finch CA, Cook JD, Labbe RF, Culala M. Effect of blood donation on iron stores as evaluated by serum ferritin. Vol. 50; 1977 [cited 2019 May 10]. Available from: www.bloodjournal.org. [PubMed]
- 2. Gropper SS. Smith JL. Cengage Learning: Advanced nutrition and human metabolism; 2012. [Google Scholar]
- 3. Kiss JE, Vassallo RR. How do we manage iron deficiency after blood donation? Br J Haematol 2018;181:590‐603. 10.1111/bjh.15136. [DOI] [PubMed] [Google Scholar]
- 4. Soppi ET. Iron deficiency without anemia ‐ a clinical challenge. Clin Case Rep 2018;6:1082‐6. 10.1002/ccr3.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kiss JE, Brambilla D, Glynn SA, et al. Oral iron supplementation after blood donation. JAMA 2015;313:575 10.1001/jama.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schotten N, Pasker‐de Jong PCM, Moretti D, et al. The donation interval of 56 days requires extension to 180 days for whole blood donors to recover from changes in iron metabolism. Blood 2016;128:2185‐8. [DOI] [PubMed] [Google Scholar]
- 7. Sweegers MG, Kraaij MGJ, van den Hurk K. First do no harm: iron loss in whole blood donors. ISBT Sci Ser 2020;15:110‐7. 10.1111/voxs.12527. [DOI] [Google Scholar]
- 8. World Health Organization . Serum ferritin concentrations for the assessment of iron status and iron deficiency in populations. Geneva, World Health Organization; 2011. [accessed 2019 May 28] Available from: http://www.who.int/vmnis/indicators/serum_ferritin.pdf [Google Scholar]
- 9. Abbaspour N, Hurrell R, Kelishadi R. Review on iron and its importance for human health. J Res Med Sci 2014;19:164‐74. [PMC free article] [PubMed] [Google Scholar]
- 10. Sanquin Blood Supply Foundation. Sanquin Annual Report 2018, Amsterdam. Available from: https://www.sanquin.nl/binaries/content/assets/sanquinen/about-sanquin/annual-reports/sanquin_annual_report_2018.pdf.
- 11. R Core Team . R: a language and environment for statistical computing. 2019. [accessed 2019 April 30] Available from: https://www.r-project.org/.
- 12. Wickham H. Ggplot2: elegant graphics for data analysis. New York: Springer‐Verlag; 2016. [accessed 2019 April 30] Available from: https://ggplot2.tidyverse.org.
- 13. Silverman BW. Density estimation for statistics and data analysis. Vol. 26. CRC press, 1986. [Google Scholar]
- 14. Milman N, Kirchhoff M, JØrgensen T. Iron status markers, serum ferritin and hemoglobin in 1359 Danish women in relation to menstruation, hormonal contraception, parity, and postmenopausal hormone treatment. Ann Hematol 1992;65:96‐102. 10.1007/BF01698138. [DOI] [PubMed] [Google Scholar]
- 15. Milman N, Kirchhoff M. Influence of blood donation on iron stores assessed by serum ferritin and haemoglobin in a population survey of 1433 Danish males. Eur J Haematol 2009;47:134‐9. 10.1111/j.1600-0609.1991.tb00136.x. [DOI] [PubMed] [Google Scholar]
- 16. Di Angelantonio E, Thompson SG, Kaptoge S, et al. Efficiency and safety of varying the frequency of whole blood donation (INTERVAL): a randomised trial of 45 000 donors. Lancet 2017;390:2360‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mittal R, Marwaha N, Basu S, Mohan H, Ravi Kumar A. Evaluation of iron stores in blood donors by serum ferritin. 2007 [cited 2019 Nov 22]. Available from: https://www.researchgate.net/publication/6516413. [PubMed]
- 18. Custer B, Schlumpf KS, Wright D, et al. Donor return after temporary deferral. Transfusion 2011;51:1188‐96. 10.1111/j.1537-2995.2010.02989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Custer B, Chinn A, Hirschler NV, et al. The consequences of temporary deferral on future whole blood donation. Transfusion 2007;47:1514‐23. 10.1111/j.1537-2995.2007.01292.x. [DOI] [PubMed] [Google Scholar]
- 20. Spekman MLC, Tilburg TG, Merz E. Do deferred donors continue their donations? A large‐scale register study on whole blood donor return in the Netherlands. Transfusion 2019;59(12):3657‐65. 10.1111/trf.15551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Falkingham M, Abdelhamid A, Curtis P, et al. The effects of oral iron supplementation on cognition in older children and adults: a systematic review and meta‐analysis. Nutr J 2010;9:4 10.1186/1475-2891-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gorlin JB. Iron replacement: precautionary principle versus risk‐based decision making. Transfusion 2019;59:1613‐5. 10.1111/trf.15274. [DOI] [PubMed] [Google Scholar]
- 23. Zalpuri S, Schoten N, Baart AM, et al. Iron deficiency‐related symptoms in whole blood donors: a systematic review. Transfusion 2019;59:3275‐87. [DOI] [PubMed] [Google Scholar]
- 24. Rigas AS, Pedersen OB, Sørensen CJ, et al. No association between iron status and self‐reported health‐related quality of life in 16,375 Danish blood donors: results from the Danish Blood Donor Study. Transfusion 2015;55:1752‐6. [DOI] [PubMed] [Google Scholar]
- 25. Smith GA, Fisher SA, Doree C, et al. Oral or parenteral iron supplementation to reduce deferral, iron deficiency and/or anaemia in blood donors. Cochrane Database Syst Rev 2014;7:81‐82. 10.1002/14651858.CD009532.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goldman M, Steele WR, Di Angelantonio E, et al. Comparison of donor and general population demographics over time: a BEST Collaborative group study. Transfusion 2017;57:2469‐76. 10.1111/trf.14307. [DOI] [PubMed] [Google Scholar]


