Abstract
Objectives
To describe in detail the techniques for transvesical robot‐assisted radical prostatectomy (RARP) using the da Vinci Si/Xi system (Intuitive Surgical, Sunnyvale, CA, USA) and to evaluate functional and oncological outcomes in 35 patients with prostate cancer.
Patients and Methods
Thirty‐five patients with localized prostate cancer were enrolled for transvesical RARP. The patients' preoperative data (mean ± sd age 63.4 ± 8.1 years, body mass index 28.6 ± 5.3 kg/m2, total prostate‐specific antigen 10.8 ± 4.9 ng/mL and prostate volume 30.6 ± 14.4 mL, and median [interquartile range {IQR}] biopsy Gleason score 6 [6–7], and International Index of Erectile Function [IIEF]‐5 score 18 [16–20]) were collected. Preoperative assessment revealed 28 cases of cT2a and seven cases of cT2b disease. All patients were continent preoperatively (defined as no pad required or one dry pad per day as a precaution). Surgical results and peri‐operative complications were assessed. All patients were followed up for at least 12 months postoperatively.
Results
The mean operating time was 150 ± 35 min. Estimated blood loss was 100 ± 45 mL. Urinary infection was noted in one patient and managed with levofloxacin. Another patient complained of nocturia on postoperative day 14, which was relieved with solifenacin succinate. Urethral catheters were removed on postoperative day 7. Thirty‐two patients achieved immediate urinary continence, with three patients returning to full continence on postoperative day 14. Postoperative pathology confirmed 24 pT2a cases, nine pT2b cases and two pT2c cases (median [IQR] Gleason score 6 [6–7]). Positive surgical margins were found in four patients (11.4%). No urethral stricture or urinary leakage was noted on urethrocystography taken 3 months after surgery. Urodynamic studies were performed preoperatively and 6 months after surgery: median (IQR) maximum urinary flow 12.2 (10.2–14.9) vs 13.7 (10.1–15.0) mL/s; bladder capacity 385.3 (351.3–410.2) vs 370.2 (330.1–395.4) mL; and voiding phase detrusor contractility 38.5 (27.8–42.3) vs 35.6 (28.3–41.3) mmH2O, respectively. During a minimum of 12 months of follow‐up, no biochemical recurrence was noted in any patient. The median (IQR) IIEF‐5 score was 17 (16–19).
Conclusions
The transvesical approach is a valid alternative to RARP in selected patients, providing promising postoperative urinary continence. Long‐term functional and oncological results require further investigation.
Keywords: prostate cancer, transvesical approach, robot assisted radical prostatectomy, urinary continence, robotic surgery
Introduction
Robot‐assisted radical prostatectomy (RARP) has become a surgical treatment favoured by many centres for localized prostate cancer, providing similar trifecta outcomes when compared to open surgery [1, 2]. Different approaches to RARP have emerged, with the aim of achieving better functional outcomes while maintaining adequate tumour control. These include the anterior [3, 4, 5], posterior [6] and lateral approach [7]. In 2008, Desai et al. [8] reported the first two cases of transvesical RARP on cadavers using the da Vinci‐S system (Intuitive Surgical, Sunnyvale, CA, USA). In the present paper, we describe detailed techniques for transvesical RARP using the da Vinci Si/Xi system on a multi‐port basis.
Patients and Methods
This study was conducted in accordance with the guidelines of the Ethics Committee of the First Affiliated Hospital of Nanchang University. Written informed consent, including a brief description of the various approaches to performing RARP (anterior approach, Retzius‐sparing/posterior approach and the experimental transvesical approach) and a clear indication of alternative choices of disease management (e.g. active surveillance as recommended by current guidelines for low‐risk patients with prostate cancer), were provided to patients harbouring naive localized prostate cancer (total PSA <20 ng/mL, Gleason score ≤7, clinical stage T1‐T2cN0M0, prostate volume <80 mL) between January 2018 and December 2018. Thirty‐five patients who provided signed consent and agreed to the transvesical approach were enrolled in this study. All patients were continent prior to surgery. No patient had a history of abdominal surgery.
Preoperative assessment included patient age, body mass index (BMI), serum total PSA, prostate volume based on TRUS, 13‐core (12 systematic cores and one suspicious core) transrectal prostate biopsy, pelvic MRI, chest X‐ray or CT, bone scan, and International Index of Erectile Function (IIEF)‐5 score.
The console time, estimated blood loss, conversion and transfusion were recorded. Peri‐operative complications were recorded and graded according to the Clavien–Dindo system [9]. Patients were encouraged to ambulate on postoperative day 1, and gradually return to normal diet once bowel function had recovered. Pelvic drainage seemed to be unnecessary based on our experience in the first five patients so was not placed in subsequent candidates. The urethral catheter was removed on postoperative day 7.
Patients were re‐evaluated one week after discharge and assigned to a 3‐month follow‐up interval for the first year, including serum total PSA, continence status (number of pads per day), erectile function (IIEF‐5 score), urethrocystography (performed 3 months after surgery), urodynamic studies (performed 6 months after surgery) and others when indicated. Biochemical recurrence was defined as total PSA >0.2 ng/mL. Continence was defined as zero pad or one dry pad for precaution.
Surgical Techniques
All surgeries were performed by a single robotic surgery team. Pure transvesical RARP was performed under general anaesthesia in all cases. A 30° lens was used throughout the procedure. Pelvic lymph node dissection was not performed in this series. Surgical techniques were as described below.
Patient preparation
Patients were placed on a clear liquid diet on the day before surgery. Two i.v. lines and one arterial line were established. A broad‐spectrum antibiotic was given i.v. 30 min prior to incision.
Patient position, trocar configuration and docking
Once the patient was intubated and properly secured in a supine position, the lower abdomen, external genitalia and upper thigh were disinfected and draped. A Foley catheter was then placed in a sterilized manner, which would be manipulated by the bedside assistant during the operation. Pneumoperitoneum was established with the Hasson open technique and maintained at 12 mmHg. A standard W configuration of the camera port, three robotic ports (for coupling with Hot Shears™ monopolar [first arm; Intuitive Surgical], Maryland bipolar forceps [second arm; Intuitive Surgical], ProGrasp™ forceps [third arm; Intuitive Surgical] and two large needle drivers [first and second arms; Intuitive Surgical]) and a 12‐mm assistant port was used, with an additional 5‐mm assistant port placed four fingerbreadths below the costal margin and between the camera port and the right‐hand robot port (Fig. 1). The patient was then placed in a 15° Trendelenburg position. The robotic cart was docked in the midline caudal position as with other transperitoneal approaches.
Fig. 1.
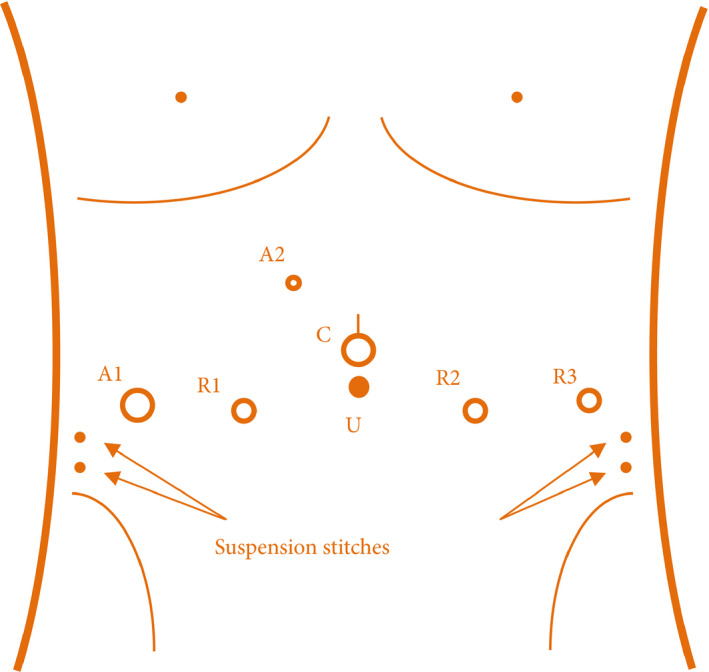
Trocar configuration. 12‐mm camera port (C): 2‐cm above the umbilicus. First‐ and second‐arm robot port (R1 and R2): lateral margin of rectus abdominis, 8 cm from camera port. Third‐arm robot port (R3) and 12‐mm assistant port (A1): anterior axillary line, 8 cm from R2 and R1, respectively. 5‐mm assistant port (A2): four fingerbreadths below the costal margin and between the camera port and first‐arm robot port. Arrows: entry and exit point of suspension stitches.
Opening the bladder
An 8‐cm longitudinal incision was made on the posteriosuperior aspect of the bladder (Fig. 2A). In order to expend the cystotomy to provide sufficient exposure of intravesical structures, suspension stitches (2‐0 monofilament polypropylene suture on ST‐1 needle; Covidien, Dublin, Ireland) were placed through the lateral abdominal wall with the entry and exit points located at 2 and 3 cm above the anterior superior iliac spines on both sides. The interureteric ridge, ureteric orifice, bladder neck and internal orifice of the urethra were identified (Fig. 2B).
Fig. 2.
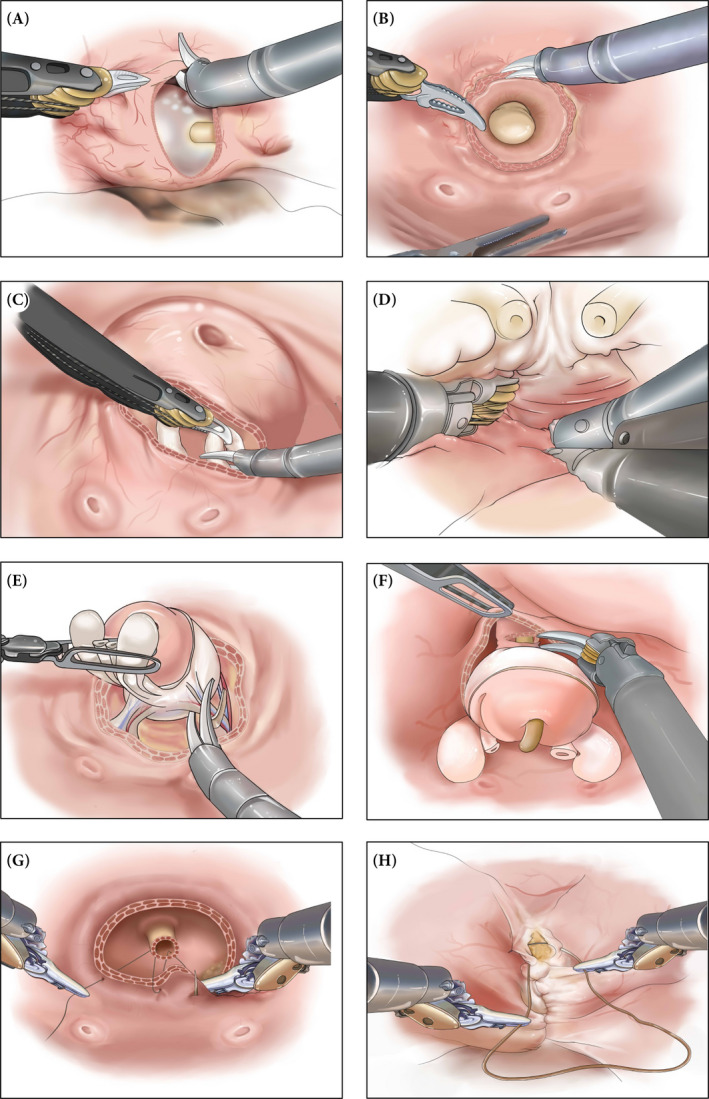
Surgical steps of transvesical robot assisted radical prostatectomy. Through a vertical cystotomy expended by suspension stitches (A), intravesical structures were exposed and a circumferential incision was made around the internal urethral orifice (B). Dissections of the vas deferens and seminal vesicles were carried out through the lower half of the circumferential incision (C). Intrafascial posterior dissection was continued towards the apex (D). Lateral dissection of prostatic pedicles and neurovascular bundles was carried out between prostatic capsule and periprostatic fascia in a nerve‐sparing manner (E). Anterior dissection continued towards the apex and urethra was exposed and transected (F). Urethrovesical anastomosis was achieved using two 4‐0 barbed polydioxanone sutures on RB‐1 needles in a running fashion (G). Bladder was closed in two layers in a running fashion (H).
Dissection of vas deferens and seminal vesicles
A circumferential incision with a radius of approximately 1.5 cm was made around the internal urethral orifice through the mucosa and muscular layer (Fig. 2B). Initial dissections of the right vas deferens and seminal vesicle were carried out through the lower half of the circumferential incision by an incision at 6 o’clock, which was then extended laterally using a combination of sharp and blunt dissection (Fig. 2C). At approximately 5–6 o’clock, the right ampullae of the vas deferens were usually the first ‘white tubular’ structure in sight. With the third‐arm Prograsp retracting the tissues around the internal urethral orifice upwards and second‐arm Maryland pulling the right ampullae leftwards, a sufficient length of the right vas deferens was then mobilized using a combination of blunt and sharp dissection. After the right vas deferens was dissected, cauterized and transected, the right seminal vesicle was then completely isolated, with special caution taken when controlling the feeding arteries at the tip where the pelvic plexus and right neurovascular bundle (NVB) run in close proximity. The left vas deferens and seminal vesicle were dissected in the same manner.
Posterior dissection
At this point, Denonvillier’s fascia was visualized. With both the vas deferens and seminal vesicles retracted upwards using the Prograsp, an intrafascial plane was then entered ventral to the Denonvillier’s fascia. Dissection was then continued along the posterior aspect of the light‐reflecting prostatic capsule (intrafascial plane), by stripping down the Denonvillier’s fascia from it. Be aware that the NVBs ran posterolaterally, the use of energy was strictly limited in the midline, and only when necessary. Mostly by blunt dissection, posterior dissection was continued towards the apex of the prostate in an antegrade manner (Fig. 2D). A blunt‐end grasper through the second assistant port pressing the Denonvillier’s fascia dorsally could be of great help. Although a frozen section should be considered to exclude the possibility of tumour invasion when significant adhesion was encountered, sometimes the adhesion might be a consequence of transrectal biopsy.
Lateral dissection
On the right side, using a combination of blunt and sharp dissection and tissue texture as a guide, a plane between the prostatic capsule and periprostatic fascia was initially entered at 3 o’clock to facilitate the exposure of the right pedicle. At this time, the light‐reflecting surface of prostatic capsule should be visualized both posteriorly and laterally. A sufficient lateral space was then created to allow the right pedicle located at 4–5 o’clock to be controlled by weck clips placed close to the prostatic capsule to avoid ‘transecting’ the NVB, and incised distally using cold scissors. A constantly applied gentle but sufficient retraction of the seminal vesicles upwards using Prograsp could be of great help during this step (Fig. 2E). When the right pedicle was completely transected, blunt dissection continued towards the apex in an antegrade fashion to relieve the prostate enclosed in the prostate capsule from the periprostatic fascia laterally (intrafascial plane dissection). It was important to avoid the use of any energy during this step, to minimize the chance of injuring the NVBs that travelled posterolaterally. The same manoeuvre was replicated on the left side.
Anterior and apex dissection
-
At this stage, the tissues on the anterior aspect of the prostate were the only attachment preventing the prostate from being rotated freely. Through the upper half of the circumferential incision around the internal urethral orifice, dissection was carried out along the prostatic capsule and towards the apex.
Three key structures were dealt with sequentially. The dorsal vein complex was the first key structure to be dissected. It was the surgeon’s decision whether or not to control it with suture ligation, which was usually not necessary. After the dorsal vein complex was detached from the anterior aspect of the prostate, slow and careful dissection, with a lateral to medial approach, was continued to reveal the conjuncture of the apex and urethral external sphincter, using tissue texture as a guide. After the external sphincter is detached from the apex, the ventral aspect of the internal sphincter of the urethra was then opened sharply and closely to the apex, to preserve a sufficient length of urethral stump (Fig. 2F). The urethral catheter should now be visualized; this was pulled out slowly by the assistant until only the tip of the catheter could be seen in the urethral stump. The exposed lateral and dorsal aspect of the urethra was then transected using cold scissors. Now the specimen was fully detached and placed in the Endocatch bag, which was securely held in place by pulling it out partly from the 12‐mm assistant trocar site and reinserting the trocar adjacent to the bag.
Urethrovesical anastomosis
-
The whole prostatic fossa was inspected for haemostasis before the anastomosis began. Using the catheter as a guide for locating the urethral stump, urethrovesical anastomosis was carried out using two 4‐0 barbed polydioxanone sutures on RB‐1 needles in a running fashion (Fig. 2G). The first suture ran clockwise from 6 o’clock to 11 o’clock using 4–6 stitches, followed by another 4–6 counterclockwise‐placed stitches from 5 o’clock to 12 o’clock. Before placing the strings on tension, an appropriate amount of absorbable haemostat material (e.g. SURGICEL® Fibrillar) was inserted laterally in the prostatic fossa. Note that all stitches were placed from outside in at the bladder, and inside out at the urethra. When the two sutures’ exit points joined and both sutures came out from the bladder, they were not cut before two knots were tied. Both sutures were then passed through the bladder and another two knots were tied outside extra‐luminally to avoid bladder irritation and stone formation. Finally, a new three‐way urethral catheter was then placed and inflated with 20 mL saline. It should be noted that we did not perform constant bladder irrigation on any patient. Intermittent bladder irrigation was performed only when necessary.
Closure of bladder and trocar wounds
Two‐layer closure of the bladder wall was achieved using 3‐0 polydioxanone barbed sutures on an SH needle in a running fashion (Fig. 2H). Watertight closure was tested by filling the bladder with 100 mL saline. The specimen was retrieved through an extended incision of the camera trocar wounds. The abdominal cavity was inspected again for any possible issues before all trocar wounds were closed in a standard fashion. Bilateral pelvic lymph node dissection was not performed and a pelvic drain was not placed.
Results
Demographics
Thirty‐five patients bearing naive localized prostate cancer administered between January 2018 and December 2018 were enrolled for transvesical RARP. Patient demographics were summarized in Table 1. The mean ± sd patient age was 63.4 ± 8.1 years, BMI 28.6 ± 5.3 kg/m2, total PSA 10.8 ± 4.9 ng/mL and prostate volume 30.6 ± 14.4 mL, and the median (interquartile range [IQR]) biopsy Gleason score was 6 (6–7) and IIEF‐5 score 18 (16–20). All patients were continent preoperatively (defined as no pad required or one dry pad per day as a precaution).
Table 1.
Patient demographics.
| Number of patients | 35 |
| Age, years | 63.4 ± 8.1 |
| BMI, kg/m2 | 28.6 ± 5.3 |
| Preoperative serum total PSA, ng/mL | 10.8 ± 4.9 |
| Prostate volume, mL | 30.6 ± 14.4 |
| Preoperative IIEF‐5 score | 18 (16, 20) |
| cTNM stage, n | |
| T2aN0M0 | 28 |
| T2bN0M0 | 7 |
| T2cN0M0 | 0 |
| Biopsy Gleason score | 6 (6, 7) |
IIEF, International Index of Erectile Function. Data are presented as mean ± sd or median (interquartile range).
Operation
All 35 operations were successfully performed without open conversion or transfusion. Peri‐operative data are summarized in Table 2. The mean ± sd operating time was 150 ± 35 min and estimated blood loss was 100 ± 45 mL. Pelvic drainage was placed in our first five patients, which yielded a negligible amount of discharge or clear fluid proven to be normal ascitic fluid. Therefore, we did not place pelvic drainage routinely thereafter.
Table 2.
Peri‐operative data.
| Number of patients | 35 |
| Operating time, min | 150 ± 35 |
| Estimated blood loss, mL | 100 ± 45 |
| Open conversion, n (%) | 0 (0) |
| Transfusion, n (%) | 0 (0) |
| Other intra‐operative complications, n (%) | 0 (0) |
| Postoperative pathology | |
| Pathological T stage | |
| T2a | 24 |
| T2b | 9 |
| T2c | 2 |
| Specimen Gleason score | 6 (6, 7) |
| Positive surgical margin, n (%) | 4 (11.4) |
| ≤Grade II postoperative complications, n (%) | 2 (5.7) |
| >Grade II postoperative complications, n (%) | 0 (0) |
| Urethral catheterization, days | 7 |
| Hospital stay, days | 7 (7, 8) |
Data were presented as mean ± sd or median (interquartile range) or otherwise indicated.
Complications
Injury of the rectum or major pelvic vessel was not encountered in our series. During the peri‐operative period, bladder rupture, urinary leakage, persistent gross haematuria, fever, intra‐abdominal infection or urinary retention after catheter removal was not observed. Asymptomatic urinary infection was noted in one patient on postoperative day 4 and was managed with oral levofloxacin. During follow‐up, one patient complained of nocturia on the first visit after discharge (postoperative day 14). Symptoms were relieved by solifenacin succinate (5 mg once daily) 1 month after surgery. Symptom complaints related to BOO were not encountered during a minimum follow‐up of 12 months of all patients.
Outcomes
Oncology
Preoperative assessment revealed 28 cases of cT2a and seven cases of cT2b disease, with a median (IQR) Gleason score of 6 (6, 7; Table 1). Postoperative pathology showed 24 pT2a cases, nine pT2b cases and two pT2c cases (median [IQR] Gleason score 6 [6, 7]). Positive surgical margins were found in four patients (three at the apex, one on the right lobe), who were all put on active surveillance after a discussion with patients for alternative options (Table 2). Biochemical recurrence was found in no patients during a minimum follow‐up of 12 months (Table 3).
Table 3.
Surgical outcomes.
| Number of patients | 35 |
| Oncology: postoperative total PSA, ng/mL | |
| 1 week | 2.105 (1.133, 3.857) |
| 3 months | 0.063 (0.010, 0.363) |
| 6 months | 0.016 (0.008, 0.030) |
| 9 months | 0.031 (0.008, 0.075) |
| Urinary continence* | |
| Continent on removal of catheter, n (%) | 32 (91.4) |
| Continent at 2 weeks, n (%) | 35 (100) |
| Urodynamic studies, preoperative vs 6 month postoperative | |
| Maximum urinary flow, mL/s | 12.2 (10.2, 14.9) vs 13.7 (10.1, 15.0) |
| Bladder capacity, mL | 385.3 (351.3, 410.2) vs 370.2 (330.1, 395.4) |
| Detrusor contractility: voiding phase, mmH2O | 38.5 (27.8, 42.3) vs 35.6 (28.3, 41.3) |
| Erectile function | |
| Postoperative IIEF‐5 score | 17 (16, 19) |
| Long‐term complications, n (%) | |
| Nocturia | 1 (2.9) |
| Dysuria | 0 (0) |
IIEF, International Index of Erectile Function. Data were presented as median (interquartile range) or otherwise indicated.
Continence was defined as no pad required or one dry pad per day as a precaution.
Urination
The Foley catheter was removed on postoperative day 7. Thirty‐two patients achieved immediate urinary continence. Three patients used two to four pads per day and returned to continence on postoperative day 14. As mentioned previously, one patient complained of nocturia 14 days after surgery and was well managed with oral intake of solifenacin succinate (5 mg once daily). All patients maintained urinary continence afterwards. No patient complained of dysuria or other signs of BOO (Table 3). No urethral stricture or urinary leakage was noted on urethrocystography taken 3 months after surgery (Fig. 3). Urodynamic studies were conducted 6 months after surgery; the median (IQR) preoperative vs 6‐month postoperative maximum urinary flow (12.2 [10.2, 14.9] vs 13.7 [10.1, 15.0] mL/s), bladder capacity (385.3 [351.3, 410.2] vs 370.2 [330.1, 395.4] mL) and voiding phase detrusor contractility (38.5 [27.8, 42.3] vs 35.6 [28.3, 41.3] mmH2O) did not appear to differ significantly (P > 0.05, paired Student t‐test; Table 3).
Fig. 3.
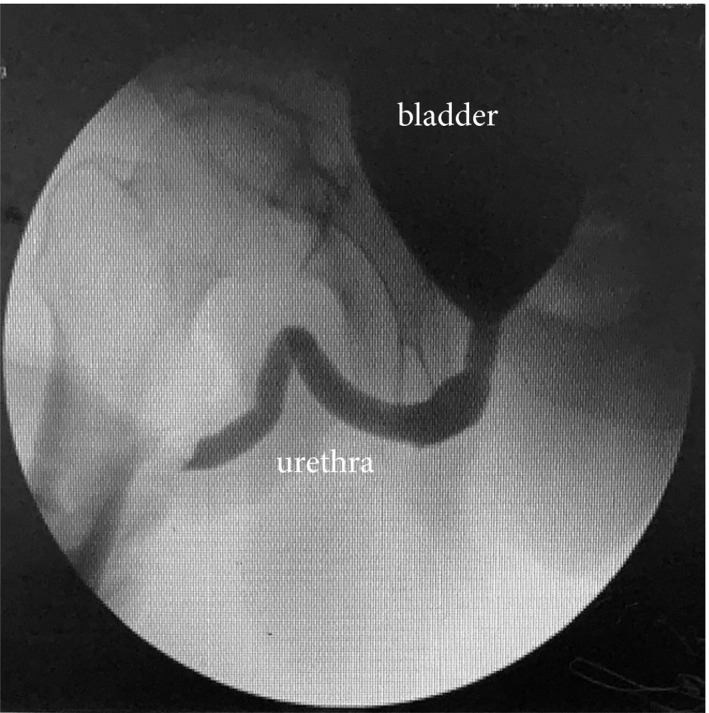
Urethrocystography taken 3 months after surgery.
Erectile Function
At 12 months postoperatively, the median (IQR) IIEF‐5 score was 17 (16, 19) and was not statistically different from the preoperative IIEF‐5 score 18 (16, 20) in a paired comparison (P > 0.05, paired Student's t‐test; Table 3).
Discussion
Various approaches to performing RARP have been described with the aim of achieving optimal functional outcomes while ensuring tumour control. The anterior approach that features a large working space and anatomical landmarks with which we are familiar is the most popular approach in both transperitoneal and extraperitoneal settings [10]. It has been extensively applied in many centres and perfected during the last 15 years, such as the VIP [11] and Veil technique [5]. To spare the retropubic structures, including the puboprostatic ligament and dorsal vein complex that contribute to postoperative urinary continence, Galfano et al. [6] proposed the posterior approach or Retzius‐sparing approach that promotes early urinary continence recovery, as demonstrated in randomized controlled trials [12, 13, 14, 15, 16].
The techniques of the transvesical approach using the da Vinci‐S robotic system were initially described by Desai et al. [8] on cadavers. Advantages of this approach include completely obviating the need to mobilize the bladder or enter the retropubic space, minimizing the dissection trauma as the operation is strictly confined to the deep bony pelvis, and not requiring a steep Trendelenburg position or any bowel retraction since the camera is inside the bladder most of the time. From a technical aspect, we made a few modifications to the transvesical technique proposed by Desai et al. Firstly, instead of using the single R‐port with four channels, our technique was based on multiple ports for better freedom of movement and the use of a third robotic arm and two assistant ports. Bladder access was gained through a vertical cystotomy expended laterally by suspension stitches. A greater working space, sufficient exposure provided by the suspension stitches and a bedside assistant may greatly reduce the learning curve. Pelvic lymph node dissection is also possible when indicated. Secondly, instead of a ‘racket‐handle’ style urethrovesical anastomosis, we simply performed direct mucosa‐to‐mucosa inline anastomosis with two running barbed sutures running to opposing directions. Signs of BOO or prolonged inconsistency (>2 weeks) were not observed during a minimum follow‐up of 12 months. No urethral stricture or urinary leakage was noted on urethrocystography taken 3 months after surgery (Fig. 3). Thirdly, we did not control the dorsal vein complex with EndoGIA or suture ligation. When necessary, we used the third‐arm Prograsp to clip it while maintaining an upward retraction before the specimen was fully detached.
The transvesical approach has the inherent advantages of the Retzius‐sparing approach [17]. Most importantly, the retropubic structures are preserved in the same manner, which translates to optimum postoperative continence. The prostate is initially approached from the posterior aspect where there are no neurovascular structures that contribute to postoperative continence and potency. An intrafascial dissection plane between the prostatic capsule and Denonvillier’s fascia can be easily found after vas deferens and seminal vesicle dissection. By using the same dissection plane as a reference and staying close to the ‘light‐reflecting’ prostatic capsule, lateral and anterior dissection are carried out intrafascially. As such, deploying a good intrafascial dissection that spares the periprostatic nerves may be easier. Differing from the Retzius‐sparing technique, direct in‐line exposure of the prostate and periprostatic structures can be obtained. Anterior or lateral dissection is therefore easier. In addition, urethrovesical anastomosis is performed in the same manner as the anterior approach (Fig. 4). All these factors may translate to a shorter learning curve. When a large median lobe is present, the relationship between the median lobe and the ureteric orifices can be clearly identified from inside the bladder (Fig. 5). Unnecessarily large bladder neck opening can therefore be avoided, lowering the risks of ureteric orifice injury. Pelvic drainage was placed in our first five patients, which yielded a negligible amount of discharge or clear fluid proven to be normal ascitic fluid. No urinary leakage to the abdominal cavity was encountered. As such, we did not place pelvic drainage routinely thereafter. We therefore believe that pelvic drainage is usually not required, as long as the cystotomy is closed in a watertight manner. Galfano et al. [18] reported that the use of a suprapubic cystostomy catheter for bladder drainage yielded less patient discomfort than a urethral catheter very recently. A cystostomy catheter placed through an 8‐mm trocar incision will be investigated in the future.
Fig. 4.

Illustration of direction of access during radical prostatectomy. (A) Retzius‐sparing approach. (B) Transvesical approach.
Fig. 5.

Intravesical structures with a large median lobe intruding the bladder during transvesical robot‐assisted radical prostatectomy.
Several technical points should be highlighted. Regarding the exposure of the operative field, percutaneous suspension stitches that expend the cystotomy from both sides are very helpful. A small prostate (<50 g) is easier to dissect in a limited working space inside the bladder. However, a ‘racket‐style’ incision by extending the circumferential incision around the internal urethral orifice upwards at 12 o’clock can be helpful when the gland is too large. When both vas deferens and seminal vesicles are isolated, a third‐arm Prograsp can be very helpful to retract these structures upwards during dissection of the posterior aspect of the prostate. As with the Retzius‐sparing approach, initial dissection starts posteriorly. Familiarity with the Retzius‐sparing approach may greatly shorten the learning curve. For urethrovesical anastomosis, the technique is almost identical to the anterior approach with which we are familiar. We routinely filled the ‘dead space’ around the anastomotic site with fibrin‐based haemostatics.
Technically, a major limitation of the transvesical approach is that the bladder is opened intentionally. The detrusor of the bladder is composed of three layers of muscles that run in different directions (excluding the trigon): an inner layer of longitudinal muscle fibres, a middle layer of circular muscle fibres and an outer layer of longitudinal muscle fibres. As such, instead of a transverse incision that transects all three layers of fibres, we chose to make a longitudinal incision on the posterosuperior aspect of the bladder that theoretically only transected the middle layer of muscle fibres that runs circumferentially. Geometrically, a longitudinal incision expended by two suspension stitches that pulls laterally generates a larger opening than a transverse incision. Still, scarring in the bladder wall resulting from an 8‐cm incision may raise concerns of possible changes in capacity and compatibility of the bladder, and in contractility of detrusor muscles. To address our concerns, urodynamic studies were undertaken 6 months after surgery. The results showed that the maximum urinary flow, bladder capacity and detrusor contractility did not appear to differ from baseline significantly (P > 0.05, paired Student t‐test), suggesting a negligible adverse impact of the intentional cystotomy on bladder function. Another limitation of the transvesical approach is not sparing the bladder neck, the preservation of which has been shown to be associated with quicker recovery of urinary function and a higher urinary continence rate in multiple studies [19, 20, 21, 22]. Although the early return of urinary continence observed in the present series can be explained by the preservation of other structures that contribute to urinary continence (e.g. retropubic structures), the impact of not sparing the bladder neck during transvesical RARP on short‐term and long‐term continence requires further investigation due to the limited number of cases. In the present study, we only included a limited number of low‐risk cases, and the follow‐up was too short to draw any conclusion on the long‐term outcomes. Lack of comparative analysis with other well established RARP techniques is another drawback of this study. We are in the process of enrolling more candidates in a registered clinical trial (ChiCTR1900024751). Finally, we restricted our inclusion criteria to low‐risk patients with prostate cancer with comparatively small prostate volume because of concerns regarding surgical margins and urinary leakage, respectively. As such, the applicability of the technique in patients with large prostates or higher‐risk disease remains to be investigated in the future.
In conclusion, transvesical RARP is feasible and represents an alternative approach to RARP in selected patients. Early urinary continence recovery and limited adverse effects on erectile function can be expected, while long‐term tumour control requires further investigation.
Conflicts of Interest
None declared.
Supporting information
Acknowledgements
We acknowledge funding from the following programmes: the Natural Science Foundation of China (NSFC 81760457 to Gongxian Wang); the Key Research and Development Programme of Jiangxi Province (20161ACG70013 to Gongxian Wang); the Natural Science Foundation of China (NSFC 81602256 to Xiaochen Zhou); and the Key Research and Development Programme of Jiangxi Province (20171ACB20029 to Xiaochen Zhou).
X.Z., B.F. and C.Z. contributed equally to this work.
References
- 1. Coughlin GD, Yaxley JW, Chambers SK et al. Robot‐assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: 24‐month outcomes from a randomised controlled study. Lancet Oncol 2018; 19: 1051–60 [DOI] [PubMed] [Google Scholar]
- 2. Yaxley JW, Coughlin GD, Chambers SK et al. Robot‐assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: early outcomes from a randomised controlled phase 3 study. Lancet 2016; 388: 1057–66 [DOI] [PubMed] [Google Scholar]
- 3. Kaul S, Savera A, Badani K, Fumo M, Bhandari A, Menon M. Functional outcomes and oncological efficacy of Vattikuti Institute prostatectomy with Veil of Aphrodite nerve‐sparing: an analysis of 154 consecutive patients. BJU Int 2006; 97: 467–72 [DOI] [PubMed] [Google Scholar]
- 4. Savera AT, Kaul S, Badani K, Stark AT, Shah NL, Menon M. Robotic radical prostatectomy with the "Veil of Aphrodite" technique: histologic evidence of enhanced nerve sparing. Eur Urol 2006; 49: 1065–73 [DOI] [PubMed] [Google Scholar]
- 5. Kaul S, Bhandari A, Hemal A, Savera A, Shrivastava A, Menon M. Robotic radical prostatectomy with preservation of the prostatic fascia: a feasibility study. Urology 2005; 66: 1261–5 [DOI] [PubMed] [Google Scholar]
- 6. Galfano A, Ascione A, Grimaldi S, Petralia G, Strada E, Bocciardi AM. A new anatomic approach for robot‐assisted laparoscopic prostatectomy: a feasibility study for completely intrafascial surgery. Eur Urol 2010; 58: 457–61 [DOI] [PubMed] [Google Scholar]
- 7. Mattei A, Naspro R, Annino F, Burke D, Guida R Jr, Gaston R. Tension and energy‐free robotic‐assisted laparoscopic radical prostatectomy with interfascial dissection of the neurovascular bundles. Eur Urol 2007; 52: 687–94 [DOI] [PubMed] [Google Scholar]
- 8. Desai MM, Aron M, Berger A et al. Transvesical robotic radical prostatectomy. BJU Int 2008; 102: 1666–9 [DOI] [PubMed] [Google Scholar]
- 9. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240: 205–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ficarra V, Wiklund PN, Rochat CH et al. The European Association of Urology Robotic Urology Section (ERUS) survey of robot‐assisted radical prostatectomy (RARP). BJU Int 2013; 111: 596–603 [DOI] [PubMed] [Google Scholar]
- 11. Menon M, Tewari A, Peabody J, VIP Team . Vattikuti Institute prostatectomy: technique. J Urol 2003; 169: 2289–92 [DOI] [PubMed] [Google Scholar]
- 12. Dalela D, Jeong W, Prasad MA et al. A pragmatic randomized controlled trial examining the impact of the Retzius‐sparing approach on early urinary continence recovery after robot‐assisted radical prostatectomy. Eur Urol 2017; 72: 677–85 [DOI] [PubMed] [Google Scholar]
- 13. Asimakopoulos AD, Topazio L, De Angelis M et al. Retzius‐sparing versus standard robot‐assisted radical prostatectomy: a prospective randomized comparison on immediate continence rates. Surg Endosc 2019; 33: 2187–96 [DOI] [PubMed] [Google Scholar]
- 14. Sood A, Abdollah F, Menon M. Retzius‐sparing robot‐assisted radical prostatectomy. BJU Int 2019; 123: 7–8 [DOI] [PubMed] [Google Scholar]
- 15. Checcucci E, Veccia A, Fiori C et al. Retzius‐sparing robot‐assisted radical prostatectomy vs the standard approach: a systematic review and analysis of comparative outcomes. BJU Int 2020; 125: 8–16 [DOI] [PubMed] [Google Scholar]
- 16. Lee J, Kim HY, Goh HJ et al. Retzius sparing robot‐assisted radical prostatectomy conveys early regain of continence over conventional robot‐assisted radical prostatectomy: a propensity score matched analysis of 1,863 patients. J Urol 2020; 203: 137–44 [DOI] [PubMed] [Google Scholar]
- 17. Secco S, Galfano A, Barbieri M et al. Technical features and the demonstrated advantages of the Retzius sparing robotic prostatectomy. Arch Esp Urol 2019; 72: 247–56 [PubMed] [Google Scholar]
- 18. Galfano A, Secco S, Panarello D et al. Pain and discomfort after Retzius‐sparing robot‐assisted radical prostatectomy: a comparative study between suprapubic cystostomy and urethral catheter as urinary drainage. Minerva Urol Nefrol 2019; 71: 381–5 [DOI] [PubMed] [Google Scholar]
- 19. Nyarangi‐Dix JN, Tichy D, Hatiboglu G, Pahernik S, Tosev G, Hohenfellner M. Complete bladder neck preservation promotes long‐term post‐prostatectomy continence without compromising midterm oncological outcome: analysis of a randomised controlled cohort. World J Urol 2018; 36: 349–55 [DOI] [PubMed] [Google Scholar]
- 20. Nyarangi‐Dix JN, Radtke JP, Hadaschik B, Pahernik S, Hohenfellner M. Impact of complete bladder neck preservation on urinary continence, quality of life and surgical margins after radical prostatectomy: a randomized, controlled, single blind trial. J Urol 2013; 189: 891–8 [DOI] [PubMed] [Google Scholar]
- 21. Freire MP, Weinberg AC, Lei Y et al. Anatomic bladder neck preservation during robotic‐assisted laparoscopic radical prostatectomy: description of technique and outcomes. Eur Urol 2009; 56: 972–80 [DOI] [PubMed] [Google Scholar]
- 22. Kim JW, Kim DK, Ahn HK, Jung HD, Lee JY, Cho KS. Effect of bladder neck preservation on long‐term urinary continence after robot‐assisted laparoscopic prostatectomy: a systematic review and meta‐analysis. J Clin Med 2019; 8: E2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


