Abstract
The Polyneuropathy And Treatment with Hizentra (PATH) study required subjects with chronic inflammatory demyelinating polyneuropathy (CIDP) to show dependency on immunoglobulin G (IgG) and then be restabilized on IgG before being randomized to placebo or one of two doses of subcutaneous immunoglobulin (SCIG). Nineteen of the 51 subjects (37%) randomized to placebo did not relapse over the next 24 weeks. This article explores the reasons for this effect. A post‐hoc analysis of the PATH placebo group was undertaken. A literature search identified other placebo‐controlled CIDP trials for review and comparison. In PATH, subjects randomized to placebo who did not relapse were significantly older, had more severe disease, and took longer to deteriorate in the IgG dependency period compared with those who relapsed. Published trials in CIDP, whose primary endpoint was stability or deterioration, had a mean non‐deterioration (placebo effect) of 43%, while trials with a primary endpoint of improvement had a placebo response of only 11%. Placebo is an important variable in the design of CIDP trials. Trials designed to show clinical improvement will have a significantly lower effect of this phenomenon than those designed to show stability or deterioration.
Keywords: CIDP, immunoglobulin, non‐relapse, placebo, relapse
1. INTRODUCTION
The double‐blind, randomized placebo‐controlled trial (RCT) is the gold standard for investigating the effect of a new therapy. The inclusion of subjects randomized to a placebo arm is supposed to measure any effect that is not due to the active ingredient of the investigational product. Placebo is considered to be a usually pharmacologically inert preparation, which may provide some benefit without having an actual effect on a disorder. 1 These effects have been shown to be related to the patient's expectations of their clinical state and desired effects of treatment, as well as conditioning from previous experiences. While the term placebo is frequently used, it actually encompasses several definitions. We use the term “placebo response” to describe when a subject reports an improvement in the condition while on a placebo. “Placebo effect” will be used when a subject does not deteriorate or, in the case of chronic inflammatory demyelinating polyneuropathy (CIDP), does not relapse as expected while on placebo. These effects of placebo are seen in clinical trials of virtually all disorders, whether the response is objective or subjective. The various factors that influence placebo response are only just beginning to be understood. 1
CIDP comprises a group of immune‐mediated disorders responsive to therapy. 2 , 3 However, in all CIDP trials, there has been recognition of a significant placebo response and effect. This has been particularly true in trials in which the primary outcome measure was based on whether a patient relapsed after an initially efficacious treatment was removed (placebo effect).
The Polyneuropathy And Treatment with Hizentra (PATH) study was a phase III RCT investigating subcutaneous immunoglobulin (SCIG) for maintenance therapy in CIDP. 4 PATH used a unique design in the desire of mitigating the placebo effect by requiring all subjects to be withdrawn in an open‐label setting from IVIG prior to entry to the blinded, randomized SCIG‐treatment period. However, 37% (19 of 51) of subjects randomized to placebo in the SCIG‐treatment period did not relapse during the study. The PATH study, therefore, provides an opportunity to explore the factors influencing this placebo effect. This information can be useful in the design and powering of future clinical trials in CIDP and other disorders.
2. METHODS
Full details of the PATH study have been published. 4 , 5 All subjects provided informed consent prior to enrolment. The PATH study is registered with ClinicalTrials.gov, number NCT01545076.
2.1. PATH placebo group: retrospective statistical analyses
In the original study, 57 subjects were randomized to placebo. 4 , 5 Six subjects were excluded from our retrospective analyses for the following reasons: two were erroneously entered into the restabilization period and then were randomized to placebo, and four subjects withdrew for a reason other than relapse. Of these, three were by subject decision and one was by physician decision. Thus, 51 subjects were analyzed. We compared the baseline characteristics and the time to deterioration in the open‐label IgG dependency test period by relapse status. All reported P‐values are two‐sided and unadjusted for multiplicity using either the Wilcoxon test or Fisher's exact test.
2.2. Literature search: selection of studies
Placebo‐controlled CIDP studies were identified in a literature search of articles indexed on PubMed. The PubMed search was conducted on 11 December 2019 with no date restrictions. See Appendix 2 for the full list of PubMed search terms used and a preferred reporting items for systematic reviews and meta‐analyses (PRISMA) flow diagram. Obvious irrelevant publications were excluded, and other publications were discussed between two individuals (RAL and AC) to agree on the eligibility and reasons for exclusion. The search aimed to capture all RCTs with a placebo arm conducted in CIDP with any active treatment. Search terms included “chronic inflammatory demyelinating polyradiculoneuropathy” and “placebo” or “sham” and “intravenous immunoglobulin” or “subcutaneous immunoglobulin” or “steroids” or “interferon beta‐1a” or “plasma exchange.” Results were filtered by analysis of the abstract to remove studies that were in a non‐English language, non‐RCTs, studies not focused on CIDP, studies with no clear placebo arm, studies without blinding, and non‐original studies (eg, reviews, study protocols, secondary publications, and letters).
2.3. Literature search: data extraction and meta‐analysis
Data on the placebo group compared with active treatment were extracted from the relevant articles. A meta‐analysis was performed to investigate the placebo response and the placebo effect. Estimates of both the percentage of subjects with improvement and stability or deterioration were calculated, and results from the different studies combined to give a single estimate for each outcome. The analyses were performed using the DerSimonian‐Laird random‐effects method regardless of the amount of heterogeneity between studies. 6 In order to stabilize variances when the proportions were close to zero and one, and a normal approximation to the binomial distribution did not hold, the Freeman‐Tukey double arcsine transformation was performed before analysis. Additionally, the amount of heterogeneity between studies was assessed based on the significance of the between‐study heterogeneity, and on the size of the I2 value. Substantial heterogeneity was assumed if the I2 value was above 50%. 7 A final analysis compared the difference in outcome between studies reporting an improvement endpoint and those with a stability or deterioration endpoint. The percentage from each meta‐analysis was extracted, along with the SE of the estimate, and using these values a z‐test was used to compare the two sets of studies.
3. RESULTS
3.1. PATH placebo group: retrospective statistical analyses
Of the 51 subjects randomized to placebo, 19 (37%) subjects did not relapse (≥1 point increase in total adjusted inflammatory neuropathy cause and treatment [INCAT]) in the subsequent 24 weeks. Thirty‐two (63%) did relapse in the same period. We investigated whether the placebo effect was specific to INCAT and found that similar non‐relapse with placebo was seen with all other outcome measures. The placebo effect was lower in the inflammatory Rasch‐built overall disability score (I‐RODS) (27%), Medical Research Council (MRC) sum sore (30%), and grip strength (30%) compared with INCAT, but the non‐relapse rate was also lower for these measures in the treatment groups.
Baseline demographics of subjects who relapsed compared with non‐relapsers revealed that non‐relapsers were older with more severe disease based on their baseline INCAT and I‐RODS (Table 1). Non‐relapsers were more likely to be males, but this difference did not reach significance. Other characteristics were similar.
TABLE 1.
Placebo subject (N = 51 a ) baseline demographics by CIDP relapse status based on adjusted INCAT score
| CIDP relapse based on adjusted INCAT (n = 32) | No CIDP relapse based on adjusted INCAT (n = 19) | Unadjusted P‐value b | |
|---|---|---|---|
| Age, years (mean [SD]) | 51.0 (12.2) | 61.6 (10.9) | .0064 |
| Weight, kg (mean [SD]) | 84.1 (18.2) | 88.7 (14.4) | .2667 |
| Sex, (% male/female) | 56/44 | 84/16 | .0647 c |
| Years since initial CIDP diagnosis (mean [SD]) | 3.7 (3.4) | 5.9 (6.5) | .8761 |
| Definite EFNS/PNS CIDP diagnosis criteria (%) | 90.6 | 90.6 | 1 c |
| Baseline INCAT total score (mean [SD]) | 1.6 (1.3) | 3.0 (2.0) | .0094 |
| Baseline I‐RODS centile score (mean [SD]) | 76.2 (17.2) d | 58.3 (21.5) e | .0081 |
| Baseline MRC sum score (mean [SD]) | 75.4 (4.3) | 71.7 (8.5) | .1924 |
| Baseline mean grip strength (dominant hand), kPa (mean [SD]) | 73.1 (28.4) | 63.4 (32.5) | .3701 |
Abbreviations: CIDP, chronic inflammatory demyelinating polyneuropathy; EFNS/PNS, European Federation of Neurological Societies/Peripheral Nerve Society; INCAT, inflammatory neuropathy cause and treatment; I‐RODS, inflammatory Rasch‐built overall disability score; MRC, Medical Research Council, SCIG, subcutaneous immunoglobulin.
Subjects with documented INCAT score at the end of the study only, the four subjects receiving placebo who withdrew from the study and the two erroneously entered the restabilization phase are not included.
All P‐values two‐sided and unadjusted for difference using the Wilcoxon test unless otherwise stated.
Unadjusted P‐value for difference calculated using the Fisher exact test.
Based on 28 subjects only.
Based on 14 subjects.
Correlation of time to show deterioration during the IgG dependency test period with CIDP relapse status in the SCIG‐treatment period revealed that those who relapsed tended to have a shorter time to a clinically meaningful deterioration in the IgG dependency test period (Figure 1). Twenty‐two of the 26 subjects (85%) who deteriorated within the first 5 weeks of the dependency test relapsed on placebo. However, only four of 12 subjects (25%) who took ≥8 weeks to deteriorate in the dependency test relapsed on placebo. Time to deterioration in the IgG dependency test period was much shorter for relapsers (mean 4.9 weeks) compared with non‐relapsers (mean of 7.6 weeks).
FIGURE 1.
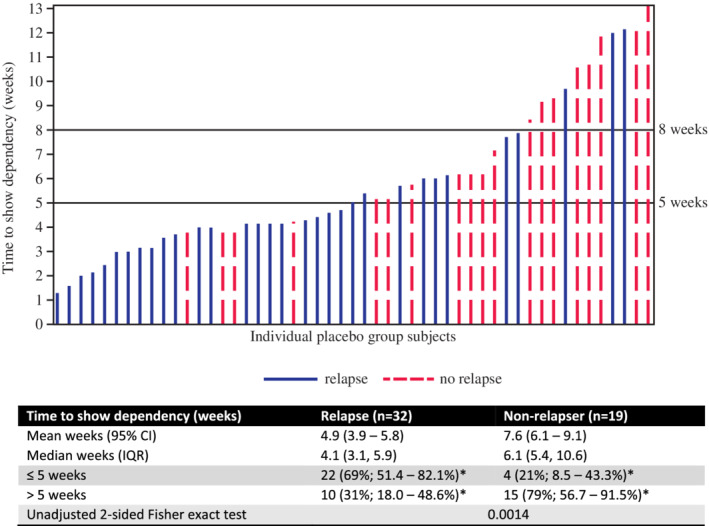
Time to show deterioration in the immunoglobulin G (IgG) dependency test period stratified by relapse status in the subcutaneous immunoglobulin (SCIG)‐treatment period for placebo group subjects (n = 51; non‐relapsers = 19; relapsers = 32). Each line represents an individual placebo group subject from the subcutaneous immunoglobulin (SCIG) randomized phase, where line colour refers to a subjects' relapse status during this phase (blue solid line, relapsed; red dashed line, did not relapse). Line height indicates time (in weeks) for that individual to show dependency (ie, deterioration) during the IgG dependency test period. *95% Wilson score CI. CI, unadjusted confidence interval
4. RESULTS OF THE LITERATURE SEARCH
Our PubMed search yielded 27 RCTs, and, of these only 15 were primary manuscripts using active treatment vs placebo (see Appendix 1). 4 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 Of these, 13 papers had useable endpoints that could be pooled for meta‐analysis (Table 2). Nine studies, encompassing 180 placebo subjects, measured improvement as the primary endpoint (Figure 2). The outcome measures in these studies were INCAT, MRC, averaged muscle score (AMS), Guillain‐Barré syndrome (GBS) disability score, neurological disability score (NDS), Rankin scale, and grip strength. The results suggested an observed improvement occurred in 11% (95% confidence interval [CI]: 4, 21) of subjects randomized to placebo (placebo response) (Table 3). Five studies, encompassing 176 placebo subjects, measured maintained stability or deterioration as the primary endpoint (Figure 2). The outcome measures in these studies were INCAT, change in isokinetic strength (IKS) of four muscles, MRC, and IVIG/steroid dose requirement. The meta‐analysis results found deterioration was observed in 57% of these subjects randomized to placebo, but 43% (95% CI: 35, 50) did not relapse (placebo effect) (Table 3). The percentage response for stability or deterioration studies was significantly higher than that for the improvement studies (P < .001).
TABLE 2.
List of CIDP studies with an improvement primary endpoint or studies with a stability vs deterioration primary endpoint 4 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 17 , 18 , 20 , 21 , 23
| Study | Active treatment | Primary endpoint measure | Sample size | Dose | Duration of study/phase | Placebo arm response | Active arm response | ||
|---|---|---|---|---|---|---|---|---|---|
| Total | Placebo | Active | |||||||
| CIDP studies with an improvement endpoint | |||||||||
| Hughes et al 14 (ICE phase 1) | Gamunex (IVIG) | Total adjusted INCAT | 117 | 58 | 59 | 1 g/kg every 3 weeks | 24 weeks | 21% improved | 54% improved |
| Mendell et al 17 | Gammar IV (IVIG) | AMS | 53 | 23 | 30 | 1 g/kg every 3 weeks | 6 weeks | 9% improved | 38% improved |
| Hadden et al 10 | Interferon beta 1a | Improvement in any 3 of 8 clinical measures a | 10 | Crossover study | 3 MIU 3x per week 2 weeks and 6 MIU for 10 weeks | 12 weeks | 20% improved | 10% improved | |
| Hahn et al 12 | IVIG | NDS, CG, and grip strength | 30 | Crossover study | 0.4 g/kg bw over 5 days | 9 weeks | 17% improved | 63% improved | |
|
Hahn et al 11 |
Plasma exchange | NDS, CG, andgrip strength | 18 | Crossover study | 10 exchanges over 4 weeks | 9 weeks | 0% improved | 80% improved | |
| Vermeulen et al 21 | IVIG | Rankin scale and MRC sum score | 28 | 13 | 15 | 0.4 g/kg bw over 5 days | 3 weeks | 23% improved | 27% improved |
| van Doorn et al 20 | IVIG | Rankin scale | 7 | Crossover study | 0.4 g/kg bw over 5 days | Unclear | 0% improved | 100% improved | |
| Dyck et al 8 | Plasma exchange | NDS | 29 | 14 | 15 | Twice a week | 3 weeks | 0% improved | 33% improved |
| Dyck et al 9 | Plasma exchange | NDS | 14 | 7 | 7 | Twice a week | 3 weeks | 43% improved | 57% improved |
| CIDP studies with a stability vs deterioration endpoint | |||||||||
| Hughes et al 13 (FORCIDP) | Fingolimod | Total adjusted INCAT | 106 | 52 | 54 | 0.5 mg daily | 3 years | 43% non‐deterioration | 42% non‐deterioration |
| van Schaik et al 4 (PATH) | Hizentra (SCIG) | Total adjusted INCAT | 172 | 51 b | 115 c | 0.2 or 0.4 g/kg weekly | 24 weeks | 37% non‐deterioration | 61%‐67% non‐deterioration |
| Markvardsen et al 16 | Subcuvia (SCIG) | IKS | 30 | 15 | 14 | 1:1 previous IVIG dose | 12 weeks | 33% non‐deterioration | 79% non‐deterioration |
|
RMC et al 18 (RMC group) |
Methotrexate | Reduction in mean weekly dose d | 59 | 32 | 27 | 7.5‐15 mg weekly | 32 weeks | 44% maintained | 52% maintained |
|
Hughes et al 14 (ICE phase 2) |
Gamunex (IVIG) | Total adjusted INCAT | 57 | 26 | 31 | 1 g/kg every 3 weeks | 24 weeks | 58% non‐deterioration | 87% non‐deterioration |
Abbreviations: AMS, average muscle score; CG, functional clinical grade; IKS, isokinetic strength; INCAT, inflammatory neuropathy cause and treatment; IVIG, intravenous immunoglobulin; MRC, Medical Research Council; NDS, neurological disability scale; SCIG, subcutaneous immunoglobulin.
Clinical measures included: timed 10‐m walk, ambulation index, expanded Medical Research Council (MRC) sum score, nine‐hole peg test time, functional independence measure, Hammersmith motor ability, a new Guy's neurological disability scale, and the EuroQoL quality of life scale.
20% reduction in mean weekly dose of IVIG or corticosteroids in last 4 weeks compared with first 4 weeks.
Of the 172 subjects randomized in the original paper, six from the placebo group were removed from our analysis due to either withdrawing without relapse (n = 4) or erroneously entering the restabilization phase (n = 2). This results in n = 51 for the placebo arm.
Combined SCIG‐treatment groups (0.2 g/kg, n = 57 and 0.4 g/kg, n = 58).
FIGURE 2.
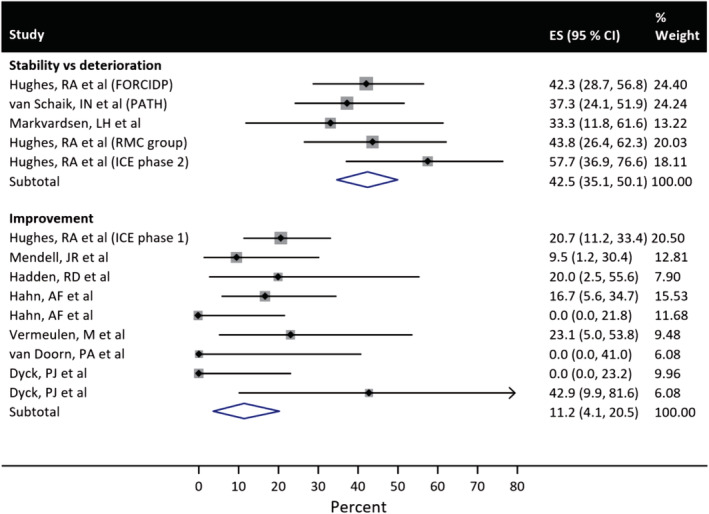
Forest plots from the meta‐analysis showing improvement in placebo subjects in the improvement endpoint studies (n = 9) and non‐deterioration in placebo subjects in the stability vs deterioration endpoint studies (n = 5). CI, confidence interval; ES, effect size
TABLE 3.
Meta‐analysis results of placebo response when improvement was the primary endpoint and placebo effect in studies where stability vs deterioration was the primary endpoint; heterogeneity was assessed based on the significance of the between‐study heterogeneity, and on the size of the I2 value
| Primary endpoint | Number of studies included | Total number of subjects included | Heterogeneity | Percent of placebo subjects meeting primary endpoint (95% CI) | |
|---|---|---|---|---|---|
| P‐value | I2 | ||||
| Improvement (placebo response) | 9 | 180 | <.01 | 51% | 11 (4, 21) |
| Stability vs deterioration (placebo effect) | 5 | 176 | .50 | 0% | 43 (35, 50) |
Abbreviation: CI, confidence interval.
5. DISCUSSION
In the PATH study, every randomized subject was taken off IVIg, proven to deteriorate and then restabilized with IVIG. Yet, 19 of 51 (37%) subjects who were then randomized to placebo remained stable for the 24‐week study period. This is a strong indication of a true placebo effect. Disease variability is unlikely to cause this as the remission period lasted 6 months when just months before these same subjects were shown to be IVIG dependent. Long‐term remissions have been noted from one or two courses of IVIG at high dose (2 g/kg), but these are almost always in newly‐diagnosed subjects, not those on long‐term treatment. 22
We found that the placebo effect in our study correlated with older age, more severe disease, and a longer time to deterioration in the IgG dependency test period. The mean age of subjects who did not relapse on placebo was 10 years greater than relapsers. The mean INCAT of non‐relapsers was almost twice that of relapsers, and the I‐RODS was 18 points lower. It may be older subjects with more severe disease who are more prone to placebo effect. The longer time to deterioration in the initial dependency phase suggests their disease was more stable at study start.
The placebo effect was not restricted to INCAT. While the placebo effect was greatest with INCAT, non‐relapse with placebo was seen with all other outcome measures. The differences between the outcome measures was also seen in the treatment arms with non‐relapse being most evident with INCAT.
Of 180 subjects across nine CIDP trials measuring improvement as the primary outcome measure, there was an 11% placebo response compared with a 43% placebo effect in 176 subjects across the five CIDP studies where relapse was the primary outcome measure. The placebo effect in the PATH study (37%) was slightly lower than in other stability or deterioration trials (see Table 2) and could be the result of having an IgG dependency test period. One possible conclusion, at least in CIDP, is clinical trials that require an improvement less prone to placebo responses compared with those, which require a relapse, or worsening.
The meta‐analysis suggested that there was a moderate to substantial degree of heterogeneity between the results for studies where improvement was the primary outcome (I2 = 51%). A relatively large degree of heterogeneity does not invalidate the analysis but reflects that there is some variation across different studies. This could be attributable to variations in the included patient population, the active treatment, outcome measure, and particularly the primary endpoints of the studies. Another potential variable is whether patients were naïve to the active treatment prior to study entry.
Two previous studies are particularly relevant. In the ICE trial, phase I required improvement and phase II required a relapse. The placebo response in phase I was 21%, but the placebo effect in phase II was 58%. The Mendell and colleagues trial included only treatment‐naïve subjects, and the primary endpoint was improvement. 17 The 9% placebo response was particularly low in this trial and likely due to all subjects being treatment naïve, thus having no experience with the effects of the therapy and therefore no conditioning.
There are many reasons for a placebo response or effect. 1 Two of the most recognised are subject expectation and conditioning. The expectation has to do with the fact that subjects may respond to a treatment based on what they expect to happen. Past experience, belief, and desire all play a role. Most subjects believe injections and invasive treatments are more likely to be effective than pills. Placebo injections are thought to have a particularly high placebo effect. 1 However, this cannot be concluded in our review of CIDP trials. The oral methotrexate and oral fingolimod trials had a similar placebo effect to the intravenous and subcutaneous treatment trials. 18 Expectation may have played a large role in the cross‐over phase of the ICE study. 14 Five of 23 (22%) subjects who did not respond to IVIG initially, responded to placebo after being crossed over, as subjects were more likely to expect the second medication to be “the real thing” if they did not respond to the first. Conditioning is related to a learned response to a medication after prolonged use. Treatment‐naïve subjects have no experience with the therapy and are not conditioned to have a response, thus leading to low placebo rates.
Our findings demonstrate the consistency of the placebo effect across CIDP studies irrespective of treatment, outcome measures, subject demographics, and study size. These findings may have important implications in the design of future clinical trials in CIDP. The difference between placebo response and placebo effect is an important distinction for power calculations and number of subjects required to demonstrate the effect of a therapy. Trials looking for improvement will need to consider lower placebo rates and therefore fewer subjects than trials in which the primary endpoint is relapsed. Studies of treatment‐naïve subjects are likely to have the lowest placebo response but will be more difficult to enrol. Recognising the potential effect of age, disease severity, and stability may also be important variables to consider. Thus, although there are psychologic factors that determine placebo response and effect, there are also aspects of the disease state that influence these responses.
Supporting information
Appendix S1. Supporting Information
ACKNOWLEDGEMENTS
We thank all investigators and the subjects for participating in the PATH study. Individual participant data will not be shared. We also wish to thank Dr Luana Colloca for her helpful advice and encouragement during preparation of this manuscript. Editorial support, funded by CSL Behring, was provided by Meridian HealthComms Ltd. CSL Behring funded the PATH study and together with a steering committee was responsible for the design of the trial, data analysis, interpretation, and writing of this report. All authors critically reviewed results and gave approval to submit this report for publication.
Lewis RA, Cornblath DR, Hartung H‐P, et al. Placebo effect in chronic inflammatory demyelinating polyneuropathy: The PATH study and a systematic review. J Peripher Nerv Syst. 2020;25:230–237. 10.1111/jns.12402
PATH study group members are listed in Appendix.
Funding information CSL Behring
Contributor Information
Richard A. Lewis, Email: richard.lewis@cshs.org.
PATH study group:
A Sabet, K George, L Roberts, R Carne, S Blum, R Henderson, P Van Damme, J Demeestere, S Larue, C D’Amour, P Kunc, M Valis, J Sussova, T Kalous, R Talab, M Bednar, T Toomsoo, I Rubanovits, K Gross‐Paju, U Sorro, M Saarela, M Auranen, J Pouget, S Attarian, G Le Masson, A Wielanek, C Desnuelle, E Delmon, P Clavelou, D Aufauvre, J Schmidt, J Zschuentzsch, C Sommer, D Kramer, O Hoffmann, C Goerlitz, J Haas, M Chatzopoulos, R Yoon, R Gold, P Berlit, A Jaspert‐Grehl, D Liebetanz, A Kutschenko, M Stangel, C Trebst, P Baum, F Bergh, J Klehmet, A Meisel, F Klostermann, J Oechtering, H Lehmann, M Schroeter, T Hagenacker, D Mueller, A Sperfeld, F Bethke, Israel V Drory, A Algom, D Yarnitsky, B Murinson, A Di Muzio, F Ciccocioppo, S Sorbi, S Mata, A Schenone, M Grandis, G Lauria, D Cazzato, G Antonini, S Morino, D Cocito, M Zibetti, T Yokota, T Ohkubo, T Kanda, M Kawai, K Kaida, H Onoue, S Kuwabara, M Mori, M Iijima, K Ohyama, M Baba, M Tomiyama, K Nishiyama, T Akutsu, K Yokoyama, K Kanai, I N van Schaik, F Eftimov, N C Notermans, N Visser, C Faber, J Hoeijmakers, K Rejdak, U Chyrchel‐Paszkiewicz, C Casanovas Pons, M Antonia, J Gamez, M Salvado, C Marquez Infante, S Benitez, M Lunn, J Morrow, D Gosal, T Lavin, I Melamed, A Testori, S Ajroud‐Driss, D Menichella, E Simpson, E Chi‐Ho Lai, M Dimachkie, R J Barohn, S Beydoun, H Johl, D Lange, A Shtilbans, S Muley, S Ladha, M Freimer, J Kissel, N Latov, R Chin, E Ubogu, S Mumfrey, T Rao, P MacDonald, K Sharma, G Gonzalez, J Allen, D Walk, L Hobson‐Webb, and K Gable
REFERENCES
- 1. Colloca L, Barsky AJ. Placebo and Nocebo effects. N Engl J Med. 2020;382(6):554‐561. [DOI] [PubMed] [Google Scholar]
- 2. Oaklander AL, Lunn MP, Hughes RA, van Schaik IN, et al. Treatments for chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): an overview of systematic reviews. Cochrane Database Syst Rev. 2017;(1):CD010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Van den Bergh PY, Hadden RD, Bouche P, et al. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society—first revision. Eur J Neurol. 2010;17(3):356‐363. [DOI] [PubMed] [Google Scholar]
- 4. van Schaik IN, Bril V, van Geloven N, et al. Subcutaneous immunoglobulin for maintenance treatment in chronic inflammatory demyelinating polyneuropathy (PATH): a randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet Neurol. 2018;17(1):35‐46. [DOI] [PubMed] [Google Scholar]
- 5. van Schaik IN, van Geloven N, Bril V, Hartung HP, et al. Subcutaneous immunoglobulin for maintenance treatment in chronic inflammatory demyelinating polyneuropathy (The PATH Study): study protocol for a randomized controlled trial. Trials. 2016;17(1):345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7(3):177‐188. [DOI] [PubMed] [Google Scholar]
- 7. Deeks JJ, Higgins JPT, Altman DG. Chapter 10: Analysing data and undertaking meta‐analyses, identifing and measuring heterogeneity In: Higgins JPT, Thomas J, Chandler J, et al., eds. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Chichester London: John Wiley & Sons; 2019. [Google Scholar]
- 8. Dyck PJ, Daube J, O'Brien P, Pineda A, et al. Plasma exchange in chronic inflammatory demyelinating polyradiculoneuropathy. N Engl J Med. 1986;314(8):461‐465. [DOI] [PubMed] [Google Scholar]
- 9. Dyck PJ, Pineda A, Swanson C, Low P, Windebank A, Daube J. The Mayo Clinic experience with plasma exchange in chronic inflammatory‐demyelinating polyneuropathy (CIDP). Prog Clin Biol Res. 1982;106:197‐204. [PubMed] [Google Scholar]
- 10. Hadden RD, Sharrack B, Bensa S, Soudain SE, et al. Randomized trial of interferon beta‐1a in chronic inflammatory demyelinating polyradiculoneuropathy. Neurology. 1999;53(1):57‐61. [DOI] [PubMed] [Google Scholar]
- 11. Hahn AF, Bolton CF, Pillay N, et al. Plasma‐exchange therapy in chronic inflammatory demyelinating polyneuropathy. A double‐blind, sham‐controlled, cross‐over study. Brain. 1996;119(4):1055‐1066. [DOI] [PubMed] [Google Scholar]
- 12. Hahn AF, Bolton CF, Zochodne D, Feasby TE. Intravenous immunoglobulin treatment in chronic inflammatory demyelinating polyneuropathy. A double‐blind, placebo‐controlled, cross‐over study. Brain. 1996;119(4):1067‐1077. [DOI] [PubMed] [Google Scholar]
- 13. Hughes RA, Dalakas MC, Merkies I, et al. Oral fingolimod for chronic inflammatory demyelinating polyradiculoneuropathy (FORCIDP trial): a double‐blind, multicentre, randomised controlled trial. Lancet Neurol. 2018;17(8):689‐698. [DOI] [PubMed] [Google Scholar]
- 14. Hughes RA, Donofrio P, Bril V, et al. ICE Study GroupIntravenous immune globulin (10% caprylate‐chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): a randomised placebo‐controlled trial. Lancet Neurol. 2008;7(2):136‐144. [DOI] [PubMed] [Google Scholar]
- 15. Hughes RA, Gorson KC, Cros D, Griffin J, et al. Intramuscular interferon beta‐1a in chronic inflammatory demyelinating polyradiculoneuropathy. Neurology. 2010;74(8):651‐657. [DOI] [PubMed] [Google Scholar]
- 16. Markvardsen LH, Debost JC, Harbo T, et al. Subcutaneous immunoglobulin in responders to intravenous therapy with chronic inflammatory demyelinating polyradiculoneuropathy. Eur J Neurol. 2013;20(5):836‐842. [DOI] [PubMed] [Google Scholar]
- 17. Mendell JR, Barohn RJ, Freimer ML, et al. Working Group on Peripheral NeuropathyRandomized controlled trial of IVIg in untreated chronic inflammatory demyelinating polyradiculoneuropathy. Neurology. 2001;56(4):445‐449. [DOI] [PubMed] [Google Scholar]
- 18.RMC Trial GroupMahdi‐Rogers M, Rutterford C, Hughes RAC, et al. Randomised controlled trial of methotrexate for chronic inflammatory demyelinating polyradiculoneuropathy (RMC trial): a pilot, multicentre study. Lancet Neurol. 2009;8(2):158‐164. [DOI] [PubMed] [Google Scholar]
- 19. Thompson N, Choudhary P, Hughes RA, Quinlivan RM. A novel trial design to study the effect of intravenous immunoglobulin in chronic inflammatory demyelinating polyradiculoneuropathy. J Neurol. 1996;243(3):280‐285. [DOI] [PubMed] [Google Scholar]
- 20. van Doorn PA, Brand A, Strengers PF, Meulstee J, et al. High‐dose intravenous immunoglobulin treatment in chronic inflammatory demyelinating polyneuropathy: a double‐blind, placebo‐controlled, crossover study. Neurology. 1990;40(2):209‐212. [DOI] [PubMed] [Google Scholar]
- 21. Vermeulen M, van Doorn PA, Brand A, Strengers PF, Jennekens FG, Busch HF. Intravenous immunoglobulin treatment in patients with chronic inflammatory demyelinating polyneuropathy: a double blind, placebo controlled study. J Neurol Neurosurg Psychiatry. 1993;56(1):36‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lunn MP, Ellis L, Hadden RD, Rajabally YA, Winer JB, Reilly MM. A proposed dosing algorithm for the individualized dosing of human immunoglobulin in chronic inflammatory neuropathies. J Peripher Nerv Syst. 2016;21(1):33‐37. [DOI] [PubMed] [Google Scholar]
- 23. Markvardsen LH, Harbo T, Sindrup SH, et al. Subcutaneous immunoglobulin preserves muscle strength in chronic inflammatory demyelinating polyneuropathy. Eur J Neurol. 2014;21(12):1465‐1470. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information


