Abstract
Background
Higher intraoperative driving pressures (ΔP) are associated with increased postoperative pulmonary complications (PPC). We hypothesised that dynamic adjustment of PEEP throughout abdominal surgery reduces ΔP, maintains positive end-expiratory transpulmonary pressures (Ptp_ee) and increases respiratory system static compliance (Crs) with PEEP levels that are variable between and within patients.
Methods
In a prospective multicentre pilot study, adults at moderate/high risk for PPC undergoing elective abdominal surgery were randomised to one of three ventilation protocols: (1) PEEP≤2 cm H2O, compared with periodic recruitment manoeuvres followed by individualised PEEP to either optimise respiratory system compliance (PEEPmaxCrs) or maintain positive end-expiratory transpulmonary pressure (PEEPPtp_ee). The composite primary outcome included intraoperative ΔP, Ptp_ee, Crs, and PEEP values (median (interquartile range) and coefficients of variation [CVPEEP]).
Results
Thirty-seven patients (48.6% female; age range: 47–73 yr) were assigned to control (PEEP≤2 cm H2O; n=13), PEEPmaxCrs (n=16), or PEEPPtp_ee (n=8) groups. The PEEPPtp_ee intervention could not be delivered in two patients. Subjects assigned to PEEPmaxCrs had lower ΔP (median8 cm H2O [7–10]), compared with the control group (12 cm H2O [10–15]; P=0.006). PEEPmaxCrs was also associated with higher Ptp_ee (2.0 cm H2O [-0.7 to 4.5] vs controls: -8.3 cm H2O [-13.0 to -4.0]; P≤0.001) and higher Crs (47.7 ml cm H2O [43.2–68.8] vs controls: 39.0 ml cm H2O [32.9–43.4]; P=0.009). Individualised PEEP (PEEPmaxCrs and PEEPPtp_ee combined) varied widely (median: 10 cm H2O [8-15]; CVPEEP=0.24 [0.14–0.35]), both between, and within, subjects throughout surgery.
Conclusions
This pilot study suggests that individualised PEEP management strategies applied during abdominal surgery reduce driving pressure, maintain positive Ptp_ee and increase static compliance. The wide range of PEEP observed suggests that an individualised approach is required to optimise respiratory mechanics during abdominal surgery.
Clinical trial registration
Keywords: lung compliance, mechanical ventilation, positive end-expiratory pressure, postoperative pulmonary complications, respiratory mechanics, ventilator-induced lung injury
Editor's key points.
-
•
Higher intraoperative driving pressures are associated with a greater risk of developing postoperative pulmonary complications.
-
•
In this pilot randomised controlled study, the authors explored whether individualised dynamic adjustment of PEEP during abdominal surgery reduces intraoperative driving pressure (amongst other measures of respiratory mechanics).
-
•
Periodic recruitment manoeuvres to optimise either respiratory system compliance or positive end-expiratory transpulmonary pressure reduced intraoperative driving pressure.
-
•
Given the wide variability in PEEP encountered, this pilot trial suggests that an individualised approach is required to optimise respiratory mechanics during abdominal surgery.
Postoperative pulmonary complications (PPCs) are associated with excess surgical morbidity and mortality.1, 2, 3, 4 The optimal PEEP level that minimises pulmonary atelectasis and overdistention, maximises oxygenation, limits hypotension, and reduces PPCs remains unclear, but is likely to be variable between individuals.5 For patients without lung injury undergoing open abdominal surgery, a PEEP≤2 cm H2O has been proposed as the standard of care.6 This recommendation was derived from a single large trial7 that compared two fixed PEEPs (≤2 vs 12 cm H2O). Although a similar incidence of PPCs occurred between the two PEEP levels, intraoperative hypotension and vasopressor requirements were more frequent in patients receiving recruitment manoeuvres and PEEP=12 cm H2O. However, lower PEEP has also been associated in other studies with higher rates of atelectasis,8 major PPCs after abdominal surgery9 and 30-day mortality.10 Thus, criteria to set intraoperative PEEP that optimises respiratory system mechanics and reduces biological injury to improve perioperative outcomes is still required.
Inadequate PEEP leads to stiffer lungs and increased driving pressures (ΔP, calculated as plateau pressure: Pplat, minus PEEP), indicating increased lung strain.11 Higher intraoperative ΔP has been associated with major PPCs, including barotrauma, lung oedema, reintubation, lung injury, and pneumonia.12,13 Obesity, surgical position, and technique contribute to highly variable mechanical loads that the respiratory system encounters throughout surgery.
Dynamic PEEP individualisation throughout surgery, instead of a fixed PEEP, may optimise perioperative respiratory mechanics. The lack of an individualised approach may explain the lack of effect associated with a single PEEP titration performed at the beginning of surgery.14,15 Two bedside approaches for PEEP individualisation have been proposed in critical care to minimise end-expiratory alveolar collapse16, 17, 18: maximisation of respiratory system static compliance (Crs)19 and maintenance of a positive end-expiratory transpulmonary pressure (Ptp_ee, defined by the difference between PEEP and end-expiratory oesophageal pressure, Pes_ee, used as a surrogate of pleural pressure). These methods have not been compared during surgery.
Therefore, we designed a pilot study to assess these two PEEP individualisation methods during major abdominal surgery. We hypothesised that periodic PEEP individualisation throughout surgery would optimise respiratory mechanics by reducing ΔP, increasing Crs and maintaining positive Ptp_ee with variable PEEP levels between and within patients. The effect of individualised PEEP strategies on circulating biomarkers for lung injury20, 21, 22, 23, 24 was explored as a secondary outcome.
Methods
Institutional Review Board approval was obtained at each institution. The study was registered at clinicaltrials.gov (NCT02671721). The frequency of PPCs was erroneously registered as the original primary endpoint. The registration was subsequently modified to clarify that the primary aim of this feasibility study was to assess respiratory mechanics and to finalise the design of a subsequent full-scale trial addressing PPCs. Signed informed consent was obtained from all patients prior to randomisation and study procedures.
Study design
This was a three-site, prospective, randomised, controlled feasibility study comparing three different PEEP management protocols during general anaesthesia for abdominal surgery: two intervention groups with distinct methods to individualise intraoperative PEEP, and a control group with a constant ≤2 cm H2O PEEP throughout surgery.7 Full details on the methods are provided in the Supplementary material.
Inclusion criteria
Patients ≥18 years undergoing elective intraperitoneal abdominal or pelvic surgery expected to last ≥2 h at intermediate to high risk of PPCs (as defined by an ‘Assess Respiratory Risk In Surgical Patients in Catalonia' (ARISCAT) risk score ≥26)25 were eligible.
Exclusion criteria
Patients with predefined significant cardiopulmonary disease26 and other conditions were ineligible.
Intraoperative ventilation
All subjects received volume-controlled ventilation (VCV) and lung protective settings6: tidal volume (VT) 7±1 ml kg−1 of predicted body weight (PBW), 20% inspiratory pause, inspired oxygen fraction (FiO2) starting at 0.4 and titrated for oxyhaemoglobin saturation (SpO2)≥92%. An oesophageal pressure (Pes) balloon was placed according to current recommendations27 in all patients for continuous monitoring of Pes and transpulmonary pressure (Ptp).16 Correct positioning of the Pes balloon was ensured by continuous Pes monitoring identifying the presence of a cardiac artefact and Ptp changes during tidal ventilation, and confirmed by similar changes of Pes and airway pressure (Paw) during an end-expiratory hold and thorax compression.27 An in vivo calibration28 was not performed. Pes was monitored continuously during PEEP titrations. Pes_ee was recorded in all patients at prespecified time points. End-inspiratory oesophageal pressure (Pes_ei) and derived variables were also studied in a subgroup of patients.
Study intervention
Randomisation was stratified by group and site. After randomisation, unblinded investigators performed the intervention. Treatment allocation was concealed from patients, postoperative care providers, and assessors of secondary outcomes. Patients randomised to the individualised PEEP groups also received preoperative education on reducing PPCs and postoperative strategies including incentive spirometry and early mobilisation. These interventions were monitored and supervised postoperatively. The control group could also receive these interventions as part of usual care, but was not supervised by the study team. Intraoperatively, patients were randomised to one of three PEEP strategies:
Control group (PEEP≤2 cm H2O)
Received PEEP≤2 cm H2O and no planned recruitment manoeuvres throughout the surgical procedure.6
Maximisation of respiratory system compliance (PEEPmaxCrs)
A recruitment manoeuvre (5 cm H2O-stepwise increase up to 20 cm H2O) was followed by a 3 cm H2O decremental stepwise PEEP titration (see Supplementary materials for details). Static Crs (=VT/[Pplat-PEEP]=VT/ΔP) was assessed at each titration step and PEEP set at the level corresponding to the maximum Crs. Pes was not used for PEEP-setting in this group. Patients receiving individualised PEEP had Crs assessed before and after each PEEP individualisation intervention. Successful interventions were those resulting in maintained or increased Crs. The successful Crs-optimisation rate was calculated for each patient as the percentage of successful interventions over the total number of interventions performed.
Positive end-expiratory transpulmonary pressure (PEEPPtp_ee)
Following the same standardised recruitment manoeuvre, absolute Pes_ee was assessed using the oesophageal balloon. PEEP was set to 1 cm H2O greater than the Pes_ee (set PEEP=Pes_ee+1 cm H2O).
Protocol
The intervention was started immediately after tracheal intubation and repeated hourly. Anaesthetic management otherwise followed routine clinical practice. Additional interventions were also performed when predefined events potentially associated with lung collapse were undertaken (application of surgical retractors, pneumoperitoneum insufflation/deflation, endotracheal tube disconnection, tracheal suctioning, Trendelenburg position). Before each PEEP adjustment, muscle paralysis was ensured and haemodynamic status were assessed. Full reversal of neuromuscular block guided by train-of-four neuromuscular monitoring was standardised.
Plasma biomarkers of lung injury
Blood was collected before (T0), at the end of (Tend) and 24 h after (T24) surgery. Plasma samples were analysed in duplicate by a blinded investigator for biomarkers of inflammatory lung injury (interleukin [IL]-6 and IL-8), epithelial injury (soluble form of the receptor for advanced glycation end-products [sRAGE] and club cell protein-16, [CC16]), endothelial injury (angiopoietin-2 [Ang-2]), and endothelial-derived coagulation activation (plasminogen activator inhibitor-1 [PAI-1]). Absolute biomarker levels and ratios relative to baseline (Tend/T0, T24/T0) were calculated.
Primary outcome
The primary outcome comprised four measurements:
-
(1)
the median intraoperative ΔP, calculated as Pplat measured at the end of the 20% inspiratory pause, minus PEEP;
-
(2)
Ptp_ee (PEEP minus Pes_ee;
-
(3)
Crs (=VT/(Pplat-PEEP)=VT/ΔP);
-
(4)
PEEP median and coefficient of variation (CVPEEP) (a joint outcome).
Of the four primary outcomes, we focused on ΔP because of its supported clinical relevance on PPCs.12,13
Secondary outcomes
Secondary outcomes were:
Statistical analyses
Collected variables (e.g. subject characteristics, comorbidities, intraoperative respiratory, and other perioperative parameters, plasma biomarkers levels) were summarised as median (first quartile [Q1]- third quartile [Q3]) or number of patients (percentage), as appropriate. Non-parametric tests were used to avoid normality assumptions. We compared the outcomes and plasma concentrations and ratios of biomarkers from the three groups using the Kruskal–Wallis test (continuous variables) or Fisher's exact test extended to three groups (categorical variables). For the four primary (composite) endpoints, we used a Bonferroni adjustment for the overall test. For PEEP values, we required both median PEEP level and CVPEEP to meet statistical significance to consider this joint endpoint as different accross the groups. We used Bonferroni adjustment for post hoc pairwise comparisons when the overall test was statistically significant. Two-tailed analyses were performed using SAS Version 9.4 (SAS Institute, Cary, NC, USA). For the four primary (composite) outcomes, a significance level of ≤0.0125 (=0.05/4) was used; otherwise 0.05 was used for overall statistical tests. For post hoc pairwise comparisons amongst the three groups after the overall test was found to be statistically significant, a significance level of <0.01667 (=0.05/3) was used.
Sample size estimation
This study was designed to include 40 subjects (minimum 13 per group) from all sites to select the individualised PEEP method that reduces intraoperative ΔP. A sample size of 13 subjects per group was estimated to have 80% power to detect a clinically relevant difference of 4 cm H2O in ΔP between the three groups, assuming a standard deviation of 3.6 cm H2O based on preliminary results from a recent publication31 (α=0.05, two-sided, analysis of variance). Our sample size estimation allowed for potential drop-outs before surgery and to adjust for the potential loss of power using a non-parametric test.
Results
Subject characteristics
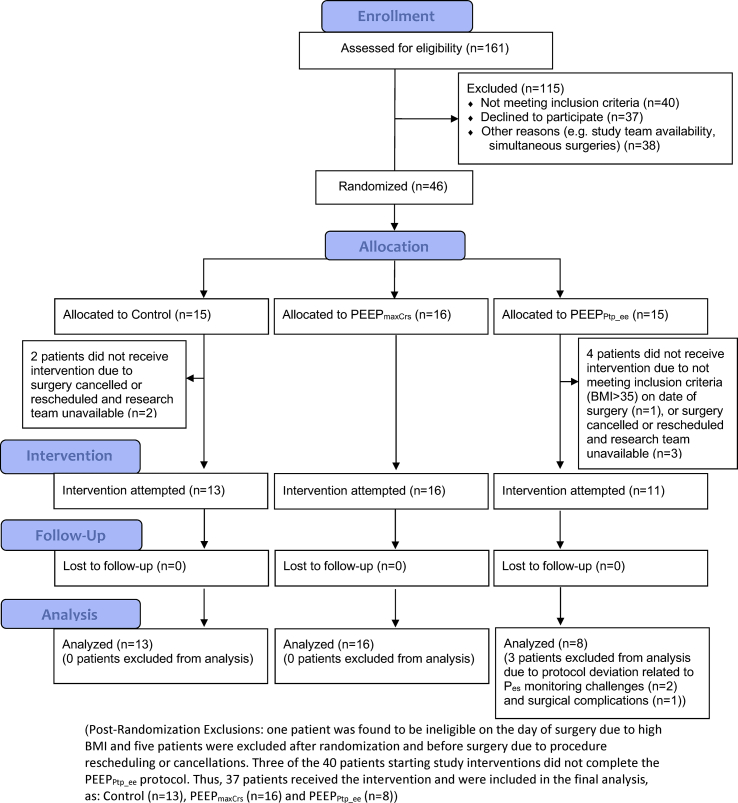
Forty-six subjects were enrolled and randomised (Fig. 1). Six patients never received the assigned intervention; three participants failed to complete the PEEPPtp_ee intervention because of surgical complications (n=1) or inability to measure Pes (n=2). There were no protocol deviations in subjects from the PEEPmaxCrs and control groups. Thus, 37 subjects were included in the final analysis (Fig. 1) comprising 13 controls, 16 PEEPmaxCrs, and eight PEEPPtp_ee subjects. Perioperative details were similar between each group (Table 1, Table 2, Supplementary Tables S1 and S2). Peak and plateau airway pressures and PETCO2 were significantly higher in the PEEPPtp_ee group than controls (Table 2). Individualised PEEP was associated with higher heart rate for both PEEPmaxCrs and PEEPPtp_ee groups (Table 2).
Fig 1.
CONSORT flow diagram.
Table 1.
Subject and procedure characteristics. Data represent median (Q1–Q3) or number of subjects (% of column). ∗P-values are calculated from Kruskal–Wallis test (for continuous variables) or Fisher's exact test (extended). aThese differences between randomised subjects are considered owing to chance and post hoc comparisons not shown.
| Variables | Control (n=13) | PEEPmaxCrs (n=16) | PEEPPtp_ee (n=8) | P-value∗ |
|---|---|---|---|---|
| Demographics | ||||
| Age (yr) | 69 (61–73) | 60 (47–75) | 55 (51–67) | 0.501 |
| Gender (male) | 7 (54) | 8 (50) | 4 (50) | 1.000 |
| Body mass index (kg m−2) | 26.4 (22.8–29.6) | 23.4 (20.8–25.4) | 24.7 (24.1–27.7) | 0.187 |
| Comorbidities | ||||
| Hypertension | 10 (77) | 2 (12) | 2 (25) | 0.002a |
| Coronary artery disease | 0 (0) | 2 (12) | 0 (0) | 0.496 |
| Heart failure | 1 (8) | 1 (6) | 0 (0) | 1.000 |
| Neurological disease | 1 (8) | 0 (0) | 1 (12) | 0.316 |
| Chronic obstructive pulmonary disease | 0 (0) | 1 (6) | 1 (12) | 0.688 |
| Asthma | 1 (8) | 2 (12) | 2 (25) | 0.695 |
| Obstructive sleep apnoea | 3 (23) | 1 (6) | 1 (12) | 0.495 |
| Smoking | 0.534 | |||
| Never | 9 (69) | 8 (50) | 7 (88) | |
| Former | 2 (15) | 5 (31) | 1 (12) | |
| Current <2 packs day−1 | 2 (15) | 3 (19) | 0 (0) | |
| Renal disease | 0 (0) | 2 (12) | 0 (0) | 0.496 |
| Liver disease | 1 (8) | 1 (6) | 1 (12) | 1.000 |
| Alcohol use (previous yr) | 0.374 | |||
| Never | 5 (38) | 5 (31) | 6 (75) | |
| Occasional | 5 (38) | 9 (56) | 2 (25) | |
| Moderate | 2 (15) | 2 (12) | 0 (0) | |
| Heavy | 1 (8) | 0 (0) | 0 (0) | |
| Cancer | 8 (62) | 11 (69) | 4 (50) | 0.629 |
| Gastro-oesophageal reflux disease | 7 (54) | 5 (31) | 3 (38) | 0.473 |
| Diabetes mellitus | 0 (0) | 1 (6) | 1 (12) | 0.688 |
| Total ARISCAT score | 41 (41–41) | 41 (35–49) | 41 (38–41) | 0.900 |
| Surgical procedure | ||||
| Surgical service | 0.717 | |||
| General surgery | 8 (62) | 12 (75) | 7 (88) | |
| Gynaecology | 2 (15) | 2 (12) | 1 (12) | |
| Urology | 3 (23) | 1 (6) | 0 (0) | |
| Other | 0 (0) | 1 (6) | 0 (0) | |
| Surgical approach | 0.416 | |||
| Supra-umbilical | 5 (38) | 9 (56) | 6 (75) | |
| Infra-umbilical | 6 (46) | 3 (19) | 1 (12) | |
| Laparoscopic | 2 (15) | 4 (25) | 1 (12) | |
| Surgery duration (min) | 185 [144–236] | 212 [179–340] | 274 [200–343] | 0.156 |
| Anaesthesia duration (min) | 243 [173–286] | 277 [217–397] | 322 [205–360] | 0.279 |
Table 2.
Intraoperative characteristics. Data represent median (Q1–Q3) of the median values of each subject during surgery. ‡P-values are calculated from the Kruskal–Wallis test (for continuous variables) or Fisher's exact test (extended). For post hoc comparisons – we consider P≤0.01667 (=0.05/3) statistically significant – allowing for multiple comparisons. However, we also indicate for completeness all two-group comparisons where 0.01667<P<0.05 as these would be considered significant if we had not adjusted for multiple comparisons. ∗P-value 0.01667<P<0.05 compared with control; ˆP-value 0.01667<P<0.05 PEEPPtp_eevs PEEPmaxCrs; ∗∗P≤0.01667 compared with control; ˆˆP≤0.01667 PEEPPtp_eevs PEEPmaxCrs. aPes values shown correspond to actual Pes measurements (absolute Pes levels). bAlbumin was the only colloid administered to any study subject. cIncludes only patients receiving any volume of albumin. PBW, predicted body weight.
| Variables | Control (n=13) | PEEPmaxCrs (n=16) | PEEPPtp_ee (n=8) | P-value‡ |
|---|---|---|---|---|
| Respiratory variables | ||||
| Driving pressure (ΔP) (cm H2O) (primary outcome) | 12.0 (10.0–15.0) | 8.0 (7.0–10.0)∗∗ | 9.0 (7.8–10.1)∗ | 0.011 |
| Respiratory system static compliance (Crs) (ml/cm H2O) | 39.0 (32.9–43.4) | 47.7 (43.2–68.8)∗∗ | 49.0 (44.6–58.7)∗∗ | 0.010 |
| Tidal volume (VT) (ml/kg PBW) | 6.7 (6.6–7.1) | 7.1 (6.6–7.5) | 6.9 (6.5–7.3) | 0.810 |
| Respiratory rate (RR) (bpm) | 10.0 (10.0–12.0) | 10.0 (10.0–12.0) | 12.5 (11.0–13.5) | 0.149 |
| Peak inspiratory pressure (PIP) (cm H2O) | 18.0 (13.0–20.0) | 21.0 (18.0–26.0)∗ | 22.5 (21.5–27.0)∗∗ | 0.007 |
| Plateau pressure (Pplat) (cm H2O) | 14.7 (10.2–17.0) | 17.3 (14.0–24.0)∗ | 20.1 (18.0–23.5)∗∗ | 0.006 |
| PEEP (cm H2O) | 2.0 (0.0–2.0) | 10.0 (6.5–15.0)∗∗ | 11.0 (9.5–14.0)∗∗ | <0.001 |
| PEEP coefficient of variation (CVPEEP) | 0.00 (0.00–0.00) | 0.25 (0.17–0.40)∗∗ | 0.17 (0.14–0.23)∗∗ | <0.001 |
| End-expiratory oesophageal pressure (Pes_ee) (cm H2O)a | 9.5 (6.0–14.8) | 7.8 (5.6–10.7) | 9.5 (8.8–12.3) | 0.269 |
| End-expiratory transpulmonary pressure (Ptp_ee) (cm H2O) | -8.3 (-13.0–-4.0) | 2.0 (-0.7–4.5)∗∗ | 1.0 (1.0–2.3)∗∗ | <0.001 |
| Inspired fraction of oxygen (FiO2) (%) | 40.0 (40.0–49.0) | 40.0 (40.0–45.5) | 43.0 (40.0–51.0) | 0.821 |
| Peripheral saturation of oxyhaemoglobin (SpO2) (%) | 98 (95–100) | 99 (98.5–100) | 99 (97.5–99.5) | 0.353 |
| Exhaled end-tidal partial pressure of CO2 PETCO2 (mmHg) | 35.0 (34.0–37.0) | 36.5 (33.5–39.0) | 38.0 (37.0–39.0)∗∗ | 0.048 |
| Other vital signs | ||||
| Temperature (°C) | 35.7 (35.5–36.2) | 35.8 (35.2–36.8) | 36.2 (35.8–36.6) | 0.483 |
| Heart rate (beats min−1) | 64 (62–66) | 70 (67–79)∗∗ | 74 (71–80)∗∗ | 0.007 |
| Mean arterial blood pressure (mmHg) | 84 (78–85) | 80 (75–84) | 82 (78–82) | 0.535 |
| Intraoperative fluid management | ||||
| Crystalloid volume (ml kg−1 h−1) | 8.5 (6.0–9.3) | 6.9 (6.1–10.6) | 5.7 (4.9–8.9) | 0.425 |
| Albumin n (%)b | 4 (31) | 5 (31) | 3 (38) | 1.000 |
| Albumin volume (ml kg−1 h−1) | 1.3 (1.2–1.7) | 2.5 (2.4–3.0)∗ | 2.1 (1.4–2.2) | 0.024 |
| Any blood product n (%) | 0.216 | |||
| Packed red blood cells | 0 (0) | 0 (0) | 0 (0) | |
| Fresh frozen plasma | 0 (0) | 0 (0) | 1 (12) | |
| Platelets | 0 (0) | 0 (0) | 0 (0) | |
| Estimated blood loss (ml) | 100 (25–750) | 188 (113–525) | 575 (138–775) | 0.407 |
| Urine output (ml kg−1 h−1) | 1.0 (0.6–1.7) | 0.9 (0.6–1.2) | 0.8 (0.5–1.7) | 0.744 |
| Vasoactive medications | ||||
| Vasoactive medications n (%) | 12 (92) | 16 (100) | 8 (100) | 0.387 |
| Phenylephrine | 9 (69) | 15 (94) | 8 (100) | 0.071 |
| Ephedrine | 9 (69) | 8 (50) | 4 (40) | 0.530 |
| Vasopressin | 2 (15) | 2 (13) | 1 (13) | 0.970 |
| Total vasoactive doses | ||||
| Phenylephrine (μg) | 850 (0–5060) | 1250 (630–3559) | 6922 (3113–20 548) | 0.064 |
| Ephedrine (mg) | 15 (0–30) | 5 (0–17.5) | 2.5 (0–17.5) | 0.455 |
| Vasopressin units | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.963 |
Primary (composite) outcomes
All four of the primary outcomes were different between the three groups (each p<0.0125) (Table 2):
-
(1)
Intraoperative ΔP: subjects randomised to PEEPmaxCrs and PEEPPtp_ee had lower intraoperative ΔP and higher Crs compared with controls (Table 2).
-
(2)
Ptp_ee: median values for Ptp_ee were higher in both PEEP individualisation groups, compared with controls (Table 2). A Ptp_ee>0 cm H2O was achieved following all PEEP titrations in the PEEPPtp_ee group and in 78 (49–96)% of the PEEPmaxCrs titrations.
-
(3)
Crs: maintained or increased Crs was achieved in 80 (64–100)% after PEEP interventions in the PEEPmaxCrs group. Crs was maintained or increased in 73 (62–92)% of the interventions undertaken in the PEEPPtp_ee group.
-
(4)
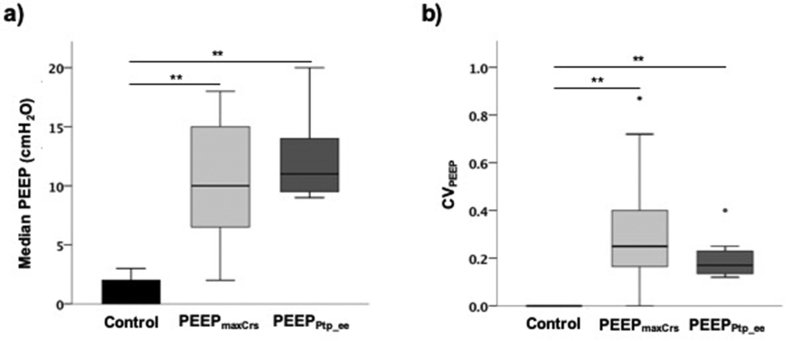
PEEP values: In both PEEPmaxCrs and PEEPPtp_ee groups, median PEEP levels were higher than those in controls (Table 2). The intraoperative individualised PEEP ranged from 2 to 20 cm H2O (median:10 cm H2O [8–15]) for combined individualised PEEP groups; Table 2; Fig. 2a). The CVPEEP in the intervention PEEP groups ranged from 0 to 0.87 (median: 0.24 [0.14–0.35]; Table 2; Fig. 2b).
Fig 2.
PEEP variability. (a) Variability of median PEEP levels between patients receiving a constant PEEP ≤2 cm H2O (control) or individualised PEEP (PEEPmaxCrs or PEEPPtp_ee); and (b) variability of PEEP levels within patients throughout surgery measured by the PEEP coefficient of variation (CVPEEP) for all PEEP levels used for each individual. (∗∗P≤0.01667 in post hoc comparison of respective group compared with control).
Secondary outcomes
Measures of respiratory mechanics
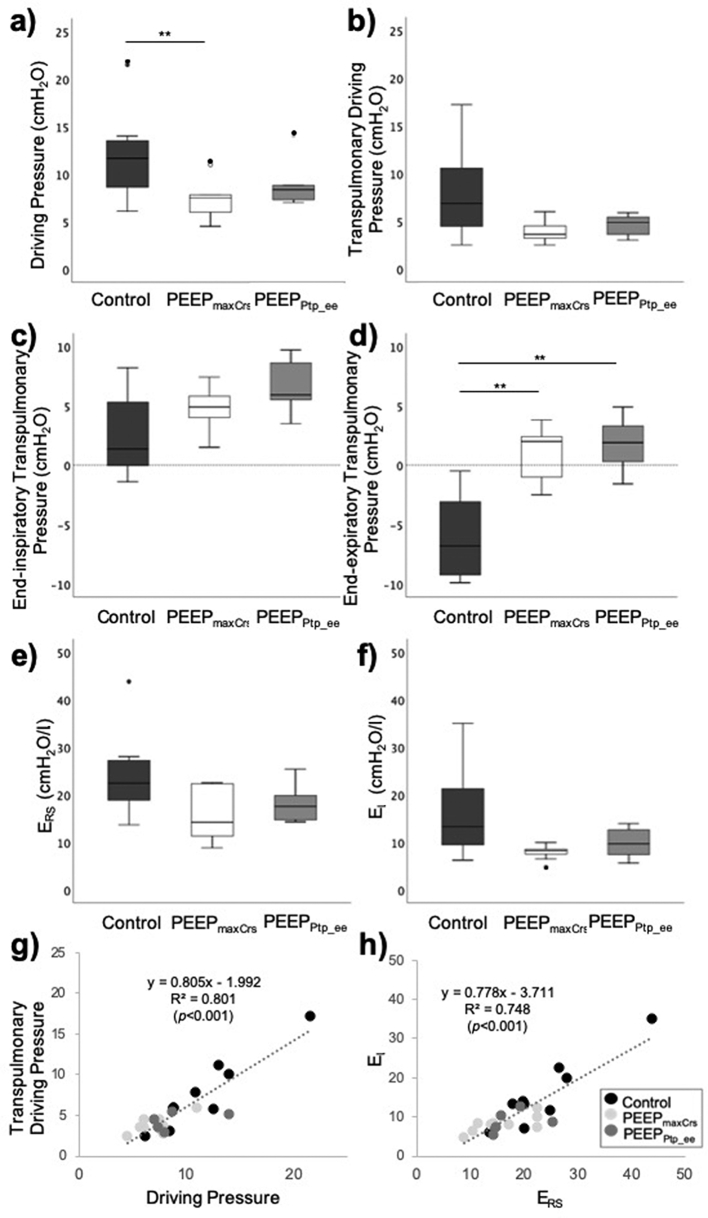
Pes_ee and other respiratory mechanic measurements were similar in both PEEP individualisation groups (Table 2). End-inspiratory respiratory parameters (Pes_ei, Ptp_ei) were also similar between each group (Fig. 3, Supplementary Table 3). We observed a significant correlation between airway and transpulmonary driving pressures (R2=0.801, P<0.001; Fig. 3) and between respiratory system and lung elastance (R2=0.748, P<0.001; Fig. 3).
Fig 3.
Intraoperative respiratory parameters in a subgroup of subjects with end-inspiratory Pes measurements. (a) Driving pressure. (b) Transpulmonary driving pressure. (c) End-inspiratory transpulmonary driving pressure. (d) End-expiratory transpulmonary driving pressure. (e) Respiratory system elastance (ERS). (f) Lung elastance (EL). (g) Correlation between driving pressure and transpulmonary driving pressure. (h) Correlation between respiratory system elastance (Ers) and lung elastance (EL). (Boxplots represent median (Q1, Q3); error bars represent minimum and maximum values; full dots identify outlier values. Results of post hoc comparisons are shown if significant differences observed between the three groups: for post hoc comparisons, P≤0.01667 (=0.05/3) statistically significant. However, we also indicate for completeness all two-group comparisons where 0.01667<P<0.05 as these would be considered significant if we had not adjusted for multiple comparisons. ∗P-value 0.01667<P<0.05 compared with control; ˆP-value 0.01667<P<0.05 PEEPPtp_eevs PEEPmaxCrs ∗∗P≤0.01667 compared with control; ˆˆP≤0.01667 PEEPPtp_eevs PEEPmaxCrs.).
Plasma lung injury biomarkers and clinical morbidity
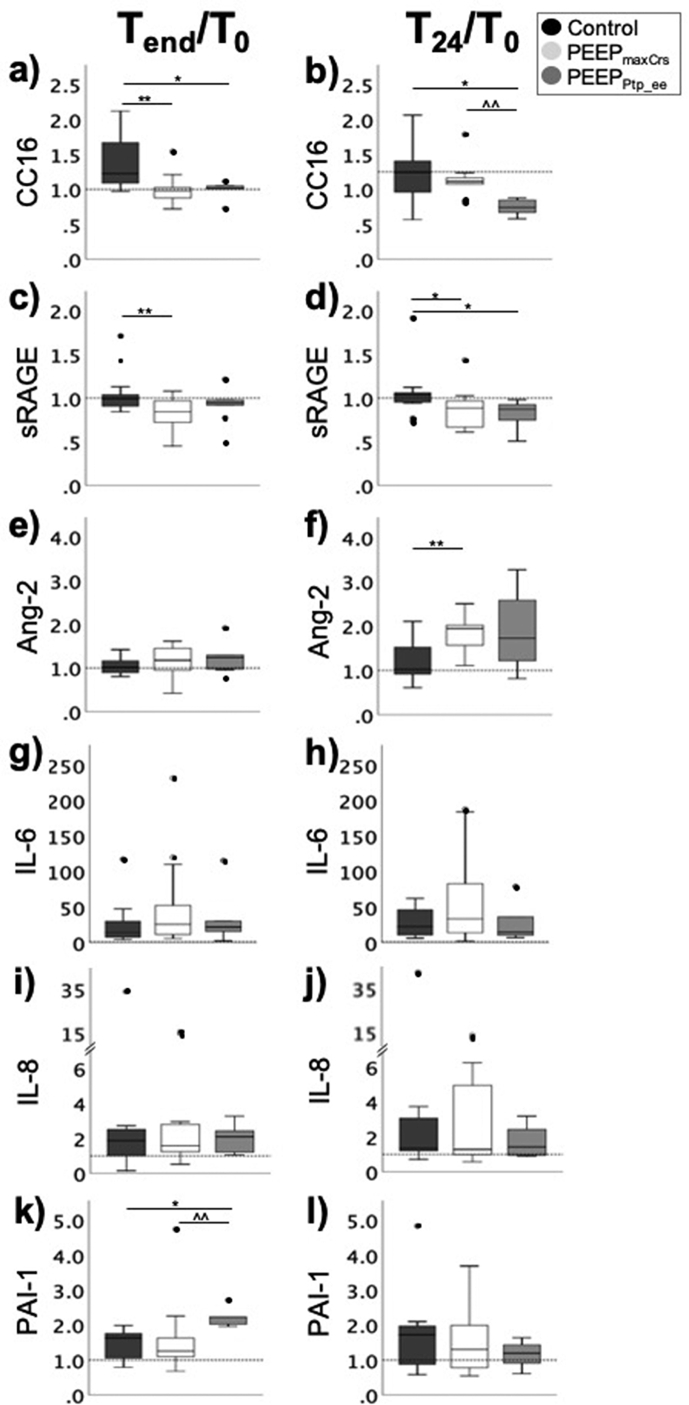
Absolute plasma levels of lung injury biomarkers were similar across groups (Fig. 4). Ratiometric measures of the epithelial injury biomarkers CC16 and sRAGE levels were lower in both PEEP-individualisation groups, compared with controls. PEEPmaxCrs subjects had higher T24h/T0 ratios for the endothelial injury biomarker Ang-2, compared with controls. Subjects randomised to PEEPPtp_ee had higher PAI-1 T24h/T0 ratios compared with controls (Supplementary Table 4). Postoperative pulmonary complications were minor and similar between groups (Supplementary Table 5).
Fig 4.
Plasma concentrations of biomarkers of lung injury. Ratios of plasma concentrations of biomarkers of: epithelial injury (club cell protein-16 [CC16] and soluble form of the receptor for advanced glycation end-products [sRAGE]) (a–d); endothelial injury (angiopoietin-2 [Ang-2]) (e–f); inflammation (interleukin [IL]-6 and IL-8) (g–h); and endothelial-derived coagulation activation (plasminogen activator inhibitor-1 [PAI-1]) (i–j) at the end of (Tend) and after 24 h (T24) compared with baseline (T0). (Boxplots represent median (Q1, Q3); error bars represent minimum and maximum values; full dots identify outlier values. Results of post hoc comparisons are shown if significant differences observed between the three groups: for post hoc comparisons, P-values≤ 0.01667 (=0.05/3) statistically significant. However, we also indicate for completeness all two-group comparisons where 0.01667<P<0.05 as these would be considered significant if we had not adjusted for multiple comparisons. ∗P-value 0.01667<P<0.05 compared with control; ˆP-value 0.01667<P<0.05 PEEPPtp_eevs PEEPmaxCrs; ∗∗P≤0.01667 compared with control; ˆˆP≤0.01667 PEEPPtp_eevs PEEPmaxCrs.).
Discussion
This prospective randomised multicentre pilot study suggests that periodic individualised PEEP aiming at optimising either intraoperative Crs or Ptp_ee during abdominal surgery results in reduced ΔP, increased Crs and maintains positive Ptp_ee, compared with a constant PEEP≤2 cm H2O. These interventions resulted in widely variable PEEP levels both between and within subjects throughout surgery.
Data on the optimal approach to set intraoperative PEEP are controversial.5,8,11,14 Our pilot results show that periodically optimising PEEP with two bedside interventions effectively reduced ΔP throughout abdominal surgery. Such a finding has physiological and clinical relevance. Physiologically, it reflects a reduction in cyclic lung strain32,33 likely because of an optimised balance between lung overdistension and derecruitment.26 Indeed, a recent study using electrical impedance tomography to individualise PEEP at the onset of abdominal surgery resulted in values similar to those in our intervention patients (∼10 cm H2O).15 Clinically, reduced ΔP may limit major PPCs including acute respiratory distress syndrome (ARDS), pneumonia, pulmonary oedema, need for reintubation, pulmonary infection, and barotrauma.12,34
PEEP adjustments required to achieve the low ΔP varied substantially between and within patients throughout abdominal surgery. Data on intraoperative variability of respiratory system mechanics are rarely available. The observed range of interindividual optimised median PEEP was 2–20 cm H2O. Consequently, based on this individualised PEEP range, had we used a constant PEEP=12 cm H2O in all our intervention patients (as in the PROtective Ventilation using HIgh vs. LOw PEEP, PROVHILO, study)7 we would have overdistended 67% (16/24) of the lungs as their individualised PEEP was <12 cm H2O, and under-recruited 33% (eight of 24) with individualised PEEP>12 cm H2O. The variability of individualised PEEP was also notable within patients, ranging from 0% to 87%. This finding emphasises the relevance of individualising PEEP settings throughout surgery, a concept often not considered in studies pursuing individualised PEEP. For example, both in a recent imaging-guided PEEP trial15 and in a major clinical trial35 including PEEP-individualisation, PEEP was titrated only once at the beginning of surgery and that PEEP was maintained unchanged until extubation. Our results clearly show that a constant PEEP, even if individualised to optimise respiratory system mechanics at the onset of surgery, is not sufficient to maintain such optimisation throughout the dynamic conditions of abdominal surgery. Our finding of large intra- and interindividual variabilities of individualised PEEP settings strongly supports this point.
Maintaining a positive Ptp_ee during mechanical ventilation aims to avoid atelectasis by ensuring end-expiratory alveolar pressures greater than pleural pressures (or their surrogate Pes).27,36 This method has been previously applied in patients with ARDS and shown to increase oxygenation and Crs.16 Of note, Ptp_ee was positive at most intervention time points while all controls presented negative Ptp_ee, suggesting that individualised PEEP resulted in less end-expiratory lung de-recruitment. There is considerable controversy on the approach to set PEEP for a positive Ptp_ee.27,36, 37, 38 The Ptp_ee method presumes equality between absolute pleural pressure and Pes. This presumption has been criticised because: (a) Ptp_ee is negative in several conditions, as in our controls, leading to an expected substantially-collapsed lung,39,40 which is inconsistent with simultaneous physiological observations; (b) absolute Pes did not correlate with CT-derived lung weight in ARDS38; (c) the use of Ptp_ee to set PEEP resulted in levels neither related to lung recruitability38 nor to ARDS severity.37 An alternative to select PEEP proposes elastance ratios (EL/Ers).41 Ptp computed with this method represents that existing in the non-dependent lung at risk for excessive strain.17 However, this elastance-based approach has also been criticised for the assumption of zero pleural pressure at relaxed functional residual capacity (FRC) with zero PEEP.42,43 Of note, our elastance ratios (EL/Ers) and elastance-derived Ptp were within normal ranges (Fig. 3, Supplementary Table S3),17,41 consistent with healthy lungs. We used absolute Pes_ee measurements based on recent use16 and on findings in lung-injured pigs and human cadavers17 indicating that they reflect pleural pressures in the dependent-to-middle lung regions, that is those most susceptible to atelectasis. It should be emphasised that Ptp_ee is a local measure, not representative of the whole lung. We speculate that this partially explains why our controls with negative Ptp_ee were not significantly hypoxaemic, as the distribution of non-aerated lung is larger in the dorso-subdiaphragmatic areas where the oesophageal balloon is positioned. The remaining volume of ventilated lung together with a normal hypoxic pulmonary vasoconstriction would have contributed to maintenance of oxygenation.
We found that Pes monitoring can present technical challenges during dynamic surgical conditions but, when available, the PEEPPtp_ee method is similarly effective to PEEPmaxCrs in reducing ΔP. The strong correlations between airway and transpulmonary driving pressures, and between respiratory system and lung elastance (Fig. 3) support the use of the clinically available measures (airway ΔP, ERS) as acceptable surrogates of more complex and Pes-dependent measures (transpulmonary driving pressures, EL) in surgical patients.
Our baseline plasma biomarker measurements were comparable to previous findings.20 PEEPmaxCrs titration reduced epithelial injury biomarkers CC-16 and sRAGE by the end of surgery (Tend/T0). Interestingly, levels of these markers on postoperative day 1 relative to baseline increased in patients receiving PEEP=12 cm H2O compared with PEEP≤2 cm H2O in the PROVHILO trial.20 Such contrasting results could reflect overdistention-related injury induced by a fixed-high PEEP rather than epithelial protection achieved with an individualised-high PEEP in our study. Further investigation will be needed to confirm our observed increased plasma biomarkers of endothelial injury Ang-2 in PEEPmaxCrs (T24h/T0) and coagulation activation PAI-1 in the PEEPPtp_ee (Tend/T0) compared with controls.
There were several major limitations in this study, with six subjects being excluded before receiving any intervention. Although the reasons (mostly surgery being rescheduled/cancelled) were beyond the control of the investigators, a possible selection bias cannot be excluded. The uneven distribution of excluded subjects within the groups reduced the power to detect differential effects in the PEEPPtp_ee group. Radiological evaluations were not mandatory, and thus positive postoperative findings of atelectasis or pleural effusion may have been affected by selection bias. This study was designed to test the impact of two individualised PEEP methods during abdominal surgery on respiratory system mechanics. It was not powered to detect differences on end-inspiratory Pes, biomarker levels, clinical outcomes, or between open vs laparoscopic surgery. Recent findings11 suggest that ΔP during abdominal surgery reflects global dynamic lung strain only when PEEP results in aerated lung volumes below the FRC. However, the relationship between ΔP and PPCs has been established in large studies for a broad range of PEEP settings.12,13,34 This supports our use of ΔP and suggests that additional factors such as the regional magnitude and interplay of static and dynamic strains44 could relate to lung injury. Repeated PEEP titrations cannot be recommended until demonstration of improved clinical outcomes, particularly considering unclear results of recent PEEP studies.14,45 We did not observe any hypotension during PEEP titration that impeded the implementation of the protocol. However, the slightly higher intraoperative HR, larger volume of administered albumin, and trend to larger phenylephrine use in the individualised PEEP groups warrant examination of the haemodynamic consequences of individualisation approaches in a larger study.
We conclude that periodic individualised PEEP targeting optimised respiratory system mechanics during abdominal surgery results in reduced ΔP, maintains positive Ptp_ee and increases Crs by using PEEP levels that vary widely between and within subjects during surgery, compared with a constant PEEP≤2 cm H2O. The Crs-guided PEEP optimisation seems preferable for intraoperative PEEP titration owing to the simplicity of implementation. Individualised PEEP reduced intraoperative ΔP and improved respiratory system mechanics without significant hypotension or other adverse events. The wide variability of optimised PEEP levels observed between subjects and during abdominal surgery suggest that a ‘one-size-fits-all’ intraoperative PEEP strategy should not be a standard of care. However, larger trials to assess clinically relevant outcomes are needed before periodic individualised PEEP can be recommended.
Authors' contributions
Study conception: AFB, JS, BTT, MFVM
Study performance: AFB, JS, KB, TW, CK, MFVM
Data collection: AFB, JS, KB, TW, CK, MFVM
Data analysis and interpretation: AFB, RAP, MFVM
Manuscript preparation: AFB, RAP, MFVM
Approval of the final version of the manuscript: JS, KB, TW, CK, BTT
Declarations of interest
The authors declare that they have no conflicts of interest.
Funding
Research reported in this article was supported by the National Heart, Lung and Blood Institute (NHLBI) of the National Institutes of Health of the United States under the awards R34HL123438 (MFVM, PI; AFB, BTT and JS, co-Investigators) and UG3HL140177 (MFVM and AFB, PIs). The funding source did not have any role in the development of the trial, or in the analysis or interpretation of the trial findings.
Handling editor: Gareth Ackland
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2020.06.030.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Boden I., Skinner E.H., Browning L. Preoperative physiotherapy for the prevention of respiratory complications after upper abdominal surgery: pragmatic, double blinded, multicentre randomised controlled trial. BMJ. 2018;360:j5916. doi: 10.1136/bmj.j5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guay J., Ochroch E.A., Kopp S. Intraoperative use of low volume ventilation to decrease postoperative mortality, mechanical ventilation, lengths of stay and lung injury in adults without acute lung injury. Cochrane Database Syst Rev. 2018;7:CD011151. doi: 10.1002/14651858.CD011151.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandez-Bustamante A., Frendl G., Sprung J. Postoperative pulmonary complications, early mortality, and hospital stay following noncardiothoracic surgery: a multicenter study by the Perioperative Research Network investigators. JAMA Surg. 2017;152:157–166. doi: 10.1001/jamasurg.2016.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The LAS VEGAS investigators Epidemiology, practice of ventilation and outcome for patients at increased risk of postoperative pulmonary complications: LAS VEGAS – an observational study in 29 countries. Eur J Anaesthesiol. 2017;34:492–507. doi: 10.1097/EJA.0000000000000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbosa F.T., Castro A.A., de Sousa-Rodrigues C.F. Positive end-expiratory pressure (PEEP) during anaesthesia for prevention of mortality and postoperative pulmonary complications. Cochrane Database Syst Rev. 2014;6:CD007922. doi: 10.1002/14651858.CD007922.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guldner A., Kiss T., Serpa Neto A. Intraoperative protective mechanical ventilation for prevention of postoperative pulmonary complications: a comprehensive review of the role of tidal volume, positive end-expiratory pressure, and lung recruitment maneuvers. Anesthesiology. 2015;123:692–713. doi: 10.1097/ALN.0000000000000754. [DOI] [PubMed] [Google Scholar]
- 7.Prove Network Investigators for the Clinical Trial Network of the European Society of Anaesthesiology. Hemmes S.N., Gama de Abreu M., Pelosi P., Schultz M.J. High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): a multicentre randomised controlled trial. Lancet. 2014;384:495–503. doi: 10.1016/S0140-6736(14)60416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ostberg E., Thorisson A., Enlund M., Zetterstrom H., Hedenstierna G., Edmark L. Positive end-expiratory pressure alone minimizes atelectasis formation in nonabdominal surgery: a randomized controlled trial. Anesthesiology. 2018;128:1117–1124. doi: 10.1097/ALN.0000000000002134. [DOI] [PubMed] [Google Scholar]
- 9.de Jong M.A.C., Ladha K.S., Vidal Melo M.F. Differential effects of intraoperative positive end-expiratory pressure (PEEP) on respiratory outcome in major abdominal surgery versuscraniotomy. Ann Surg. 2016;264:362–369. doi: 10.1097/SLA.0000000000001499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levin M.A., McCormick P.J., Lin H.M., Hosseinian L., Fischer G.W. Low intraoperative tidal volume ventilation with minimal PEEP is associated with increased mortality. Br J Anaesth. 2014;113:97–108. doi: 10.1093/bja/aeu054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grieco D.L., Russo A., Romano B. Lung volumes, respiratory mechanics and dynamic strain during general anaesthesia. Br J Anaesth. 2018;121:1156–1165. doi: 10.1016/j.bja.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 12.Serpa Neto A., Hemmes S.N., Barbas C.S. Association between driving pressure and development of postoperative pulmonary complications in patients undergoing mechanical ventilation for general anaesthesia: a meta-analysis of individual patient data. Lancet Respir Med. 2016;4:272–280. doi: 10.1016/S2213-2600(16)00057-6. [DOI] [PubMed] [Google Scholar]
- 13.Ladha K., Vidal Melo M.F., McLean D.J. Intraoperative protective mechanical ventilation and risk of postoperative respiratory complications: hospital based registry study. BMJ. 2015;351:h3646. doi: 10.1136/bmj.h3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrando C., Soro M., Unzueta C. Individualised perioperative open-lung approach versus standard protective ventilation in abdominal surgery (iPROVE): a randomised controlled trial. Lancet Respir Med. 2018;6:193–203. doi: 10.1016/S2213-2600(18)30024-9. [DOI] [PubMed] [Google Scholar]
- 15.Pereira S.M., Tucci M.R., Morais C.C.A. Individual positive end-expiratory pressure settings optimize intraoperative mechanical ventilation and reduce postoperative atelectasis. Anesthesiology. 2018;129:1070–1081. doi: 10.1097/ALN.0000000000002435. [DOI] [PubMed] [Google Scholar]
- 16.Talmor D., Sarge T., Malhotra A. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med. 2008;359:2095–2104. doi: 10.1056/NEJMoa0708638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshida T., Amato M.B.P., Grieco D.L. Esophageal manometry and regional transpulmonary pressure in lung injury. Am J Respir Crit Care Med. 2018;197:1018–1026. doi: 10.1164/rccm.201709-1806OC. [DOI] [PubMed] [Google Scholar]
- 18.Williams E.C., Motta-Ribeiro G.C., Vidal Melo M.F. Driving pressure and transpulmonary pressure: how do we guide safe mechanical ventilation? Anesthesiology. 2019;131:155–163. doi: 10.1097/ALN.0000000000002731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrando C., Mugarra A., Gutierrez A. Setting individualized positive end-expiratory pressure level with a positive end-expiratory pressure decrement trial after a recruitment maneuver improves oxygenation and lung mechanics during one-lung ventilation. Anesth Analg. 2014;118:657–665. doi: 10.1213/ANE.0000000000000105. [DOI] [PubMed] [Google Scholar]
- 20.Serpa Neto A., Campos P.P., Hemmes S.N. Kinetics of plasma biomarkers of inflammation and lung injury in surgical patients with or without postoperative pulmonary complications. Eur J Anaesthesiol. 2017;34:229–238. doi: 10.1097/EJA.0000000000000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agrawal A., Matthay M.A., Kangelaris K.N. Plasma angiopoietin-2 predicts the onset of acute lung injury in critically ill patients. Am J Respir Crit Care Med. 2013;187:736–742. doi: 10.1164/rccm.201208-1460OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agrawal A., Zhuo H., Brady S. Pathogenetic and predictive value of biomarkers in patients with ALI and lower severity of illness: results from two clinical trials. Am J Physiol Lung Cell Mol Physiol. 2012;303:L634–L639. doi: 10.1152/ajplung.00195.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bos L.D., Schouten L.R., van Vught L.A. Identification and validation of distinct biological phenotypes in patients with acute respiratory distress syndrome by cluster analysis. Thorax. 2017;72:876–883. doi: 10.1136/thoraxjnl-2016-209719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mrozek S., Jabaudon M., Jaber S. Elevated plasma levels of sRAGE are associated with nonfocal CT-based lung imaging in patients with ARDS: a prospective multicenter study. Chest. 2016;150:998–1007. doi: 10.1016/j.chest.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 25.Canet J., Gallart L., Gomar C. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology. 2010;113:1338–1350. doi: 10.1097/ALN.0b013e3181fc6e0a. [DOI] [PubMed] [Google Scholar]
- 26.Reilly D.F., McNeely M.J., Doerner D. Self-reported exercise tolerance and the risk of serious perioperative complications. Arch Intern Med. 1999;159:2185–2192. doi: 10.1001/archinte.159.18.2185. [DOI] [PubMed] [Google Scholar]
- 27.Mauri T., Yoshida T., Bellani G. Esophageal and transpulmonary pressure in the clinical setting: meaning, usefulness and perspectives. Intensive Care Med. 2016;42:1360–1373. doi: 10.1007/s00134-016-4400-x. [DOI] [PubMed] [Google Scholar]
- 28.Mojoli F., Iotti G.A., Torriglia F. In vivo calibration of esophageal pressure in the mechanically ventilated patient makes measurements reliable. Crit Care. 2016;20:98. doi: 10.1186/s13054-016-1278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kroenke K., Lawrence V.A., Theroux J.F., Tuley M.R. Operative risk in patients with severe obstructive pulmonary disease. Arch Intern Med. 1992;152:967–971. [PubMed] [Google Scholar]
- 30.Force A.D.T., Ranieri V.M., Rubenfeld G.D. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 31.Brandao J.C., Lessa M.A., Motta-Ribeiro G. Global and regional respiratory mechanics during robotic-assisted laparoscopic lurgery: a randomized study. Anesth Analg. 2019;129:1564–1573. doi: 10.1213/ANE.0000000000004289. [DOI] [PubMed] [Google Scholar]
- 32.Bugedo G., Retamal J., Bruhn A. Driving pressure: a marker of severity, a safety limit, or a goal for mechanical ventilation? Crit Care. 2017;21:199. doi: 10.1186/s13054-017-1779-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goligher E.C., Ferguson N.D., Brochard L.J. Clinical challenges in mechanical ventilation. Lancet. 2016;387:1856–1866. doi: 10.1016/S0140-6736(16)30176-3. [DOI] [PubMed] [Google Scholar]
- 34.Blum J.M., Stentz M.J., Dechert R. Preoperative and intraoperative predictors of postoperative acute respiratory distress syndrome in a general surgical population. Anesthesiology. 2013;118:19–29. doi: 10.1097/ALN.0b013e3182794975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferrando C., Suarez-Sipmann F., Tusman G. Open lung approach versus standard protective strategies: effects on driving pressure and ventilatory efficiency during anesthesia – a pilot, randomized controlled trial. PLoS One. 2017;12 doi: 10.1371/journal.pone.0177399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grieco D.L., Chen L., Brochard L. Transpulmonary pressure: importance and limits. Ann Transl Med. 2017;5:285. doi: 10.21037/atm.2017.07.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiumello D., Cressoni M., Carlesso E. Bedside selection of positive end-expiratory pressure in mild, moderate, and severe acute respiratory distress syndrome. Crit Care Med. 2014;42:252–264. doi: 10.1097/CCM.0b013e3182a6384f. [DOI] [PubMed] [Google Scholar]
- 38.Chiumello D., Cressoni M., Colombo A. The assessment of transpulmonary pressure in mechanically ventilated ARDS patients. Intensive Care Med. 2014;40:1670–1678. doi: 10.1007/s00134-014-3415-4. [DOI] [PubMed] [Google Scholar]
- 39.Chiumello D., Guerin C. Understanding the setting of PEEP from esophageal pressure in patients with ARDS. Intensive Care Med. 2015;41:1465–1467. doi: 10.1007/s00134-015-3776-3. [DOI] [PubMed] [Google Scholar]
- 40.Lundin S., Persson P., Larsson A. Strategies to adjust positive end-expiratory pressure in patients with ARDS. JAMA. 2019;322:580–581. doi: 10.1001/jama.2019.7884. [DOI] [PubMed] [Google Scholar]
- 41.Gattinoni L., Giosa L., Bonifazi M. Targeting transpulmonary pressure to prevent ventilator-induced lung injury. Expert Rev Respir Med. 2019;1–10 doi: 10.1080/17476348.2019.1638767. [DOI] [PubMed] [Google Scholar]
- 42.Loring S.H., Topulos G.P., Hubmayr R.D. Transpulmonary pressure: the importance of precise definitions and limiting assumptions. Am J Respir Crit Care Med. 2016;194:1452–1457. doi: 10.1164/rccm.201512-2448CP. [DOI] [PubMed] [Google Scholar]
- 43.Gulati G., Novero A., Loring S.H., Talmor D. Pleural pressure and optimal positive end-expiratory pressure based on esophageal pressure versus chest wall elastance: incompatible results. Crit Care Med. 2013;41:1951–1957. doi: 10.1097/CCM.0b013e31828a3de5. [DOI] [PubMed] [Google Scholar]
- 44.Motta-Ribeiro G.C., Hashimoto S., Winkler T. Deterioration of regional lung strain and inflammation during early lung injury. Am J Respir Crit Care Med. 2018;198:891–902. doi: 10.1164/rccm.201710-2038OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial I. Cavalcanti A.B., Suzumura E.A. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2017;318:1335–1345. doi: 10.1001/jama.2017.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.