Summary
For patients with untreated hepatic veno‐occlusive disease (VOD)/sinusoidal obstruction syndrome (SOS) with multi‐organ dysfunction (MOD), mortality is >80%. We conducted a pooled analysis of three studies that assessed Day 100 survival in relationship to MOD severity, with dialysis and/or ventilator dependence representing the most severe organ dysfunction. All patients in the analysis were diagnosed using Baltimore criteria/biopsy. This analysis of patients with VOD/SOS and MOD after haematopoietic cell transplantation (HCT; n = 651) demonstrated higher Day 100 survival rates amongst defibrotide‐treated patients with VOD/SOS with less versus more severe forms of MOD. Even patients with severe forms of MOD post‐HCT benefitted from defibrotide.
Keywords: defibrotide, veno‐occlusive disease/sinusoidal obstruction syndrome, multi‐organ dysfunction, ventilator, dialysis
Hepatic veno‐occlusive disease (VOD), also known as sinusoidal obstruction syndrome (SOS), is a potentially life‐threatening complication of conditioning regimens for haematopoietic cell transplantation (HCT) and has been reported to have an overall incidence of 14% after HCT (Coppell et al., 2010), although this rate is heavily dependent on prior therapy, transplant‐related factors (e.g. type of transplant or intensity of the transplant conditioning) and patient‐related factors (e.g. Eastern Cooperative Oncology Group status). Mortality associated with untreated VOD/SOS with multi‐organ dysfunction (MOD) is reported to be >80% (Coppell et al., 2010). It has been suggested that the response to VOD/SOS treatment may be more favourable if treatment is initiated before serious organ damage occurs (Mohty et al., 2016). Defibrotide is approved in the European Union for treatment of severe hepatic VOD/SOS after HCT in patients aged >1 month (Defitelio, 2018). Defibrotide is also approved to treat adult and paediatric patients with hepatic VOD/SOS with renal or pulmonary dysfunction after HCT in the USA and Canada (Defitelio, 2016, 2017). A pooled analysis of data from patients with VOD/SOS and MOD after HCT was conducted to examine Day 100 survival in relationship to MOD severity, with dialysis or ventilator dependence at study entry indicating the most severe dysfunction.
Patients and methods
The analysis included data from a phase 2 dose‐finding study (NCT00003966; 75 patients treated with defibrotide 25 mg/kg/day and 74 treated with 40 mg/kg/day [Richardson et al., 2010]), a phase 3 historically controlled study (NCT00358501; 102 patients treated with defibrotide 25 mg/kg/day and 32 historical controls [Richardson et al., 2016]), and final results from the expanded‐access Defibrotide for Patients With Hepatic Veno‐occlusive Disease: A Treatment IND Study (T‐IND, NCT00628498; 1000 post‐HCT patients treated with defibrotide 25 mg/kg/day [Kernan et al., 2018b; Richardson et al., 2017]). The definitions of renal and pulmonary dysfunction were similar in the phase 3 and T‐IND studies but varied slightly in the phase 2 study (Table SI). In the phase 3 and T‐IND studies, renal dysfunction was characterised as serum creatinine ≥3× baseline, or creatinine clearance (CrCl) or estimated glomerular filtration rate (eGFR) ≤40% of baseline, or dialysis dependence (Richardson et al., 2016; Richardson et al., 2017); the phase 2 study defined renal dysfunction as serum creatinine ≥2× baseline, or CrCl/eGFR ≤50% of baseline, or dialysis dependence (Richardson et al., 2010). In both studies, patients who received continuous veno‐venous haemofiltration were included among dialysis‐dependent patients. In the phase 3 and T‐IND studies, pulmonary dysfunction criteria included oxygen saturation ≤90% on room air or oxygen supplementation/ventilator dependence; the phase 2 study defined pulmonary dysfunction as oxygen saturation ≤90% on room air and/or as positive pressure/ventilator dependence.
All three studies used Baltimore criteria or biopsy for the diagnosis of VOD/SOS, while the T‐IND study also allowed diagnosis using modified Seattle criteria (Richardson et al., 2010; Richardson et al., 2016; Richardson et al., 2017; Kernan et al., 2018a). The current pooled analysis included only patients diagnosed by Baltimore criteria (bilirubin ≥2 mg/l and at least two of the following symptoms: hepatomegaly, ascites, and weight gain ≥5%, within 21 days of transplant) or by biopsy.
The Kaplan–Meier method was used to estimate overall survival at Day 100 and beyond. Logistic regression modelling was used to calculate odds ratios (ORs) and 95% confidence intervals (CIs) for survival at Day 100 for patients with various levels of dependency on supportive care versus patients without dependencies.
Results
The total pooled population from the three studies was 651 patients with VOD/SOS plus subsequent MOD post‐HCT diagnosed by Baltimore criteria or by biopsy (phase 2 study, n = 149; phase 3 study, n = 102; T‐IND study, n = 400). The median (range) age was 18 (0–72) years in the pooled treatment population and 18 (1–57) years in the historical controls. Of the 651 patients, 418 (64%) did not develop ventilator or dialysis dependence. The remaining 233 patients had at least one dependency: 137 had only one dependency and 96 had both dependencies. Overall, 185 (28%) patients had ventilator dependence (irrespective of dialysis dependence) and 144 (22%) patients had dialysis dependence (irrespective of ventilator dependence; Table SII). The historical control patients (n = 32) from the phase 3 study were analysed separately, as they did not receive defibrotide. Of these patients, 25 (78%) did not develop ventilator or dialysis dependency. The remaining seven patients had at least one dependency: six had only one dependency and one had both dependencies [six (19%) patients had ventilator dependence and two (6%) had dialysis dependence].
In the total pooled population (n = 651), the overall Kaplan–Meier Day 100 estimated survival rate was 44% (95% CI 40–48%; Fig 1A). Among subgroups, the Kaplan–Meier Day 100 estimated survival rate was highest in patients who did not develop either dependency (48%; 95% CI 44–53%) and lowest in patients with both ventilator and dialysis dependence (28%; 95% CI 19–37%); in patients with only one dependence, the Kaplan–Meier Day 100 estimated survival rate was 40% (95% CI 32–48%). The Kaplan–Meier Day 100 estimated survival rate was 33% (95% CI 26–41%) in dialysis‐dependent patients and 33% (95% CI 27–40%) in ventilator‐dependent patients, noting that patients in each of these groups could also have the other dependency. In the historical control group (n = 32), the Kaplan–Meier Day 100 estimated survival was 25% (95% CI 12–40%) overall, 28% (95% CI 12–46%) in patients who did not develop either dependency and 14% (95% CI <1–47%) in patients with only one dependency (Fig 1B). One patient in the historical control group had both ventilator and dialysis dependence and died prior to Day 100. Kaplan–Meier estimates of data up to 1 year post‐HCT were supportive of the Day 100 endpoint analysis (Fig 1C).
Figure 1.
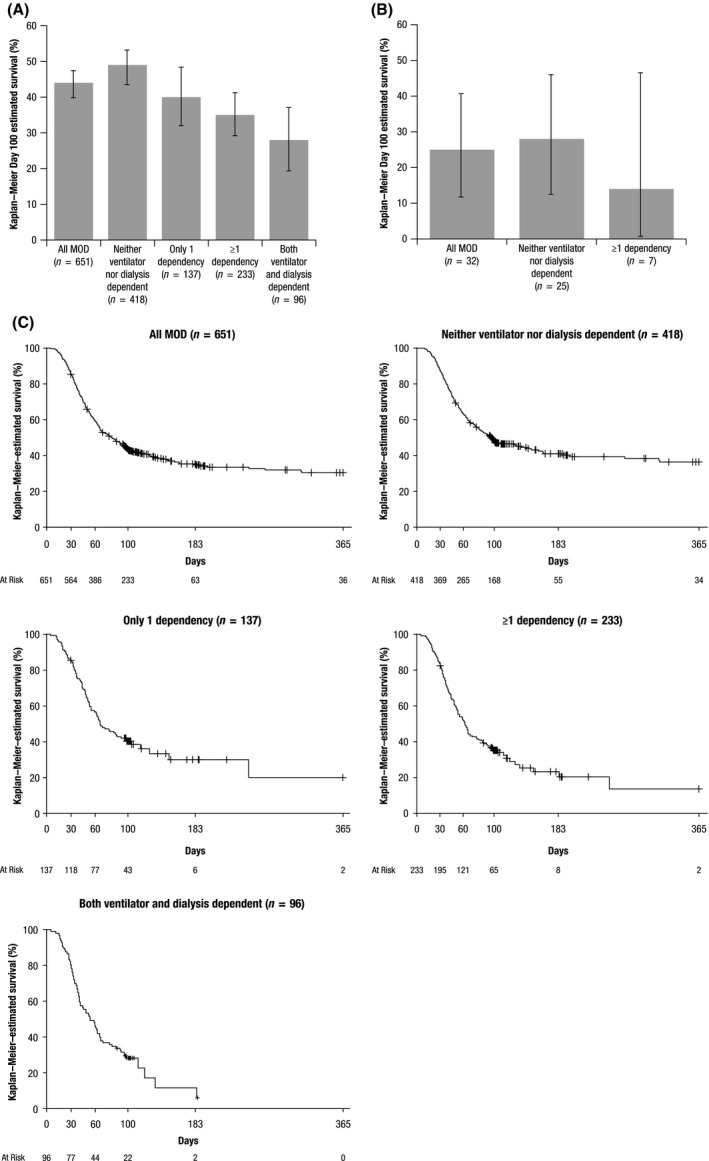
Kaplan–Meier–estimated Day 100 survival in defibrotide‐treated patients (A) and historical controls (B) and Kaplan–Meier–estimated overall survival by severity of MOD in a pooled analysis of defibrotide‐treated patients (C). MOD, multi‐organ dysfunction. Error bars denote 95% confidence intervals. Censored patients are represented with a + symbol.
Compared to patients who did not develop either dependency, the OR of survival at Day 100 was 0·68 (95% CI 0·45–1·04), 0·56 (95% CI 0·39–0·80), and 0·41 (95% CI 0·24–0·69) in patients with only one dependency, patients with one or more dependencies, and patients with both dependencies, respectively.
In the total pooled population, 546 (84%) had at least one treatment‐emergent adverse event (TEAE), with 167 (26%) having a treatment‐related adverse event, as assessed by the investigator (Table 1). TEAEs occurred in 96 (23%) patients who did not develop either dependency and in 24 (25%) patients with both ventilator and dialysis dependence. Haemorrhagic events (any grade) were reported in 276 (42%) patients in the total pooled population, 176 (42%) patients who did not develop either dependency, and 37 (39%) patients with both dependencies. Hypotensive events were reported in 160 (25%) patients in the pooled population, 98 (23%) patients who did not develop either dependency, and 18 (19%) patients with both dependencies. All (100%) historical control group patients had at least one adverse event; among them, 24 (75%) had haemorrhagic events and 16 (50%) had hypotensive events.
Table 1.
Common treatment‐emergent adverse events (≥2% in the all MOD population).
| Adverse event, n (%) |
All MOD (n = 651) |
Neither ventilator nor dialysis dependent (n = 418) |
Only 1 dependency (n = 137) |
≥1 dependency* (n = 233) |
Both ventilator and dialysis dependent (n = 96) |
|---|---|---|---|---|---|
| Any | 167 (26) | 96 (23) | 47 (34) | 71 (31) | 24 (25) |
| Hypotension | 29 (5) | 13 (3) | 14 (10) | 16 (7) | 2 (2) |
| Gastrointestinal haemorrhage | 27 (4) | 17 (4) | 8 (6) | 10 (4) | 2 (2) |
| Pulmonary haemorrhage | 25 (4) | 12 (3) | 7 (5) | 13 (6) | 6 (6) |
| Epistaxis | 19 (3) | 12 (3) | 3 (2) | 7 (3) | 4 (4) |
| Pulmonary alveolar haemorrhage | 14 (2) | 10 (2) | 3 (2) | 4 (2) | 1 (1) |
MOD, multi‐organ dysfunction.
Patients with ≥1 dependency had at least 1 dependency (ventilator or dialysis) and could have both dependencies.
Discussion
The present analysis supports the indication for defibrotide in the treatment of patients with VOD/SOS and MOD post‐HCT, including those with ventilator and/or dialysis dependence. In addition, Day 100 survival was higher in patients with MOD without ventilator or dialysis dependence versus those with these dependencies. These results were supported by analysis of overall survival, which extended beyond Day 100. It is uncertain whether patients without ventilator or dialysis dependence in these studies would have progressed to more severe forms of the disease if they had not received defibrotide treatment. At the same time, the results suggest that administering defibrotide at an earlier stage of disease may improve outcomes, as opposed to waiting for patients to progress to the point where a ventilator or dialysis is required. This idea is supported by a retrospective study of paediatric patients with VOD/SOS post‐HCT, which identified early intervention with defibrotide as a predictor of a complete response (Corbacioglu et al., 2004; Mohty et al., 2016).
The present pooled analysis is limited by the lack of a prospective control arm. Data from the non–defibrotide‐treated historical control population from the phase 3 study were provided for reference. The uniformity of dismal outcomes in patients with VOD/SOS with MOD is well established and therefore the benchmark provided by the historical control group is useful, despite its small number of patients with ventilator/dialysis dependency. Further, it is worth noting that the eligibility criteria from the phase 3 study differed slightly from those used in the phase 2 study.
In summary, results from this pooled analysis of three defibrotide studies of patients with VOD/SOS and MOD post‐HCT (n = 651) demonstrated that Day 100 survival rates were higher among defibrotide‐treated patients with VOD/SOS with less severe versus more severe forms of MOD (e.g. ventilator and/or dialysis dependence). However, even patients with VOD/SOS with severe forms of MOD post‐HCT benefitted from defibrotide treatment.
Conflict of interest disclosures
Paul G. Richardson has served on advisory committees for Jazz Pharmaceuticals. Nancy A. Kernan received grants from Gentium during the conduct of the study, and her research was supported by the National Cancer Institute of the National Institutes of Health under award number P30 CA008748; the content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health. Robert J. Soiffer has consulted for Celgene, Gilead, Jazz Pharmaceuticals, Mana Therapeutics, and Neovii; he has served on the data and safety advisory board for Juno and is on the board of directors for Kiadis. William Tappe is a former employee of Jazz Pharmaceuticals and holds stock ownership in Jazz Pharmaceuticals. Robert J. Ryan is an employee of and holds stock ownership in Jazz Pharmaceuticals. Stephan Grupp receives study support from Novartis, Kite, and Servier; he has consulted for Novartis, Roche, GSK, Cure Genetics, Humanigen, and CBMG and is on study steering committees or scientific advisory boards for Jazz, Adaptimmune, TCR2, Eureka, Cellectis, Juno, and Vertex; he holds a patent (Toxicity management for anti‐tumor activity of CARs, WO 2014011984 A1) that is managed according to the University of Pennsylvania patent policy. Angela R. Smith and Leslie Lehmann have nothing to disclose.
Supporting information
Table SI. Definitions of MOD.
Table SII. Baseline demographics of pooled patients and historical controls with MOD.
Acknowledgements
All authors contributed to the acquisition, analysis or interpretation of the data, provided critical review of the manuscript, and gave final approval of the manuscript. This analysis was funded by Jazz Pharmaceuticals. Medical writing and editorial support were provided by William Perlman, PhD, and Erica Chevalier‐Larsen, PhD, of SciFluent Communications, and were financially supported by Jazz Pharmaceuticals.
References
- Coppell, J.A. , Richardson, P.G. , Soiffer, R. , Martin, P.L. , Kernan, N.A. , Chen, A. , Guinan, E. , Vogelsang, G. , Krishnan, A. , Giralt, S. & Revta, C. (2010) Hepatic veno‐occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biology of Blood and Marrow Transplantation, 16, 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbacioglu, S. , Greil, J. , Peters, C. , Wulffraat, N. , Laws, H.J. , Dilloo, D. , Strahm, B. , Gross‐Wieltsch, U. , Sykora, K.W. , Ridolfi‐Lüthy, A. & Basu, O. (2004) Defibrotide in the treatment of children with veno‐occlusive disease (VOD): a retrospective multicentre study demonstrates therapeutic efficacy upon early intervention. Bone Marrow Transplantation, 33, 189–195. [DOI] [PubMed] [Google Scholar]
- Defitelio (defibrotide sodium) (2016) Prescribing Information. Jazz Pharmaceuticals, Inc., Palo Alto, CA: Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208114lbl.pdf [Google Scholar]
- Defitelio (defibrotide sodium) (2017) Product Monograph. Jazz Pharmaceuticals Ireland, Limited, Dublin, Ireland: Available at: http://pp.jazzpharma.com/pi/defitelio.ca.PM‐en.pdf [Google Scholar]
- Defitelio (defibrotide) (2018) Summary of Product Characteristics. Gentium SpA, Villa Guardia, Italy: Available at: https://www.ema.europa.eu/documents/product‐infromation/defitelio‐epar‐product‐information_en.pdf [Google Scholar]
- Kernan, N.A. , Grupp, S. , Smith, A.R. , Arai, S. , Triplett, B. , Antin, J.H. , Lehmann, L. , Shore, T. , Ho, V.T. , Bunin, N. & Iacobelli, M. (2018a) Final results from a defibrotide treatment‐IND study for patients with hepatic veno‐occlusive disease/sinusoidal obstruction syndrome. British Journal of Haematology, 181, 816–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernan, N.A. , Richardson, P.G. , Smith, A.R. , Triplett, B.M. , Antin, J.H. , Lehmann, L. , Messinger, Y. , Liang, W. , Hume, R. , Tappe, W. & Soiffer, R.J. (2018b) Defibrotide for the treatment of hepatic veno‐occlusive disease/sinusoidal obstruction syndrome following nontransplant‐associated chemotherapy: final results from a post hoc analysis of data from an expanded‐access program. Pediatric Blood & Cancer, 65, pe27269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohty, M. , Malard, F. , Abecassis, M. , Aerts, E. , Alaskar, A.S. , Aljurf, M. , Arat, M. , Bader, P. , Baron, F. , Bazarbachi, A. & Blaise, D. (2016) Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno‐occlusive disease in adult patients: a new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplantation, 51, 906–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson, P.G. , Soiffer, R.J. , Antin, J.H. , Uno, H. , Jin, Z. , Kurtzberg, J. , Martin, P.L. , Steinbach, G. , Murray, K.F. , Vogelsang, G.B. & Chen, A.R. (2010) Defibrotide for the treatment of severe hepatic veno‐occlusive disease and multiorgan failure after stem cell transplantation: a multicenter, randomized, dose‐finding trial. Biology of Blood and Marrow Transplantation, 16, 1005–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson, P.G. , Riches, M.L. , Kernan, N.A. , Brochstein, J.A. , Mineishi, S. , Termuhlen, A.M. , Arai, S. , Grupp, S.A. , Guinan, E.C. , Martin, P.L. & Steinbach, G. (2016) Phase 3 trial of defibrotide for the treatment of severe veno‐occlusive disease and multi‐organ failure. Blood, 127, 1656–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson, P.G. , Smith, A.R. , Triplett, B.M. , Kernan, N.A. , Grupp, S.A. , Antin, J.H. , Lehmann, L. , Shore, T. , Iacobelli, M. , Miloslavsky, M. & Hume, R. (2017) Defibrotide for patients with hepatic veno‐occlusive disease/sinusoidal obstruction syndrome: interim results from a treatment IND study. Biology of Blood and Marrow Transplantation, 23, 997–1004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI. Definitions of MOD.
Table SII. Baseline demographics of pooled patients and historical controls with MOD.


