Abstract
Aim
Transanal minimally invasive surgery (TAMIS) is used increasingly often as an organ‐preserving treatment for early rectal cancer. If final pathology reveals unfavourable histological prognostic features, completion total mesorectal excision (cTME) is recommended. This study is the first to investigate the results of cTME after TAMIS.
Method
Data were retrieved from the prospective database of the Elisabeth‐TweeSteden Hospital. Completion TME patients were case matched with a control group of patients undergoing primary TME (pTME). Primary and secondary outcomes were surgical outcomes and oncological outcomes, respectively.
Results
From 2011 to 2017, 20 patients underwent cTME and were compared with 40 patients undergoing pTME. There were no significant differences in operating time (238 min vs 226 min, P = 0.53), blood loss (137 ml vs. 158 ml, P = 0.88) or complications (45% vs 55%, P = 0.07) between both groups. There was no 90‐day mortality in the cTME group. The mesorectal fascia was incomplete in three patients (15%) in the cTME group compared with no breaches in the pTME group (P = 0.083). There were no local recurrences in either group. In three patients (15%), distant metastases were detected after cTME compared with one patient (2.5%) in the pTME group (P = 0.069). After cTME patients had a 1‐ and 5‐year disease‐free survival of 85% compared with 97.5% for the pTME group (P = 0.062).
Conclusion
Completion TME surgery after TAMIS is not associated with increased peri‐ or postoperative morbidity or mortality compared with pTME surgery. After cTME surgery patients have a similar disease‐free and overall survival when compared with patients undergoing pTME.
Keywords: Transanal minimally invasive surgery, completion, TEMS, organ preservation
What does this paper add to the literature?
The literature reporting the oncological results of completion total mesorectal excision (TME) surgery after local excision of early rectal cancer is scarce and contradictory. This is the first study on completion TME after transanal minimally invasive surgery. The present study shows that overall and disease‐specific survival of patients after complete TME surgery is not compromised.
Introduction
Rectal resection according to the principles of total mesorectal excision (TME) is still considered to be the gold standard for the treatment of rectal cancer 1. Since its introduction in 2010 by Attalah and co‐workers, transanal minimally invasive surgery (TAMIS) has been used often as an alternative to TME for local excision of early rectal cancer 2. Local excision is associated with good quality of life and functional outcomes, as well as a decreased morbidity, short hospital stay and cost 3, 4, 5, 6. Furthermore, local excision can be considered in the treatment of elderly patients with rectal cancer who are unfit (or unwilling) to undergo conventional TME surgery 7.
Unfortunately, histopathological examination after TAMIS can reveal unfavourable histological prognostic features, including tumour invasion beyond the muscularis propria (>T1), poorly differentiated tumour grading, lymphovascular invasion or incomplete resection 8. Several studies have shown that this occurs in up to 24% of patients treated by local excision 3, 9, 10, 11. In these cases, completion total mesorectal excision (cTME) is recommended.
This treatment strategy can only be considered when cTME yields similar outcomes to primary TME (pTME) 8, 10, 12, 13, 14. However, contradictory results in terms of perioperative complications 15 as well as high colostomy rates and high abdominoperineal excision (APR) rates are reported when completion surgery is performed after TEMS 16, 17.
To the best of our knowledge, no literature exists on the outcomes of cTME surgery following previous TAMIS compared with primary TME (pTME) surgery for rectal cancer. The aim of the present study was therefore to evaluate the effects and consequences of previous TAMIS when followed by cTME, specifically focusing on surgical and oncological outcomes.
Method
All operations were performed in the ETZ (Elisabeth‐TweeSteden Hospital) in Tilburg, the Netherlands, which is a large teaching hospital.
Indications for TAMIS were: early stage rectal cancer defined as well‐differentiated T1 cancers, tumour size < 30 mm without lymph node, (lympho)vascular or perineural involvement on MRI. Furthermore, TAMIS was performed in patients with a radiological response after chemoradiation (yc1‐2), to prove or disprove pathological complete response (ypT0). Patients were excluded if they had previously undergone conventional transanal excision (e.g. with a Lonestar retractor or Parks speculum). All patients were assessed preoperatively with digital rectal examination, flexible colonoscopy, tumour biopsy and rigid rectoscopy; the latter to determine the height and location of the lesion. Patients were also staged preoperatively with pelvic MRI to evaluate invasion (T‐stage) and lymph node involvement (N‐stage). All TAMIS procedures were full‐thickness resections performed under general anaesthesia, as described earlier 18.
The decision to perform cTME surgery after TAMIS was based on tumour invasion beyond the muscularis propria (> T1), lymphovascular or perineural involvement, poor tumour differentiation and cases with incomplete resection margins. The decision to perform initial TAMIS and cTME was made during multidisciplinary team meetings and after extensive shared decision‐making with the patient at the outpatient clinic (Fig. 1).
Figure 1.
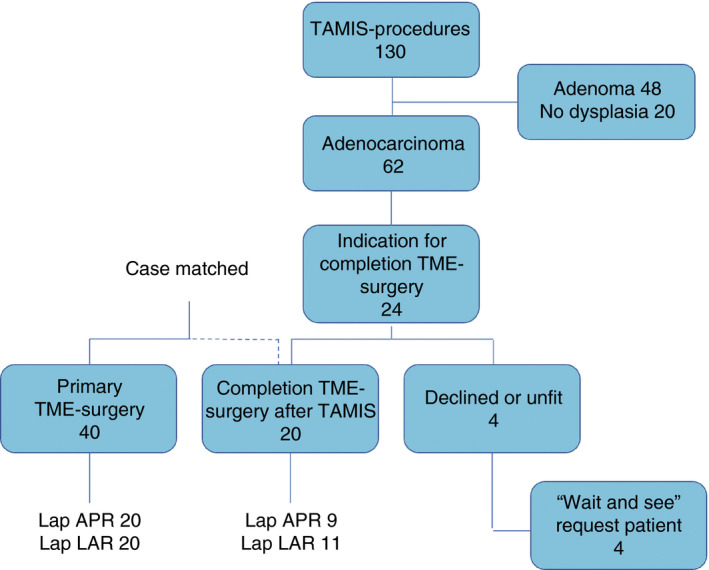
Study design (TAMIS, transanal minimally invasive surgery; TME, total mesorectal excision).
A minimum convalescence period of 6–8 weeks was maintained in order to facilitate safe secondary surgery. Follow‐up was carried out in accordance with Dutch national guidelines on colorectal cancer 19.
Primary end‐points are surgical outcomes defined as total procedure time, intra‐operative blood loss, peri‐ and postoperative morbidity and mortality. Secondary end‐points are oncological outcomes defined as mesorectal integrity of the resected specimen, local and distant recurrence and disease‐free survival. All resection specimens were sent fresh (without fixation) to pathology. Mesorectal integrity was judged by the pathologist according to the Quirke classification 20.
Statistical analysis
Every patient in the cTME group was matched with two patients who underwent pTME surgery during the same time period and at the same hospital. We case‐matched patients on the basis of age, American Society of Anesthesiologists classification, gender, body mass index, distance of the tumour from the anal verge, tumour size, clinical T‐stage (cT/TNM stage) and whether or not they underwent neoadjuvant chemoradiotherapy.
Descriptive statistics were expressed as median and range (minimum, maximum) for continuous variables. Differences between groups were calculated using the Mann–Whitney U‐test for continuous variables. The Pearson chi‐square test or Fisher’s exact test, if appropriate, were used for categorical variables. We used Kaplan–Meier analyses to calculate the estimated survival rates. Differences in recurrence rates were analysed by using the log‐rank test. Statistical significance was considered at P < 0.05. Statistical analyses were performed by IBM spss Statistics v.19 (IBM Corp, Armonk, New York, USA) and sas v.9.4 (SAS Institute, Cary, North Carolina, USA).
Results
From October 2011 to October 2017, 130 patients underwent a TAMIS procedure. Of these patients, 20 (15.4%) [female:male 30%:70%; median age 70 (range 46–84) years] patients underwent cTME surgery after previous TAMIS. Detailed patient characteristics are summarized in Table 1 .
Table 1.
Patient characteristics.
| cTME (n = 20) | pTME (n = 40) | P‐value | |
|---|---|---|---|
| Age (years), median ± SD [range] | 70 ± 9.9 [46–84] | 73 ± 8.3 [54–87] | 0.11 |
| Gender, n (%) | F = 6 (30), M = 14 (70) | F = 16 (40), M = 24 (60) | 0.457 |
| ASA grade, n (%) | |||
| I | 6 (30) | 11 (27.5) | 0.327 |
| II | 7 (35) | 26 (65) | |
| III | 6 (30) | 2 (5) | |
| IV | 1 (5) | 1 (2.5) | |
| BMI (kg/m2), median ± SD [range] | 25.3 ± 3.5 [21.3–33.2] | 25.8 ± 3.1 [22.0–32.8] | 0.249 |
| Distance from anal verge (mm), median ± SD [range] | 85.0 ± 37.3 [20–140] | 80.0 ± 35.7 [20–110] | 0.058 |
| < 50 mm | 6 (30) | 17 (42.5) | |
| 60–100 mm | 6 (30) | 17 (42.5) | |
| > 110 mm | 8 (40) | 6 (15.0) | |
| Tumour size (mm2), median ± SD [range] | 39.0 ± 12.6 [20–65] | 33.0 ± 12.4 [10–55] | 0.062 |
| T‐stage | |||
| Scar, n (%) | 0 (0) | 0 (0) | 0.312 |
| T1, n (%) | 1 (5) | 3 (7.5) | |
| T2, n (%) | 17 (85) | 32 (80) | |
| T3, n (%) | 2 (10) | 5 (12.5) | |
| Neoadjuvant radiation therapy, n(%) | 4 (20) | 15 (37.5) | 0.152 |
| Follow‐up (years), median [range] | 2.5 [1.1–6.3] | 3.22 [1.6–7.6] | 0.057 |
ASA, American Society of Anesthesiologists; BMI, body mass index; cTME, completion TME; pTME, primary TME; TAMIS, transanal minimally invasive surgery; TME total mesorectal excision.
During the primary TAMIS procedure, the peritoneal cavity was entered inadvertently in three patients (15%), in two of these endoluminal closure was possible and in one case the defect was closed laparoscopically due to an unstable pneumorectum. Four patients (20%) encountered postoperative complications within 90 days after TAMIS: in one case a rectal stricture which could be dilated endoscopically formed at the location of the TAMIS resection, two patients developed fever for which they were treated with oral antibiotics and one patient developed urinary retention for which a urethral catheter was inserted.
The median time to cTME was 8 (range 6–16) weeks. In the present cohort, one completion TME procedure was performed after 11 weeks and one after 16 weeks. In both cases, patients needed more time to decide whether or not they wanted to undergo the cTME procedure.
Procedural features are summarized in Table 2. The control group consisted of 40 patients [female:male 16:24; median age 73 (range 54–87) years] who underwent pTME surgery for rectal cancer within the same time frame. There were no statistically significant differences at baseline between the groups.
Table 2.
Procedural results.
| cTME (n = 20) | pTME (n = 40) | P‐value | |
|---|---|---|---|
| Type of procedure, n (%) | |||
| APR | 9 (45) | 20 (50) | 0.720 |
| LAR | 10 (50) | 15 (37.5) | |
| LAR‐i | 1 (5) | 5 (12.5) | |
| Interval between TAMIS and TME (weeks), mean [range] | 8.0 [6–16] | NA | |
| Operative time (min), mean ± SD [range] | 238 ± 69.6 [143–369] | 226 ± 67.0 [105–377] | 0.531 |
| Intra‐operative blood loss (ml), mean ± SD [range] | 137 ± 248.4 [0–1000] | 158.33 ± 259.0 [0–1000] | 0.883 |
| Intra‐operative complications, n (%) | 2 (10) | 3 (7.5) | 0.069 |
| Bleeding | 1 | 1 | |
| Iatrogenic bowel perforation | 2 | 2 | |
| Conversion to open surgery, n(%) | 1 (5) | 1 (2.5) | 1 |
| Obesity | 0 | 1 | |
| Bleeding | 1 | 0 | |
APR, abdominoperineal excision; cTME, completion TME; LAR, low anterior resection; LAR‐i, low anterior resection with protective ileostomy; pTME, primary TME; NA, not applicable; TAMIS, transanal minimally invasive surgery; TME, total mesorectal excision.
All patients in both groups underwent laparoscopic TME surgery. The decision to perform an APR in nine patients (45%) was based on distal tumour location diagnosed on preoperative work‐up. No statistically significant differences were found concerning mean operation time and intra‐operative blood loss. In the cTME group two patients (10%) endured an intra‐operative complication, in the pTME group such complications occurred in three patients (7.5%) (P = 0.069). In both groups, one conversion to conventional open anterior resection was necessary. In the cTME this was a reactive conversion due to a retroperitoneal haematoma (puncture of the abdominal aorta) that occurred after open insertion of Hasson trocar. The conversion to open surgery in the pTME group was an early strategic conversion due to obesity of the patient.
Postoperative outcomes are summarized in Table 3. Complications occurred in 9 (45%) of the cTME cases and 22 (55%) of the pTME cases (P = 0.473). No statistically significant differences could be discerned for wound infection, anastomotic dehiscence, pelvic abscess or sepsis between the groups. There was no difference in the severity of complications according to the Clavien–Dindo severity index. A higher incidence of readmissions was noted in the cTME group [6 (30%) vs 4 (10%), P = 0.032]. Surgical site infections and perineal wound dehiscence were the main reasons for readmittance in both groups. We found a 2.5% mortality rate (one patient) within 90 days after pTME surgery. This patient developed severe sepsis on the basis of bowel perforation. Reoperation was performed with resection of the perforated bowel segment and extensive rinsing of the abdominal cavity with saline solution. The patient was admitted to the ICU but died of irreversible multiple organ failure at the third postoperative day. No mortality was observed in the completion group during the study period (P = 0.317). Furthermore, there was no statistically significant difference in hospital stay. After pTME patients stayed for a median of 6 days while after cTME patients stayed for a median of 5 days (P = 0.988).
Table 3.
Postoperative outcomes.
| cTME (n = 20) | pTME (n = 40) | P‐value | |
|---|---|---|---|
| TAMIS complication* | 6 (30) | NA | |
| Morbidity† | 9 (45) | 22 (55.0) | 0.473 |
| Arrhythmia | 3 (15) | 0 (0) | 0.011 |
| Urinary retention | 4 (20) | 1 (2.5) | 0.079 |
| Perineal wound dehiscence | 4 (20) | 3 (7.5) | 0.746 |
| Superficial wound infection | 3 (15) | 2 (5) | 0.193 |
| Ileus | 1 (5) | 6 (15) | 0.370 |
| Bowel ischaemia | 0 (0) | 2 (5) | 0.317 |
| Sepsis | 0 (0) | 2 (5) | 0.317 |
| Pneumonia | 0 (0) | 4 (10) | 0.148 |
| Anastomotic dehiscence | 3 (15) | 4 (10) | 0.291 |
| Pelvic abscess | 3 (15) | 6 (15) | 1 |
| High‐output ileostomy | 1 (5) | 3 (7.5) | 0.216 |
| Haemorrhage | 1 (5) | 1 (5) | 0.484 |
| Bowel evisceration | 0 (0) | 1 (5) | 0.312 |
| Clavien–Dindo grade | |||
| I + II | 8 (40) | 14 (35) | 0.646 |
| IIIa | 1 (5) | 2 (5) | 1 |
| ≥ IIIb | 4 (20) | 12 (30) | 0.192 |
| 30‐day mortality | 0 (0) | 1 (2.5) | 0.317 |
| Hospital stay (days), mean [range] | 5.0 [3–21] | 6.0 [2–33] | 0.998 |
| Readmission | 6 (30) | 4 (10) | 0.032 |
Values are number (%) unless otherwise stated.
cTME, completion TME; NA, not applicable; pTME, primary TME; TAMIS, transanal minimally invasive surgery; TME, total mesorectal excision.
Initial TAMIS procedure.
Number of procedures (%) with postoperative complications.
Bold values are statistically significant.
Mesorectal integrity was judged by the pathologist according to the Quirke classification. Breach of the mesorectal fascia was found in three (15%) of the cTME cases. All defects in the mesorectal fascia were found at the previous TAMIS site; these resection specimens were classified as Quirke 2 (moderate) and 100% mesorectal integrity (Quirke 1) was found in the pTME group (P = 0.083). No residual tumour was found in four patients (20%) in the cTME group. It is noteworthy that no residual tumour was found in all cases where the mesorectal fascia was breached. None of the patients with an inferior resection specimen developed local or distant metastasis.
Median follow‐up was 36 (13–91.2) months. No local recurrences were observed in either group. Three patients in the cTME group and one patient in the pTME group (15% vs 2.5%, P = 0.069) developed distant metastases. In the cTME group, this included one patient (ypT2N1) with lung and liver metastases, discovered after 6 months’ follow‐up. Additional resection of both metastases was performed successfully. Two patients (ypT2N1 and yT3N0) developed liver metastases after 3 and 4 months, respectively, for which they underwent successful additional resection as well.
In the pTME group, one patient (ypT3N2) developed liver metastases at 7 months of follow‐up. Additional liver resection was performed successfully. All patients with metastases were alive at the time of preparation of this manuscript.
Patients after cTME surgery had 1 and 5‐year disease‐free survival of 85%. Patients in the pTME group had 1 and 5‐year disease free survival of 97.5% (P = 0.062, log rank). The overall survival for patients undergoing cTME was 100% at 1 and 5 years. Overall survival in the pTME group was 100% at 1 year and 86% at 5 years.
Oncological outcomes are summarized in Table 4 and Fig. 2.
Table 4.
Oncological outcomes.
| cTME (n = 20) | pTME (n = 40) | P‐value | |
|---|---|---|---|
| Definitive tumour stage* | 0.359 | ||
| Complete regression | 4 (20) | 0 (0) | |
| I | 9 (45) | 32 (80) | |
| IIa | 4 (20) | 2 (5) | |
| IIb | 0 (0) | 1 (2.5) | |
| IIIa | 3 (15) | 5 (12.5) | |
| Mesorectal integrity | 17 (85) | 40 (100) | 0.083 |
| Length of specimen (cm), mean ± SD [range] | 37.9 ± 53 [9–225] | 26.7 ± 35.1 [7–195] | 0.383 |
| Harvested lymph nodes, n ± SD [range] | 15 ± 6 [2–30] | 18 ± 6.8 [5–33] | 0.060 |
| Local recurrence | 0 (0) | 0 (0) | NA |
| Metastasis | 3 (15) | 1 (2.5) | 0.069 |
Values are number (%) unless otherwise stated.
cTME, completion TME; pTME, primary TME; TAMIS, transanal minimally invasive surgery; TME, total mesorectal excision.
American Joint Committee on Cancer staging system.
Figure 2.
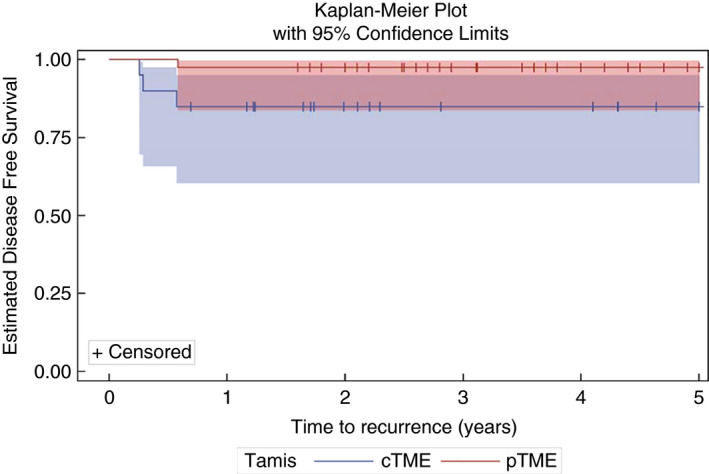
Disease‐free survival. Kaplan–Meier curves for 5‐year estimated disease‐free survival with strata comparison completion TME (cTME) versus primary TME (pTME) (P = 0.0618, log‐rank test). Survival probability at 5 years was 85% (cTME) and 97.5% (pTME).
Discussion
Local excision is considered by many surgeons to be the gold standard for the treatment of selected and early stage low‐risk neoplasms in the lower two‐thirds of the rectum 5, 21. Techniques such as TAMIS are associated with low morbidity and are considered as curative treatment in case of pT1 early rectal cancers without unfavourable characteristics 10, 22. However, these treatment modalities require careful patient selection and detailed preoperative radiological staging to meet the criteria for treatment with TAMIS. Imaging tools like endorectal ultrasound and MRI are indispensable in preoperative staging and analysing tumour invasion of the rectal wall 23, 24, nonetheless there is often a discrepancy between preoperative T‐stage and the definitive T‐stage after pathological examination 25, 26. This discrepancy results in up to 23% of the patients requiring a cTME procedure 27. In our analysis, 12.3% of patients underwent cTME surgery after primary TAMIS.
With the increasing incidence of early stage rectal cancer and a trend towards organ‐preserving surgery, it is likely that the total amount of cTME surgery is going to increase drastically. Furthermore, the benefits of adjuvant chemoradiotherapy or intensive watch and wait protocols after local excision are under investigation 28.
Completion TME surgery after primary local excision is considered by many surgeons to be a challenging procedure, with high incidence of APR 13, 14, 16, 17, 29, 30, 31. Dissection is considered to be more difficult due to fibrotic changes to the bowel wall after primary full thickness resection which tend to obscure the normal pelvic dissection planes. These anatomical changes could potentially lead to difficult surgery (e.g. increased blood loss, increased morbidity) but could also affect oncological results due to increased risk of violating surgical dissection planes and making radical surgery more difficult. Interestingly, the present data do not support this perception of difficulty. Operating time is not significantly longer, no increase in perioperative complications is observed and no difference in blood loss is encountered. We can only surmise that the complexity of cTME is mainly subjective.
Few studies have evaluated the surgical and oncological outcomes of cTME surgery after previous TEM. Morino et al. 17 retrospectively analysed patients who underwent laparoscopic cTME after TEM; they found a significantly longer procedural time and a significantly increased APR rate in the cTME group (intra‐operative difficulties due to severe inflammation were the reason to convert to APR). In contrast, Levic et al. 31 showed a shorter total operation time for the cTME group. Morbidity rates were 52% for both groups. APR was required in 44% of patients in both groups. An intra‐operative perforation rate of 20% was reported, all at the previous TEM site. Even though intra‐operative rectal wall perforation is one of the most important risk factors for local and distant recurrence in radical rectal surgery 32, 33 no local recurrences and only one distant metastasis were reported during a median of 25 months of follow‐up. The study by Hompes et al. 29 shows similar surgical outcomes to Levic et al. 31. They suggest that mesorectal integrity during cTME is the most important predictor for disease‐free survival. The largest study by van Gijn et al. 16 retrospectively compared 59 patients undergoing cTME after TEM with 881 pTME procedures. They report a colostomy rate of 50.8% in the cTME group versus 45.9% in the pTME group. In the cTME group 10.2% of patients developed local recurrence compared with 5.2% in the pTME group. Even though the present study identified the same difference in mesorectal integrity, this difference did not reach statistical significance. Moreover, it did not translate to an increased incidence of local recurrences. It has to be noted, however, that follow‐up of the present series is relatively short.
In our cTME surgery group we did not find a higher APR rate (45% in the cTME group versus 50% in the pTME group, P = 0.720). None of the APRs in the cTME group were performed due to mesorectal fibrosis or perforation of the resection specimen during dissection. All APRs in the cTME group were planned preoperatively based on the height of the tumour from the anal verge. On the other hand, in cases where cTME surgery is warranted, we postulate that a certain percentage of the APRs are performed in order to ascertain complete and oncologically safe resection, since no surgeon is willing to run the risk of needing to perform a third operation. Moreover, it is possible that the decision to proceed with initial local excision by whatever means can be influenced by the eventual ablative procedure. In patients in whom APR would be necessary, both surgeon and patient will be more likely to choose primary local resection in order to prevent a definitive stoma.
The 1‐ and 5‐year disease‐free survival of 85% after completion surgery in this patient population is comparable to the disease‐free survival in patients after pTME surgery when compared with the literature 29. There were no statistically significant differences in the overall survival between both groups. The disease‐free survival in this study is determined solely by distant metastatic disease. The increased incidence of distant metastases in the cTME group is a matter for concern. This phenomenon was also encountered by Levic et al. 31 and Doornebosch et al. 34 in their cTME groups. They postulated that completion therapy should therefore be upgraded by adding neoadjuvant radiotherapy and/or adjuvant chemotherapy in an effort to improve oncological outcomes. Currently there are no data on neoadjuvant radiotherapy and/or adjuvant chemotherapy in relation to cTME, so the potential benefit on disease‐free survival remains hypothetical 28.
As mentioned before, pathological analysis in the present study revealed three cTME cases that showed violation of the mesorectal fascia versus no mesorectal fascia breaches in the pTME group. Similar observations were done made Hompes et al. 29. Whether an inferior resection specimen will result in increased metastatic disease remains unanswered based on our analysis, since we found no recurrent disease in those patients with an inferior resection specimen. Recently two studies have suggested using the transanal TME (TaTME) technique for cTME surgery after local excision 30, 35. Both studies show significantly better‐quality resection specimen compared with standard ‘top‐down’ cTME. Unfortunately, both studies lack long‐term follow‐up and oncological results. Long‐term follow‐up needs to be awaited in order to prove that mesorectal integrity plays a role in disease‐free survival after cTME.
To our knowledge, this is the only published study on patients undergoing cTME surgery after TAMIS. The foremost limitation of this study is the small study population. This could result in statistical bias of the surgical and oncological results. The limitation of the relatively small group is that statistics are relative; even though no statistically significant differences can be shown it is not fully clear whether there are no differences or whether the relatively small groups contribute to apparent equality. Due to the absence of local recurrences in this study, no multivariate analysis for prognostic factors could be performed. Furthermore, the shorter follow‐up time for the cTME group and the retrospective nature of the collection of the control group in this study are considered to be limitations.
Conclusion
Laparoscopic cTME surgery after TAMIS is feasible. In the present study, cTME surgery after TAMIS is not associated with increased morbidity and mortality when compared with pTME surgery, neither are local recurrence rates influenced. However, the suggestion that patients have an increased risk of developing distant metastasis necessitates vigilance and further investigation.
Funding
The research for this manuscript was not financially supported and none of the authors had any relevant financial relationships.
Conflicts of interest
The authors report no conflicts of interest.
References
- 1. MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet 1993; 341: 457–60. [DOI] [PubMed] [Google Scholar]
- 2. Atallah S, Albert M, Larach S. Transanal minimally invasive surgery: a giant leap forward. Surg Endosc 2010; 24: 2200–5. [DOI] [PubMed] [Google Scholar]
- 3. Clermonts S, van Loon YT, Schiphorst AHW, Wasowicz DK, Zimmerman DDE. Transanal minimally invasive surgery for rectal polyps and selected malignant tumors: caution concerning intermediate‐term functional results. Int J Colorectal Dis 2017; 32: 1677–85. [DOI] [PubMed] [Google Scholar]
- 4. Clermonts S, van Loon YT, Wasowicz DK, Langenhoff BS, Zimmerman DDE. Comparative quality of life in patients following transanal minimally invasive surgery and healthy control subjects. J Gastrointest Surg 2018; 22: 1089–97. [DOI] [PubMed] [Google Scholar]
- 5. De Graaf EJ, Doornebosch PG, Tollenaar RA et al Transanal endoscopic microsurgery versus total mesorectal excision of T1 rectal adenocarcinomas with curative intention. Eur J Surg Oncol 2009; 35: 1280–5. [DOI] [PubMed] [Google Scholar]
- 6. Lee W, Lee D, Choi S, Chun H. Transanal endoscopic microsurgery and radical surgery for T1 and T2 rectal cancer. Surg Endosc 2003; 17: 1283–7. [DOI] [PubMed] [Google Scholar]
- 7. Leijtens JWA, Koedam TWA, Borstlap WAA et al Transanal endoscopic microsurgery with or without completion total mesorectal excision for T2 and T3 rectal carcinoma. Dig Surg 2018; 36: 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morino M, Allaix ME, Caldart M, Scozzari G, Arezzo A. Risk factors for recurrence after transanal endoscopic microsurgery for rectal malignant neoplasm. Surg Endosc 2011; 25: 3683–90. [DOI] [PubMed] [Google Scholar]
- 9. Schiphorst AH, Langenhoff BS, Maring J, Pronk A, Zimmerman DD. Transanal minimally invasive surgery: initial experience and short‐term functional results. Dis Colon Rectum 2014; 57: 927–32. [DOI] [PubMed] [Google Scholar]
- 10. Borschitz T, Heintz A, Junginger T. The influence of histopathologic criteria on the long‐term prognosis of locally excised pT1 rectal carcinomas: results of local excision (transanal endoscopic microsurgery) and immediate reoperation. Dis Colon Rectum. 2006; 49: 1492–506. discussion 500–5. [DOI] [PubMed] [Google Scholar]
- 11. Baatrup G, Breum B, Qvist N et al Transanal endoscopic microsurgery in 143 consecutive patients with rectal adenocarcinoma: results from a Danish multicenter study. Colorectal Dis 2009; 11: 270–5. [DOI] [PubMed] [Google Scholar]
- 12. Baron PL, Enker WE, Zakowski MF, Urmacher C. Immediate vs. salvage resection after local treatment for early rectal cancer. Dis Colon Rectum 1995; 38: 177–81. [DOI] [PubMed] [Google Scholar]
- 13. Hahnloser D, Wolff BG, Larson DW, Ping J, Nivatvongs S. Immediate radical resection after local excision of rectal cancer: an oncologic compromise? Dis Colon Rectum 2005; 48: 429–37. [DOI] [PubMed] [Google Scholar]
- 14. Lee WY, Lee WS, Yun SH, Shin SH, Chun HK. Decision for salvage treatment after transanal endoscopic microsurgery. Surg Endosc 2007; 21: 975–9. [DOI] [PubMed] [Google Scholar]
- 15. Piessen G, Cabral C, Benoist S, Penna C, Nordlinger B. Previous transanal full‐thickness excision increases the morbidity of radical resection for rectal cancer. Colorectal Dis 2012; 14: 445–52. [DOI] [PubMed] [Google Scholar]
- 16. van Gijn W, Brehm V, de Graaf E et al Unexpected rectal cancer after TEM: outcome of completion surgery compared with primary TME. Eur J Surg Oncol 2013; 39: 1225–9. [DOI] [PubMed] [Google Scholar]
- 17. Morino M, Allaix ME, Arolfo S, Arezzo A. Previous transanal endoscopic microsurgery for rectal cancer represents a risk factor for an increased abdominoperineal resection rate. Surg Endosc 2013; 27: 3315–21. [DOI] [PubMed] [Google Scholar]
- 18. Clermonts S, van Loon YT, Schiphorst AHW, Wasowicz DK, Zimmerman DDE. Transanal minimally invasive surgery for rectal polyps and selected malignant tumors: caution concerning intermediate‐term functional results. Int J Colorectal Dis 2017. [DOI] [PubMed] [Google Scholar]
- 19. https://www.oncoline.nl/colorectaalcarcinoom
- 20. Campa‐Thompson M, Weir R, Calcetera N, Quirke P, Carmack S. Pathologic processing of the total mesorectal excision. Clin Colon Rectal Surg 2015; 28: 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marijnen CA. Organ preservation in rectal cancer: have all questions been answered? Lancet Oncol 2015; 16: e13–22. [DOI] [PubMed] [Google Scholar]
- 22. Ptok H, Marusch F, Meyer F et al Oncological outcome of local vs radical resection of low‐risk pT1 rectal cancer. Arch Surg 2007; 142: 649–55; discussion 56. [DOI] [PubMed] [Google Scholar]
- 23. Beets‐Tan RG, Beets GL. Rectal cancer: review with emphasis on MR imaging. Radiology 2004; 232: 335–46. [DOI] [PubMed] [Google Scholar]
- 24. Lahaye MJ, Engelen SM, Nelemans PJ et al Imaging for predicting the risk factors–the circumferential resection margin and nodal disease–of local recurrence in rectal cancer: a meta‐analysis. Semin Ultrasound CT MR 2005; 26: 259–68. [DOI] [PubMed] [Google Scholar]
- 25. Marusch F, Ptok H, Sahm M et al Endorectal ultrasound in rectal carcinoma–do the literature results really correspond to the realities of routine clinical care? Endoscopy 2011; 43: 425–31. [DOI] [PubMed] [Google Scholar]
- 26. Ashraf S, Hompes R, Slater A et al A critical appraisal of endorectal ultrasound and transanal endoscopic microsurgery and decision‐making in early rectal cancer. Colorectal Dis 2012; 14: 821–6. [DOI] [PubMed] [Google Scholar]
- 27. Bach SP, Hill J, Monson JR et al A predictive model for local recurrence after transanal endoscopic microsurgery for rectal cancer. Br J Surg 2009; 96: 280–90. [DOI] [PubMed] [Google Scholar]
- 28. Borstlap WA, Tanis PJ, Koedam TW et al A multi‐centred randomised trial of radical surgery versus adjuvant chemoradiotherapy after local excision for early rectal cancer. BMC Cancer 2016; 16: 513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hompes R, McDonald R, Buskens C et al Completion surgery following transanal endoscopic microsurgery: assessment of quality and short‐ and long‐term outcome. Colorectal Dis 2013; 15: e576–81. [DOI] [PubMed] [Google Scholar]
- 30. Letarte F, Raval M, Karimuddin A, Phang PT, Brown CJ. Salvage TME following TEM: a possible indication for TaTME. Tech Coloproctol 2018; 22: 355–61. [DOI] [PubMed] [Google Scholar]
- 31. Levic K, Bulut O, Hesselfeldt P, Bulow S. The outcome of rectal cancer after early salvage TME following TEM compared with primary TME: a case‐matched study. Tech Coloproctol 2013; 17: 397–403. [DOI] [PubMed] [Google Scholar]
- 32. Bulow S, Christensen IJ, Iversen LH, Harling H; Danish Colorectal Cancer Group . Intra‐operative perforation is an important predictor of local recurrence and impaired survival after abdominoperineal resection for rectal cancer. Colorectal Dis 2011; 13: 1256–64. [DOI] [PubMed] [Google Scholar]
- 33. Jorgren F, Johansson R, Damber L, Lindmark G. Oncological outcome after incidental perforation in radical rectal cancer surgery. Int J Colorectal Dis 2010; 25: 731–40. [DOI] [PubMed] [Google Scholar]
- 34. Doornebosch PG, Ferenschild FT, de Wilt JH, Dawson I, Tetteroo GW, de Graaf EJ. Treatment of recurrence after transanal endoscopic microsurgery (TEM) for T1 rectal cancer. Dis Colon Rectum 2010; 53: 1234–9. [DOI] [PubMed] [Google Scholar]
- 35. Koedam TWA, Veltcamp Helbach M, Penna M et al Short‐term outcomes of transanal completion total mesorectal excision (cTaTME) for rectal cancer: a case‐matched analysis. Surg Endosc 2019; 33: 103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


