Abstract
Background
Coronavirus disease 2019 (COVID‐19) can lead to systemic coagulation activation and thrombotic complications.
Objectives
To investigate the incidence of objectively confirmed venous thromboembolism (VTE) in hospitalized patients with COVID‐19.
Methods
Single‐center cohort study of 198 hospitalized patients with COVID‐19.
Results
Seventy‐five patients (38%) were admitted to the intensive care unit (ICU). At time of data collection, 16 (8%) were still hospitalized and 19% had died. During a median follow‐up of 7 days (IQR, 3‐13), 39 patients (20%) were diagnosed with VTE of whom 25 (13%) had symptomatic VTE, despite routine thrombosis prophylaxis. The cumulative incidences of VTE at 7, 14 and 21 days were 16% (95% CI, 10‐22), 33% (95% CI, 23‐43) and 42% (95% CI 30‐54) respectively. For symptomatic VTE, these were 10% (95% CI, 5.8‐16), 21% (95% CI, 14‐30) and 25% (95% CI 16‐36). VTE appeared to be associated with death (adjusted HR, 2.4; 95% CI, 1.02‐5.5). The cumulative incidence of VTE was higher in the ICU (26% (95% CI, 17‐37), 47% (95% CI, 34‐58), and 59% (95% CI, 42‐72) at 7, 14 and 21 days) than on the wards (any VTE and symptomatic VTE 5.8% (95% CI, 1.4‐15), 9.2% (95% CI, 2.6‐21), and 9.2% (2.6‐21) at 7, 14, and 21 days).
Conclusions
The observed risk for VTE in COVID‐19 is high, particularly in ICU patients, which should lead to a high level of clinical suspicion and low threshold for diagnostic imaging for DVT or PE. Future research should focus on optimal diagnostic and prophylactic strategies to prevent VTE and potentially improve survival.
Keywords: COVID‐19, critically ill, low‐molecular‐weight heparin, pulmonary embolism, venous thrombosis
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) is caused by the severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) and can lead to systemic coagulation activation. Initial studies from China report increased D‐dimers (0.5 mg/L or higher) in 46% to 63% of patients, as well as other signs of coagulation activation including mild thrombocytopenia and a moderately prolonged prothrombin time.1., 2. Additionally, more pronounced coagulation activation seems to be correlated with a severe disease course, including admission to the intensive care unit (ICU) and death. For example, patients who died of COVID‐19 had higher D‐dimers on admission compared with those who survived, whereas D‐dimer levels increased further during hospital stay in patients who died, but not in survivors.3 In another study, patients with D‐dimer levels of 1.0 µg/L or higher had an 18‐fold increased risk of death.2 One study used the International Society on Thrombosis and Haemostasis definition of disseminated intravascular coagulation and found that a score of ≥5 points was present in 71% of those who died compared with 0.6% in survivors.4 None of these studies reported on the number of patients with thrombotic complications.
Since the pandemic spread of SARS‐CoV‐2, there have been several anecdotal reports from colleagues on a high incidence of thrombotic complications, including thrombosis of extracorporeal circuits for continuous venovenous hemofiltration, central venous catheter‐associated thrombosis, and deep venous thrombosis (DVT) and pulmonary embolism (PE). Most but not all of these complications occurred in patients admitted to the ICU, with most patients receiving routine thrombosis prophylaxis.
Diagnosis of DVT and PE may be particularly challenging in patients with COVID‐19. Symptoms of PE overlap with symptoms of COVID‐19 and mild symptoms may be overlooked in a patient already suffering from shortness of breath. Similarly, clinical signs and symptoms of DVT may be harder to detect, especially in ICU patients, and when treating clinicians primarily focus on respiratory status and do not systematically assess lower extremities for signs of DVT.
In early April 2020, a large number of venous thromboembolic events were diagnosed in COVID‐19 patients admitted to our ICU, based on a clinical suspicion of DVT in the lower extremities. These observations have led us to intensify the dose of low‐molecular‐weight heparin to prevent VTE in COVID‐19 patients in the ICU. In the present study, we report on the incidence and risk factors of VTE in COVID‐19 patients admitted to the ICU or general ward.
2. METHODS
2.1. Patients
We identified consecutive patients admitted for COVID‐19 to the Amsterdam University Medical Centers, location Academic Medical Center, until April 12, 2020. COVID‐19 was confirmed by a reverse transcription polymerase chain reaction (RT‐PCR) test on a nose/throat swab or sputum sample positive for SARS‐CoV‐2. Given the sensitivity of RT‐PCR of only 50% to 80%,5 a daily multidisciplinary team also considered COVID‐19 confirmed in patients with a negative RT‐PCR but with symptoms and disease course consistent with COVID‐19, the absence of an alternative diagnosis, as well as a computed tomography (CT) scan of the chest showing abnormalities highly suspicious of typical pulmonary involvement of COVID 19 (COVID‐19 Reporting and Data System [CO‐RADS] 4 or 5 per the Dutch Radiology Society).6., 7. We did not include patients who were diagnosed with COVID‐19 during hospital stay for other medical conditions.
Hospitalized patients were categorized as ICU patients or as ward patients. Patients were categorized as ward patients if they had not been transferred to the ICU at any time during the course of their disease. All ICU patients were admitted on the ICU for mechanical ventilation.
Thrombosis prophylaxis was part of standard of care in all COVID‐19 patients. Ward patients received thrombosis prophylaxis with nadroparin 2850 IU once daily or 5700 IU for patients with a body weight of ≥100 kg. From April 3 onwards, patients in ICU received a double dose of nadroparin compared with patients on the wards, which was nadroparin 2850 IU twice daily for patients with a body weight <100 kg and 5700 IU twice daily for those ≥100 kg.
2.2. Outcomes
The primary outcome was an objectively confirmed diagnosis of distal or proximal DVT, PE, or venous thrombosis at other sites including catheter‐related thrombosis. The secondary outcome was symptomatic VTE, excluding events detected by bilateral leg ultrasound screening. All outcomes were adjudicated by two of the authors (M.C. and N.v.E.). We did not adjudicate deaths to identify fatal PE because almost all deaths were due to hypoxemic respiratory failure, which can be indistinguishable from fatal PE, whereas autopsies were rarely performed in COVID‐19 patients.
2.3. Data collection
Patient data were retrospectively reviewed from the day of admission to our hospital (also in case a patient was transferred from another hospital) until death, hospital discharge, transfer to another hospital, or end of data collection on April 30, 2020. We collected data on demographics and blood tests on admission. D‐dimer levels were included if measured on or within 72 hours of admission. Formal approval from the Medical Ethics Review Committee was not required as the Medical Research Involving Human Subjects Act does not apply for this observational study.
2.4. Statistical analysis
Patient characteristics were compared between ICU and ward patients using standard descriptive statistics. The proportion of ICU and ward patients with VTE was assessed, with 95% confidence intervals (CI) calculated using Wilson's score interval. In addition, the cumulative incidence, overall and for symptomatic VTE only, was calculated at 7, 14, and 21 days using a competing risk approach considering death as a competing risk. Risk factors for VTE were evaluated by calculating subdistribution hazard ratios (SHR) in Fine & Gray competing risk regression models. A sensitivity analysis was performed in which missing values were imputed 20 times using multiple imputation with chained equations, assuming a missing at random pattern. The multiple imputation model included all patient characteristics, laboratory values, radiology information, and outcome data. Estimates across the imputation datasets were combined using Rubin's rule. The association between VTE and mortality and between ICU stay and VTE were analyzed by calculating a time‐varying hazard ratio in Cox proportional hazards model. Analyses were performed in R, version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria; http://www.R‐project.org/).
3. RESULTS
Between March 2 and April 12, 2020, 199 patients who were hospitalized because of COVID‐19 were identified. One patient was excluded because he was immediately transferred to another hospital from the emergency department. Of the remaining 198 patients, 148 (75%) were hospitalized after an emergency department visit, whereas 50 (25%) were transferred from another hospital. Seventy‐five patients (38%) were admitted to the ICU after being transferred from the ICU of another hospital (n = 44), our general ward (n = 20), or directly from the emergency department (n = 11). COVID‐19 was confirmed by a positive RT‐PCR in 173 patients (87%) and considered confirmed by clinical features consistent with COVID‐19 in combination with a CT of the chest with highly suspicious or typical features (CO‐RADS 4 or 5) and no alternative diagnosis in 25 (13%).
3.1. Characteristics
Patient characteristics are shown in Table 1 . Mean age was 61 years (standard deviation, 14) and 130 (66%) were male. Median body mass index was 27 kg/m2 (interquartile range [IQR], 24, 31). Compared with ward patients, ICU patients were more often male (77% vs 59%; P = .011) and had higher D‐dimer levels on admission (median 2.0 vs 1.1 mg/L; P = .006). The median time between symptom onset and admission to our hospital was 7 days (IQR, 5, 10) for patients presenting at the emergency department and 11 days (IQR, 6, 14) for those transferred from another hospital. Thrombosis prophylaxis was initiated in 167 patients (84%), whereas 19 (9.6%) continued therapeutic anticoagulation for an indication that was present at the time of admission (eg, atrial fibrillation).
Table 1.
Baseline characteristics
| All Patients N = 198 | Patients Admitted to ICU N = 75 | Patients Admitted to Regular Ward N = 123 | P value | |
|---|---|---|---|---|
| Mean age, y (SD) | 61 (14) | 62 (10) | 60 (16) | .28 |
| Male sex, n (%) | 130 (66) | 58 (77) | 72 (59) | .011 |
| Body weight ≥ 100 kg, n (%) | 22/157 (14) | 12/73 (16) | 10/84 (12) | .56 |
| Median body mass index, kg/m2 (IQR) | 27 (24, 31) | 27 (24, 29) | 28 (25, 31) | .17 |
| History of venous thromboembolism, n (%) | 11 (5.6) | 2 (2.7) | 9 (7.3) | .27 |
| Active cancer, n (%) | 7 (3.5) | 3 (4.0) | 4 (3.3) | 1.0 |
| Anticoagulant therapy at admission | 19 (9.6) | 7 (9.3) | 12 (9.8) | 1.0 |
| Antiplatelet therapy at baseline | 29 (15) | 8 (11) | 21 (17) | .30 |
| Platelet count | ||||
| Mean, ×109/L (SD) | 239 (93) | 251 (89) | 231 (95) | .15 |
| <150 × 109/L, n (%) | 27/196 (14) | 7 (9.5) | 20/122 (16) | .23 |
| D‐dimer | ||||
| Median, mg/L (IQR) | 1.1 (0.7, 2.3) | 2.0 (0.8, 8.1) | 1.1 (0.7, 1.6) | .006 |
| >0.5 mg/L, n (%) | 110/131 (84) | 40/48 (83) | 70/83 (84) | 1.0 |
| >1.0 mg/L, n (%) | 75/131 (57) | 31/48 (65) | 44/83 (53) | .27 |
At the end of data collection (April 30, 2020), 136 patients (69%) had been discharged, 8 (4.0%) transferred to another hospital, 38 had died (19%), and 16 (8%) were still hospitalized. All patients still hospitalized were followed for at least 17 days. The median times from admission to discharge or death were 5 days (IQR, 3, 9) and 9 days (IQR, 5, 14), respectively. Thirteen patients (6.6%) were rehospitalized after a median of 4 days (IQR, 2, 16) after discharge.
3.2. Venous thromboembolism
During a median follow‐up of 7 days (IQR, 3, 13; range, 1‐43), 39 patients (20%; 95% CI, 15‐26) were diagnosed with VTE and 2 (1.0%; 95% CI, 0.28‐3.6) with extensive symptomatic thrombophlebitis for which therapeutic anticoagulation was initiated. Type of VTE was PE with or without DVT in 13 patients (6.6%), proximal DVT in 14 (7.1%), distal DVT in 11 (5.6%), and upper extremity DVT in 1 (0.5%) (Table 2 ). VTE was symptomatic in 25 patients (13%) and detected incidentally or by screening in 14 (7.1%). Of note, screening for lower extremity DVT was performed in 55 patients (28%) during hospital stay (ICU, n = 38; ward, n = 17), whereas CT pulmonary angiography for PE was only performed on indication (eg, sudden worsening hypoxemia). VTE was diagnosed after a median of 7 days after admission (IQR, 4, 10) and symptomatic VTE also after a median of 7 days (IQR, 5, 9).
Table 2.
Clinical outcomes
| All Patients (N = 198) n (%) | ICU Patients (N = 75) n (%) | Patients in Wards (N = 123) n (%) | |
|---|---|---|---|
| Venous thromboembolism | 39 (20) | 35 (47) | 4 (3.3) |
| Pulmonary embolism | 13 (6.6) | 11 (15) | 2 (1.6) |
| Central or lobar | 1 (0.5) | 1 (1.3) | 0 |
| Segmental | 10 (5.1) | 9 (12) | 1 (0.8) |
| Subsegmental | 2 (1.0) | 1 (1.3) | 1 (0.8) |
| DVT | 26 (13) | 24 (32) | 2 (1.6) |
| Proximal leg DVT | 14 (7.1) | 14 (19) | 0 |
| Distal leg DVT | 11 (5.6) | 9 (12) | 2 (1.6) |
| Upper extremity DVT | 1 (0.5) | 1 (1.3) | 0 |
| Symptomatic VTE | 25 (13) | 21 (28) | 4 (3.3) |
| Pulmonary embolism | 13 (6.6) | 11 (15) | 2 (1.6) |
| Proximal DVT | 8 (4.0) | 8 (11) | 0 |
| Distal DVT | 4 (2.0) | 2 (2.7) | 2 (1.6) |
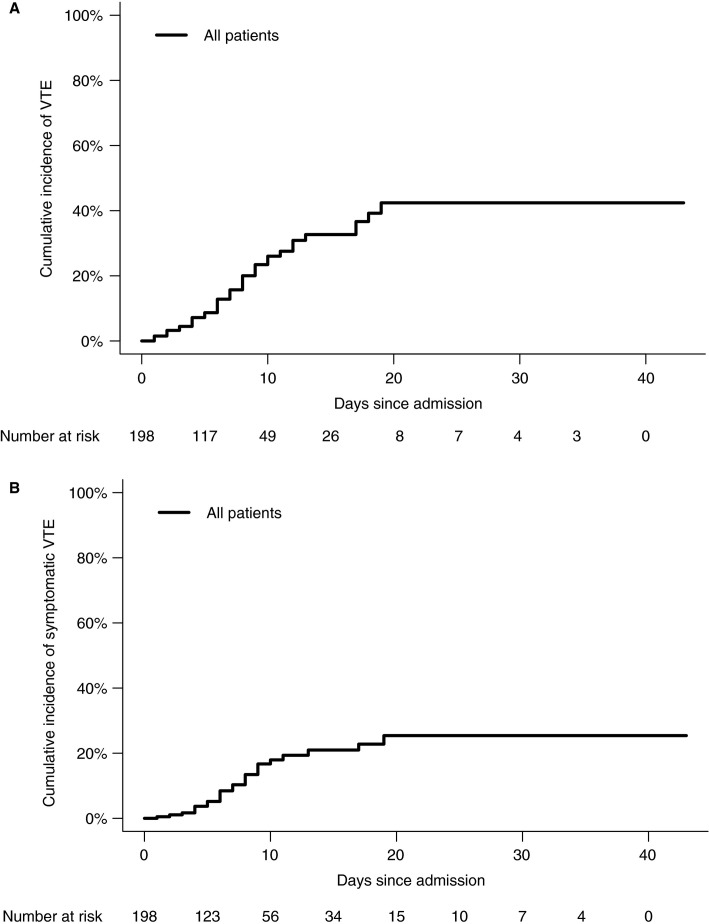
In the competing risk model, the cumulative incidences of VTE at 7, 14, and 21 days were 16% (95% CI, 10‐22), 33% (95% CI, 23‐43), and 42% (95% CI, 30‐54), respectively (Figure 1A ). When only considering symptomatic VTE, the cumulative incidences were 10% (95% CI, 5.8‐16), 21% (95% CI, 14‐30), and 25% (95% CI, 16‐36) at 7, 14, and 21 days, respectively (Figure 1B). When analyzed as a time‐varying variable, VTE was significantly associated with death (hazard ratio [HR], 2.7; 95% CI, 1.3‐5.8), also when adjusted for age, sex, and ICU stay as time‐varying variable (adjusted HR, 2.4; 95% CI, 1.02‐5.5).
Figure 1.
A, Venous thromboembolism. B, Symptomatic venous thromboembolism. ICU, intensive care unit; VTE, venous thromboembolism
All VTE were diagnosed in patients receiving thrombosis prophylaxis. The risk of VTE in ICU patients was not lower during the period when the standard dose of nadroparin prophylaxis was doubled (58%) vs in the first follow‐up period (41%).
3.3. ICU vs ward patients
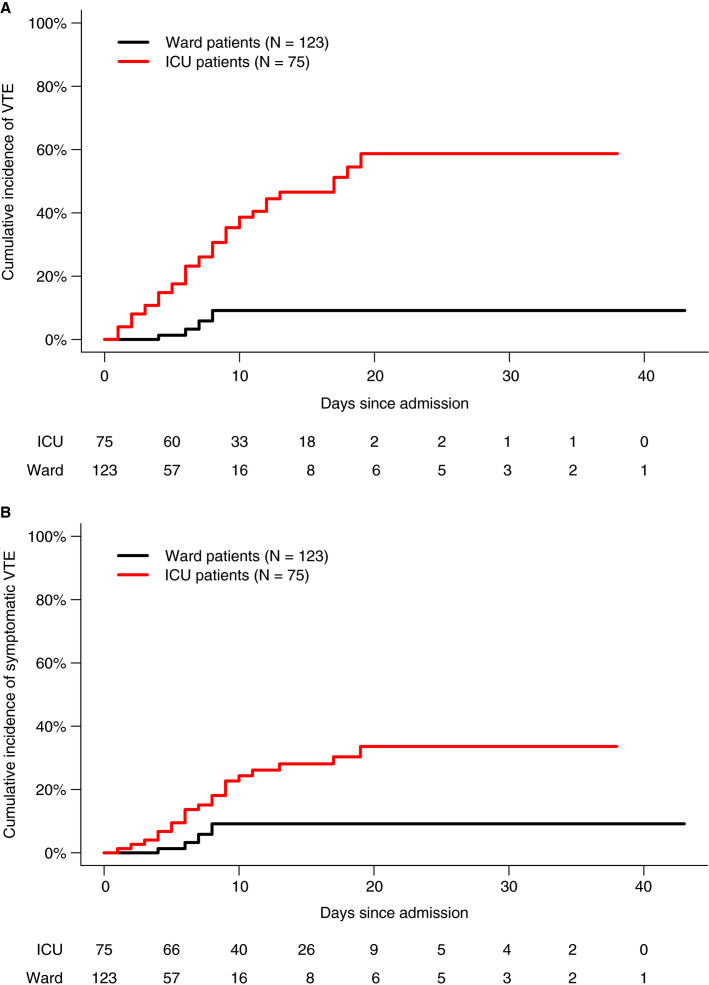
Median follow‐up duration was 15 days in ICU patients (IQR, 9, 20) and 4 days in ward patients (IQR, 2, 7). The proportion of patients with VTE was significantly higher in ICU patients (47%; 95% CI, 36‐58) than in ward patients (3.3%; 95% CI, 1.3‐8.1), corresponding to an SHR of 7.9 (95% CI, 2.8‐23). The cumulative incidences of any VTE in ICU patients at 7, 14, and 21 days were 26% (95% CI, 17‐37), 47% (95% CI, 34‐58), and 59% (95% CI, 42‐72), respectively (Figure 2A ).
Figure 2.
A, Venous thromboembolism in ICU and ward patients. B, Symptomatic venous thromboembolism in ICU and ward patients. ICU, intensive care unit; VTE, venous thromboembolism
Symptomatic VTE was detected in 21 (28%) ICU patients and 4 (3.3%) ward patients (SHR, 3.9; 95 CI, 1.3‐12). The cumulative incidences of symptomatic VTE in ICU patients at 7, 14, and 21 days were 15% (95% CI, 8.0‐24), 28% (95% CI, 18‐39), and 34% (95% CI, 21‐46) (Figure 2B). The cumulative incidences of both any VTE and symptomatic VTE in ward patients at 7, 14, and 21 days were 5.8% (95% CI, 1.4‐15), 9.2% (95% CI, 2.6‐21), and 9.2% (2.6‐21) (Figure 2).
The difference between ICU and ward patients was comparable when ICU stay was modelled as a time‐varying variable (HR for any VTE, 7.1; 95% CI, 3.1‐16). The higher risk in ICU patients was consistent in the sensitivity analysis excluding patients transferred from another hospital (50% vs 3.4%; SHR, 7.2; 95% CI, 2.3‐23).
3.4. Risk factors for venous thromboembolism
Besides ICU stay, other risk factors associated with VTE in univariable regression analyses were a higher white blood cell count (SHR, 1.9 for every log‐transformed unit increase; 95% CI, 1.1‐3.2), higher neutrophil‐to‐lymphocyte ratio (SHR, 2.0 for every log‐transformed unit increase; 95% CI, 1.3‐3.1), and a higher D‐dimer level (SHR, 1.6 for every log‐transformed unit increase; 95% CI, 1.2‐2.1) (Table 3 ). These associations remained materially unchanged when adjusted for age, sex, and ICU stay (Table 3), when excluding patients transferred from another hospital (data not shown), and when missing values were imputed (data not shown). Notably, none of the 19 patients (0%) who continued therapeutic anticoagulation that they used for other indications developed VTE compared with 39 of 179 of the remaining patients (22%; SHR, not estimable; Fisher exact test P = .03).
Table 3.
Risk factors for venous thromboembolism
| VTE (N = 39) | No VTE (N = 159) | Univariable SHR (95% CI)c | Multivariable SHR (95% CI)d | |
|---|---|---|---|---|
| Mean age, years (SD) | 62 (10) | 60 (15) | 0.98 (0.8‐1.2)a | 1.05 (0.82‐1.4)a |
| Male sex | 27 (69) | 103 (65) | 0.7 (0.4‐1.5) | 0.53 (0.27‐1.0) |
| Intensive care unit | 35 (89) | 40 (25) | 7.9 (2.8‐23) | 8.9 (3.2‐25) |
| Median body weight, kg/m2 (IQR) | 82 (74, 93) | 84 (75, 95) | 0.6 (0.2, 2.3)b | 0.9 (0.2, 3.9)b |
| History of venous thromboembolism | 3 (7.9) | 8 (5.2) | 1.1 (0.3‐3.0) | 1.6 (0.4‐7.2) |
| Anticoagulant use at admission | 0 (0) | 19 (12) | ||
| Mean hemoglobin, mmol/L (SD) | 8.0 (1.4) | 7.9 (1.2) | 1.04 (0.8‐1.4)b | 1.1 (0.8‐1.5)b |
| Median white blood cell count, ×109/L (IQR) | 7.6 (5.9, 11) | 6.9 (5.4, 9.3) | 1.9 (1.1, 3.2) | 1.9 (0.9, 4.1)b |
| Median neutrophil count, ×109/L | 6.0 (4.4‐8.1) | 5.2 (3.8‐7.1) | 2.0 (0.99‐4.0) | 1.7 (0.8‐3.7)b |
| Median lymphocyte count, ×109/L | 0.59 (0.47‐0.83) | 1.0 (0.8‐1.3) | 0.66 (0.43‐1.02) | 0.7 (0.4‐0.95)b |
| Median neutrophil‐to‐lymphocyte ratio | 11 (7.0‐15) | 5.4(3.5‐8.1) | 2.0 (1.3‐3.1)b | 1.7 (1.2‐2.5)b |
| Mean platelet count, ×109/L | 246 (87) | 237 (95) | 1.02 (0.99‐1.1)a | 1.002 (0.97‐1.04)a |
| Median D‐dimer, mg/L (IQR) | 2.6 (1.1, 18) | 1.0 (0.7, 1.7) | 1.6 (1.2, 2.1)b | 1.4 (1.1, 1.9) |
Abbreviations: IQR, interquartile range; SD, standard deviation; SHR, subdistribution hazard ratio; VTE, venous thromboembolism.
Per 10‐unit increase.
Per 1‐unit increase.
Variables with a non‐normal distribution (ie, body weight, white blood cell count, neutrophil count, lymphocyte count, neutrophil‐to‐lymphocyte ratio, and D‐dimer) were analyzed log‐transformed.
Multivariable analysis were adjusted for age, sex, and intensive care unit admission.
4. DISCUSSION
We observed a very high risk of VTE in patients with COVID‐19. Although the profound coagulopathy associated with COVID‐19 has been described soon after start of the pandemic, few data on clinical VTE have been reported. In a cohort of 81 ICU patients in China, in which routine thromboprophylaxis was not the standard of care, the proportion of patients who were diagnosed with DVT was 25%; a follow‐up duration or cumulative incidence was not reported.8 In a study of 184 ICU patients in 3 Dutch hospitals, where routine low‐molecular‐weight heparin prophylaxis was applied, 68 (37%) patients had VTE, with a reported cumulative incidence of 49%.9 Similar observations have now been reported in ICU patients in France and Italy.10., 11. In our hospital, where thrombosis prophylaxis in patients admitted with COVID‐19 is standard of care, VTE was observed in 35 of 75 (47%) ICU patients, with a cumulative incidence of 59% at 21 days. The very high incidence in ICU patients in the present study may partially be explained by the initiation of a screening approach, although the risk remained high if only symptomatic VTE was considered (28% of patients; cumulative incidence 34% at 21 days). In non‐ICU COVID‐19 patients admitted to the regular ward, 4 of 123 patients (3%) were diagnosed with symptomatic VTE despite thrombosis prophylaxis.
Our study has some limitations and strengths. First, this was a single‐center cohort study with a relatively small sample size, and 8% of patients were still hospitalized at the time of data collection. Second, including patients transferred from other hospitals may lead to immortal time bias because they need to survive until transfer, thereby potentially biasing the VTE cumulative incidence. However, restricting the analysis to patients admitted directly from our own emergency department did not substantially affect the results. Although immortal time bias could also have been introduced by placing patients who were transferred from the ward to the ICU in the ICU group, results were consistent when analyzing ICU stay in a time‐varying model. There appeared to be a considerable difference between the crude proportion of patients with VTE and the cumulative incidence estimate from the survival model, despite the use of a competing risk model to mitigate the influence of death. Likely explanations include the relatively short median follow‐up duration, the number of patients still hospitalized, and the (informative) censoring of patients when discharged from the hospital; the risk of VTE in the latter group is likely to be lower than that of patients remaining in the cohort. Finally, based on concerns of a high risk of (fatal) VTE following early observations, we changed our practice during the follow‐up period by performing screening compression ultrasound in the ICU every 5 days, while also performing a single cross‐sectional round of compression ultrasounds at the ward in the 10 days before data collection. This screening led to diagnosis of asymptomatic DVT, all in the ICU group, which may be clinically less relevant than symptomatic DVT. Strengths include the inclusion of consecutive patients, the follow‐up duration of at least 17 daWANys, no loss to follow‐up, and the objectively confirmed and adjudicated diagnosis of VTE.
Whether the high incidence of VTE observed in the ICU justifies higher or therapeutic doses of pharmacological prophylaxis at an acceptable bleeding risk and whether this would improve the outcome of severe COVID‐19 pneumonia is unknown. One observational study from China that included 449 hospitalized COVID‐19 patients suggested that thrombosis prophylaxis was associated with a 56% to 63% reduction in mortality in patients with sepsis‐induced coagulopathy, but not in other patients.12 Only 22% of COVID‐19 patients received thrombosis prophylaxis, which is much less than expected according to guidelines on thrombosis prophylaxis in medical patients.13 Currently, several randomized controlled trials are being planned or have started in which the optimal dose of thrombosis prophylaxis will be investigated. Some of these trials use an elevated D‐dimer level as an entry criterion (eg, Coagulopathy of COVID‐19: A Pragmatic Randomized Controlled Trial of Therapeutic Anticoagulation Versus Standard Care [RAPID COVID COAG]; clinicalTrials.gov identifier NCT04362085). This approach is supported by the present observation that higher D‐dimer levels at baseline are associated with VTE during follow‐up. It is not known whether VTE contributes to respiratory deterioration or death in COVID‐19 pneumonia, although VTE during the course of disease appeared to be associated with mortality in an exploratory analysis in our cohort. Interestingly, none of the patients who were receiving therapeutic anticoagulation at admission (for other indications) developed VTE.
The 3% risk of VTE among patients who were not admitted to ICU is considerable, despite the standard use of thrombosis prophylaxis. In an Italian single‐center retrospective cohort study, the proportion of COVID‐19 patients with VTE was 6% in ward patients, corresponding to a cumulative incidence of 7%.11 These reported risks appear to be higher than expected in medical hospitalized patients who are not critically ill.13
Based on the present findings, we believe the threshold of suspicion of VTE in COVID‐19 patients should be low and elicit appropriate diagnostic testing and treatment if VTE is diagnosed. The clinical value of ultrasound screening of the lower extremities in ICU patients with COVID‐19 is a matter of debate. However, given the high risk of symptomatic VTE in ICU patients, screening followed by initiating therapeutic anticoagulation may be justified in patients diagnosed with asymptomatic (proximal) DVT to prevent extension and embolization. It is possible that a higher intensity of thrombosis prophylaxis, both in ICU and ward patients, not only decreases VTE but also decreases mortality. Future research should therefore focus on optimal diagnostic and prophylactic strategies for VTE in hospitalized patients with COVID‐19.
CONFLICT OF INTEREST
Dr. Middeldorp reports grants and fees paid to her institution, outside the present work, from Abbvie, BMS/Pfizer, Aspen, Daiichi Sankyo, Bayer, Boehringer Ingelheim, Sanofi, and Portola. Dr. Coppens reports research support and lecturing or consultancy fees, outside the present work, from Bayer, CSL Behring, Daiichi Sankyo, Novo Nordisk, Sanquin Blood Supply, Sobi, and Portola. Dr. van Es reports fees paid to his institution, outside the present work, from Bayer, LEO Pharma, and Daiichi Sankyo. The other authors have no disclosures.
AUTHOR CONTRIBUTIONS
All authors contributed substantially to the study design, acquisition, analysis, and interpretation of the data. Saskia Middeldorp, Michiel Coppens, and Nick van Es drafted the first version of the manuscript. All authors revised the manuscript critically and approved the final version.
ACKNOWLEDGMENTS
We thank Maeke J. Scheerder, Aart Terpstra, and Lisette Koehorst for organizing and performing screening compression ultrasounds. The authors thank all colleagues involved in the care of the COVID‐19 patients in Amsterdam UMC.
Footnotes
Manuscript handled by: David Lillicrap
Final decision: David Lillicrap, 1 May 2020
REFERENCES
- 1.Guan W., Ni Z., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2019;2020:1–13. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang W., Xu Y., Gao R., et al. Detection of SARS‐CoV‐2 in different types of clinical specimens. JAMA. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ai T., Yang Z., Xia L. Correlation of chest CT and RT‐PCR testing in coronavirus disease. Radiology. 2020;2019:1–8. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prokop M., van Everdingen W., van Rees V.T., et al. CO‐RADS – a categorical CT assessment scheme for patients with suspected COVID‐19: definition and evaluation. Radiology. 2020;1 doi: 10.1148/radiol.2020201473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1–4. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID‐19: an updated analysis. Thromb Res. 2020:113391. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helms J. High risk of thrombosis in patients in severe SARS‐CoV‐2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020 doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lodigiani C., Iapichino G., Carenzo L., et al. Venous and arterial thromboembolic complications in COVID‐19 patients admitted to an academic hospital in Milan. Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schünemann H.J., Cushman M., Burnett A.E., et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2(22):3198–3225. doi: 10.1182/bloodadvances.2018022954. [DOI] [PMC free article] [PubMed] [Google Scholar]