Summary
As COVID–19 disease escalates globally, optimising patient outcome during this catastrophic healthcare crisis is the number one priority. The principles of patient blood management are fundamental strategies to improve patient outcomes and should be given high priority in this crisis situation. The aim of this expert review is to provide clinicians and healthcare authorities with information regarding how to apply established principles of patient blood management during the COVID–19 pandemic. In particular, this review considers the impact of the COVID–19 pandemic on blood supply and specifies important aspects of donor management. We discuss how preventative and control measures implemented during the COVID–19 crisis could affect the prevalence of anaemia, and highlight issues regarding the diagnosis and treatment of anaemia in patients requiring elective or emergency surgery. In addition, we review aspects related to patient blood management of critically ill patients with known or suspected COVID–19, and discuss important alterations of the coagulation system in patients hospitalised due to COVID–19. Finally, we address special considerations pertaining to supply‐demand and cost‐benefit issues of patient blood management during the COVID–19 pandemic.
Keywords: anaemia, coagulation, coronavirus, COVID‐19, patient blood management
Introduction
In December 2019, the first reports of patients with coronavirus disease 2019 (COVID‐19) emerged, a disease caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) 1. Over the course of a few months, COVID‐19 has spread globally, with more than 2.8 million confirmed cases at the time of writing 2. This prompted the World Health Organization (WHO) to declare the COVID‐19 outbreak a pandemic 3. During times of crisis, clinicians are constantly being pushed to their limits. As numbers of patients with COVID‐19 are continuing to increase, new scientific findings need to be integrated into routine clinical practice. However, established evidence‐based medical concepts should still be followed when treating patients.
Patient blood management is a multimodal, multidisciplinary approach based on timely application of a bundle of evidence‐based medical and surgical concepts aimed at improving the outcome of patients at risk 4. The three pillars of patient blood management encompass measures to optimise the patient's red cell mass, reduce peri‐operative blood loss and enhance anaemia tolerance 5. In addition, patient blood management implementation results in reduced transfusion rates and lower healthcare costs 6, 7. These aspects are crucial for national medical systems during times when resources and funding are limited. The aim of this expert review is to provide clinicians and healthcare authorities with information on how to apply the principles of patient blood management during the COVID‐19 pandemic.
Methods
This article is a focused expert review. The authors used PubMed, Scopus and Web of Science for their literature search. No standardised protocol for the literature search was defined.
Impact on blood supply
In most countries around the world, COVID‐19 has generated an unprecedented healthcare and socio‐economic emergency. As a consequence of social restrictions made by governments to fight the spread of COVID–19, the number of blood donations has significantly decreased during the last few weeks. In addition, although to our knowledge there has been no scientifically documented evidence yet of the transmission of COVID–19 infection through transfusion of blood components, the current outbreak has produced a heated debate regarding the safety of blood donations in endemic countries 8.
Following the indications from the European Centre for Disease Prevention and Control regarding the supply of substances of human origin 9, most European National Health Competent Authorities have issued some recommendations on the prevention of transmission of COVID–19 infection through transfusion of labile blood components. Beside the recommendation of employing the hospitals’ patient blood management‐based programmes of blood saving, several actions have been implemented in the setting of blood donor selection.
Specific donor management
The Italian National Blood Centre (Centro Nazionale Sangue, CNS), the Health Ministry's technical and scientific advisory body on matters related to blood and blood products, has issued a number of measures aimed at maintaining high standards of blood donation and blood safety in Italy, one of the countries with a higher incidence of COVID‐19‐related casualties worldwide 10:
Strengthen surveillance measures of individuals in contact with subjects with documented COVID–19;
Defer blood donations from donors returning from any national or international territory with a travel health notice for 14 days, donors who have possibly been exposed to the risk of infection by contact with subjects with COVID–19 documented infection, and donors with a history of COVID–19 infection (documented infection or onset of symptoms compatible with COVID–19 infection) who are convalescent;
Require donors to inform their blood collection centres in case of symptoms compatible with COVID–19 infection or in the case of diagnosis of COVID–19 infection within 14 days after donation (post‐donation information);
Promote the implementation of simple triage processes during the reception of donors at blood collection units, aiming to avoid the possible spread of the virus in waiting rooms. This includes the measurement of body temperature outside the collection area. A body temperature of 37.5°C or higher is defined as a criterion for the temporary deferral of the donor;
Request all personnel working at blood collection units to comply scrupulously with behavioural protocols designed to prevent the spread of respiratory infections, including COVID–19 infection.
Additional actions directed towards the management of voluntary blood donors to ensure continuous and safe donation of blood components include a timely appeal to donate blood when shortage is foreseeable; careful planning of the donor schedule to avoid an excessive number of donors at blood collection centres as well as compliance with social distancing measures; use of adequate personal protective equipment; and meticulous adherence to hygiene regulations. In addition, travel to donate blood should be considered as essential by governments or – if deemed non‐essential – provision of a certificate allowing travel to the collection centre should be provided. The application of these recommendations has facilitated the safer management of blood donations and, following an initial 10% decrease in whole blood collection, has maintained a stable volume of total blood donations (approximately 50,000 units per week), thus guaranteeing blood component self‐sufficiency and safety in Italy.
Anaemia
In major elective surgery, patients can be exposed to the effects of pre‐operative anaemia, blood loss and red cell transfusion, all of which adversely influence postoperative outcome 11, 12. In patients undergoing major surgical procedures, it is recommended that pre‐operative anaemia be defined by haemoglobin < 130 g.l−1, irrespective of sex 13, 14. Using this definition, in a large cohort of major elective procedures, the overall prevalence of pre‐operative anaemia was 36%. Over 70% of anaemic patients presented with absolute or functional iron deficiency, resulting in iron‐restricted erythropoiesis 15. In addition, pre‐operative iron deficiency in patients undergoing cardiac surgery is associated with a three‐fold increased 90‐day mortality 16. Non‐anaemic haematinic deficiencies are also prevalent and may hamper pre‐operative haemoglobin optimisation and/or recovery from postoperative anaemia 5, 15. As for non‐elective procedures, up to 75% of patients undergoing hip fracture repair surgery presented with haemoglobin < 130 g.l−1 on admission 17.
These figures could be expected to increase during the COVID‐19 pandemic due to changes in diet and lifestyle, aggravated by a reduced purchasing power and decreased incomes. Possible consequences include: a reduced consumption of fresh food, including fruit and vegetables (vitamin C, folic acid), dairy products (vitamin D), fish and meat (iron, vitamin B6, vitamin B12); and a lack of sunlight exposure and muscle atrophy resulting from curfew restrictions and social distancing. Consequently, proliferation of red cell progenitors, iron homeostasis and haemoglobin synthesis, as well as overall physical and mental performance, could be affected 18, 19. Depending on their general health condition and the duration of the COVID‐19 pandemic, the elderly are likely to be the most affected 20. Most importantly, this population has the greatest comorbidity load and represents the largest proportion of hospitalised medical and surgical patients.
Diagnosis and treatment of anaemia in patients awaiting elective surgery
During the COVID‐19 pandemic, elective medical activity is markedly reduced and elective surgery is frequently postponed, with priority given to urgent and emergency surgery. Pre‐operative anaemia clinics, which function to screen, diagnose, and treat iron deficiency and other causes of anaemia are mostly closed and appointments cancelled. As a result, blood testing and diagnostic tools are less readily available, hampering the diagnosis and treatment of anaemia of various causes. Remote (telephone or video) consultations are possible and have become much more widespread. It is certainly possible to elicit the most common symptoms of severe iron deficiency: fatigue/exhaustion; brain fog; palpitations, shortness of breath; anxiety; low mood/depression; aching and restless legs; alopecia, brittle/ridged fingernails; and pica (appetite for non‐nutritive substances). Patients with these symptoms should be expedited to iron therapy. Iron deficiency anaemia may worsen over time, so early treatment is favoured. Intravenous (i.v.) iron administration is preferred, either via anaemia clinics or through general practitioners as it yields rapid results. However, as administering i.v. iron might not be feasible during the pandemic, oral iron may be the treatment of choice to correct iron deficiency and treat anaemia when non‐urgent surgery is delayed and waiting periods are prolonged. Alternate‐day treatment with oral iron is recommended, rather than daily doses, to improve uptake and compliance 21. Newer oral iron formulations with enhanced absorption and gastro‐intestinal tolerability, such as sucrosomial iron, should be considered 22. This line of action is also practicable in individuals who cannot leave their home due to quarantine measures. Once surgery is rescheduled, diagnostic testing for haemoglobin, ferritin, C‐reactive protein (CRP), and transferrin saturation should be undertaken during pre‐operative assessment.
Diagnosis and treatment of anaemia in patients admitted for emergency surgery
As stated above, the incidence and severity of anaemia is likely to increase during the pandemic due to changes in diet and lifestyle. Thus, we may experience a greater proportion of patients admitted for urgent or emergency surgery being anaemic. Low ferritin (< 30 ng.ml−1) is often used as an indicator of very low iron stores and iron deficiency. However, ferritin is increased during acute phase inflammation as may be seen with viral infections such as COVID–19. Consequently, the use of ferritin to diagnose iron deficiency may be problematic in patients with COVID‐19 disease, who may have normal or high ferritin levels despite very low iron stores 23. Hence, we recommend concurrent measurement of CRP and transferrin saturations. Transferrin saturation < 20% strongly suggests iron deficiency, especially when ferritin is < 100 ng.l−1 13. Increased CRP (> 4 mg.l−1) indicates that ferritin measurement alone is unreliable due to acute inflammation or viraemia. The bone marrow response to i.v. iron starts early after infusion, with a peak haemoglobin concentration within the following 4–6 weeks 24. If urgent surgery is necessary in patients with iron deficiency anaemia, i.v. iron should be favoured over oral iron 13. The benefit of short‐term treatment with i.v. iron has been demonstrated in patients undergoing orthopaedic 25 and cancer surgery 26, 27, 28. In addition, administering high‐dose i.v. iron pre‐operatively, even on the day of surgery, could help prevent postoperative anaemia 29. This approach is also feasible in iron‐deficient COVID‐19 patients admitted for urgent procedures. In patients with more profound anaemia, an ultra‐short‐term treatment course (1–2 days before surgery) of erythropoietin and i.v. iron may be more effective. A regimen of 40,000 IU epoetin alpha and 1000 mg i.v. iron, together with 1 mg subcutaneous vitamin B12 and 5 mg oral folic acid, has been shown to reduce blood transfusion in cardiac surgery patients 30. The beneficial effects of very short‐term treatment with i.v. iron and epoetin alpha have also been observed in elective and non‐elective orthopaedic surgery 25. Intravenous iron is also effective for treating postoperative anaemia, and should be considered, even in cancer patients who are undergoing surgery during the COVID‐19 crisis 26, 31, 32.
Patient blood management in critically ill patients with known or suspected COVID‐19
Early reports have described mild anaemia in COVID‐19 patients admitted to the ICU 33, 34. In general, the most common aetiologies for anaemia in the ICU are inflammation and iron deficiency. Just as in surgical patients, iron, vitamin B12, folic acid and erythropoietin can be administered in critically ill patients 35. Intravenous iron formulations are superior to oral formulations in such patients, as enteral iron uptake is reduced in inflammatory states due to increased expression of hepcidin. Moreover, a recent meta‐analysis suggested that therapy with erythropoietin may decrease mortality in critically ill adults, even though results were described as hypothesis‐generating by the authors 36. In COVID‐19 positive critically ill patients, the risk of thrombosis associated with erythropoiesis‐stimulating agents has to be individually considered before each administration, and mitigated with anticoagulation. When a shortage of blood supply is anticipated, treatment should be initiated early, before anaemia reaches critical levels. To balance the risks, haemoglobin should be maintained at levels sufficient to enable adequate oxygenation. This process can be achieved with below‐normal haemoglobin values in most patients. In addition, patients should be anticoagulated adequately whenever possible, and special consideration given to the possible prothrombotic nature of COVID‐19 in critically ill patients 37. Prevention of anaemia is just as important as treatment of anaemia in critically ill patients. Anaemia is aggravated by repeated blood tests in non‐bleeding critically ill patients, especially those with acute respiratory distress syndrome (ARDS) or sepsis 38. Blood sampling is increased in severely affected patients due to frequent blood gas analyses, laboratory testing, and blood culture testing. Two simple ways to prevent iatrogenic blood loss are micro‐sampling and the use of blood conservation devices to reduce the amount of discarded blood 39. For each blood test, the lowest possible amount of blood necessary to perform testing should be drawn, and only tests that are essential for clinical decisions should be ordered.
Acute respiratory distress syndrome is common in COVID‐19 patients who develop pneumonia 34, 40. Transfusion of red cells has been associated with negative clinical effects on the lung, such as transfusion‐related acute lung injury or pulmonary hypertension 41. These side‐effects might enhance the severity of ARDS in affected patients 42. Thus, it is important to carefully consider each indication for transfusion, taking into account individual factors such as age, intravascular volume status of the patient and concomitant diseases. Before transfusing allogeneic red cells, measures that increase oxygen delivery should be utilised, including improvement of oxygen saturation and cardiac output. Total erythrocyte mass is not always reflected by haemoglobin values and it is not advisable to merely focus on transfusion thresholds. Whenever possible, a single‐unit policy should be followed in order to limit volume transfused and multiple donor exposure, except in patients with active massive bleeding.
Alterations of coagulation in COVID‐19 patients
Several reports describe coagulation alterations in patients hospitalised with COVID‐19 infection 40, 43, 44, 45. In these reports, prothrombin time and activated partial thromboplastin time were longer in COVID‐19‐infected vs. non‐infected individuals. In addition, more abnormal coagulation parameters were found in advanced disease states and in non‐survivors compared with survivors 40, 44, 45, together with elevated fibrinogen concentrations 43, 44. Markedly elevated D‐dimers were described as the most prominent characteristic, a finding interpreted as overt disseminated intravascular coagulation (DIC) 44. A sub‐set of patients with the most severe COVID‐19 are prone to develop consumptive coagulopathy and a hypercoagulable state. Furthermore, the most severely affected COVID‐19 patients may require extracorporeal membrane oxygenation (ECMO) for respiratory (or cardiac) failure. This treatment can be life‐saving, but also transfusion‐intensive with anecdotal reports of frequent clotting within the ECMO circuits. As new insights are gained about the pathophysiology of these prothrombotic alterations in COVID‐19 patients, it will be important to adapt therapy accordingly 46. Early prehospital low‐dose aspirin or early in‐hospital low molecular weight heparin have been proposed as pre‐emptive treatment strategies. However, premature and unsubstantiated changes in therapeutic concepts should be avoided when based merely on anecdotal reports, as the consequences may be deleterious. Patients should be enrolled in ethically approved trials, and the results of these ongoing randomised trials should guide future therapeutic advances in treatment.
To our knowledge, there is no study yet published in which thrombo‐elastographic or thrombo‐elastometric techniques are used to assess coagulation in COVID‐19 infected patients. Monitoring coagulation with viscoelastic point‐of‐care devices and quick laboratory tests is crucial in all bleeding patients and is recommended by the European Society of Anaesthesiology 47, the European Association for Cardiothoracic Surgery, the European Association of Cardiothoracic Anesthesiology 48, and by the European Guidelines on management of major bleeding and coagulopathy following trauma 49. Such timely coagulation monitoring allows rapid detection of disturbances in the coagulation system, and to accurately diagnose the abnormality. On this basis, an individualised goal‐directed coagulation treatment using coagulation factors according to an algorithm is possible. The success of such treatment algorithms has been shown in cardiac surgery 50, trauma 51, and major postpartum haemorrhage 52. Of particular relevance during the current COVID‐19 pandemic is that the use of treatment algorithms can reduce transfusions of red cells, plasma, and platelets as well as admission to ICU, and shorten ventilation time and ICU length of stay 50, 51, 52. A simple and pragmatic coagulation algorithm is depicted in Fig. 1. The success of viscoelastic point‐of‐care based algorithms is largely independent of the use of thrombo‐elastographic or thrombo‐elastometric techniques 53. In addition, in a recent study, the latest models of both types of viscoelastic techniques were found to be largely comparable 54.
Figure 1.
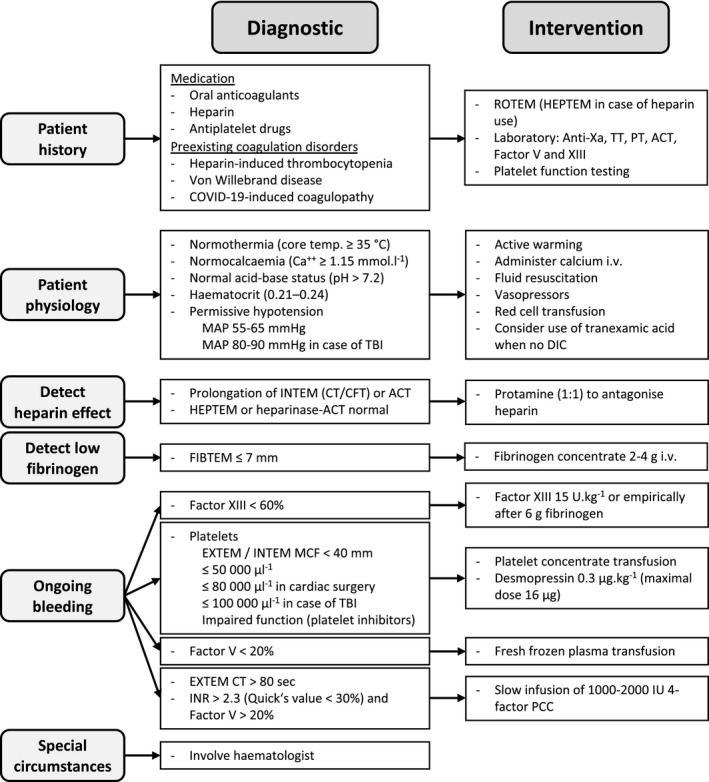
Individualised goal‐directed coagulation and transfusion algorithm in the case of blood loss ≥ 50% or diffuse bleeding (adapted from an algorithm of the Institute of Anaesthesiology, University Hospital Zurich, Switzerland). ACT, activated clotting time; COVID‐19, coronavirus disease 2019; CFT, clot formation time; CT, clotting time; DIC, disseminated intravascular coagulation; INR, international normalised ratio; IU, international units; MAP, mean arterial pressure; MCF, maximum clot firmness; PCC, prothrombin complex concentrate; PT, prothrombin time; ROTEM, rotational thrombo‐elastometry; TBI, traumatic brain injury; TT, thrombin time.
Irrespective of the type of coagulation algorithm used, the early use of tranexamic acid may be recommended to manage the bleeding patient provided there is no contraindication (active DIC or active hypercoagulable state being absolute contraindications) 55. At present, there are close to 200 meta‐analyses that show the success of tranexamic acid in reducing bleeding and the use of allogeneic blood products in surgery and in non‐surgical fields. These meta‐analyses also document the absence of an increased incidence of thromboembolic adverse outcomes following tranexamic acid administration 4.
Some COVID‐19 infected patients need surgery during their ICU stay. In an early phase, most patients are likely to be in a distinct acute phase response with very high levels of platelets, fibrinogen concentration, Factor VIII and von Willebrand factor. Later, some may develop DIC. However, the current level of knowledge is very limited and the difference between patients at different stages of the disease is likely to be significant. Therefore, monitoring coagulation with viscoelastic point‐of‐care devices and quick laboratory tests is particularly important in COVID‐19 infected patients. At the present time we propose using the established coagulation algorithms (Fig. 1) including the use of tranexamic acid in COVID‐19 positive patients with less severe disease suffering from trauma.
Special considerations
During disasters, work forces are overstrained, supply chains are disrupted, manufacturing capacities are reduced or diverted, and infrastructures are undermined in the face of increased demand. These circumstances lead to shortages of crucial medical resources. This reality is exemplified during the COVID‐19 pandemic by the shortage of personal protective equipment, ventilators, ICU beds, vital medications and other critical equipment for healthcare workers and frontline responders. All this puts medical personnel at great personal risk and forces difficult decisions to be made regarding the allocation of scarce patient resources 56. Considering the many uncertainties of the COVID‐19 pandemic, the full extent of consequences remains unknown. Some models suggest a direct human toll in the tens of thousands in US alone, extrapolating to hundreds of thousands of deaths globally. Indirectly, as economies around the world suffer, populations face risks due to disrupted healthcare systems, reduced access to care and preventative measures, impaired mental health and poverty. There is often a sinister inverse relationship between measures of economic prosperity and various health and well‐being indicators 57. Patient blood management can play an important role in mitigating short‐term shortages in the midst of the acute phase of the pandemic (and subsequent waves until herd immunity is established through widespread infection or vaccination) and longer‐term socio‐economic health challenges in the post‐COVID‐19 world. The main focus of patient blood management on improving patients’ outcomes can directly translate into a reduced burden of illness and severity in individual patients, releasing limited resources for other needs. Patient blood management practices prevent anaemia (a global health problem) and reduce allogeneic blood transfusion, resulting not only in the best allocation of resources and cost savings, but also decreased patient morbidity and mortality 6, 58. These improved outcomes and the concomitant cost savings are urgently needed as we deal with a pandemic yet to reach its peak, and the ongoing challenges thereafter.
During the COVID‐19 pandemic, the most fundamental aspect of patient blood management is the prevention and management of anaemia. While the exact impact of anaemia on the outcomes of COVID‐19 patients is not yet fully understood, data show unequivocally that the prognosis of COVID‐19 patients with pre‐existing and chronic conditions is significantly worse. As such, it is not unreasonable to expect that anaemia will also have a negative impact on the outcomes of COVID‐19 patients and widespread prevention and management of anaemia might confer some protection against more severe cases of COVID‐19.
Conclusion
As we are facing the biggest global health challenge of our lives, only a major collaborative effort will allow us to achieve a positive outcome. Using a patient‐centred approach, proven evidence‐based principles should be applied and established expert‐consensus good practice concepts continued. Patient blood management is one piece of the puzzle needed to save patients’ lives. Every drop of blood saved can be decisive, even more so in the critical situation we are currently facing.
Acknowledgements
DB has received honoraria for lectures and educational grant funding from Vifor Pharma. MJ has been a consultant for SABM and Gauss Surgical. AK has received educational grant funding, honoraria or travel expenses in the last 3 years from Pharmacosmos, Vifor Pharma, Fisher and Paykel, Massimo, Hemosonics and Hemonetics. PM has received research support from Vifor, Fresenius Medical, B.Braun Melsungen and CSL Behring. MM has received honoraria for lectures and/or consultancies from Pharmacosmos (Denmark), Vifor Pharma (Spain & Switzerland), PharmaNutra (Italy), and Zambon (Spain). AS has received consulting fee from Pharmacosmos, Vifor Pharma, CSL Behring, Octaphama, Masimo and Baxter. DRS is receiving departmental grant support from the Swiss National Science Foundation, Berne, Switzerland, the Swiss Society of Anesthesiology and Reanimation (SGAR), Berne, Switzerland, the Swiss Foundation for Anesthesia Research, Zurich, Switzerland, Vifor SA, Villars‐sur‐Glâne, Switzerland; is co‐chair of the ABC‐Trauma Faculty, sponsored by unrestricted educational grants from Novo Nordisk Health Care AG, Zurich, Switzerland, CSL Behring GmbH, Marburg, Germany, LFB Biomédicaments, Courtaboeuf Cedex, France and Octapharma AG, Lachen, Switzerland; received honoraria/travel support for consulting or lecturing from: Danube University of Krems, Austria, US Department of Defense, Washington, USA, European Society of Anesthesiology, Brussels, BE, Korean Society for Patient Blood Management, Seoul, Korea, Korean Society of Anesthesiologists, Seoul, Korea, Baxter AG, Volketswil, Switzerland, Baxter S.p.A., Roma, Italy, Bayer AG, Zürich, Switzerland, Bayer Pharma AG, Berlin, Germany, B. Braun Melsungen AG, Melsungen, Germany, Boehringer Ingelheim GmbH, Basel, Switzerland, Bristol‐Myers‐Squibb, Rueil‐Malmaison Cedex, France and Baar, Switzerland, CSL Behring GmbH, Hattersheim am Main, Germany and Berne, Switzerland, Celgene International II Sàrl, Couvet, Switzerland, Curacyte AG, Munich, Germany, Daiichi Sankyo AG, Thalwil, Switzerland, GlaxoSmithKline GmbH & Co. KG, Hamburg, Germany, Haemonetics, Braintree, MA, USA, Instrumentation Laboratory (Werfen), Bedford, MA, USA, LFB Biomédicaments, Courtaboeuf Cedex, France, Merck Sharp & Dohme, Kenilworth, New Jersey, USA, Octapharma AG, Lachen, Switzerland, Organon AG, Pfäffikon/SZ, Switzerland, PAION Deutschland GmbH, Aachen, Germany, Pharmacosmos A/S, Holbaek, Denmark, Photonics Healthcare B.V., Utrecht, Netherlands, Pierre Fabre Pharma, Alschwil, Switzerland, Roche Diagnostics International Ltd, Reinach, Switzerland, Roche Pharma AG, Reinach, Switzerland, Sarstedt AG & Co., Sevelen, Switzerland and Nümbrecht, Germany, Schering‐Plough International, Inc., Kenilworth, New Jersey, USA, Tem International GmbH, Munich, Germany, Verum Diagnostica GmbH, Munich, Germany, Vifor Pharma, Munich, Germany, Vienna, Austria and Villars‐sur‐Glâne, Switzerland, Vifor (International) AG, St. Gallen, Switzerland, Zuellig Pharma Holdings, Singapore, Singapore. KZ has received research support from Vifor, Fresenius Medical, B.Braun Melsungen and CSL Behring. No other external funding or competing interests declared.
References
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. New England Journal of Medicine 2020; 382: 727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. CSSE . COVID‐19 dashboard by the center for systems science and engineering (CSSE) at Johns Hopkins University (JHU). 2020. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 (accessed 25/4/2020).
- 3. WHO . Coronavirus disease (COVID‐19) pandemic. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed 16/4/2020).
- 4. Spahn DR, Muñoz M, Klein AA, et al. Patient blood management: effectiveness and future potential. Anesthesiology 2020. Epub 24 February. doi.org/10.1097/ALN.00000000 00003198 [DOI] [PubMed] [Google Scholar]
- 5. Muñoz M, Acheson AG, Bisbe E, et al. An international consensus statement on the management of postoperative anaemia after major surgical procedures. Anaesthesia 2018; 73: 1418–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leahy MF, Hofmann A, Towler S, et al. Improved outcomes and reduced costs associated with a health‐system‐wide patient blood management program: a retrospective observational study in four major adult tertiary‐care hospitals. Transfusion 2017; 57: 1347–58. [DOI] [PubMed] [Google Scholar]
- 7. Kaserer A, Rossler J, Braun J, et al. Impact of a Patient Blood Management monitoring and feedback programme on allogeneic blood transfusions and related costs. Anaesthesia 2019; 74: 1534–41. [DOI] [PubMed] [Google Scholar]
- 8. Chang L, Yan Y, Wang L. Coronavirus disease 2019: coronaviruses and blood safety. Transfusion Medicine Reviews 2020. Epub 21 February. doi.org/10.1016/j.tmrv.2020.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. ECDC . Coronavirus disease 2019 (COVID‐19) and supply of substances of human origin in the EU/EEA. 2020. https://www.ecdc.europa.eu/en/publications-data/coronavirus-disease-2019-covid-19-and-supply-substances-human-origin-eueea (accessed 16/04/2020).
- 10. Franchini M, Farrugia A, Velati C, et al. The impact of the SARS‐CoV‐2 outbreak on the safety and availability of blood transfusion in Italy. Vox Sanguinis 2020. Epub 2 April. doi.org/10.1111/vox.1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ranucci M, Baryshnikova E, Castelvecchio S, et al. Major bleeding, transfusions, and anemia: the deadly triad of cardiac surgery. Annals of Thoracic Surgery 2013; 96: 478–85. [DOI] [PubMed] [Google Scholar]
- 12. Baron DM, Hochrieser H, Posch M, et al. Preoperative anaemia is associated with poor clinical outcome in non‐cardiac surgery patients. British Journal of Anaesthesia 2014; 113: 416–23. [DOI] [PubMed] [Google Scholar]
- 13. Muñoz M, Acheson AG, Auerbach M, et al. International consensus statement on the peri‐operative management of anaemia and iron deficiency. Anaesthesia 2017; 72: 233–47. [DOI] [PubMed] [Google Scholar]
- 14. Blaudszun G, Munting KE, Butchart A, et al. The association between borderline pre‐operative anaemia in women and outcomes after cardiac surgery: a cohort study. Anaesthesia 2018; 73: 572–8. [DOI] [PubMed] [Google Scholar]
- 15. Muñoz M, Laso‐Morales MJ, Gómez‐Ramírez S, et al. Pre‐operative haemoglobin levels and iron status in a large multicentre cohort of patients undergoing major elective surgery. Anaesthesia 2017; 72: 826–34. [DOI] [PubMed] [Google Scholar]
- 16. Rossler J, Schoenrath F, Seifert B, et al. Iron deficiency is associated with higher mortality in patients undergoing cardiac surgery: a prospective study. British Journal of Anaesthesia 2020; 124: 25–34. [DOI] [PubMed] [Google Scholar]
- 17. Muñoz M, Gómez‐Ramírez S, Cuenca J, et al. Very‐short‐term perioperative intravenous iron administration and postoperative outcome in major orthopedic surgery: a pooled analysis of observational data from 2547 patients. Transfusion 2014; 54: 289–99. [DOI] [PubMed] [Google Scholar]
- 18. Camaschella C, Nai A, Silvestri L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica 2020; 105: 260–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tardy AL, Pouteau E, Marquez D, et al. Vitamins and minerals for energy, fatigue and cognition: a narrative review of the biochemical and clinical evidence. Nutrients 2020; 12: E228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stauder R, Valent P, Theurl I. Anemia at older age: etiologies, clinical implications, and management. Blood 2018; 131: 505–14. [DOI] [PubMed] [Google Scholar]
- 21. Stoffel NU, Zeder C, Brittenham GM, et al. Iron absorption from supplements is greater with alternate day than with consecutive day dosing in iron‐deficient anemic women. Haematologica 2019. Epub 14 August. doi.org/10.3324/haematol. 2019. 220830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gómez‐Ramírez S, Brilli E, Tarantino G, Muñoz M. Sucrosomial((R)) iron: a new generation iron for improving oral supplementation. Pharmaceuticals 2018; 11: E97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Medicine 2020. Epub 3 March. doi.org/10.1007/s00134‐020‐5991‐x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wolf M, Rubin J, Achebe M, et al. Effects of iron isomaltoside vs ferric carboxymaltose on hypophosphatemia in iron‐deficiency anemia: two randomized clinical trials. Journal of the American Medical Association 2020; 323: 432–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gómez‐Ramírez S, Maldonado‐Ruiz MA, Campos‐Garrigues A, et al. Short‐term perioperative iron in major orthopedic surgery: state of the art. Vox Sanguinis 2019; 114: 3–16. [DOI] [PubMed] [Google Scholar]
- 26. Froessler B, Palm P, Weber I, et al. The important role for intravenous iron in perioperative patient blood management in major abdominal surgery: a randomized controlled trial. Annals of Surgery 2016; 264: 41–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Keeler BD, Simpson JA, Ng O, et al. Randomized clinical trial of preoperative oral versus intravenous iron in anaemic patients with colorectal cancer. British Journal of Surgery 2017; 104: 214–21. [DOI] [PubMed] [Google Scholar]
- 28. Laso‐Morales M, Jerico C, Gomez‐Ramirez S, et al. Preoperative management of colorectal cancer‐induced iron deficiency anemia in clinical practice: data from a large observational cohort. Transfusion 2017; 57: 3040–8. [DOI] [PubMed] [Google Scholar]
- 29. Johansson PI, Rasmussen AS, Thomsen LL. Intravenous iron isomaltoside 1000 (Monofer(R)) reduces postoperative anaemia in preoperatively non‐anaemic patients undergoing elective or subacute coronary artery bypass graft, valve replacement or a combination thereof: a randomized double‐blind placebo‐controlled clinical trial (the PROTECT trial). Vox Sanguinis 2015; 109: 257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Spahn DR, Schoenrath F, Spahn GH, et al. Effect of ultra‐short‐term treatment of patients with iron deficiency or anaemia undergoing cardiac surgery: a prospective randomised trial. Lancet 2019; 393: 2201–12. [DOI] [PubMed] [Google Scholar]
- 31. Khalafallah AA, Yan C, Al‐Badri R, et al. Intravenous ferric carboxymaltose versus standard care in the management of postoperative anaemia: a prospective, open‐label, randomised controlled trial. Lancet Haematology 2016; 3: e415–25. [DOI] [PubMed] [Google Scholar]
- 32. Kim YW, Bae JM, Park YK, et al. Effect of intravenous ferric carboxymaltose on hemoglobin response among patients with acute isovolemic anemia following gastrectomy: the FAIRY randomized clinical trial. Journal of the American Medical Association 2017; 317: 2097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Medicine 2020. Epub 28 February. doi.org/10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shander A, Javidroozi M, Lobel G. Patient blood management in the intensive care unit. Transfusion Medicine Reviews 2017; 31: 264–71. [DOI] [PubMed] [Google Scholar]
- 36. Litton E, Latham P, Inman J, et al. Safety and efficacy of erythropoiesis‐stimulating agents in critically ill patients admitted to the intensive care unit: a systematic review and meta‐analysis. Intensive Care Medicine 2019; 45: 1190–9. [DOI] [PubMed] [Google Scholar]
- 37. Hunt B, Retter A, McClintock C. Practical guidance for the prevention of thrombosis and management of coagulopathy and disseminated intravascular coagulation of patients infected with COVID‐19. 2020. https://thrombosisuk.org/covid-19-thrombosis.php (accessed 16/4/2020).
- 38. Nguyen BV, Bota DP, Melot C, Vincent JL. Time course of hemoglobin concentrations in nonbleeding intensive care unit patients. Critical Care Medicine 2003; 31: 406–10. [DOI] [PubMed] [Google Scholar]
- 39. Whitehead NS, Williams LO, Meleth S, et al. Interventions to prevent iatrogenic anemia: a Laboratory Medicine Best Practices systematic review. Critical Care 2019; 23: 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. Journal of the American Medical Association: Internal Medicine 2020; 180: 934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Baron DM, Lei C, Berra L. Old, older, the oldest: red blood cell storage and the potential harm of using older red blood cell concentrates. Current Opinion in Anaesthesiology 2020; 33: 234–9. [DOI] [PubMed] [Google Scholar]
- 42. Vlaar APJ, Toy P, Fung M, et al. A consensus redefinition of transfusion‐related acute lung injury. Transfusion 2019; 59: 2465–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Han H, Yang L, Liu R, et al. Prominent changes in blood coagulation of patients with SARS‐CoV‐2 infection. Clinical Chemistry and Laboratory Medicine 2020. Epub 16 March. doi.org/10.1515/cclm‐2020‐0188 [DOI] [PubMed] [Google Scholar]
- 44. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. Journal of Thrombosis and Haemostasis 2020; 18: 844–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Luks A, Freer L, Grissom C, et al. COVID‐19 lung injury is not high altitude pulmonary edema. High Altitude Medicine and Biology 2020. Epub 13 April. doi.org/10.1089/ham.2020.0055 [DOI] [PubMed] [Google Scholar]
- 47. Kozek‐Langenecker SA, Ahmed AB, Afshari A, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology: first update 2016. European Journal of Anaesthesiology 2017; 34: 332–95. [DOI] [PubMed] [Google Scholar]
- 48. Boer C, Meesters MI, Milojevic M, et al. 2017 EACTS/EACTA Guidelines on patient blood management for adult cardiac surgery. Journal of Cardiothoracic and Vascular Anesthesia 2018; 32: 88–120. [DOI] [PubMed] [Google Scholar]
- 49. Spahn DR, Bouillon B, Cerny V, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fifth edition. Critical Care 2019; 23: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Weber CF, Gorlinger K, Meininger D, et al. Point‐of‐care testing: a prospective, randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anesthesiology 2012; 117: 531–47. [DOI] [PubMed] [Google Scholar]
- 51. Stein P, Kaserer A, Sprengel K, et al. Change of transfusion and treatment paradigm in major trauma patients. Anaesthesia 2017; 72: 1317–26. [DOI] [PubMed] [Google Scholar]
- 52. Mallaiah S, Barclay P, Harrod I, et al. Introduction of an algorithm for ROTEM‐guided fibrinogen concentrate administration in major obstetric haemorrhage. Anaesthesia 2015; 70: 166–75. [DOI] [PubMed] [Google Scholar]
- 53. Deppe AC, Weber C, Zimmermann J, et al. Point‐of‐care thromboelastography/thromboelastometry‐based coagulation management in cardiac surgery: a meta‐analysis of 8332 patients. Journal of Surgical Research 2016; 203: 424–33. [DOI] [PubMed] [Google Scholar]
- 54. Ziegler B, Voelckel W, Zipperle J, et al. Comparison between the new fully automated viscoelastic coagulation analysers TEG 6s and ROTEM Sigma in trauma patients: a prospective observational study. European Journal of Anaesthesiology 2019; 36: 834–42. [DOI] [PubMed] [Google Scholar]
- 55. Goobie SM, Faraoni D. Tranexamic acid and perioperative bleeding in children: what do we still need to know? Current Opinion in Anaesthesiology 2019; 32: 343–52. [DOI] [PubMed] [Google Scholar]
- 56. Rombola G, Heidempergher M, Pedrini L, et al. Practical indications for the prevention and management of SARS‐CoV‐2 in ambulatory dialysis patients: lessons from the first phase of the epidemics in Lombardy. Journal of Nephrology 2020; 33: 193–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Costa D. Health and the economy in the United States, from 1750 to the present. Journal of Economic Literature 2015; 53: 503–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Meybohm P, Straub N, Fullenbach C, et al. Health economics of patient blood management: a cost‐benefit analysis based on a meta‐analysis. Vox Sanguinis 2020; 115: 182–8. [DOI] [PubMed] [Google Scholar]


