Abstract
Objective
This post hoc analysis evaluated long‐term efficacy and safety in patients with focal to bilateral tonic‐clonic seizures (FBTCS) or generalized tonic‐clonic seizures (GTCS) who entered open‐label extension (OLEx) studies to receive long‐term adjunctive perampanel.
Methods
Patients aged 12 years and older who completed phase II or III randomized, double‐blind, placebo‐controlled studies could enter an OLEx study, each comprising a blinded conversion period followed by an open‐label maintenance period (32‐424 weeks; maximum perampanel dose = 12 mg/d). Exposure, seizure outcomes, and treatment‐emergent adverse events (TEAEs) were analyzed.
Results
Baseline characteristics were generally balanced between patients with FBTCS (n = 720) and GTCS (n = 138). Mean (standard deviation) cumulative duration of perampanel exposure was 102.3 (70.3) weeks (FBTCS) and 83.9 (38.4) weeks (GTCS). Retention rates were 50.0% for up to 4 years (FBTCS) and 49.2% for up to 2 years (GTCS). Across OLEx treatment durations, median reductions in seizure frequency per 28 days were 66.7% (FBTCS) and 80.6% (GTCS). Fifty percent and 75% responder and seizure‐freedom rates were 59.5%, 45.3%, and 18.4%, respectively (FBTCS), and 72.5%, 51.5%, and 16.7%, respectively (GTCS). Efficacy was sustained for up to 4 years (FBTCS) and up to 3 years (GTCS), even when accounting for early dropouts. TEAE incidence was highest during Year 1 (FBTCS, 85.3%; GTCS, 86.2%); most common were dizziness and somnolence. During Year 1, serious TEAEs were reported in 81 (11.3%; FBTCS) and 10 (7.2%; GTCS) patients. TEAEs were consistent with the known safety profile of perampanel; no new safety signals were identified with long‐term treatment.
Significance
This post hoc analysis suggests long‐term (up to 4 years) adjunctive perampanel (up to 12 mg/d) is efficacious and well tolerated in patients (aged 12 years and older) with FBTCS or GTCS.
Keywords: AMPA receptor antagonist, antiseizure medication, focal to bilateral tonic‐clonic seizures, generalized tonic‐clonic seizures
Key Points.
This post hoc analysis reports long‐term efficacy and safety outcomes with adjunctive perampanel in patients with tonic‐clonic seizures
Patients were from open‐label extension studies and had FBTCS or GTCS
Retention rates were 50.0% for up to 4 years in patients with FBTCS and 49.2% for up to 2 years in patients with GTCS
Median percent reductions in seizure frequency per 28 days were maintained for up to 4 years (FBTCS) and up to 3 years (GTCS)
The safety profile was consistent with the known safety profile of perampanel; no new safety signals emerged with up to 4 years of exposure
1. INTRODUCTION
Perampanel is a once‐daily oral antiseizure medication (ASM) for focal seizures (previously known as partial onset seizures), with or without focal to bilateral tonic‐clonic seizures (FBTCS; previously secondarily generalized seizures), and generalized tonic‐clonic seizures (GTCS; previously primary generalized tonic‐clonic seizures). 1 , 2 The clinical development of adjunctive perampanel included multiple phase II and III randomized, double‐blind, placebo‐controlled studies, in which perampanel (up to 12 mg/d) demonstrated clinical efficacy and favorable tolerability. Studies 206 (NCT00144690; conducted in Australia, Europe, USA) and 208 (NCT00416195; Australia, Europe) were phase II studies involving patients aged 18‐70 years inclusively, with uncontrolled focal seizures, with or without FBTCS. 3 Studies 304 (NCT00699972; North and Latin America), 305 (NCT00699582; global), 306 (NCT00700310; Europe, Asia, Australia), and 335 (NCT01618695; Asia‐Pacific) were phase III studies in patients aged 12 years and above with uncontrolled focal seizures, with or without FBTCS. 4 , 5 , 6 , 7 Lastly, Study 332 (NCT01393743; Europe, Asia‐Pacific, USA) was a phase III study in patients aged 12 years and above with idiopathic generalized epilepsy (IGE) and GTCS. 8
Randomized trials offer relatively short exposures to investigational ASMs (typically 8‐12 weeks); therefore, postmarketing and long‐term follow‐up studies are important to monitor adverse side effects that may only occur after long‐term exposure and to assess long‐term efficacy. 9 Open‐label extension (OLEx) studies provide opportunities to assess safety during chronic exposure and evaluate patient retention rates over a longer time period, which can help inform physicians of treatment success over several years under clinical conditions. 9 To assess the long‐term efficacy and safety of adjunctive perampanel, patients who completed the phase II and III double‐blind studies could enter an OLEx study: OLEx Study 207 (NCT00368472; patients from Studies 206 or 208), 10 OLEx Study 307 (NCT00735397; Studies 304, 305, and 306), 11 Study 335 OLEx (NCT01618695), 12 and Study 332 OLEx (NCT01393743). 13
At the time that the double‐blind and OLEx studies were conducted, the International League Against Epilepsy (ILAE) 1981 seizure classification was in use; however, in accordance with the new ILAE 2017 seizure classification, “secondarily generalized seizures” and “primary generalized tonic‐clonic seizures” have been updated to FBTCS and GTCS, respectively, throughout this article. 14 The updated terminology has also been applied to any other seizure types that were observed in these patients.
Patients with epilepsy are at risk of seizure‐related complications and comorbidities, including sudden unexpected death in epilepsy (SUDEP). 15 , 16 , 17 Previous studies have shown that risk of seizure‐related injuries and SUDEP are greater in patients with tonic‐clonic seizures, including FBTCS and GTCS. 15 , 18 The therapeutic management of tonic‐clonic seizures is important to reduce these risks and achieve seizure control. 19 It can sometimes be challenging for physicians to distinguish between FBTCS and GTCS due to similarities in their presentation. 20 However, this is essential for their management and the selection of appropriate ASMs, because some ASMs that are efficacious against FBTCS may exacerbate other generalized seizure types (eg, absence, myoclonic) in patients with IGE. 21 , 22 , 23 To improve the therapeutic management of tonic‐clonic seizures and reduce the risk of exacerbating other generalized seizures types in patients with GTCS, it would be advantageous to have an approved ASM that is efficacious against both FBTCS and GTCS. We report a post hoc analysis to evaluate the long‐term (up to 4 years) efficacy and safety of adjunctive perampanel (up to 12 mg/d) in patients with FBTCS or GTCS who participated in the OLEx studies.
2. MATERIALS AND METHODS
2.1. Study designs
The designs of the double‐blind studies (206, 208, 304, 305, 306, 335, and 332) have been described elsewhere. 3 , 4 , 5 , 6 , 7 , 8 The OLEx studies were performed in different patient populations across various regions (OLEx Study 207: Australia, Europe, USA; OLEx Study 307: global; Study 335 OLEx: Asia‐Pacific; Study 332 OLEx: Europe, Asia‐Pacific, USA). All OLEx studies comprised a blinded conversion period (6‐16 weeks across studies) where perampanel dose optimization was achieved (maximum 12 mg/d), followed by a 32‐ to 424‐week maintenance period, resulting in up to 1‐8 years of perampanel exposure (Table S1).
Patients who received placebo during the double‐blind studies were blindly converted to adjunctive perampanel during the OLEx conversion period; patients who received perampanel continued on this treatment. During the conversion period, patients had their perampanel doses uptitrated in 2‐mg increments weekly (Studies 335 OLEx and 332 OLEx) or every 2 weeks (Studies 207 and 307) from the dose on which they completed the double‐blind study (or from 2 mg for patients previously receiving placebo), to a maximum of 12 mg/d, based on tolerability and seizure control. During the maintenance period, patients were unblinded to study treatment and remained on the optimal perampanel dose established during the blinded conversion period. Adjustment of perampanel dose during the maintenance period was permitted at the investigator’s discretion, based on tolerability. Patients entered the OLEx studies on the same concomitant ASMs as they were on during the double‐blind studies; however, in Studies 207, 307, and 332 OLEx, the dose and types of ASM could be adjusted, changed, or discontinued during the OLEx period. In Study 335 OLEx, concomitant ASMs were to be used at a stable dose and administration during the OLEx period.
Although some patients with FBTCS were exposed to perampanel for up to 8 years during OLEx studies, this post hoc analysis only reports data for up to 4 years due to the small number of patients who received perampanel at later timepoints (eg, 5 years, n = 24).
2.2. Efficacy assessments
Efficacy assessments were based on the Full Analysis Set, which comprised all patients who received at least one dose of perampanel during the OLEx period, and had baseline seizure frequency data and valid seizure data during the OLEx treatment duration or perampanel treatment duration (defined under 2.4 Statistical Analysis). Assessments conducted based on seizure data collected for up to 4 years of perampanel treatment included median percent change in FBTCS (Studies 207, 307, 335 OLEx) or GTCS (Study 332 OLEx) frequency per 28 days between preperampanel baseline and the OLEx treatment duration or perampanel treatment duration, 50% and 75% responder rates for FBTCS or GTCS (defined as the proportion of patients who achieved a 50% or greater reduction or a 75% or greater reduction in FBTCS or GTCS frequency between preperampanel baseline and the OLEx treatment duration or each respective year of the perampanel treatment duration), and seizure‐freedom rates for FBTCS or GTCS (defined as the proportion of patients who completed the period of analysis and were free from FBTCS or GTCS during that period of the OLEx treatment duration or perampanel treatment duration).
2.3. Safety assessments
Safety assessments were based on the Safety Analysis Set, which included patients who received at least one dose of perampanel during the OLEx studies and had any on‐treatment safety data during the OLEx period. Safety assessments conducted based on data collected for up to 4 years of perampanel treatment included retention and discontinuation rates, monitoring of treatment‐emergent adverse events (TEAEs; defined as an adverse event [AE] that began either between the first day of perampanel administration and up to 30 days after the last dose, or before the date of first perampanel dose and worsened in severity during perampanel treatment) and serious TEAEs (those that resulted in death, were life‐threatening, required hospitalization/prolongation of hospitalization, resulted in persistent or significant disability/incapacity, or were congenital anomalies/birth defects in children of patients who received perampanel), and dose adjustments (withdrawals, reductions, interruptions). AEs were summarized across the entire perampanel exposure. For patients who received perampanel in the double‐blind study, perampanel exposure comprised the double‐blind plus the OLEx treatment duration. For patients who received placebo in the double‐blind study, perampanel exposure comprised the OLEx treatment duration.
2.4. Statistical analysis
For patients who received placebo during the double‐blind studies, preperampanel baseline included seizure diary data from the double‐blind study. For patients who received perampanel during the double‐blind studies, preperampanel baseline included seizure diary data from the baseline period (prerandomization monitoring phase) of the double‐blind study plus 4 weeks prior.
The OLEx treatment duration started from the first perampanel dose in the OLEx period to the last perampanel dose in the OLEx period. The perampanel treatment duration started from the first perampanel dose in the double‐blind study to the last perampanel dose in the OLEx period (with the exception of patients who had a gap in perampanel exposure from the double‐blind study to the OLEx period of at least 14 days; for these patients, the perampanel treatment duration was the OLEx exposure).
For any respective year, only patients who could have received perampanel for the full year of treatment were included in the retention rate analysis. Kaplan–Meier analysis was used to estimate time to discontinuation. Efficacy and safety assessments were based on 52‐week treatment intervals from the initiation of perampanel treatment.
To account for patients who dropped out of the study early, sensitivity analyses were conducted for all efficacy assessments. For these, the last observation carried forward approach was used for all patients, meaning that patients who completed or withdrew from the study had their last year of treatment carried forward to later timepoints; for patients who were treated for less than 1 year, their entire treatment period was carried forward to later timepoints. Analyses for efficacy assessments were also conducted excluding patients who dropped out; for 50% and 75% responder rates, each year of treatment included only those patients for whom efficacy data were recorded during that corresponding year, even if they did not complete the full year of treatment.
3. RESULTS
3.1. Patients and treatment exposure
The Full and Safety Analysis Sets included 720 patients with FBTCS (Studies 207, 307, 335 OLEx) and 138 patients with GTCS (Study 332 OLEx 24 , 25 ). Patient demographics and clinical characteristics were comparable, regardless of seizure type (Table 1). For patients with FBTCS, 105 (14.6%), 334 (46.4%), and 281 (39.0%) patients received 1, 2, and ≥3 concomitant ASMs during baseline, respectively; for patients with GTCS, 48 (34.8%), 61 (44.2%), and 28 (20.3%) received 1, 2, and 3 concomitant ASMs, respectively. During baseline, the most common concomitant ASMs taken by patients with FBTCS were valproic acid (including Ergenyl Chrono; n = 296 [41.1%]), carbamazepine (n = 244 [33.9%]), and lamotrigine (n = 214 [29.7%]); for patients with GTCS, these were valproic acid (including Ergenyl Chrono; n = 59 [42.8%]), lamotrigine (n = 57 [41.3%]), and levetiracetam (n = 40 [29.0%]).
TABLE 1.
Demographic and clinical characteristics at double‐blind study baseline (Safety Analysis Set)
| FBTCS, n = 720 | GTCS, n = 138 | |
|---|---|---|
| Mean age, y (SD) | 32.5 (12.8) | 27.9 (11.1) |
| Female, n (%) | 352 (48.9) | 79 (57.3) |
| Race, n (%) | ||
| Caucasian | 407 (56.5) | 71 (51.5) |
| Asian a | 275 (38.2) | 61 (44.2) |
| Black or African American | 16 (2.2) | 3 (2.2) |
| Other b | 22 (3.1) | 3 (2.2) |
| Seizure types, c n (%) | ||
| Focal aware without motor signs [previously simple partial without motor signs] | 197 (27.4) | 0 (0.0) |
| Focal aware with motor signs [previously simple partial with motor signs] | 194 (26.9) | 0 (0.0) |
| Focal impaired awareness [previously complex partial] | 570 (79.2) | 0 (0.0) |
| FBTCS [previously secondarily generalized] | 714 (99.2) | 0 (0.0) |
| GTCS [previously PGTC] | 0 (0.0) | 138 (100.0) |
| Myoclonic | 0 (0.0) | 59 (42.8) |
| Absence | 0 (0.0) | 69 (50.0) |
| Tonic | 0 (0.0) | 2 (1.5) |
| Clonic | 0 (0.0) | 1 (0.7) |
| Most common concomitant ASMs [at least 10% patients], n (%) | ||
| Valproic acid d , e | 296 (41.1) | 59 (42.8) |
| Carbamazepine f | 244 (33.9) | 13 (9.4) |
| Lamotrigine e | 214 (29.7) | 57 (41.3) |
| Levetiracetam e | 188 (26.1) | 40 (29.0) |
| Topiramate e | 148 (20.6) | 23 (16.7) |
| Oxcarbazepine f | 122 (16.9) | 5 (3.6) |
| Phenytoin f | 80 (11.1) | 7 (5.1) |
| Zonisamide e | 44 (6.1) | 15 (10.9) |
Abbreviations: ASM, antiseizure medication; FBTCS, focal to bilateral tonic‐clonic seizures; GTCS, generalized tonic‐clonic seizures; PGTC, primary generalized tonic‐clonic; SD, standard deviation.
Includes Chinese, Japanese, and other Asian.
Includes American Indian or Alaskan Native.
Based on epilepsy‐specific medical history as recorded during the baseline period of the preceding double‐blind study.
Includes Ergenyl Chrono.
Noninducing ASMs.
Enzyme‐inducing ASMs.
The mean modal (standard deviation [SD], range) perampanel dose received was 9.5 (3.8, 0‐12) mg/d for patients with FBTCS and 8.2 (2.3, 2‐12) mg/d for patients with GTCS. The mean (SD) cumulative duration of perampanel exposure was 102.3 (70.3) weeks and 83.9 (38.4) weeks 24 for patients with FBTCS and GTCS, respectively. Excluding administrative/other reasons for discontinuation, 340 (47.2%) patients with FBTCS and 46 (33.3%) patients with GTCS discontinued perampanel (Table 2). The most common primary reasons for discontinuation were patient choice (16.3% with FBTCS and 11.6% with GTCS), inadequate therapeutic effect (14.2% and 5.1%, respectively), and AEs (11.0% and 8.7%, respectively). Administrative/other reasons led to the discontinuation of 208 (28.9%) patients and 14 (10.1%) patients with FBTCS and GTCS, respectively. Retention rates on perampanel during Years 1, 2, 3, and 4 are shown in Figure 1A, and the estimated time to discontinuation for patients with either seizure type is shown in Figure 1B. Retention rates for patients with FBTCS were 77.7% during Year 1 and 50.0% during Year 4. For patients with GTCS, retention rates were 74.6% during Year 1 and 49.2% during Year 2.
TABLE 2.
Patient disposition and primary reasons for discontinuation for patients with FBTCS (Studies 207/307/335 OLEx) or GTCS (Study 332 OLEx)
| FBTCS, n = 720 | GTCS, n = 138 | |
|---|---|---|
| Treated, n (%) | 720 (100.0) | 138 (100.0) 24 , 25 |
| Completed | 22 (3.1) | 78 (56.5) |
| Discontinued [overall] a | 548 (76.1) | 60 (43.5) 24 , 25 |
| Discontinued [excluding administrative/other reasons] | 340 (47.2) | 46 (33.3) |
| Ongoing b | 150 (20.8) | 0 (0.0) |
| Primary reason for discontinuation from therapy, n (%) | ||
| Administrative/other c | 208 (28.9) | 14 (10.1) |
| Patient choice | 117 (16.3) | 16 (11.6) |
| Inadequate therapeutic effect | 102 (14.2) | 7 (5.1) |
| AE | 79 (11.0) | 12 (8.7) |
| Withdrawal of consent | 2 (0.3) | 8 (5.8) |
| Lost to follow‐up | 16 (2.2) | 2 (1.4) |
| Pregnancy | 2 (0.3) | 1 (0.7) |
Abbreviations: AE, adverse event;FBTCS, focal to bilateral tonic‐clonic seizures; GTCS, generalized tonic‐clonic seizures; OLEx, open‐label extension.
For Study 307, most patients were listed as “discontinued” because Eisai stopped the study following the approval of perampanel.
At time of analysis.
“Administrative/other” reasons for discontinuation include sponsor request for study closure (Study 307, after receipt of a positive opinion for perampanel from the Committee for Medicinal Products for Human Use; Study 332 OLEx, after closure of sites in China at the end of the double‐blind study and these sites declined continued access to perampanel on the expanded access program); continuation of treatment on Study 341 (NCT02427607), 37 an expanded access program, or commercial perampanel; lack of efficacy; noncompliance with protocol; and no longer meeting inclusion criteria.
FIGURE 1.
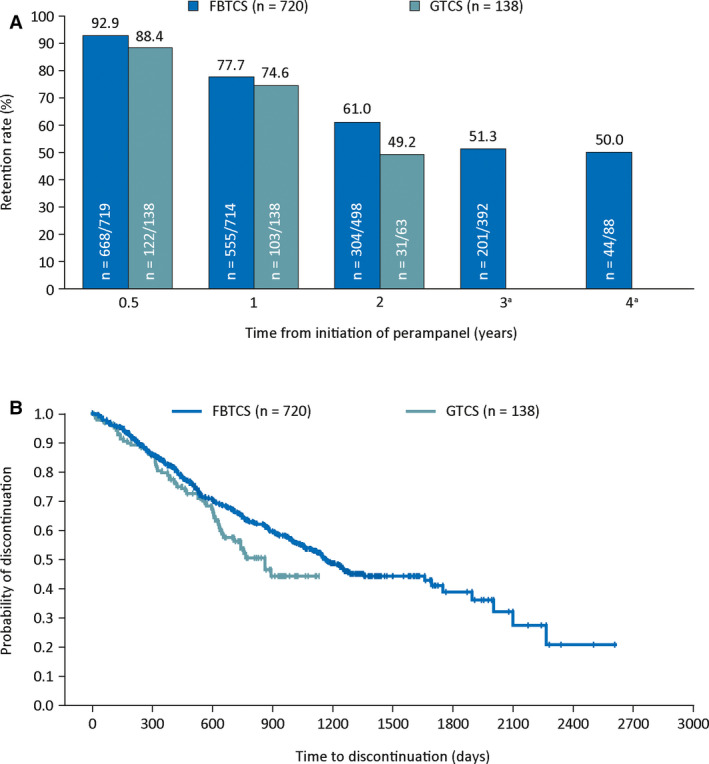
A, Retention rates on perampanel for up to 4 years; and B, Kaplan–Meier plot of estimated time to discontinuation for patients with FBTCS (Studies 207/307/335 OLEx) or GTCS (Study 332 OLEx). Retention rate is defined as the number of patients on treatment for at least x years/the number of patients who could have been on treatment for at least x years. Only patients who could have completed the full respective year of treatment based on when they entered the study were included in the retention rate analysis. aAt 3 years, 0/7 patients with GTCS were retained on perampanel treatment. At 4 years, zero patients with GTCS could have been retained on perampanel treatment. FBTCS, focal to bilateral tonic‐clonic seizures; GTCS, generalized tonic‐clonic seizures; OLEx, open‐label extension
3.2. Efficacy outcomes
Across the OLEx treatment duration, median percent reductions in seizure frequency per 28 days were 66.7% for FBTCS and 80.6% for GTCS. In the analysis including early dropouts, seizure frequency reductions during Year 1 were 56.9% and 74.7% for FBTCS and GTCS, respectively, and during Year 3 were 59.0% and 82.9%, respectively (Figure 2A). In the analysis excluding dropouts, seizure frequency reductions were maintained for up to 4 years for FBTCS and up to 3 years for GTCS, across the perampanel treatment duration (Figure 2B).
FIGURE 2.
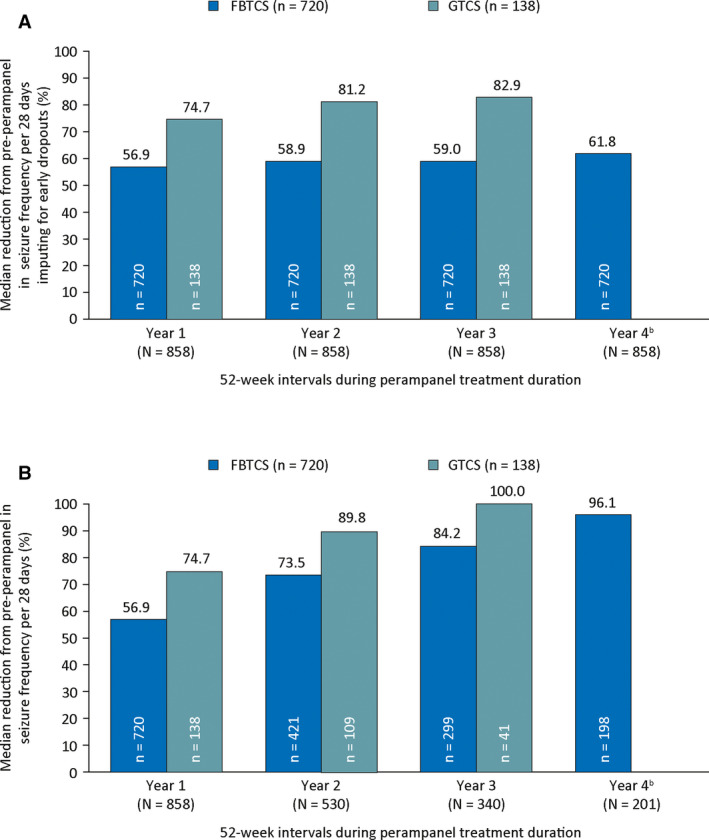
Median percent reduction in seizure frequency per 28 days during the perampanel treatment duration by 52‐week treatment intervals A, including dropouts a and B, excluding dropouts ( Full Analysis Set). All patients with any recorded efficacy data during a single respective year were included in the efficacy analysis for that year. a The analysis including early dropouts used the last observation carried forward approach, in which patients who completed or withdrew from the study had their last year of treatment carried forward to later timepoints. bWhen including dropouts, the median reduction from preperampanel in GTCS frequency per 28 days was 85.5% at Year 4 (n = 138). However, efficacy data were only recorded for three patients with GTCS at Year 4, and median reduction from preperampanel in GTCS frequency per 28 days was 100.0% when excluding dropouts. FBTCS, focal to bilateral tonic‐clonic seizures; GTCS, generalized tonic‐clonic seizures
Fifty percent and 75% responder and seizure‐freedom rates across the OLEx treatment duration were 59.5%, 45.3%, and 18.4%, respectively, for FBTCS, and 72.5%, 51.5%, and 16.7%, respectively, for GTCS. For analyses including dropouts and those excluding dropouts, responder and seizure‐freedom rates were also consistently observed for up to 4 years for FBTCS and up to 3 years for GTCS (Figure 3).
FIGURE 3.
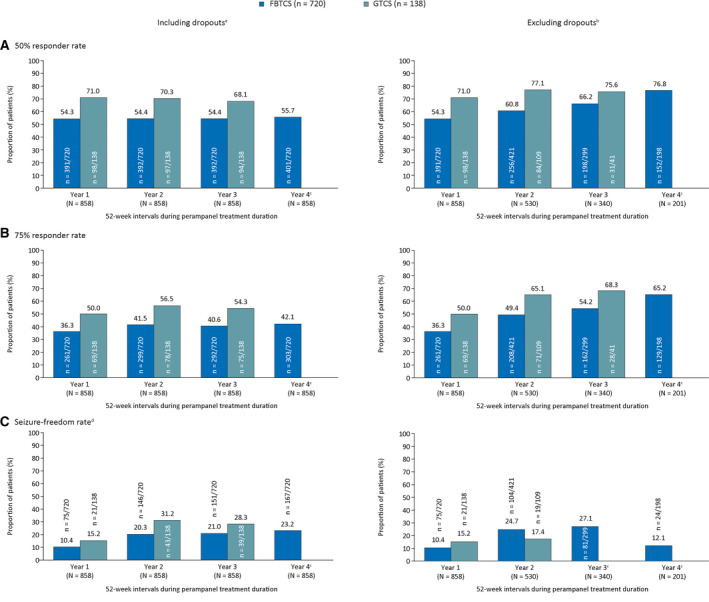
A, 50% responder rates; B, 75% responder rates; and C, seizure‐freedom rates during the perampanel treatment duration by 52‐week treatment intervals including dropoutsa and excluding dropoutsb (Full Analysis Set). aThese analyses used the last observation carried forward approach, in which patients who completed or withdrew from the study had their last full year of treatment carried forward to later timepoints. bFor 50% and 75% responder rates, all patients with any recorded efficacy data during a single respective year were included in the efficacy analysis for that year. cAt Year 4, efficacy data were only recorded for three patients with GTCS; all three achieved a 75% or greater response. At Years 3 and 4, when excluding dropouts, no patients achieved GTCS seizure freedom (0/41 [0.0%] and 0/3 [0.0%] patients, respectively). dFor seizure‐freedom rates, patients must have completed the corresponding 52‐week interval to be classed as seizure‐free. FBTCS, focal to bilateral tonic‐clonic seizures; GTCS, generalized tonic‐clonic seizures
For both seizure types, improvements in seizure frequency from preperampanel baseline were observed, irrespective of prior treatment received during the double‐blind studies (placebo/perampanel; data not shown).
3.3. Safety outcomes
Across the entire perampanel exposure, TEAEs were experienced by 650 (90.3%) patients with FBTCS and 120 (87.0%) 24 , 25 patients with GTCS. For both seizure types, overall incidence of TEAEs was highest during the first year of perampanel exposure (Table 3). During Years 2 and 3, the incidence of TEAEs was lower for patients with GTCS compared with those with FBTCS (36.2% vs 52.9%, respectively), and was most notable for TEAEs of dizziness and somnolence. The most frequently reported TEAEs (occurring in at least 10% of patients during any year of treatment) are listed in Table 3; during Year 1, the two most frequently reported TEAEs for both seizure types were dizziness and somnolence. The most common TEAEs were observed more frequently during the maintenance period compared with the titration period, with the exception of fatigue in patients with GTCS, which was higher during titration (7.2%) than during maintenance (3.7%; Table S2). However, it should be noted that the maintenance period was considerably longer than the titration period in the majority of cases.
TABLE 3.
Overall incidence of TEAEs and most frequent TEAEs (occurring in at least 10% of patients with either seizure type) by years of perampanel exposure (Safety Analysis Set)
| Year 1 | Year 2 | Year 3 | Year 4 | |||||
|---|---|---|---|---|---|---|---|---|
| FBTCS, n = 720 | GTCS, n = 138 | FBTCS, n = 511 | GTCS, n = 109 | FBTCS, n = 305 | GTCS, n = 43 | FBTCS, n = 200 | GTCS, n = 3 | |
| TEAEs, n (%) | 614 (85.3) | 119 (86.2) | 269 (52.6) | 42 (38.5) | 163 (53.4) | 13 (30.2) | 67 (33.5) | 0 (0.0) |
| Treatment‐related TEAEs | 540 (75.0) | 96 (69.6) | 144 (28.2) | 16 (14.7) | 75 (24.6) | 1 (2.3) | 24 (12.0) | 0 (0.0) |
| Severe TEAEs | 79 (11.0) | 14 (10.1) | 36 (7.0) | 5 (4.6) | 22 (7.2) | 2 (4.7) | 8 (4.0) | 0 (0.0) |
| Serious TEAEs | 81 (11.3) | 10 (7.2) | 45 (8.8) | 7 (6.4) | 33 (10.8) | 2 (4.7) | 8 (4.0) | 0 (0.0) |
| Deaths | 4 (0.6) | 1 (0.7) | 1 (0.2) | 0 (0.0) | 1 (0.3) | 0 (0.0) | 1 (0.5) | 0 (0.0) |
| Other | 77 (10.7) | 9 (6.5) | 45 (8.8) | 7 (6.4) | 32 (10.5) | 2 (4.7) | 7 (3.5) | 0 (0.0) |
| TEAEs leading to study drug dose adjustment, n (%) | 404 (56.1) | 50 (36.2) | 79 (15.5) | 6 (5.5) | 24 (7.9) | 0 (0.0) | 4 (2.0) | 0 (0.0) |
| Withdrawal | 67 (9.3) | 12 (8.7) | 17 (3.3) | 1 (0.9) | 7 (2.3) | 0 (0.0) | 2 (1.0) | 0 (0.0) |
| Dose reductions | 261 (36.3) | 40 (29.0) | 29 (5.7) | 4 (3.7) | 18 (5.9) | 0 (0.0) | 1 (0.5) | 0 (0.0) |
| Dose interruptions | 191 (26.5) | 3 (2.2) | 36 (7.0) | 1 (0.9) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 0 (0.0) |
| Most frequent TEAEs [occurring in at least 10% of patients], n (%) a | ||||||||
| Dizziness | 300 (41.7) | 50 (36.2) | 31 (6.1) | 3 (2.8) | 7 (2.3) | 0 (0.0) | 6 (3.0) | 0 (0.0) |
| Somnolence | 137 (19.0) | 18 (13.0) | 16 (3.1) | 0 (0.0) | 6 (2.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Headache | 87 (12.1) | 15 (10.9) | 20 (3.9) | 6 (5.5) | 13 (4.3) | 2 (4.7) | 8 (4.0) | 0 (0.0) |
| Nasopharyngitis | 76 (10.6) | 17 (12.3) | 29 (5.7) | 4 (3.7) | 9 (3.0) | 1 (2.3) | 7 (3.5) | 0 (0.0) |
| Fatigue | 63 (8.8) | 14 (10.1) | 8 (1.6) | 2 (1.8) | 7 (2.3) | 0 (0.0) | 4 (2.0) | 0 (0.0) |
| Irritability | 59 (8.2) | 17 (12.3) | 10 (2.0) | 2 (1.8) | 9 (3.0) | 0 (0.0) | 4 (2.0) | 0 (0.0) |
| Upper respiratory tract infection | 47 (6.5) | 18 (13.0) | 10 (2.0) | 3 (2.8) | 9 (3.0) | 0 (0.0) | 2 (1.0) | 0 (0.0) |
| Vertigo | 31 (4.3) | 14 (10.1) | 2 (0.4) | 1 (0.9) | 1 (0.3) | 0 (0.0) | 2 (1.0) | 0 (0.0) |
Abbreviations: AE, adverse event; FBTCS, focal to bilateral tonic‐clonic seizures; GTCS, generalized tonic‐clonic seizures; TEAE, treatment‐emergent adverse event.
A patient with two or more AEs in the same system organ class (or preferred term) is counted only once for that system organ class (or preferred term).
Across Years 1, 2, 3, and 4 of perampanel treatment, psychiatric TEAEs occurred in 324 (45.0%), 89 (17.4%), 61 (20.0%), and 32 (16.0%) patients with FBTCS, respectively, and in 90 (65.2%), 16 (14.7%), 4 (9.3%), and 0 (0.0%) patients with GTCS, respectively (Table S3). Severe TEAEs were reported in 127 (17.6%) and 20 (14.5%) patients with FBTCS and GTCS, respectively; incidence of severe TEAEs was highest during the first year of perampanel exposure for both seizure types (Table 3). Convulsion and dizziness were the two most common severe TEAEs for FBTCS (n = 15 [2.1%] and n = 13 [1.8%], respectively) and GTCS (n = 2 [1.4%] and n = 3 [2.2%], respectively; Table S4). Concomitant ASMs taken by patients with severe TEAEs are shown in Table S5; the most common in patients with FBTCS was valproic acid (n = 37 [29.1%]) and with GTCS was lamotrigine (n = 10 [50.0%]).
The numbers of TEAEs leading to dose adjustments, withdrawals, reductions, or interruptions were highest during the first year of perampanel exposure for both seizure types (Table 3). During Year 1, TEAEs leading to withdrawal were reported in 67 (9.3%) patients with FBTCS and 12 (8.7%) patients with GTCS. Overall, serious TEAEs were reported in 147 (20.4%) patients with FBTCS and 18 (13.0%) 24 , 25 patients with GTCS; the most common were convulsion (n = 29 [4.0%; FBTCS]; n = 3 [2.2%; GTCS]), status epilepticus (n = 14 [1.9%; FBTCS]; n = 0 [0.0%; GTCS]), and pneumonia (n = 11 [1.5%; FBTCS]; n = 1 [0.7%; GTCS]). Overall, 46 (6.4%) patients with FBTCS and seven (5.1%) patients with GTCS had serious TEAEs that were considered treatment‐related. Treatment‐related serious TEAEs that occurred in two or more patients were convulsion (n = 10 [1.4%]), status epilepticus (n = 6 [0.8%]), aggression (n = 4 [0.6%]), epilepsy and psychotic disorder (both n = 3 [0.4%]), and GTCS (investigator term: grand mal convulsion), postictal psychosis, and suicide attempt (all n = 2 [0.3%]) for FBTCS, and suicide attempt (n = 2 [1.4%]) for GTCS. SUDEP was reported in one patient with FBTCS (aged 45 years; receiving concomitant lamotrigine) and no patients with GTCS; an additional patient with FBTCS also reported “sudden death with unknown cause” (aged 27 years; receiving concomitant levetiracetam, phenobarbital, and oxcarbazepine).
In a subgroup of adolescent patients (aged ≥12 to <18 years), TEAEs were reported in 76/81 (93.8%) patients with FBTCS and 15/19 (78.9%) patients with GTCS; for both seizure types, TEAE incidence was highest during the first year of perampanel exposure (data not shown). The most common TEAEs, occurring in ≥15% of adolescent patients with FBTCS, were nasopharyngitis (n = 26 [32.1%]), dizziness (n = 25 [30.9%]), somnolence (n = 20 [24.7%]), aggression (n = 18 [22.2%]), headache (n = 16 [19.8%]), convulsion (n = 15 [18.5%]), and irritability (n = 13 [16.0%]). In adolescents with GTCS, these were dizziness (n = 5 [26.3%]), somnolence (n = 4 [21.1%]), nasopharyngitis (n = 3 [15.8%]), upper respiratory tract infection (n = 3 [15.8%]), and cough (n = 3 [15.8%]). Overall, 11/81 (13.6%) adolescent patients with FBTCS had TEAEs leading to discontinuation; those leading to discontinuation in two or more patients were convulsion, aggression, and anger (n = 2 each). Only 1/19 (5.3%) adolescent patients with GTCS had TEAEs leading to discontinuation (altered visual depth perception, nausea, and dizziness).
4. DISCUSSION
Long‐term, once‐daily, adjunctive perampanel was efficacious and safe in patients aged 12 years and above with FBTCS or GTCS. Retention rates remained high for up to 4 years (FBTCS; 50.0%) and 2 years (GTCS; 49.2%); most discontinuations were due to administrative/other reasons or patient choice. Discontinuations were higher for FBTCS (76.1%) vs GTCS (43.5%), which may be explained by most patients with FBTCS in Study 307 being listed as discontinued after study closure following perampanel approval. When administrative/other reasons were excluded, discontinuation rates were still slightly higher for FBTCS (47.2%) than GTCS (33.3%), potentially due to the longer duration of FBTCS studies and patients with FBTCS possibly experiencing other frequent focal seizures. The higher proportion of FBTCS patients receiving up to three concomitant baseline ASMs (39.0%) vs GTCS patients (20.3%) suggests increased refractoriness in the FBTCS population, and polytherapy may have resulted in greater AE incidences, leading to increased discontinuations.
As cohorts persisting at later treatment intervals may have included patients who tolerated perampanel and responded well to treatment, and therefore were more likely to continue treatment, dropout analyses were conducted to account for potential selection bias. These analyses demonstrated improvements in seizure outcomes that were maintained for up to 4 (FBTCS) and 3 years (GTCS). During Year 4 of adjunctive perampanel, median reductions in FBTCS frequency per 28 days from preperampanel baseline were 61.8% (including dropouts) and 96.1% (excluding dropouts). Corresponding reductions for GTCS during Year 3 were 82.9% and 100.0%, respectively. Therefore, seizure frequency reductions were observed for up to 4 and 3 years for FBTCS and GTCS, respectively, when including dropouts, although these were diminished at later timepoints compared with the analysis excluding dropouts.
OLEx studies provide information on long‐term safety/tolerability, particularly regarding new safety signals that may not emerge during shorter trials. 9 , 10 The safety profile reported here was generally consistent with perampanel’s known safety profile and the double‐blind studies; no new safety signals emerged with long‐term treatment. 3 , 4 , 5 , 6 , 7 , 8 TEAE incidence reported during Year 1 for FBTCS (85.3%) was similar to those reported for all focal seizures in pooled analyses of extension studies of adjunctive treatment with perampanel (91.5%), lacosamide (81.9%), eslicarbazepine (72.9%), and brivaracetam (84.5%). 9
TEAE incidence was lower for GTCS vs FBTCS across Years 2 and 3. Interpretation of these data should consider the smaller patient numbers for GTCS vs FBTCS. During Year 1, serious TEAEs were only reported in 81 (11.3%) and 10 (7.2%) patients with FBTCS and GTCS, respectively. It is unsurprising that TEAE incidence and frequency of the most common TEAEs (excluding fatigue in patients with GTCS) were higher during maintenance than titration, because the duration of follow‐up and perampanel exposure was longer during the former. A TEAE exposure‐adjusted analysis may provide a more representative comparison of TEAE frequency during these periods. These differences may reflect differences in the number, types, and flexible dosing of concomitant ASMs during the study periods. As patients received treatment with combinations of ASMs, it would be challenging to draw conclusions on the effect of interactions between perampanel and other ASMs on safety across studies.
The most common TEAEs reported in our adolescent subgroup are similar to those previously described in an Italian report of patients aged ≤18 years with refractory epilepsy treated with long‐term perampanel for 5‐13 months, which found that irritability, aggressiveness, reduced vigilance/fatigue, and dizziness were the most commonly reported. 26 There were very few adolescent patients who had TEAEs leading to discontinuation, and although behavioral disturbances have previously been reported to be common in adolescent patients, events such as aggression and anger only led to the discontinuation of two adolescent patients each. These data suggest that long‐term perampanel is well tolerated in adolescent patients with FBTCS or GTCS; however, the small patient numbers in these subgroups should be noted.
As tonic‐clonic seizures are disabling, resulting in injuries, 15 long postictal periods of confusion, 20 and increased risk of SUDEP, 18 , 27 control of these seizures is critically important. It can be challenging to distinguish between FBTCS and GTCS. 20 Few ASMs are licensed to treat both, and some ASMs that are efficacious against FBTCS aggravate seizures in IGE. 21 , 22 , 23 Perampanel is a selective antagonist of α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid (AMPA) receptors. 28 AMPA receptors are rational targets for epilepsy treatment, as they are activated by glutamate, the major excitatory neurotransmitter, which can subsequently induce seizures via initiation of glutamatergic transmission. 28 , 29 AMPA administration provokes seizures in preclinical models, 30 and AMPA receptors are implicated in various disorders characterized by neuronal overexcitation. 31 , 32 Evidence suggests tonic‐clonic seizures are characterized by abnormalities in cortical hyperexcitability that can be affected by ASMs. 33 , 34 In preclinical models, perampanel protected mice from tonic‐clonic seizures in audiogenic and maximal electroshock‐induced seizure tests with higher potency than carbamazepine and sodium valproate. 28 , 32 In phase III clinical trials, perampanel was associated with reductions in FBTCS and GTCS frequency. 4 , 5 , 6 , 8 , 11 Our results, together with previous preclinical and clinical data, indicate perampanel offers long‐term efficacy for both seizure types, suggesting perampanel may be a viable treatment option for cases where physicians are unable to distinguish between FBTCS and GTCS.
Real‐world studies of perampanel have reported retention (1 year) and seizure‐freedom rates (at least 6 months) in epilepsy patients. 35 , 36 A pooled analysis of perampanel observational studies demonstrated 1117/2332 (47.9%) patients were continuing on perampanel at 12 months; similar retention rates were reported for patients with focal seizures only (49.5% [856/1728]) and generalized seizures only (57.0% [61/107]). 35 Seizure freedom for at least 6 months was reported in 74/803 (9.2%) patients 1 year after perampanel initiation. 35 In a retrospective, 1‐year observational study in patients with IGE and GTCS, the perampanel 12‐month retention rate was 83.2% (124/149). 36 At 12 months, 88/149 (59.1%) and 72/115 (62.6%) patients had been free from all seizures or GTCS, respectively, for at least 6 months prior. 36 While these studies support long‐term (1‐year) perampanel use, our analysis builds upon this by evaluating retention, efficacy, and safety for tonic‐clonic seizure types separately (FBTCS and GTCS) over a longer treatment duration. We also compared outcomes to explore whether perampanel offers similar efficacy and tolerability for both FBTCS and GTCS, because an approved ASM that is efficacious against both would be advantageous. Therefore, in the context of long‐term treatment of a chronic disease, our results may be particularly important.
Limitations of this analysis include those inherent to post hoc analyses, and the open‐label nature of the studies, meaning no placebo data are available. The analysis was conducted across four studies with different populations and study designs, and concomitant ASM dose adjustments were permitted in three studies, potentially influencing outcomes. Lastly, the small GTCS patient population limits interpretation of the impact of efficacy outcomes from Years 3 and 4. Despite these limitations, we show that large proportions of patients with FBTCS or GTCS had reductions in seizures during long‐term adjunctive perampanel therapy with no new safety signals, which is encouraging given the refractory nature of the patient populations and seizure types.
The finding that retention rates and improvements in seizure responses were relatively stable over treatment periods of up to 4 years (FBTCS) and 3 years (GTCS) is promising, and taken alongside the tolerability profile, suggests perampanel demonstrates a favorable risk/benefit ratio as long‐term adjunctive therapy in patients with FBTCS or GTCS. Consequently, and owing to the severity of these seizure types, adjunctive perampanel may be a suitable long‐term treatment for FBTCS or GTCS.
CONFLICT OF INTEREST
G.L.K. has served as a consultant for Eisai, Otsuka, and Shire Pharmaceuticals, and has received research support from Eisai, SK Life Science, and UCB Pharma. B.W. is a former employee of Eisai Inc., and has carried out consultancy work for Eisai Inc. A.P. is an employee of Eisai Ltd. M.M. is an employee of Eisai Inc. A.L. is a former employee of Eisai Inc. R.T.W. has been a clinical trial investigator for Aquestive, Biogen, Cavion, Cerevel, Eisai, Engage Pharma, Greenwich Biosciences, Lundbeck, Otsuka, Pfizer, SK Life Science, Sunovion, UCB Pharma, Xenon, and Zogenix; has served on advisory boards and/or carried out consulting work for Brain Sentinel, Cerevel, Eisai, Engage Pharma, Greenwich Biosciences, Lundbeck, Otsuka, SK Life Science, Sunovion, and UCB Pharma; has received speaker bureau honoraria from Aquestive, Eisai, Greenwich Biosciences, LivaNova, SK Life Science, Sunovion, and UCB Pharma; and is a member of the Epilepsy Study Consortium. The remaining authors have no conflicts of interest. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
AUTHOR CONTRIBUTIONS
All authors provided substantial contributions to the conception, study design, acquisition of data, or data analysis. All authors were involved in the interpretation of the results, the reviewing and approval of the manuscript, and the decision to submit the article for publication. All authors also confirm accountability for the accuracy and integrity of the work.
Supporting information
Table S1‐S5
ACKNOWLEDGMENTS
The authors would like to thank the patients who participated in these studies and their families, as well as all study investigators and their teams. Francesco Bibbiani, formerly an employee of Eisai Inc., contributed to the study design and interpretation of the results. Medical writing support, under the direction of the authors, was provided by David Pertab, PhD, of CMC AFFINITY, McCann Health Medical Communications, and Fiona Scott, PhD, on behalf of CMC AFFINITY, funded by Eisai Inc., in accordance with Good Publication Practice (GPP3) guidelines. Studies 207, 307, 335 OLEx, and 332 OLEx, as well as the present analysis, were funded by Eisai Inc. The data reported in this paper have previously been presented at the 70th Annual Meeting of the American Epilepsy Society, Houston, Texas, December 2‐6, 2016; the 69th Annual Meeting of the American Academy of Neurology, Boston, Massachusetts, April 22‐28, 2017; the Association of British Neurologists Annual Meeting, Liverpool, UK, May 3‐5, 2017; and the 32nd International Epilepsy Congress, Barcelona, Spain, September 2‐6, 2017. In addition, some of the data for GTCS were previously presented at the 12th European Congress on Epileptology, Prague, Czech Republic, September 11‐15, 2016.
Rektor I, Krauss GL, Inoue Y, et al. Assessment of the long‐term efficacy and safety of adjunctive perampanel in tonic‐clonic seizures: Analysis of four open‐label extension studies. Epilepsia. 2020;61:1491–1502. 10.1111/epi.16573
REFERENCES
- 1. Food and Drug Administration . Fycompa® prescribing information. May 2019. Available at: https://www.fycompa.com/‐/media/Files/Fycompa/Fycompa_Prescribing_Information.pdf. Accessed May 11, 2020.
- 2. European Medicines Agency . Fycompa® annex I: summary of product characteristics. April 2017. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Information/human/002434/WC500130815.pdf. Accessed May 11, 2020.
- 3. Krauss GL, Bar M, Biton V, et al. Tolerability and safety of perampanel: two randomized dose‐escalation studies. Acta Neurol Scand. 2012;125:8–15. [DOI] [PubMed] [Google Scholar]
- 4. French JA, Krauss GL, Biton V, et al. Adjunctive perampanel for refractory partial‐onset seizures: randomized phase III study 304. Neurology. 2012;79:589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. French JA, Krauss GL, Steinhoff BJ, et al. Evaluation of adjunctive perampanel in patients with refractory partial‐onset seizures: results of randomized global phase III study 305. Epilepsia. 2013;54:117–25. [DOI] [PubMed] [Google Scholar]
- 6. Krauss GL, Serratosa JM, Villanueva V, et al. Randomized phase III study 306: adjunctive perampanel for refractory partial‐onset seizures. Neurology. 2012;78:1408–15. [DOI] [PubMed] [Google Scholar]
- 7. Nishida T, Lee SK, Inoue Y, et al. Adjunctive perampanel in partial‐onset seizures: Asia‐Pacific, randomized phase III study. Acta Neurol Scand. 2018;137:392–9. [DOI] [PubMed] [Google Scholar]
- 8. French JA, Krauss GL, Wechsler RT, et al. Perampanel for tonic‐clonic seizures in idiopathic generalized epilepsy: a randomized trial. Neurology. 2015;85:950–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kwok CS, Johnson EL, Krauss GL. Comparing safety and efficacy of "third‐generation" antiepileptic drugs: long‐term extension and post‐marketing treatment. CNS Drugs. 2017;31:959–74. [DOI] [PubMed] [Google Scholar]
- 10. Rektor I, Krauss GL, Bar M, et al. Perampanel Study 207: long‐term open‐label evaluation in patients with epilepsy. Acta Neurol Scand. 2012;126:263–9. [DOI] [PubMed] [Google Scholar]
- 11. Krauss GL, Perucca E, Ben‐Menachem E, et al. Long‐term safety of perampanel and seizure outcomes in refractory partial‐onset seizures and secondarily generalized seizures: results from phase III extension study 307. Epilepsia. 2014;55:1058–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. ClinicalTrials.gov . A study with an open‐label extension phase to evaluate the efficacy and safety of perampanel (E2007) administered as an adjunctive therapy in subjects with refractory partial‐onset seizures. 2018. Available at: https://clinicaltrials.gov/ct2/show/NCT01618695. Accessed May 11, 2020.
- 13. ClinicalTrials.gov . A efficacy and safety study of adjunctive perampanel in primary generalized tonic clonic seizures. Available at: http://www.clinicaltrials.gov/ct2/show/NCT01393743. Accessed May 11, 2020.
- 14. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:522–30. [DOI] [PubMed] [Google Scholar]
- 15. Asadi‐Pooya AA, Nikseresht A, Yaghoubi E, Nei M. Physical injuries in patients with epilepsy and their associated risk factors. Seizure. 2012;21:165–8. [DOI] [PubMed] [Google Scholar]
- 16. Holst AG, Winkel BG, Risgaard B, et al. Epilepsy and risk of death and sudden unexpected death in the young: a nationwide study. Epilepsia. 2013;54:1613–20. [DOI] [PubMed] [Google Scholar]
- 17. Thurman DJ, Hesdorffer DC, French JA. Sudden unexpected death in epilepsy: assessing the public health burden. Epilepsia. 2014;55:1479–85. [DOI] [PubMed] [Google Scholar]
- 18. Hesdorffer DC, Tomson T, Benn E, et al. Do antiepileptic drugs or generalized tonic‐clonic seizure frequency increase SUDEP risk? A combined analysis. Epilepsia. 2012;53:249–52. [DOI] [PubMed] [Google Scholar]
- 19. Rheims S, Ryvlin P. Pharmacotherapy for tonic‐clonic seizures. Expert Opin Pharmacother. 2014;15:1417–26. [DOI] [PubMed] [Google Scholar]
- 20. Wyllie EE. Treatment of Epilepsy: Principles and Practice. 5th ed Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 21. Thomas P, Valton L, Genton P. Absence and myoclonic status epilepticus precipitated by antiepileptic drugs in idiopathic generalized epilepsy. Brain. 2006;129:1281–92. [DOI] [PubMed] [Google Scholar]
- 22. Shields WD, Saslow E. Myoclonic, atonic, and absence seizures following institution of carbamazepine therapy in children. Neurology. 1983;33:1487–9. [DOI] [PubMed] [Google Scholar]
- 23. Vendrame M, Khurana DS, Cruz M, et al. Aggravation of seizures and/or EEG features in children treated with oxcarbazepine monotherapy. Epilepsia. 2007;48:2116–20. [DOI] [PubMed] [Google Scholar]
- 24. Wechsler RT, French J, Trinka E, et al. Long‐term safety and efficacy outcomes of adjunctive perampanel: an open‐label extension (OLEx) of a phase III study in patients with drug‐resistant primary generalized tonic‐clonic (PGTC) seizures in idiopathic generalized epilepsy (IGE) (P5.233). Neurology. 2017;88(P5):233. [Google Scholar]
- 25. Wechsler RT, French J, Trinka E, et al. Long‐term safety and efficacy of adjunctive perampanel in patients with drug‐resistant primary generalised tonic‐clonic seizures in idiopathic generalised epilepsy: results of an open‐label extension. Epilepsia. 2016;57(S2):168. [Google Scholar]
- 26. De Liso P, Vigevano F, Specchio N, et al. Effectiveness and tolerability of perampanel in children and adolescents with refractory epilepsies—an Italian observational multicenter study. Epilepsy Res. 2016;127:93–100. [DOI] [PubMed] [Google Scholar]
- 27. Harden C, Tomson T, Gloss D, et al. Practice guideline summary: Sudden unexpected death in epilepsy incidence rates and risk factors: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsy Curr. 2017;17:180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hanada T, Hashizume Y, Tokuhara N, et al. Perampanel: a novel, orally active, noncompetitive AMPA‐receptor antagonist that reduces seizure activity in rodent models of epilepsy. Epilepsia. 2011;52:1331–40. [DOI] [PubMed] [Google Scholar]
- 29. Scharfman HE. The neurobiology of epilepsy. Curr Neurol Neurosci Rep. 2007;7:348–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yamaguchi S, Donevan SD, Rogawski MA. Anticonvulsant activity of AMPA/kainate antagonists: comparison of GYKI 52466 and NBOX in maximal electroshock and chemoconvulsant seizure models. Epilepsy Res. 1993;15:179–84. [DOI] [PubMed] [Google Scholar]
- 31. Seeburg PH. The TINS/TiPS lecture. The molecular biology of mammalian glutamate receptor channels. Trends Neurosci. 1993;16:359–65. [DOI] [PubMed] [Google Scholar]
- 32. Di Bonaventura C, Labate A, Maschio M, et al. AMPA receptors and perampanel behind selected epilepsies: current evidence and future perspectives. Expert Opin Pharmacother. 2017;18:1751–64. [DOI] [PubMed] [Google Scholar]
- 33. Badawy RAB, Vogrin SJ, Lai A, et al. Capturing the epileptic trait: cortical excitability measures in patients and their unaffected siblings. Brain. 2013;136:1177–91. [DOI] [PubMed] [Google Scholar]
- 34. Badawy RAB, Vogrin SJ, Lai A, et al. Patterns of cortical hyperexcitability in adolescent/adult‐onset generalized epilepsies. Epilepsia. 2013;54:871–8. [DOI] [PubMed] [Google Scholar]
- 35. Rohracher A, Zimmermann G, Villanueva V, et al. Perampanel in routine clinical use across Europe: pooled, multicenter, observational data. Epilepsia. 2018;59:1727–39. [DOI] [PubMed] [Google Scholar]
- 36. Villanueva V, Montoya J, Castillo A, et al. Perampanel in routine clinical use in idiopathic generalized epilepsy: the 12‐month GENERAL study. Epilepsia. 2018;59:1740–52. [DOI] [PubMed] [Google Scholar]
- 37. ClinicalTrials.gov . An open‐label extension study to evaluate the safety and tolerability of perampanel (E2007) administered as an adjunctive therapy in epilepsy subjects. Available at: https://clinicaltrials.gov/ct2/show/record/NCT02427607. Accessed May 11, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S5


