Abstract
Objective
Interleukin‐17A (IL‐17A) and tumor necrosis factor (TNF) contribute to the pathogenesis of psoriatic arthritis (PsA). However, their functional relationship in PsA synovitis has not been fully elucidated. Additionally, although CD8+ T cells in PsA have been recognized via flow cytometry as a source of IL‐17A production, it is not clear whether CD8+ T cells secrete IL‐17A under more physiologically relevant conditions in the context from PsA synovitis. This study was undertaken to clarify the roles of IL‐17A and TNF in the synovial fluid (SF) from patients with PsA and investigate the impact of CD8+ T cells on IL‐17A production.
Methods
IL‐17A+ T cells were identified by flow cytometry in SF samples from 20 patients with active PsA, blood samples from 22 treatment‐naive patients with PsA, and blood samples from 22 healthy donors. IL‐17A+ T cells were sorted from 12 PsA SF samples and stimulated using anti‐CD3/anti‐CD28 or phorbol myristate acetate (PMA) and ionomycin ex vivo, alone (n = 3) or together with autologous monocytes (n = 3) or PsA fibroblast‐like synoviocytes (FLS) (n = 5–6). To evaluate the differential allogeneic effects of neutralizing IL‐17A and TNF, SF CD4+ T cells and PsA FLS cocultures were also used (n = 5–6).
Results
Flow cytometry analyses of SF samples from patients with PsA showed IL‐17A positivity for CD4+ and CD8+ T cells (IL‐17A, median 0.71% [interquartile range 0.35–1.50%] in CD4+ cells; median 0.44% [interquartile range 0.17–1.86%] in CD8+ T cells).
However, only CD4+ T cells secreted IL‐17A after anti‐CD3/anti‐CD28 activation, when cultured alone and in cocultures with PsA monocytes or PsA FLS (each P < 0.05). Remarkably, CD8+ T cells only secreted IL‐17A after 4‐ or 72‐hour stimulation with PMA/ionomycin. Anti–IL‐17A and anti‐TNF treatments both inhibited PsA synovitis ex vivo. Neutralizing IL‐17A strongly inhibited IL‐6 (P < 0.05) and IL‐1β (P < 0.01), while anti‐TNF treatment was more potent in reducing matrix metalloproteinase 3 (MMP‐3) (P < 0.05) and MMP‐13.
Conclusion
CD8+ T cells, in contrast to CD4+ T cells, in SF specimens obtained from PsA patients did not secrete IL‐17A following T cell receptor activation. Overlapping, but distinct, effects at the level of inflammatory cytokines and MMPs were found after neutralizing IL‐17A or TNF ex vivo in a human model of PsA synovitis.
Introduction
Psoriatic arthritis (PsA) is a chronic inflammatory arthritis that develops in up to 30% of patients with active psoriasis or a history of psoriasis 1. Activated T cells have long been reported to contribute to arthropathies, including PsA pathogenesis 2, and therapies that deplete lymphocytes have been tested in PsA patients with limited clinical response 3, with lack of efficacy during depletion therapy attributed to the presence of modest lymphopenia in the synovial fluid (SF) despite a significant reduction in lymphocytes in the peripheral blood. This pinpoints the pathogenic role of local T cells in PsA joints. Moreover, enhanced local clonal expansions of CD4+ and CD8+ T cells were identified in PsA SF compared to PsA peripheral blood 4, further suggesting that intraarticular T cell activation drives PsA joint inflammation.
Activated T cells excrete a wide range of proinflammatory cytokines including interleukin‐17A (IL‐17A) and tumor necrosis factor (TNF), both of which have been shown to be elevated in PsA SF or synovium 5, 6, 7. Evidence from studies of PsA patients and other arthropathies points to the involvement of IL‐17A in the pathogenesis of arthritis 8, 9. It has been suggested that CD4+ T cells 10, 11, CD8+ T cells 12, 13, 14, and group 3 innate lymphoid cells (ILCs) 15 can be potential sources of IL‐17A in PsA SF or synovium. However, it is still not clear which of the above cell types is the main producer of IL‐17A in local joints affected with PsA. Recently, it was reported that ILC3s fail to express IL‐17A upon in vitro stimulation in joints affected with spondyloarthritis 16. Nevertheless, direct ex vivo comparison of IL‐17A production upon T cell receptor (TCR) activation by CD4+ and CD8+ T cells has yet to be performed using PsA SF specimens.
TNF is a proinflammatory cytokine present at high levels in PsA 5, 6. Neutralization of TNF has been proven to be effective in reducing local inflammation while slowing or halting joint destruction in individuals with PsA 17. However, with success rates of ~60–70% in patients with active PsA, standard anti‐TNF therapies fail to achieve satisfactory results in the remaining nonresponders 18, 19. Even among responders, initial efficacy with TNF inhibition fails to sustain a response in some subgroups of patients 18. Interestingly, in psoriasis patients who are not responsive to the TNF blocker, etanercept, persistent serum levels of IL‐17A were observed 20. Recent studies have shown that antibodies targeting IL‐17A, including secukinumab and ixekizumab, were effective in treating PsA patients 21, 22, and both have been approved for treatment of active PsA. Similar to anti‐TNF biologic agents, IL‐17A blockade also successfully suppresses joint inflammation and prevents radiographic progression 21, 22. Patients intolerant or nonresponsive to TNF inhibition still showed disease improvement with anti–IL‐17A therapy 23, 24. Further understanding of the potential overlapping and distinct effects of anti–IL‐17A and anti‐TNF treatments is still relevant, and additional insights may help guide clinical practice and potentially lead to achievement of more sustainable therapeutic effects for individuals with PsA.
Patients and methods
Study design
IL‐17A+ CD4+ and CD8+ T cells were first identified using flow cytometry in the following groups: 1) SF from patients with active PsA, 2) peripheral blood from treatment‐naive patients with early PsA, and 3) peripheral blood from age‐ and sex‐matched healthy volunteers as controls. CD4+ and CD8+ T cells from SF samples were then sorted to compare ex vivo IL‐17A secretion in the absence or presence of stimulation with anti‐CD3/anti‐CD28 or phorbol myristate acetate (PMA)/ionomycin. Sorted CD4+ and CD8+ T cells were further cocultured with autologous PsA monocytes or allogeneic PsA fibroblasts to examine ex vivo IL‐17A secretion. With TCR activation, sorted SF CD4+ T cells were cocultured with PsA fibroblasts to evaluate the effects of IL‐17A neutralization compared to TNF blockade.
Patients
SF samples from 20 patients with active PsA were collected. Peripheral blood from 22 treatment‐naive patients with early PsA and 22 age‐ and sex‐matched healthy volunteers were also included in the analysis. PsA was diagnosed by rheumatologists according to the Classification of Psoriatic Arthritis (CASPAR) Study Group criteria 25. This study is part of the Dutch Southwest Psoriatic Arthritis Register (DEPAR) and is approved by the Ethics Review Board of Erasmus University Medical Center Rotterdam. Details on the demographic and clinical characteristics of the PsA patients and healthy volunteers are summarized in Supplementary Table 1, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41271/abstract.
Flow cytometry and cell sorting
Cell pellets from PsA SF samples were stained for surface markers with or without density gradient centrifugation in accordance with standard practice. Peripheral blood from patients with early PsA and healthy volunteers were also lysed for red blood cells and stained for surface markers in the same manner as described above. For intracellular staining, parts of the cell pellets were stimulated for 4 hours with 50 ng/ml of PMA (Sigma‐Aldrich), 500 ng/ml of ionomycin, and GolgiStop (BD Biosciences). Cells were then stained for surface markers and Fixable Viability Dye eF506 (eBioscience no. 65‐0866‐14) according to the manufacturer's instructions. Cells were then fixed with 2% paraformaldehyde in phosphate buffered saline (PBS) and permeabilized with 0.5% saponin buffer (0.5% bovine serum albumin, 0.05% NaN3 in PBS).
Sorted CD4+ and CD8+ T cells were cultured or cocultured ex vivo for 72 hours and restimulated to examine intracellular staining as described above. An LSRII flow cytometer (BD Biosciences) was used to analyze samples, and results were processed with FlowJo software (TreeStar). Cells were sorted with a FACSAria cell sorter (BD Biosciences), and the purity of the sorted cell populations was found to be ≥98%.
Flow cytometry antibodies
The following antibodies were used during staining: phycoerythrin (PE)–CF594–conjugated CD8 (clone RPA‐T8; BD Biosciences), PE–Cy7–conjugated TCRγδ (clone 11F2; BD Biosciences), PE–Cy7–conjugated CD19 (clone SJ25C1; BD Biosciences), PE–CF594–conjugated CD45 (clone HI30; BD Biosciences), allophycocyanin (APC)–H7–conjugated CD14 (clone MϕP9; BD Biosciences), PerCP–Cy5.5–conjugated CD45RO (clone UCHL1; BD Biosciences), Alexa Fluor 700–conjugated CD56 (clone 5.1H11; BioLegend), Alexa Fluor 700–conjugated CD15 (clone W6D3; BioLegend), BV785‐conjugated CD16 (clone 3G8; BioLegend), Alexa Fluor 488–conjugated interferon‐γ (IFNγ) (clone 4S.B3; BioLegend), BV421‐conjugated TNF (clone MAb11; BioLegend), PE–Cy7–conjugated CD25 (clone BC96; BioLegend), PE‐conjugated IL‐17A (clone eBio64DEC17; eBioscience), eF660‐conjugated IL‐22 (clone 22URTI; eBioscience), PerCP–Cy5.5–conjugated CD45 (clone HI30; Sony Biotechnology), BV785‐conjugated CD3 (clone OKT3; Sony Biotechnology), BV711‐conjugated CD4 (clone OKT4; Sony Biotechnology), and fluorescein isothiocyanate–conjugated CD4 (clone RPA‐T4; Sony Biotechnology).
Cell culture
Sorted T cells were seeded at 2.5 × 104/well in 96‐well round‐bottomed culture plates and stimulated with 0.3 μg/ml of soluble anti‐CD3 (Sanquin) and 0.4 μg/ml of soluble anti‐CD28 (Sanquin) for 4 hours or 72 hours in Iscove's modified Dulbecco's medium (Lonza) supplemented with 10% fetal calf serum (Invitrogen), 100 units/ml penicillin/streptomycin (Lonza), 2 mM l‐glutamine (Lonza), and 50 μM β‐mercaptoethanol (Merck). PsA fibroblast‐like synoviocytes (FLS) were isolated and cultured as described previously 26. After reaching 90% confluence, allogeneic PsA FLS were seeded at 1.0 × 104/well in 96‐well flat‐bottomed culture plates, and after overnight incubation, T cells were added at 2.5 × 104/well following incubation with soluble anti‐CD3/anti‐CD28 for 72 hours as described above. In cocultures with autologous monocytes, CD14+ monocytes at 1.0 × 104/well were seeded with T cells at 2.5 × 104/well in 96‐well round‐bottomed culture plates and incubated with soluble anti‐CD3/anti‐CD28 for 72 hours.
During neutralization experiments, 100 μg/ml of anti–IL‐17 (secukinumab; Novartis) and 1 μg/ml of anti‐TNF (adalimumab; AbbVie) were used. An isotype (IgG1κ; Sigma‐Aldrich) was included as control, and soluble anti‐CD3/anti‐CD28 activation was used as stimulation.
Enzyme‐linked immunosorbent assay (ELISA)
IFNγ, IL‐6, and IL‐8 in culture supernatants were measured by ELISA (Invitrogen) according to the manufacturer's instructions. IL‐17A, TNF, matrix metalloproteinase 1 (MMP‐1), and MMP‐3 were measured with ELISA DuoSet (R&D Systems) according to the manufacturer's instructions.
Real‐time reverse transcription–polymerase chain reaction (RT‐PCR)
RNA was isolated with a Total RNA Miniprep Kit (Sigma‐Aldrich). Complementary DNA was synthesized with 10 units/μl of Superscript II (Invitrogen) following treatment with 0.1 units/μl of DNAse (Invitrogen). RT‐PCR was performed with a ViiA 7 Sequence Detecting System (Life Technologies). Probes were chosen from the universal probe library (Roche Applied Science), and primers were designed using ProbeFinder software. Gene expression data were normalized to the expression values for the housekeeping gene hypoxanthine guanine phosphoribosyltransferase (HPRT). Primer sequences are available upon request from the corresponding author.
Statistical analysis
Flow cytometry results are shown as the median (interquartile range [IQR]), and ex vivo stimulation data are shown as the mean ± SEM. Statistical differences were determined with Student's paired or unpaired t‐test. All data analyses were performed with GraphPad Prism software version 5.0. P values less than 0.05 were considered significant.
Results
Comparable subsets of lymphoid and myeloid cells, but not B cells or natural killer (NK) cells, in PsA SF and peripheral blood
Peripheral blood samples from patients with early PsA and matched healthy volunteers (n = 22 each) as well as SF samples from patients with active PsA (n = 20) were stained for surface markers to identify immune cells. Gating strategies for cell populations are shown in Supplementary Figure 1, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41271/abstract. The median percentages of CD3+ T cells were 44.8% (IQR 8.6–55.4%) in SF from PsA patients, 43.3% (IQR 25.6–58.2%) in peripheral blood from PsA patients, and 47.5% (IQR 26.7–60.3%) in blood from healthy controls (Figure 1A). For myeloid cells, the median percentages of CD14+ monocytes in SF from PsA patients, peripheral blood from PsA patients, and peripheral blood from healthy controls were 6.2% (IQR 2.4–9.7%), 5.2% (IQR 2.7–9.9%), and 6.2% (IQR 3.7–9.9%), respectively. Median percentages of CD15+CD16+ neutrophils in SF from PsA patients, peripheral blood from PsA patients, and peripheral blood from healthy controls were 22.8% (IQR 4.3–38.3%), median 17.3% (IQR 3.3–50.4%), and median 10.2% (IQR 3.1–40.6%), respectively (Figure 1A).
Figure 1.
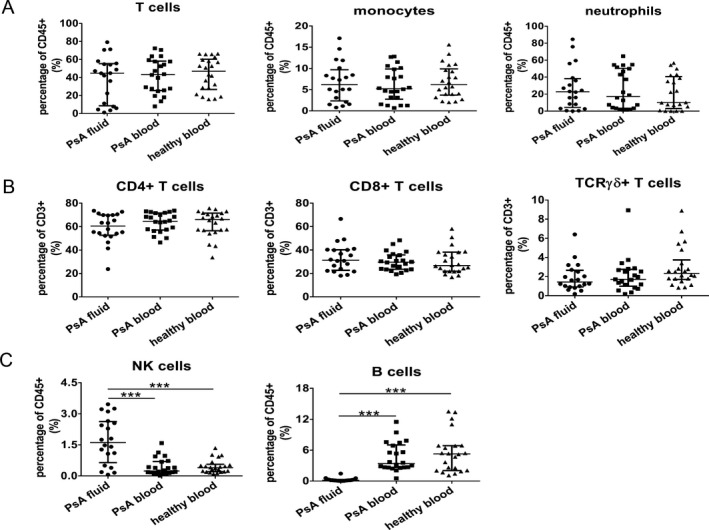
Differences in the percentages of B cells and natural killer (NK) cells in synovial fluid (SF) from patients with psoriatic arthritis (PsA) compared to peripheral blood from PsA patients and healthy controls. SF samples from 20 patients with active PsA and peripheral blood samples from 22 patients with early PsA and 22 age‐ and sex‐matched healthy volunteers were stained for surface markers. Gating strategies for cell populations are shown in Supplementary Figure 1, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41271/abstract. A, Frequency of T cells (CD3+), myeloid cells (CD14+ monocytes), and neutrophils (CD15+CD16+) among CD45+ immune cells. B, CD4+, CD8+, and T cell receptor γδ (TCRγδ) T cell subsets among CD3+ immune cells. C, Levels of NK cells (CD3–CD56bright) and B cells (CD3−CD19+) among CD45+ immune cells. Symbols represent individual samples; horizontal lines and error bars show the median and interquartile range. *** = P < 0.001.
Among CD3+ T cell subsets, the median percentages of CD4+ T cells accounted for >60% of the T cell population (60.5% [IQR 52.7–69.9%] in SF from PsA patients, 64.6% [IQR 57.1–71.7%] in peripheral blood from PsA patients, and 66.2% [IQR 56.5–1.6] in peripheral blood from healthy controls) (Figure 1B). Approximately 30% of total T cells were CD8+ cells (median 31.3% [IQR 22.6–40.3%]) in SF from PsA patients, median 29.7% [IQR 23.5–35.8%] in peripheral blood from PsA patients, and median 26.7% [21.9–38.2%] in peripheral blood from healthy controls (Figure 1B). Median percentages of TCRγδ+ T cells were ~1.5–2.5% among all groups (1.4% [IQR 0.92–2.7%] in SF from PsA patients, 1.7% [IQR 0.99–2.8%] in peripheral blood from PsA patients, and 2.3% [IQR 1.7–3.7%] in peripheral blood from healthy controls) (Figure 1B).
As shown in Figure 1C, significantly enhanced accumulation of CD56bright NK cells in the SF from PsA patients was observed when compared to peripheral blood from either treatment‐naive patients with early PsA or healthy controls (median 1.62% [IQR 0.64–2.62%] in SF from PsA patients compared to median 0.24% [IQR 0.13–0.69%] in peripheral blood from PsA patients and median 0.39% [IQR 0.20–0.56%] in peripheral blood from healthy controls). In contrast, the median percentages of CD19+ B cells in SF from PsA patients were significantly lower compared to either blood samples, with 0.17% (IQR 0.08–0.30%) in SF from PsA patients, 3.38% (IQR 2.65–7.71%) in peripheral blood from PsA patients, and 5.26% (IQR 2.05–6.87%) in peripheral blood from healthy controls (Figure 1C).
Enrichment of IL‐17A+CD8+ T cells in PsA SF compared to peripheral blood
Cell pellets from the SF from patients with active PsA (n = 20) and peripheral blood from treatment‐naive patients with early PsA and healthy volunteers (n = 22 each) were intracellularly stained for IL‐17A, IFNγ, TNF, and IL‐22. Intracellular staining showed that surface marker CD4, but not CD8, was down‐regulated in the PsA SF following stimulation with PMA and ionomycin (Supplementary Figure 2, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41271/abstract). Therefore, the CD45+CD3+CD8− T cell population was considered as CD4+ T cells. In Figure 2A, representative results of intracellular staining for IL‐17A, IFNγ, TNF, and IL‐22 among CD4+ and CD8+ T cells in SF from PsA patients, peripheral blood from PsA patients, and peripheral blood from healthy volunteers are shown.
Figure 2.
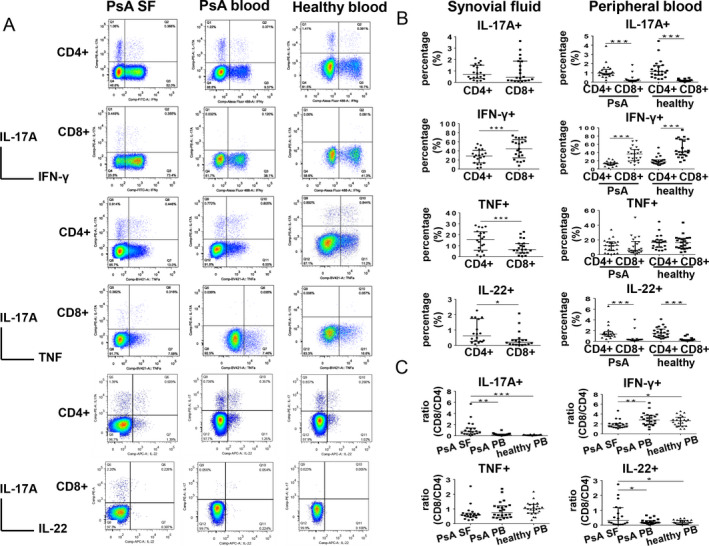
Enrichment of interleukin‐17A+ (IL‐17A+) CD8+ T cells in synovial fluid (SF) samples from patients with psoriatic arthritis (PsA) compared to peripheral blood (PB) samples. A, Representative intracellular staining for IL‐17A, interferon‐γ (IFNγ), tumor necrosis factor (TNF), and IL‐22 among SF samples from PsA patients (n = 20), peripheral blood samples from PsA patients (n = 22), and peripheral blood samples from healthy controls (n = 22) after 4 hours of stimulation with phorbol myristate acetate/ionomycin. B, IL‐17A, IFNγ, TNF, and IL‐22 staining of CD4+ T cells and CD8+ T cells in PsA SF (left) and peripheral blood (right). C, Percentage ratios of CD8+ T cells to CD4+ T cells summarized for each cytokine staining in individual samples. Symbols represent individual samples; horizontal lines and error bars show the median and interquartile range. * = P < 0.05; ** = P < 0.01; *** = P < 0.001.
In PsA SF samples, the median percentages of IL‐17A expression were not significantly different between CD4+ and CD8+ T cells (in CD4+ T cells, median 0.71% [IQR 0.35–1.50%]; in CD8+ T cells, median 0.44% [IQR 0.17–1.86%]) (Figure 2B). In peripheral blood from PsA patients, however, the median percentages of IL‐17A expression were significantly lower in CD8+ T cells than in CD4+ T cells (in CD8+ T cells, median 0.13% [IQR 0.09–0.22%]; in CD4+ T cells, median 0.95% [IQR 0.77–1.36%]) (Figure 2B). A similar result with regard to IL‐17A expression within the T cell subsets was found in the peripheral blood from healthy controls (in CD4+ T cells, median 1.15% [IQR 0.73–1.78%]; in CD8+ T cells, median 0.13% [IQR 0.08–0.21%]) (Figure 2B).
In the SF from patients PsA, the percentage of IFNγ+ CD8+ T cells was significantly higher than the percentage of IFNγ+ CD4+ T cells. In PsA SF, the median proportion of IFNγ+ cells among CD4+ T cells was 44.4% (IQR 24.7–64.8%) compared to a median proportion of IFNγ+ cells among CD4+ T cells of 28.8% (IQR 13.5–41.2%). Similar differences were observed in peripheral blood samples from PsA patients in regard to the percentage of IFNγ+CD4+ cells (median 13.1% [IQR 10.0–15.2%]) compared to the percentage of IFNγ+CD8+ cells (median 36.4% [IQR 20.3–47.2%]) as well as in peripheral blood from healthy controls (median 17.6% [IQR 13.3–22.2%] for IFNγ+CD4+ cells versus median 42.1% [IQR 34.0–72.0%] for IFNγ+CD8+ cells) (Figure 2B).
In the SF from PsA patients, the median percentage of TNF+CD4+ T cells was significantly higher than percentage of TNF+CD8+ T cells (for CD4+ T cells, median 15.7% [IQR 5.8–22.7%]; for CD8+ T cells, 6.5% [IQR 3.4–12.1%]) (Figure 2B). However, findings were similar in the peripheral blood from PsA patients (median 12.5% [IQR 5.3–17.4%] for TNF+CD4+ T cells versus median 5.9% [IQR 3.5–17.9%] for TNF+CD8+ T cells) and in the peripheral blood from healthy controls (median 16.5% [IQR 9.9–20.1%] for TNF+CD4+ T cells versus median 16.2% [IQR 9.1–22.0%] for TNF+CD8+ T cells) (Figure 2B).
With regard to IL‐22+ cells, the proportion of IL‐22+CD4+ T cells was higher than the proportion of IL‐22+CD8+ T cells in the SF from patients with PsA (median 0.61% [IQR 0.26–1.74%] for IL‐22+CD4+ T cells median 0.20 [IQR 0.06–0.38%] for IL‐22+CD8+ T cells) (Figure 2B). Similar findings were observed in the peripheral blood from PsA patients, with the median percentages of IL‐22+CD4+ T cells and IL‐22+CD8+ T cells differing significantly (median 1.35% [IQR 0.96–1.82%] for IL‐22+CD4+ T cells versus median 0.27% [IQR 0.16–0.32%] for IL‐22+CD8+ T cells) as well as in the peripheral blood from healthy controls (median 1.51% [IQR 0.81–2.30%] for IL‐22+CD4+ T cells versus median 0.20% [IQR 0.15–0.34%] for IL‐22+CD8+ T cells) (Figure 2B).
For direct comparison of differences between percentages of CD4+ and CD8+ T cells in SF from PsA patients, peripheral blood from PsA patients, and peripheral blood from healthy controls, percentage ratios were calculated. The percentage of IL‐17A+CD8+ T cells was divided by the percentage of IL‐17A+CD4+ T cells, with similar calculations performed for IFNγ, TNF, and IL‐22 expression between both T cell subsets. Percentage ratios close to 1 in PsA SF (median 0.77 [IQR 0.33–1.39]) indicated that nearly equal percentages of CD8+ and CD4+ T cells were IL‐17A+ (Figure 2C). In contrast, percentage ratios in the peripheral blood from PsA patients (median 0.12 [IQR 0.09–0.30]) and peripheral blood from healthy controls (median 0.11 [IQR 0.08–0.18]) were both significantly lower than the percentage ratios in SF from PsA patients (Figure 2C). This suggests that a specific enrichment of IL‐17A+CD8+ T cells over IL‐17A+CD4+ T cells in PsA SF compared to peripheral blood from PsA patients and healthy controls.
With regard to IFNγ+ cells, the percentage ratios were a median 1.72 (IQR 1.34–2.10) in PsA SF, 2.81 (IQR 1.90–3.75) in peripheral blood from PsA patients, and 2.67 (IQR 1.59–3.36) in peripheral blood from healthy controls (Figure 2C), indicating 2–3 times more IFNγ+CD8+ T cells than IFNγ+CD4+ T cells in all groups.
TNF+ percentage ratios were comparable between SF from PsA patients (0.60 [IQR 0.46–0.71]), peripheral blood from PsA patients (0.75 [IQR 0.44–1.21]), and peripheral blood from healthy controls (1.02 [IQR 0.72–1.31]) (Figure 2C). This suggests that almost equal percentages of CD8+ and CD4+ T cells were TNF+ in PsA SF and peripheral blood.
Similar to IL‐17A, median IL‐22+ percentage ratios were significantly higher in SF from PsA patients (0.33 [IQR 0.07–1.17]) than in peripheral blood from PsA patients (0.17 [IQR 0.14–0.31]) and peripheral blood from healthy controls (0.19 [IQR 0.09–0.32]), indicating enhanced accumulation of IL‐22+CD8+ T cells when compared to IL‐22+CD4+ T cells in PsA SF compared to peripheral blood from PsA patients and healthy controls.
Secretion of IL‐17A upon TCR activation of CD4+ T cells, but not CD8+ T cells, in PsA SF
To compare IL‐17A production and secretion between CD4+ and CD8+ T cells, PsA SF CD4+ and CD8+ T cells were sorted from SF mononuclear cells (SFMCs). These sorted cells were stimulated with anti‐CD3/anti‐CD28 and cultured ex vivo for 72 hours. Sorted CD4+ T cells did not include CD4+CD14+ monocytes or CD4+CD25high T regulatory cells. After 72 hours, the concentration of IL‐17A was measured in culture supernatants using ELISA. Cells were also assessed by intracellular staining for IL‐17A, in which cells were stimulated with PMA/ionomycin for another 4 hours. As shown in Figure 3A, intracellular staining for IL‐17A was positive in both CD4+ and CD8+ T cells. However, in contrast to the results of intracellular IL‐17A staining, measurement of IL‐17A secretion in the supernatants showed that IL‐17A was only secreted by CD4+ T cells, but not CD8+ T cells. Similar results were observed for IL‐17F, as shown in Supplementary Figure 3, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41271/abstract.
Figure 3.
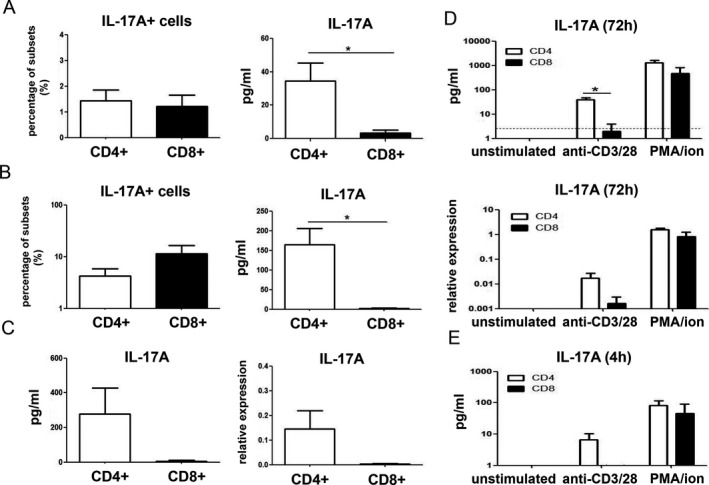
Production of IL‐17A by CD4+ T cells, but not CD8+ T cells, upon anti‐CD3/anti‐CD28 activation in PsA SF. A, IL‐17A staining in cells and protein level in culture supernatants from sorted CD4+ and CD8+ T cells from SF samples after 72 hours of ex vivo anti‐CD3/anti‐CD28 activation. B, IL‐17A staining in cells and protein levels in culture supernatants from SF CD4+ and CD8+ T cells after 72 hours of coculture with allogeneic PsA fibroblast‐like synoviocytes and anti‐CD3/anti‐CD28 activation. In A and B, n = 5 or 6 samples per group from 2 independent experiments. C, IL‐17A in culture supernatants and mRNA expression in SF CD4+ and CD8+ T cells after 72 hours of ex vivo coculture with autologous CD14+ monocytes and anti‐CD3/anti‐CD28 activation (n = 3; different patients assessed than those in A and B). D and E, IL‐17A in culture supernatants and mRNA expression in sorted SF CD4+ and CD8+ T cells that were left unstimulated or after ex vivo stimulation with anti‐CD3/anti‐CD28 or phorbol myristate acetate/ionomycin (PMA/ion), after 72 hours (n = 3; different patients assessed than those in A and B) (broken line indicates IL‐17A detection limit) (D) and IL‐17A in culture supernatants subjected to the same culture conditions as in D for 4 hours (n = 3) (E). Values are the mean ± SEM. * = P < 0.05. See Figure 2 for other definitions.
To further confirm that anti‐CD3/anti‐CD28 activation did not result in secretion of IL‐17A from CD8+ T cells, CD4+ and CD8+ T cells were sorted from PsA SFMCs and cocultured with allogeneic PsA FLS or autologous CD14+ PsA monocytes. Consistent with the findings described above, flow cytometry revealed that CD8+ T cells stained positive for IL‐17A after 3 days of coculture with PsA FLS (Figure 3B). However, excreted IL‐17A levels were not detectable in supernatants of anti‐CD3/anti‐CD28–activated CD8+ T cell cocultures with PSA FLS (Figure 3B). Similarly, in CD4+ and CD8+ T cells that were cocultured for 72 hours with autologous monocytes, anti‐CD3/anti‐CD28 activation led to IL‐17A secretion in coculture supernatants and expression of IL‐17A messenger RNA (mRNA) only in CD4+ T cells, but not in CD8+ T cells (Figure 3C).
To further investigate this discrepancy, PsA SF CD4+ and CD8+ T cells were cultured with anti‐CD3/anti‐CD28 or PMA/ionomycin, or left unstimulated, for 4 hours and 72 hours. As shown in Figure 3D, at 72 hours, only CD4+ T cells produced IL‐17A after anti‐CD3/anti‐CD28 activation at both protein and mRNA levels, while PMA/ionomycin stimulated both CD4+ and CD8+ T cells to produce IL‐17A. Similar results were confirmed with 72‐hour cocultures of T cells and autologous monocytes (Supplementary Figure 4, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41271/abstract). More importantly, as shown in Figure 3E, even after 4 hours of PMA/ionomycin stimulation, CD8+ T cells produced as much IL‐17A as CD4+ T cells, but no IL‐17A secretion was detectable by CD8+ T cells after 4 hours of anti‐CD3/anti‐CD28 stimulation. Time course analysis evaluating IL‐17A expression in CD4+ and CD8+ T cells at 24, 48, and 72 hours after stimulation with anti‐CD3/anti‐CD28 or PMA/ionomycin in SF samples from 3 other PsA patients confirmed the above findings (data not shown).
Overlapping and differential effects of IL‐17A–induced inflammation compared to TNF‐induced inflammation in PsA FLS
As established above, CD4+ T cells in PSA SF are the main IL‐17A producers with anti‐CD3/anti‐CD28 activation. To evaluate overlapping and differential therapeutic effects of TNF and IL‐17A blockers, CD4+ T cells were isolated from PsA SFMCs, stimulated with anti‐CD3/anti‐CD28, and cocultured with allogeneic PsA FLS. Expression of IL‐17RA and IL‐17RC was confirmed on both PsA FLS and CD14+ PsA monocytes (Supplementary Figure 5, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41271/abstract). As shown in Supplementary Figure 6 (available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41271/abstract), pre‐titrations of antibodies were performed, and concentrations were chosen to comparably achieve 60–70% inhibition for each cytokine.
Anti–IL‐17A and anti‐TNF antibody treatment each specifically and significantly reduced the production of either IL‐17A or TNF, respectively, in the coculture system (Figure 4A). Compared to an isotype antibody control, IFNγ was not affected by anti–IL‐17A and anti‐TNF antibody treatment (Figure 4A). Both anti–IL‐17A and anti‐TNF treatments significantly reduced IL‐8 levels compared to the isotype antibody group (Figure 4B). Combining both antibodies yielded a significant additive effect compared to anti–IL‐17A alone (Figure 4B). Interestingly, anti‐TNF did not significantly influence IL‐6 production in these cocultures compared to the isotype control, while anti–IL‐17A significantly suppressed the levels of IL‐6 compared to both isotype controls and anti‐TNF (Figure 4B). Similar results were observed during antibody titrations (Supplementary Figure 6, http://onlinelibrary.wiley.com/doi/10.1002/art.41271/abstract). Although anti‐TNF treatment significantly reduced mRNA expression of IL‐1β, it was significantly less effective compared to anti–IL‐17A treatment (Figure 4B).
Figure 4.
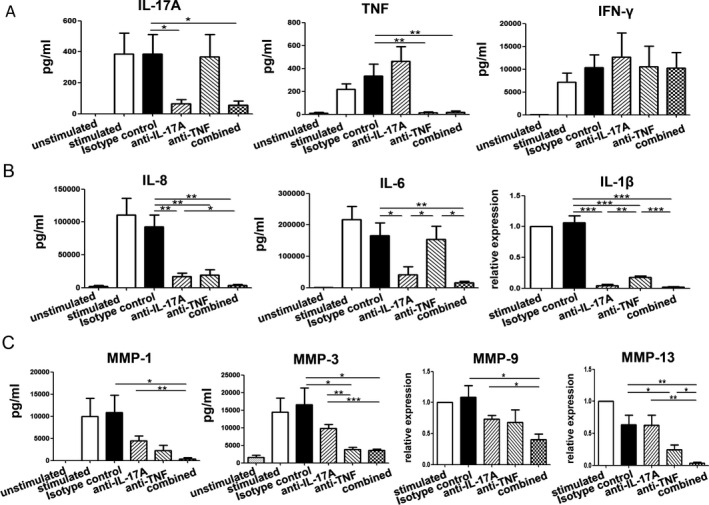
Induction of differential effects by IL‐17A compared to TNF in PsA fibroblast‐like synoviocytes (FLS). CD4+ T cells were sorted from PsA SF and cocultured with allogeneic PsA FLS for 72 hours in the absence or presence of anti‐CD3/anti‐CD28 stimulation. A, Reduced levels of IL‐17A and TNF, but not IFNγ, with anti–IL‐17A and anti‐TNF treatment compared to treatment with isotype control antibody, in culture supernatants of cocultured cells. B, IL‐6 and IL‐8 levels in culture supernatants and IL‐1β mRNA expression levels in cocultured cells after 72 hours. Levels of IL‐8 were significantly reduced by both anti–IL‐17A and anti‐TNF treatments compared to treatment with isotype antibody. IL‐6 production was significantly reduced by anti–IL‐17A therapy compared to both isotype control and anti‐TNF therapy. Anti‐TNF treatment significantly reduced expression of mRNA for IL‐1β, though it was significantly less effective compared to anti–IL‐17A treatment. C, Matrix metalloproteinase 1 (MMP‐1) and MMP‐3 levels in culture supernatants and MMP‐9 and MMP‐13 mRNA expression levels in cocultured cells after 72 hours. Levels of MMP‐1 and MMP‐9 were significantly down‐regulated by the combination treatment of anti–IL‐17A and anti‐TNF compared to isotype control or anti–IL‐17A alone. MMP‐3 levels were significantly reduced after anti‐TNF therapy compared to treatment with anti–IL‐17A or isotype control, and MMP‐13 levels were significantly reduced after anti‐TNF therapy compared to therapy with anti–IL‐17A/anti‐TNF combination therapy or isotype control. Values are the mean ± SEM of 5 or 6 samples and are pooled from 2 independent experiments. * = P < 0.05; ** = P < 0.01; *** = P < 0.001. See Figure 2 for other definitions.
Levels of MMP‐1 were significantly down‐regulated by the combination treatment of anti–IL‐17A and anti‐TNF compared to isotype control or anti–IL‐17A alone (Figure 4C). MMP‐3 levels were significantly suppressed after neutralizing TNF compared to both anti–IL‐17A and isotype control (Figure 4C). Similar to the effects on MMP‐1, expression of MMP‐9 was significantly reduced only by the combination of anti–IL‐17A and anti‐TNF compared to isotype control (Figure 4C). Similar to the effects on MMP‐3, anti‐TNF treatment significantly down‐regulated the expression of MMP‐13 compared to isotype control, and in combination with anti–IL‐17A, achieved a significant level of further reduction of MMP‐13 expression compared to treatment with either antibody alone (Figure 4C).
Discussion
Currently, the role of IL‐17A in the pathogenesis of PsA is widely accepted following the clinical success of biologic agents targeting the IL‐17 pathway 21, 22. Though various cell types have been suggested to be potential sources of IL‐17A in PsA, a detailed ex vivo analysis comparing IL‐17A–producing cells using various techniques is still of relevance. Here, we present evidence that CD4+ T cells, but not CD8+ T cells, in the SF from PsA patients excrete IL‐17A upon ex vivo activation with anti‐CD3/anti‐CD28. This indicates that upon TCR activation, CD4+ T cells will be the main producer of IL‐17A in local joints affected with PsA despite the fact that both CD4 and CD8 T cells produce IL‐17A with PMA and ionomycin stimulation. To better reflect in‐tissue conditions, SF T cells were cocultured with FLS. With this ex vivo PsA coculture assay, we showed that anti–IL‐17A and anti‐TNF both have therapeutic effects in inhibiting the proinflammatory activation loop between T cells and stromal cells. Interestingly, differential effects between anti–IL‐17A and anti‐TNF were also observed. Anti–IL‐17A treatment demonstrates stronger inhibition of inflammatory cytokines such as IL‐6 and IL‐1β while anti‐TNF therapy has a more potent response in reducing the production of MMPs.
Earlier reports have suggested that CD8+ T cells are predominant in PsA 27. However, our findings show that CD4+ T cells are the major T cell subset in PsA SF, which is similar to PsA blood. Possible explanations for this finding may lie in the methods of handling samples. First, during our experiments, surface staining was performed directly after cell pelleting without density gradient separation, in order to maintain the T cell subsets in genuine conditions resembling those found in vivo. Second, percentages of cell subsets were analyzed without stimulation ex vivo and separated from intracellular staining. PMA and ionomycin stimulation conventionally used during intracellular staining can strongly down‐regulate CD4, but not CD8, surface expression, as shown in Supplementary Figure 1, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41271/abstract. Therefore, research conditions should represent the infiltrated immune cell characteristics in SF from patients with PsA as closely as possible.
IL‐17A+CD8+ T cells have been observed in both psoriasis and PsA 12, 13, 14, 28, 29, and particularly in PsA, it was found that the percentage of IL‐17A+CD8+ T cells correlates with disease activity and progression of joint damage 12. Our results confirmed the enrichment of IL‐17A+CD8+ T cells in PsA SF. However, most of the results from previously published studies on IL‐17A+ were obtained via intracellular staining with PMA and ionomycin stimulation 11, 12, 13, 14, 28, 29. Such strong stimuli do not resemble the physiologic state of T cell activation.
In contrast, anti‐CD3/anti‐CD28 stimulation used in our study activated T cells in vitro by providing both primary TCR signal and secondary costimulatory signal. Our findings prove that in contrast to CD4+ T cells, CD8+ T cells activated with anti‐CD3/anti‐CD28 ex vivo did not produce measurable amounts of IL‐17A. Of note, both CD4+ and CD8+ T cells were positive for IL‐17A during intracellular staining. Further, in a study by Raychaudhuri et al, anti‐CD3/anti‐CD28 activation for 3 days, instead of PMA and ionomycin, was used during intracellular staining, and it was found that levels of IL‐17A+CD8+ T cells were negligible in PsA SF compared to distinct IL‐17A+CD4+ T cell staining 10. However, in Raychaudhuri and colleagues’ study, mixed T cell subsets were activated simultaneously, and the possibility of IL‐17A endocytosis cannot be excluded. We avoided this limitation by sorting CD4+ and CD8+ T cells from PsA SF, and thus clearly showed that CD4+ T cells, but not CD8+ T cells, secrete IL‐17A upon ex vivo anti‐CD3/anti‐CD28 activation. This indicated that in joints affected with PsA, TCR activation promotes CD4+ T cells, but not CD8+ T cells, to produce IL‐17A.
Similar results were observed for IL‐17F (Supplementary Figure 3, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41271/abstract), and it implies that CD4+ T cells may be the dominant source of both IL‐17A and IL‐17F in PsA SF upon TCR activation. In a recent study by Glatt et al, a dual specific antibody targeting both IL‐17A and IL‐17F was tested in PsA and the results supported the notion that neutralizing both IL‐17A and IL‐17F could achieve rapid and sustained therapeutic effects 30. Group 3 ILCs were another cell type that has been reported as a potential source of IL‐17A in PsA SF 15. However, a recent study showed that although these group 3 ILCs are expanded in inflamed arthritis joints, they have failed to express IL‐17A upon in vitro stimulation 16. These findings are similar to our observations with regard to CD8+ T cells. Therefore, in contrast to CD4+ T cells, the contribution of CD8+ T cells and group 3 ILCs to IL‐17 production in PsA joints may be limited under physiologic conditions.
Biologic agents targeting IL‐17A or TNF have been proven effective in treating PsA patients, but clinical response rates vary considerably. Failed treatment with 1 biologic drug would necessitate the need to switch to an alternative, which can be a different biologic from the same class or a biologic with a different mode of action. We used an ex vivo human model of PsA synovitis to explain differential effects and showed that although both biologic agents efficiently inhibited the proinflammatory loop in the coculture system, distinct strengths existed between anti–IL‐17A and anti‐TNF treatment. We provided evidence that neutralizing IL‐17A strongly reduced the level of inflammatory cytokines, such as IL‐6 and IL‐1β, while anti‐TNF therapy was potent in reducing MMPs, like MMP‐3 and MMP‐13. Therefore, in order to achieve better and sustainable treatment results, it is rational to target both IL‐17A and TNF. A bispecific antibody, ABT‐122, was recently developed, and its first phase II study in PsA showed that dual inhibition had efficacy and safety similar to adalimumab, a TNF blocker 31, 32. Synergistic effects of anti–IL‐17A and anti‐TNF therapies were also observed in our experiments and by other investigators 33. Combination therapy or sequential therapy using both neutralizing agents may be another option in treating PsA. However, adverse effects such as infection should be closely monitored during all dual neutralization strategies.
Compared to IL‐17A, the percentages of IFNγ+ CD4+ and CD8+ T cells are relatively high in the blood of patients with PsA. Additionally, levels of IFNγ were much higher compared to IL‐17A and TNF in the ex vivo human model of PsA synovitis. However, neutralization of IL‐17A or TNF did not influence the level of IFNγ in these coculture experiments, but significantly decreased inflammation in our model of PsA synovitis, indicating that IFNγ does not play a major role in T cell–PsA synovial fibroblast activation.
In PsA SF, we found a lower percentages of CD19+ B cells, but a higher percentage of CD56bright NK cells, which is consistent with data from other groups 34, 35, 36. B cells tend to function in a protective way in psoriasis or PsA and can be a source of antiinflammatory cytokines, such as IL‐10 35, 37. Interestingly, an increased level of CD56bright NK cell cells was reported to trigger differentiation of monocytes into dendritic cells in PsA SF 38. Monocytes and dendritic cells are major sources of IL‐23, and the IL‐23/IL‐17 axis plays a pivotal role in inflammatory arthritis 39, 40. Biologic agents targeting the IL‐23 subunits p40 and p19 were shown to be a successful treatment strategy in PsA clinical trials 41, 42. Whether SF CD56bright NK cells modulate IL‐23 expression by monocytes/dendritic cells, thereby influencing the IL‐23/IL‐17 axis, is still not known and warrants further research.
In summary, our study shows that the contribution of CD8+ T cells to the local production of IL‐17A in PsA SF needs further examination as TCR activation, mimicked by anti‐CD3/anti‐CD28 stimulation, did not induce the secretion of IL‐17A from CD8+ T cells, in contrast to CD4+ T cells. IL‐17A and TNF blockade showed differential effects in our ex vivo human model of PsA synovial inflammation, with IL‐17A strongly inhibiting inflammatory cytokines and TNF blockade having a more potent response in reducing MMPs. We acknowledged the limitations of our ex vivo study systems, and further validation of combination therapy or sequential therapy in patients with IL‐17A and TNF neutralizing agents is needed. Our study demonstrates that targeting both IL‐17A and TNF may be a complementary therapeutic strategy that confers additive benefits in coping with destructive synovitis in patients with PsA.
Author contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Lubberts had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Xu, Hazes, Baeten, Vis, Bisoendial, Prens, Lubberts.
Acquisition of data
Xu, Davelaar, Mus, Asmawidjaja.
Analysis and interpretation of data
Xu, Davelaar, Mus, Asmawidjaja.
Supporting information
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure S4
Supplementary Figure S5
Supplementary Figure S6
Supplementary Table S1
Dr. Baeten's work was supported by a Netherlands Scientific Organization NWO Vici grant and a European Research Council consolidator grant. Dr. Lubberts’ work was supported by the Dutch Arthritis Association.
1Xiaofei Xu, MD, Nadine Davelaar, BSc, Anne‐Marie Mus, BSc, Patrick S. Asmawidjaja, BSc, Johanna M. W. Hazes, MD, PhD, Marijn Vis, MD, PhD, Radjesh J. Bisoendial, MD, PhD, Errol P. Prens, MD, PhD, Erik Lubberts, PhD: Erasmus Medical Center, Rotterdam, The Netherlands; 2Dominique L. P. Baeten, MD, PhD: Academic Medical Center, Amsterdam, The Netherlands.
Dr. Lubberts received research support from Novartis. No other disclosures relevant to this article were reported.
References
- 1. Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med 2017;376:957–70. [DOI] [PubMed] [Google Scholar]
- 2. Poulter LW, Duke O, Panayi GS, Hobbs S, Raftery MJ, Janossy G. Activated T lymphocytes of the synovial membrane in rheumatoid arthritis and other arthropathies. Scand J Immunol 1985;22:683–90. [DOI] [PubMed] [Google Scholar]
- 3. Takada K, Danning CL, Kuroiwa T, Schlimgen R, Tassiulas IO, Davis JC Jr, et al. Lymphocyte depletion with fludarabine in patients with psoriatic arthritis: clinical and immunological effects. Ann Rheum Dis 2003;62:1112–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Costello PJ, Winchester RJ, Curran SA, Peterson KS, Kane DJ, Bresnihan B, et al. Psoriatic arthritis joint fluids are characterized by CD8 and CD4 T cell clonal expansions appear antigen driven. J Immunol 2001;166:2878–86. [DOI] [PubMed] [Google Scholar]
- 5. Partsch G, Steiner G, Leeb BF, Dunky A, Bröll H, Smolen JS. Highly increased levels of tumor necrosis factor‐α and other proinflammatory cytokines in psoriatic arthritis synovial fluid. J Rheumatol 1997;24:518–23. [PubMed] [Google Scholar]
- 6. Ritchlin C, Haas‐Smith SA, Hicks D, Cappuccio J, Osterland CK, Looney RJ. Patterns of cytokine production in psoriatic synovium. J Rheumatol 1998;25:1544–52. [PubMed] [Google Scholar]
- 7. Van Baarsen LG, Lebre MC, van der Coelen D, Aarrass S, Tang MW, Ramwadhdoebe TH, et al. Heterogeneous expression pattern of interleukin 17A (IL‐17A), IL‐17F and their receptors in synovium of rheumatoid arthritis, psoriatic arthritis and osteoarthritis: possible explanation for nonresponse to anti‐IL‐17 therapy? Arthritis Res Ther 2014;16:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lubberts E. Th17 cytokines and arthritis. Semin Immunopathol 2010;32:43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang EA, Suzuki E, Maverakis E, Adamopoulos IE. Targeting IL‐17 in psoriatic arthritis. Eur J Rheumatol 2017;4:272–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Raychaudhuri SP, Raychaudhuri SK, Genovese MC. IL‐17 receptor and its functional significance in psoriatic arthritis. Mol Cell Biochem 2012;359:419–29. [DOI] [PubMed] [Google Scholar]
- 11. Wade SM, Canavan M, McGarry T, Low C, Wade SC, Mullan RH, et al. Association of synovial tissue polyfunctional T‐cells with DAPSA in psoriatic arthritis. Ann Rheum Dis 2019;78:350–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Menon B, Gullick NJ, Walter GJ, Rajasekhar M, Garrood T, Evans HG, et al. Interleukin‐17+CD8+ T cells are enriched in the joints of patients with psoriatic arthritis and correlate with disease activity and joint damage progression. Arthritis Rheumatol 2014;66:1272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diani M, Casciano F, Marongiu L, Longhi M, Altomare A, Pigatto PD, et al. Increased frequencey of activated CD8+ T cell effectors in patients with psoriatic arthritis. Sci Rep 2019;9:10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Steel KJ, Srenathan U, Ridley M, Durham LE, Wu SY, Ryan SE, et al. Polyfunctional, proinflammatory, tissue‐resident memory phenotype and function of synovial interleukin‐17A+CD8+ T cells in psoriatic arthritis. Arthritis Rheumatol 2020;72:435–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leijten EF, van Kempen TS, Boes M, Michels‐van Amelsfort JM, Hijnen D, Hartgring SA, et al. Enrichment of activated group 3 innate lymphoid cells in psoriatic arthritis synovial fluid. Arthritis Rheumatol 2015;67:2673–8. [DOI] [PubMed] [Google Scholar]
- 16. Blijdorp IC, Menegatti S, van Mens LJ, van de Sand MG, Chen S, Hreggvidsdottir HS, et al. Expansion of interleukin‐22–and granulocyte–macrophage colony‐stimulating factor–expressing, but not interleukin‐17a–expressing, group 3 innate lymphoid cells in the inflamed joints of patients with spondyloarthritis. Arthritis Rheumatol 2019;71:392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mease PJ. Tumour necrosis factor (TNF) in psoriatic arthritis: pathophysiology and treatment with TNF inhibitors [review]. Ann Rheum Dis 2002;61:298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aaltonen K, Heinonen A, Joensuu J, Parmanne P, Karjalainen A, Varjolahti‐Lehtinen T, et al. Effectiveness and drug survival of TNF‐inhibitors in the treatment of psoriatic arthritis: a prospective cohort study. Semin Arthritis Rheum 2017;46:732–9. [DOI] [PubMed] [Google Scholar]
- 19. Taurog JD, Chhabra A, Colbert RA. Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med 2016;374:2563–74. [DOI] [PubMed] [Google Scholar]
- 20. Fitz L, Zhang W, Soderstrom C, Fraser S, Lee J, Quazi A, et al. Association between serum interleukin‐17A and clinical response to tofacitinib and etanercept in moderate to severe psoriasis. Clin Exp Dermatol 2018;43:790–7. [DOI] [PubMed] [Google Scholar]
- 21. Van der Heijde D, Landewé RB, Mease PJ, McInnes IB, Conaghan PG, Pricop L, et al. Secukinumab provides significant and sustained inhibition of joint structural damage in a phase III study of active psoriatic arthritis. Arthritis Rheumatol 2016;68:1914–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mease PJ, van der Heijde D, Ritchlin CT, Okada M, Cuchacovich RS, Shuler CL, et al. Ixekizumab, an interleukin‐17A specific monoclonal antibody, for the treatment of biologic‐naive patients with active psoriatic arthritis: results from the 24‐week randomised, double‐blind, placebo‐controlled and active (adalimumab)‐controlled period of the phase III trial SPIRIT‐P1. Ann Rheum Dis 2017;76:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pelechas E, Memi T, Voulgari PV, Drosos AA. A case of recalcitrant psoriatic arthritis to TNF inhibitors improved after administration of secukinumab, an IL‐17A inhibitor. Rheumatol Ther 2017;4:509–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nash P, Kirkham B, Okada M, Rahman P, Combe B, Burmester GR, et al. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24‐week randomised, double‐blind, placebo‐controlled period of the SPIRIT‐P2 phase 3 trial. Lancet 2017;389:2317–27. [DOI] [PubMed] [Google Scholar]
- 25. Classification of Psoriatic Arthritis (CASPAR) Study Group Criteria for PsA . Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H, and the CASPAR Study Group . Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–73. [DOI] [PubMed] [Google Scholar]
- 26. Van Hamburg JP, Asmawidjaja PS, Davelaar N, Mus AM, Colin EM, Hazes JM, et al. Th17 cells, but not Th1 cells, from patients with early rheumatoid arthritis are potent inducers of matrix metalloproteinases and proinflammatory cytokines upon synovial fibroblast interaction, including autocrine interleukin‐17A production. Arthritis Rheum 2011;63:73–83. [DOI] [PubMed] [Google Scholar]
- 27. Costello P, Bresnihan B, O'Farrelly C, FitzGerald O. Predominance of CD8+ T lymphocytes in psoriatic arthritis. J Rheumatol 1999;26:1117–24. [PubMed] [Google Scholar]
- 28. Ortega C, Fernández‐A S, Carrillo JM, Romero P, Molina IJ, Moreno JC, et al. IL‐17‐producing CD8+ T lymphocytes from psoriasis skin plaques are cytotoxic effector cells that secrete Th17‐related cytokines. J Leukoc Biol 2009;86:435–43. [DOI] [PubMed] [Google Scholar]
- 29. Res PC, Piskin G, de Boer OJ, van der Loos CM, Teeling P, Bos JD, et al. Overrepresentation of IL‐17A and IL‐22 producing CD8 T cells in lesional skin suggests their involvement in the pathogenesis of psoriasis. PLoS One 2010;5:e14108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Glatt S, Baeten D, Baker T, Griffiths M, Ionescu L, Lawson AD, et al. Dual IL‐17A and IL‐17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo‐controlled clinical trial that IL‐17F contributes to human chronic tissue inflammation. Ann Rheum Dis 2018;77:523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Genovese MC, Weinblatt ME, Mease PJ, Aelion JA, Peloso PM, Chen K, et al. Dual inhibition of tumour necrosis factor and interleukin‐17A with ABT‐122: open‐label long‐term extension studies in rheumatoid arthritis or psoriatic arthritis. Rheumatology (Oxford) 2018;57:1972–81. [DOI] [PubMed] [Google Scholar]
- 32. Mease PJ, Genovese MC, Weinblatt ME, Peloso PM, Chen K, Othman AA, et al. Phase II study of ABT‐122, a tumor necrosis factor‐ and interleukin‐17A‐targeted dual variable domain immunoglobulin, in patients with psoriatic arthritis with an inadequate response to methotrexate. Arthritis Rheumatol 2018;70:1778–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fischer JA, Hueber AJ, Wilson S, Galm M, Baum W, Kitson C, et al. Combined inhibition of tumor necrosis factor α and interleukin‐17 as a therapeutic opportunity in rheumatoid arthritis: development and characterization of a novel bispecific antibody. Arthritis Rheumatol 2015;67:51–62. [DOI] [PubMed] [Google Scholar]
- 34. Spadaro A, Scrivo R, Moretti T, Bernardini G, Riccieri V, Taccari E, et al. Natural killer cells and γ/δ T cells in synovial fluid and in peripheral blood of patients with psoriatic arthritis. Clin Exp Rheumatol 2004;22:389–94. [PubMed] [Google Scholar]
- 35. Mavropoulos A, Varna A, Zafiriou E, Liaskos C, Alexiou I, Roussaki‐Schulze A, et al. IL‐10 producing Bregs are impaired in psoriatic arthritis and psoriasis and inversely correlate with IL‐17‐ and IFNγ‐producing T cells. Clin Immunol 2017;184:33–41. [DOI] [PubMed] [Google Scholar]
- 36. Dalbeth N, Callan MF. A subset of natural killer cells is greatly expanded within inflamed joints. Arthritis Rheum 2002;46:1763–72. [DOI] [PubMed] [Google Scholar]
- 37. Dass S, Vital EM, Emery P. Development of psoriasis after B cell depletion with rituximab. Arthritis Rheum 2007;56:2715–8. [DOI] [PubMed] [Google Scholar]
- 38. Zhang AL, Colmenero P, Purath U, Teixeira de Matos C, Hueber W, Klareskog L, et al. Natural killer cells trigger differentiation of monocytes into dendritic cells. Blood 2007;110:2484–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, et al. Novel p19 protein engages IL‐12p40 to form a cytokine, IL‐23, with biological activities similar as well as distinct from IL‐12. Immunity 2000;13:715–25. [DOI] [PubMed] [Google Scholar]
- 40. Lubberts E. The IL‐23‐IL‐17 axis in inflammatory arthritis [review]. Nat Rev Rheumatol 2015;11:415–29. [DOI] [PubMed] [Google Scholar]
- 41. Kavanaugh A, Ritchlin C, Rahman P, Puig L, Gottlieb AB, Li S, et al. Ustekinumab, an anti‐IL‐12/23 p40 monoclonal antibody, inhibits radiographic progression in patients with active psoriatic arthritis: results of an integrated analysis of radiographic data from the phase 3, multicentre, randomised, double‐blind, placebo‐controlled PSUMMIT‐1 and PSUMMIT‐2 trials. Ann Rheum Dis 2014;73:1000–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Deodhar A, Gottlieb AB, Boehncke WH, Dong B, Wang Y, Zhuang Y, et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double‐blind, placebo‐controlled, phase 2 study. Lancet 2018;391:2213–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure S4
Supplementary Figure S5
Supplementary Figure S6
Supplementary Table S1


