Abstract
Lately, the incidence of overweight, obesity, and type 2 diabetes has shown a staggering increase. To prevent and treat these conditions, one must look at their etiology. As life on earth has evolved under the conditions of nature’s 24‐hour light/dark cycle, it seems likely that exposure to artificial light at night (LAN) would affect physiology. Indeed, ample evidence has shown that LAN impacts many metabolic parameters, at least partly via the biological clock in the suprachiasmatic nucleus of the hypothalamus. This review focuses on the impact of chronic and acute effects of LAN of different wavelengths on locomotor activity, food intake, the sleep/wake cycle, body temperature, melatonin, glucocorticoids, and glucose and lipid metabolism. While chronic LAN disturbs daily rhythms in these parameters, experiments using short‐term LAN exposure also have shown acute negative effects in metabolically active peripheral tissues. Experiments using LAN of different wavelengths not only have indicated an important role for melanopsin, the photopigment found in intrinsically photosensitive retinal ganglion cells, but also provided evidence that each wavelength may have a specific impact on energy metabolism. Importantly, exposure to LAN has been shown to impact glucose homeostasis also in humans and to be associated with an increased incidence of overweight, obesity, and atherosclerosis.
Study Importance.
What is already known?
-
►
Evidence is increasing that exposure to (artificial) light at night (LAN) has a negative impact on metabolic health in humans and animals by causing circadian disruption. What does this review add?
-
►
Effects of chronic exposure to LAN are mainly caused by its disruptive effects on the central brain clock in the suprachiasmatic nucleus of the hypothalamus (SCN); however, the effects of acute exposure to LAN may be driven by both SCN and non-SCN neural pathways.
-
►
The specific effects of LAN exposure are dependent on both wavelength and timing.
How might these results change the direction of research?
-
►
Further research using the principle of silent substitution, together with more standardized protocols in terms of light nomenclature, measurements, and reporting, is necessary to determine which type of light has the most (or least) harmful health consequences.
Introduction
In 2016, nearly 40% of adults worldwide were reported to have overweight and 13% were reported to have obesity (1). Overweight and obesity are associated with type 2 diabetes and cardiovascular disease. As the development of these diseases can in large part be ascribed to lifestyle, it is important to look at societal changes. Over the past century, we have gotten used to abundant food often rich in fat and refined carbohydrates. Cars, public transportation, and office jobs have reduced the need for physical activity. Moreover, we have shifted away from nature’s 24‐hour day/night rhythm toward a society in which people work around the clock, stay out late, and have their screens on until the early hours.
Most organisms, including mammals, have developed an endogenous circadian timing system under the earth’s natural 24‐hour rhythm that is adapted to the regular alternation of light and dark phases. Thus, it seems likely that changing these conditions will impact physiology. The aim of this review is to summarize the current evidence on the impact of exposure to light at night (LAN) on metabolic parameters and present an anatomical framework through which this may happen. Our modern society exposes us to various types of artificial LAN. Outdoor artificial LAN caused by street lighting is usually low in intensity and chronically present. Shift work, on the contrary, exposes us to single nights of bright light. The usage of screens exposes us to light of shorter wavelengths and might thus impact metabolism differently. The aim of this review is to summarize the current evidence of the impact of exposure to LAN on metabolic parameters as well as an anatomical framework through which this may happen. To properly map the effects of different exposures of LAN, a distinction will be made between chronic and acute LAN exposures and the impact of different wavelengths.
Light’s Connection to Metabolism: An Anatomical Framework
Both rodents and humans have, apart from rods and cones, a third type of ocular photoreceptor, the so‐called intrinsically photosensitive retinal ganglion cells (ipRGCs). These ipRGCs contain the photopigment melanopsin, which is optimally sensitive to light at a wavelength of 484 nm in rodents (2). Human ipRGC subtypes have shown peak activity in response to 457, 459, and 470 nm light with a maximal sensitivity at 480 nm (2, 3). Rods and cones, with peak sensitivities varying within a range of 440 to 580 nm (4), provide input to the ipRGCs as well, lowering ipRGC response thresholds and increasing their action potential discharge rates (2). Several brain areas are responsible for coordinating energy homeostasis by regulating locomotor activity, food intake, energy expenditure, hormone levels, and activity in metabolic tissues. Some of these areas receive direct input from the ipRGCs, which contain the photopigment melanopsin (5, 6). One of the areas receiving input from the ipRGCs is the suprachiasmatic nucleus of the hypothalamus (SCN) (6) or master biological clock (7). The molecular mechanism of this biological clock consists of negative transcription and translation feedback loops, causing oscillations in gene and protein expression with a period close to 24 hours (i.e., a circadian rhythm) (8).
Daily alternations of light and dark synchronize the circadian clock in the SCN neurons to the exact 24‐hour cycle in our environment. Light information reaching the SCN via ipRGCs is the most important synchronizer or “Zeitgeber” for the SCN neurons. Depending on the timing, light exposure will enhance or dampen the expression of certain clock genes. Light exposure at the end of the night will advance the clock’s phase, whereas light at the beginning of the night will delay it (9).
The SCN has reciprocal interactions with the arcuate nucleus of the hypothalamus, which regulates daily rhythms in food intake (10) and locomotor activity (11). The dorsomedial nucleus of the hypothalamus receives projections from the SCN and is involved in coordinating daily rhythms of food intake and locomotor activity with the sleep‐wake cycle (12). Furthermore, the SCN interacts with the intergeniculate leaflet, which receives a direct input from the ipRGCs and further helps coordinate circadian rhythms (6). Moreover, the SCN projects to the lateral habenula, a structure involved in several brain functions, including reward, memory, learning, mood, and sleep (13). The lateral habenula also receives projections from the lateral hypothalamic area that regulates feeding and reward (14).
The SCN, the dorsomedial nucleus of the hypothalamus, and ipRGCs also project to the paraventricular nucleus of the hypothalamus (PVN) and via this connection transmit the time‐of‐day signal to other brain areas and periphery. The PVN projects to the intermediolateral column of the spinal cord, regulating the secretion of melatonin from the pineal gland (15). It also has sympathetic projections to the adrenal gland, via which it modulates the sensitivity of the adrenal cortex to ACTH (adrenocorticotropic hormone). (16), and sympathetic and parasympathetic projections to the thyroid gland (17), the pancreas (18), the liver (19), and white adipose tissue (WAT) (20). Furthermore, the PVN controls the activity of the hypothalamo‐pituitary‐thyroid and hypothalamo‐pituitary‐adrenal axis via the release of thyrotropin‐releasing hormone (21) and corticotrophin‐releasing hormone (22). Thus, by means of its influence on the autonomic and neuroendocrine output of the hypothalamus, the SCN’s circadian rhythm is transmitted to other brain areas, endocrine glands, and peripheral tissues (20). Likewise, peripheral tissues themselves also show a circadian rhythm in clock gene expression. Thus, the molecular clock mechanism is present not just in SCN neurons but also in virtually every cell. As peripheral cells cannot be entrained by light directly, they depend on the SCN to keep their clock synchronized with the environment (23). Additionally, peripheral clocks have been shown to respond to other Zeitgebers, including glucocorticoids (24), glucose (25), body temperature (26), melatonin (27), activity rhythms (28), food intake (29), and the microbiome (30). Altogether, the SCN has a vast reach, and therefore, through its impact on the SCN, the effects of light are likely to be widespread too (Figure 1).
Figure 1.
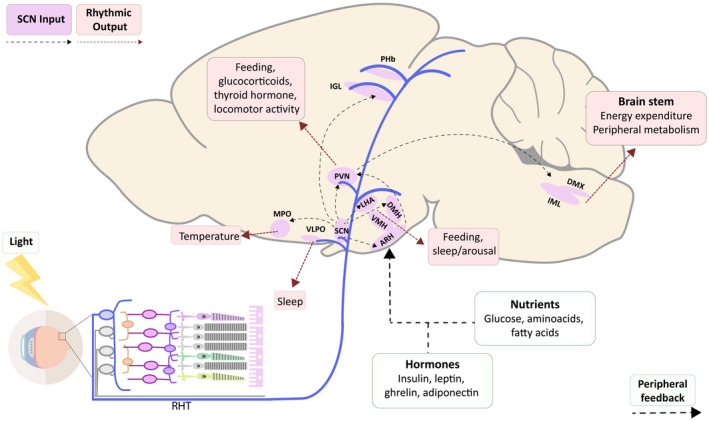
Sagittal view of a mouse brain providing a schematic overview of brain areas involved in the control of energy metabolism that receive input from the master clock located in the SCN or direct light input via the ipRGCs. RHT, retinohypothalamic tract; SCN, suprachiasmatic nucleus; VLPO, ventrolateral preoptic nucleus; MPO, medial preoptic area; ARH, arcuate nucleus of the hypothalamus; VMH, ventromedial nucleus of the hypothalamus; DMH, dorsomedial nucleus of the hypothalamus; LHA, lateral hypothalamic area; PVN, paraventricular nucleus of the hypothalamus; IGL, intergeniculate leaflet; PHb, perihabenular complex; IML, intermediolateral column of the spinal cord; DMX, dorsal motor nucleus of the vagus.
Moreover, the ipRGCs also project directly to many of the SCN target areas mentioned above. Hence, light exposure could also affect energy metabolism, feeding behavior, and reward directly (i.e., independent) from the SCN.
Light: Definitions and Nomenclature
In order to facilitate comparison of the effects of light among different studies, it is important to report the spectral sensitivity within each retinal photopigment complement using the guidelines proposed by the International Commission on Illumination (31) and the suggestions described extensively elsewhere by Spitschan et al. (32). Furthermore, in principle, color terms are not applicable to rodents, but even in humans, they are rarely meaningful in the kind of studies described below. For example, both monochromatic 460 nm and 495 nm light could be described as “blue,” as could a cloudless sky, which is not monochromatic. Thus, there are multiple ways to generate “blue appearing” light (33). However, importantly, the fact that a light appears more or less blue does not uniquely specify its melanopic effect. On the contrary, different combinations of light can have a similar effect on melanopsin. In addition, referring to the color of monochromatic light is not useful without intensity information. Because of the principle of univariance, i.e., a single photopigment cannot distinguish between a change in wavelength and a change in intensity, different combinations of light (i.e., wavelengths) can elicit the same photoreceptor response (34). Therefore, in principle, the intensity of light of any wavelength can be increased to evoke a certain response, such as by the method of silent substitution (35). Unfortunately, in most studies, light conditions are only defined by color/wavelength and amount of lux as a measure of intensity. The kind of necessary information detailed above only started to be reported more consistently in the last few years. In view of this large variation in information provided and in order to maintain readability of this review, we maintained the color information as mentioned in the different manuscripts and provided all the available physical light details in [Link], [Link], [Link]. Light information from the references cited in the paragraphs on the chronic effects of bright light and dim LAN (dLAN) is presented in Supporting Information Table S1. Supporting Information Table S2 contains the information from the references reporting on the acute effects of light, and in Supporting Information Table S3, light information from the studies on the LAN effects of different wavelengths is presented.
The Impact of Exposure to Constant Bright Light on Rodent Metabolism
Although exposure to constant bright light (LL) is usually not a natural condition, it is an interesting experimental condition to study light effects on clock function. In mice, in vivo recordings of SCN electrical output showed that LL caused an immediate reduction in rhythm amplitude (36). Also, the amplitude of Period 1 (Per1) expression in the SCN was shown to be blunted, and although SCN neurons still oscillated with a phase of roughly 24 hours in LL conditions, individual neurons had a different phase in the expression of Per1 (37). Experiments also showed a loss of daily locomotor activity rhythms (36), with no impact on total daily locomotor activity (38). One experiment showed the disturbance in locomotor activity rhythms to correlate with asynchrony among individual SCN neuronal Per1 rhythms (37). Some mice exhibited activity rhythm splitting. In these mice, neurons in the left and right half of the SCN oscillated in antiphase (37). Multiple experiments in rats showed that circadian rhythms in locomotor activity were abolished by LL (39) and replaced with ultradian rhythms (36, 37), with restoration of this rhythm after 1 week in constant darkness, suggesting clock function might have been suppressed by LL (40).
LL caused the circadian rhythm in body temperature in rats to dampen and be replaced with ultradian rhythms, although the circadian rhythm in body temperature persisted longer than the rhythm in locomotor activity (40). Likewise, 1 week of constant darkness restored the circadian rhythm of body temperature. This coincided with a restoration of the circadian rhythm and absolute levels of plasma melatonin (40), which were shown to be flattened and decreased in LL conditions (41). In mice and rats, the circadian rhythm in dietary and water intake was shown to be abolished. Total daily food intake was increased in one experiment in mice (36), whereas in another, there was no impact of LL on total food intake (38).
Rats exposed to LL conditions had a disturbed circadian rhythm in plasma glucocorticoid levels, and its absolute levels were elevated (41, 42). In mice, the rhythm in plasma glucocorticoids was found to be disturbed (38), yet absolute plasma glucocorticoid levels were found to be decreased (38, 43). Plasma total fatty acid levels, free fatty acids, phospholipids, and cholesterol esters were found to be continuously elevated in one rat study, while circadian rhythms in all plasma fatty acid levels were abolished (41). In both mice and rats, LL was shown to cause weight gain (36, 38, 39) and increased epididymal fat pad mass (38).
LL rats showed disturbance of the circadian rhythm in basal plasma glucose levels and hyperglycemia as opposed to nearly constant normoglycemic basal plasma glucose levels in rats in a regular light/dark (LD) cycle (41). Elevated plasma glucose levels were found in mice during an intraperitoneal glucose tolerance test, indicating LL reduced glucose tolerance (38); in another mouse experiment, it was found that the diurnal variation in insulin sensitivity was abolished by LL (36). An in vitro study using rat pancreatic islet cells from animals exposed to LL showed reduced glucose sensitivity and reduced total insulin secretion. Per1 expression in pancreatic islet cells still showed circadian oscillations in LL conditions, with dampened amplitude and individual islets oscillating out of phase (44).
Overall, exposure to constant light caused altered clock function, which translated to a wide array of changes in metabolic parameters. Circadian rhythms in locomotor activity, body temperature, food intake, plasma glucocorticoids, lipids and glucose, and insulin sensitivity were shown to be disturbed, and total levels of plasma melatonin, glucocorticoids, fatty acids, and glucose were altered. Furthermore, clock gene expression in the pancreas was dampened. Most likely, the LL‐induced disturbances in the central clock cause perturbed circadian rhythms in brain regions and peripheral organs, resulting in misalignment and altered function among organs. Changes in glucose metabolism illustrate particularly well how such a disturbed interplay between different peripheral tissues could cause serious impairment of metabolic efficiency. As peripheral clocks have been shown to also rely on behavioral and metabolic Zeitgebers, changes in feeding and locomotor activity and in the output of metabolically active peripheral tissues will further add to the alterations observed in peripheral clock gene expression. Moreover, it was recently shown that light may also have a direct effect on peripheral clocks, without the need of an oscillating SCN (45).
The Impact of Exposure to dLAN on Rodent Metabolism
Although exposure to dLAN provides constant exposure to light over the course of the 24‐hour day, the difference in light intensity between day and night still provides a temporal cue to discern the 24‐hour rhythm of the environment. Therefore, dLAN might affect circadian rhythms to a lesser or different extent than LL. Expression of Per1, Per2, and cryptochrome 2 (Cry2) in the SCN was altered at multiple time points during dLAN compared with LD in mice (46). In hamsters, SCN Per1 expression was altered at the beginning of the dim phase (47), while in rats, dLAN decreased diurnal variation in SCN Per1 and aryl hydrocarbon receptor nuclear translocator (Arntl) expression (48).
The 24‐hour rhythm in locomotor activity was less pronounced in rats in dLAN conditions, although still present. Interestingly, exposure to dLAN gave rise to a second interacting rhythm in locomotor activity, with a period of 25.1 hours (48), possibly reflecting different parts of the SCN expressing their own rhythm. In mice, dLAN did not affect the daily locomotor activity rhythm (38, 49). Results on the locomotor activity pattern of hamsters are ambiguous (47, 50, 51). Total locomotor activity was not affected in either rodent species (38, 47, 48, 49, 50). In a recent mouse study, long‐term dLAN induced pronounced alterations in sleep architecture, enhancing age‐associated changes (52).
dLAN did not affect diurnal variation in plasma glucocorticoid levels in mice (38), while the opposite was observed in hamsters (50). The daily rhythm in plasma glucocorticoids was maintained in rats, but the phase of the rhythm was delayed by 4 hours. Absolute plasma glucocorticoid levels were not affected (41). Interestingly, in mice, dLAN advanced the rhythm in body temperature. The amplitude of the rhythm was dampened, with peak temperature being decreased and occurring 3 hours earlier (49). Nocturnal melatonin levels were shown to be suppressed by dLAN in rats (41, 53).
Exposure to dLAN induced a shift in the timing of food intake toward daytime in mice and rats (38, 48). Total daily food intake was not affected in mice (38, 49). In rats, there was a small reduction in food intake during dLAN in one experiment (48), whereas in another, there was no difference (54). The importance of the timing of food intake in the metabolic effects of dLAN was demonstrated by Fonken et al., when they showed that restricting food intake to subjective nighttime prevented the dLAN‐induced body weight increase in mice (38).
Previously, it was shown that dLAN causes an increase in body weight in regular (38, 46, 55, 56) and TALLYHO mice (genetically prone to develop type 2 diabetes) (57) as well as in hamsters (47) but not in regular (48) or spontaneously hypertensive rats (54). Multiple experiments showed increased epididymal fat pad mass in mice (38, 46, 55). Interestingly, returning to an LD schedule after dLAN reversed body weight increase in TALLYHO mice (57). An experiment in regular mice found that dLAN induced a shift from lipid to carbohydrate metabolism and reduced energy expenditure without changing locomotor activity (49). In mice, dLAN changed the expression of Rev‐erb in WAT (46).
Spontaneously hypertensive rats showed increased hepatic triglycerides during dLAN, while there was no effect on basal plasma triglycerides, total and low‐density lipoprotein cholesterol, or leptin levels. Hepatic expression of PPARγ was elevated by dLAN in these rats. Epididymal expression of peroxisome proliferator‐activated receptors (PPAR) PPARγ and of PPARα was also elevated (54). In regular rats, there was no effect of dLAN on absolute values or the daily rhythm in plasma lipid levels (41).
Plasma glucose during an intraperitoneal glucose tolerance test was found to be elevated in both TALLYHO (57) and regular mice (38, 55). Furthermore, plasma glucose levels during an intraperitoneal insulin tolerance test were elevated in TALLYHO mice in dLAN conditions. Returning to an LD schedule reversed the reduction in insulin sensitivity. Furthermore, basal plasma glucose levels in TALLYHO mice were elevated in dLAN conditions, and a larger percentage of dLAN TALLYHO mice acquired diabetes compared with TALLYHO dark controls. Additionally, dLAN TALLYHO mice had a lower survival rate compared with dark controls (57). In an experiment in regular mice, basal plasma insulin levels were elevated and diurnal variation in plasma insulin levels was abolished (55). Basal plasma glucose levels were not affected in another experiment in regular mice. However, in this experiment, liver expression of Per1, Per2, Cry1, Cry2, brain and muscle ARNT‐like 1, and Rev‐erb was altered (46). In both regular and spontaneously hypertensive rats, there was no effect of dLAN on absolute basal plasma glucose levels (41, 54), although dLAN did induce a shift in the daily rhythm in plasma glucose in regular rats (41). There was no effect on basal plasma insulin levels in spontaneously hypertensive rats. While hepatic expression of PPARγ and epididymal expression of PPARγ and PPARα were increased by dLAN in spontaneously hypertensive rats, cardiac expression of glucose transporter 4 (Glut4) was decreased (54).
Summarizing, dLAN caused shifts in circadian rhythms and extension of circadian periods on multiple parameters rather than completely abolishing rhythms. It has been established that the ventral SCN region is more susceptible to acute changes in the LD cycle compared with the dorsal SCN (58). Possibly, continuous activity of the ventral SCN region in response to dLAN, combined with a steady generation of a 24‐hour rhythm by the dorsal SCN, results in a dampened but intact circadian rhythm in SCN output, which in turn translates to blunted circadian rhythms in locomotor activity, food intake, body temperature, and other parameters. On the contrary, in LL conditions, both the ventral and dorsal SCN region are disturbed, abolishing all rhythms.
The dLAN‐induced increase in body weight could be prevented by restoring the nocturnal rhythm in food intake. Thus, it seems likely that dLAN‐induced alteration of circadian rhythms gives rise to metabolic disturbance. Altered expression of proteins such as PPARγ, PPARα, and Glut4, however, indicates dLAN also impacts glucose and lipid homeostasis on a tissue‐specific level. It is likely that both perturbed behavioral rhythms and tissue‐specific changes contribute to the vast range of metabolic disturbances found in dLAN‐exposed animals. Importantly, metabolic disturbances observed in TALLYHO mice indicate that dLAN might be especially harmful to individuals that have or are prone to develop diabetes.
The Effects of Chronic Exposure to LAN on Human Metabolism
It has been established that 99% of the population of the European Union and the United States lives in areas where nighttime illumination is above the threshold for light pollution (59). It is therefore essential to determine the extent to which LAN is a health hazard and how it affects metabolic parameters in humans. Cross‐sectional studies have found that high outdoor LAN was associated with disturbances in the daily sleep/wake cycles and in sleep duration (60, 61). Obayashi et al. (62) performed a cross‐sectional study in elderly individuals and measured LAN intensity in people’s bedrooms. They found that exposure to LAN correlated with elevated plasma triglycerides and LDL and lowered high‐density lipoprotein, although urinary melatonin levels were not affected. In another study, this same group found LAN to be associated with subclinical atherosclerosis (63).
Park et al. (64) analyzed data of 43,722 women and found that those who slept in light rooms had higher body weight compared with women sleeping in dark rooms. Similarly, a cross‐sectional analysis of over 100,000 women in the Breakthrough Generations Study showed that the odds of having overweight and obesity were higher when sleeping in a light room (65). The odds of having an increased waist circumference, waist‐hip ratio, and waist‐height ratio were also higher in people who slept in light rooms (62, 64, 65). When satellite data of outdoor LAN were matched with data on body weight, it was observed that LAN was a strong positive predictor for overweight and obesity in both men and women (60, 66, 67).
Several experiments conducted in men living on Antarctica found that in December, when outdoor light exposure is continuous, plasma levels of glucose, insulin, and thyroid‐stimulating hormone were altered compared with other months (68, 69, 70, 71). Unfortunately, in these experiments, the indoor lighting regiment was not specified. In addition, variations in temperature, physical activity, and food intake between months likely will also have impacted the changes in physiology mentioned above.
Overall, ample evidence has suggested that high LAN levels, either outdoor or in the bedroom, correlate with increased body weight and obesity in humans. Furthermore, increased incidence in dyslipidemia, subclinical atherosclerosis, and central obesity suggest LAN might be an important risk factor for the development and deterioration of cardiovascular disease.
Acute Effects of Exposure to LAN on Rodent Metabolism
Experiments using short‐term exposure to LAN in experimental animals are useful to determine whether light has acute effects on energy metabolism and clock function, as it is well known that even very short exposures to nocturnal light will inhibit melatonin release. Many studies have shown acute alterations in clock gene expression and increased c‐Fos expression in the SCN upon short‐term nocturnal light exposure (51, 72, 73, 74), an indirect marker of neuronal activity. Experiments in rats have shown nocturnal light pulses to acutely alter Per1 and Per2 expression in the ventrolateral SCN but not in the dorsomedial SCN. The dorsomedial SCN continued its robust circadian oscillation in clock gene expression (75), indicating that the ventrolateral SCN readily adapts to the environment, whereas the intrinsic core clock rhythmicity is maintained in the dorsomedial SCN. Interestingly, exposure to a light pulse during late subjective nighttime elicited greater c‐Fos expression in the SCN and PVN of hamsters compared with a pulse early in the subjective night, indicating that SCN sensitivity to light is dependent on its timing within the circadian cycle (76).
Light pulses also affected metabolism in a timing‐ and intensity‐dependent manner. For instance, locomotor activity was acutely decreased by a pulse of bright light in rats and mice but not by a pulse of dim light in rats (77, 78). In the rat pineal gland, expression of Per1 and arylalkylamine‐N‐acetyltransferase, a key enzyme to produce melatonin, was decreased by a pulse in the early subjective night and by a pulse in the late subjective night. However, expression of Per2 and Per3 in the pineal was decreased exclusively by a pulse in the late subjective night (79).
Effects of light on plasma glucocorticoid levels are controversial. In the rat, Cailotto et al. (79) found increased ACTH receptor mRNA expression in the adrenal and increased plasma glucocorticoid levels in the early subjective night, whereas adrenal Cry2 expression was altered in the late subjective nighttime (79). Mohawk et al. (80), on the contrary, found increased plasma glucocorticoid levels in the middle and end of subjective nighttime, along with increased plasma ACTH levels. Yet another experiment in rats using light pulses of a lower intensity showed plasma glucocorticoid levels were not affected (78). In mice, a pulse in the middle of the dark phase increased plasma glucocorticoid levels in an intensity‐dependent manner as well as increased Per1 and Per2 expression without alterations in ACTH levels (81, 82). Interestingly, the elevated plasma glucocorticoid levels were accompanied by increased activity in the adrenal nerve, suggesting light information was primarily transmitted to the mouse adrenal via the autonomic nervous system (82).
A light pulse in the early dark phase elevated basal plasma glucose levels and decreased expression of GLUT4 in mice skeletal muscle (72). In rats, plasma glucose levels during an intravenous glucose tolerance test (IVGTT) were elevated by a pulse in the early dark phase, while insulin levels were not affected. On the contrary, in the late dark phase, no effect of light on plasma glucose levels was found, but insulin levels were elevated. Surprisingly, an intravenous insulin tolerance test showed insulin sensitivity was not affected at either time point. Possibly, elevations in plasma glucose levels were caused by altered insulin‐independent glucose uptake or increased glucose production by the liver. The elevation in plasma glucose levels in rats was found as a result of bright light pulses but not dim light pulses (78). In the rat liver, expression of Per1, Per 2, GLUT2, and phosphoenolpyruvate carboxykinase was increased, while glucokinase was decreased by pulses at different time points depending on the specific clock genes and enzymes (83). Interestingly, hepatic denervation prevented light‐induced changes in clock gene and enzyme expression, supporting the notion that the autonomic nervous system is essential in transmitting these acute effects of light to peripheral tissues (79, 84, 85).
It appears light has an acute effect on peripheral tissues first via the ventral region of the SCN and then via the autonomic nervous system. As the acute effects of light differ among and within peripheral tissues, it is likely that either the transduction of the light signal or the receptivity of the peripheral tissue to light is modulated at a level downstream of the SCN or at the level of the peripheral tissue itself. The effects of LAN on glucose metabolism are ambiguous, as LAN seems to promote both mobilization and conservation of glucose. Possibly, daily variations in the receptivity of the liver, skeletal muscle, and the pancreas to light explain the variety of effects LAN has at different circadian time points. Counterintuitively, LAN acutely decreased locomotor activity yet increased plasma glucocorticoid levels, again suggesting LAN has both an energy conserving and an arousing effect on these nocturnal animals.
Acute Effects of Exposure to LAN on Human Metabolism
In an experiment in which 48 participants were exposed to a night of dim light, sleep duration was decreased without affecting salivary melatonin levels (86). In another study in 14 men, salivary cortisol levels were acutely increased by a light pulse in the morning but not in the evening (87). Similarly, in an experiment involving 17 men, exposure to light acutely increased heart rate in the morning and middle of the night but not in the evening. Furthermore, the increase in heart rate was intensity dependent in the morning but not in the evening (88). Increased heart rate during morning light exposure has been shown to likely rely on an increase in sympathetic activity (89, 90). Interestingly, morning light has also been shown to cause elevated plasma triglycerides levels in healthy men and elevated plasma glucose levels and plasma TAG levels in men with type 2 diabetes, indicating exposure to light in the morning increases energy availability (89).
A study in 17 participants found that salivary melatonin levels as well as evening preprandial plasma free‐fatty acid levels were decreased in individuals exposed to a night of bright light compared with a night of dim light. Evening postprandial plasma glucose and insulin levels were elevated in individuals exposed to a night of bright light. Basal TAG levels and basal plasma glucose and insulin levels were similar between lighting conditions (91). Another study in eight male participants also found that LAN elevated postprandial plasma insulin levels in the evening. Interestingly, plasma insulin and glucagon‐like peptide 1 levels were also elevated after a meal in the morning following LAN exposure. Postprandial glucose levels were not affected at any time point in this study. Plasma glucocorticoid levels were briefly elevated by LAN in the middle of the night. Plasma melatonin levels were decreased (92).
Results of experiments using LAN and light in the morning indicate light has an arousing effect on humans at the onset of the activity period, which is most likely mediated by the autonomic nervous system. Results of LAN exposure on plasma cortisol levels and heart rate at the beginning of the subjective night differed from results found in the morning, indicating the arousing effects of LAN are circadian‐phase dependent. Contrarily, effects of LAN on postprandial plasma glucose and insulin levels at the beginning of the subjective night and the following morning were similar, suggesting acute effects of LAN on glucose metabolism are more constant. Impairment of glucose metabolism indicates LAN might especially propose a risk to individuals that are prone to or suffer from type 2 diabetes.
The Effects of LAN of Different Wavelengths on Rodent Metabolism
As multiple photoreceptors contribute to signaling light information to the circadian system, each with their own peak sensitivity (5, 6) it is to be expected that light of different spectral compositions will impact clock function and energy metabolism differently. Indeed, in the hamster SCN, a 30‐minute pulse of dim blue light elicited c‐Fos expression, whereas a pulse of dim red light did not. Interestingly, dim blue light also elicited greater c‐Fos induction in the hamster SCN than dim white light (51). Similarly, in the mouse SCN, a 30‐minute pulse of blue enriched white light caused greater Per2 expression than a pulse of blue‐filtered white light (72). Furthermore, Blue light also induced greater c‐Fos expression in the mouse SCN compared with green and violet light. Contrastingly, in the mouse ventrolateral preoptic nucleus, green light elicited greater c‐Fos expression than blue light, suggesting that especially the SCN is sensitive to blue light. In melanopsin‐knockout mice, expression of c‐Fos, Per1, and Per2 in the SCN in response to blue light was reduced compared with wild‐type mice but elevated compared with dark controls, indicating that the melanopsin‐containing ipRGCs amplify the effects of blue light in the SCN (93).
In mice, a 1‐hour pulse of green light in the early dark phase rapidly induced sleep, whereas blue light induced sleep with a delayed onset. Interestingly, a corticosteroid receptor antagonist advanced sleep onset in blue light conditions, indicating blue light increased arousal, whereas green light did not (93). In rats, 2‐hour pulses of green and blue light acutely decreased locomotor activity, whereas a pulse of red light did not (78). In hamsters, 8 hours of blue dLAN reduced nocturnal locomotor activity, whereas red dLAN increased both total and nocturnal locomotor activity (51). Constant dim green light disturbed the circadian locomotor activity rhythm in hamsters and lengthened the circadian period by 0.3 hours (94).
In mice, both a 1‐hour pulse of green and blue light elevated plasma glucocorticoid levels, although the increase was significantly greater in blue light. Expression of both Per1 and Per2 in the adrenal was elevated by blue light but not by green light. The response of both plasma glucocorticoid levels and adrenal Per1 and Per2 expression to blue light was decreased in melanopsin‐knockout mice compared with wild‐type mice. Contrarily, in green light, the response of plasma glucocorticoid levels was increased in melanopsin‐knockout mice and comparable to plasma glucocorticoid levels in blue‐light–exposed melanopsin‐knockout mice. Thus, melanopsin most likely modulated the effects of light, translating into a different impact of green and blue light on the adrenal gland (93).
In Microtus Socialis, a nocturnal nonlaboratory rodent, 30‐minute pulses of both blue and yellow light increased urinary corticosteroid levels, with higher levels in blue light, whereas red light did not impact urinary corticosteroid levels (95). In rats, however, plasma glucocorticoid levels were unaffected by a pulse of either red, green, or blue light (78), but when 8 hours of chronic red dLAN was used for 6 weeks, a decrease in total plasma glucocorticoid levels and an 8‐hour advance in the phase of its daily rhythm was found (96). In our study with the diurnal rodent Arvicanthis ansorgei, a 1‐hour pulse of blue light reduced plasma glucocorticoid levels in male animals fed a chow diet and increased plasma glucocorticoid levels in female animals fed a high‐fat high‐sucrose (HFHS) diet (97).
Plasma melatonin levels in hamsters were reduced after 8 hours of dim green light but not after 2 hours (94). In rats, 4 hours of red dLAN reduced plasma melatonin levels (96). Both a blue and a yellow 30‐minute light pulse reduced urinary melatonin levels in M. Socialis and caused weight loss, whereas red light did not. Interestingly though, daily energy expenditure was reduced in blue light as well as in red light, but not in yellow light, in M. Socialis (95). Exposure to 8 hours of red dLAN over the course of 6 weeks did not impact food and water intake or body weight in rats. The daily rhythm in plasma total fatty acid levels in rats persisted during 6 weeks under red dLAN, but the amplitude was dampened with a disturbed daily rhythm. Absolute levels of plasma leptin levels were higher in these conditions(96). Contrastingly, a 1‐hour pulse of blue light had no effect on plasma leptin levels in Arvicanthis ansorgei, a diurnal rat species (97). In the mouse liver, expression of ATP binding cassette subfamily G member 1, a protein involved in cholesterol and lipid transport, was elevated by a 30‐minute pulse of white light that contained blue light but not by blue‐filtered white light (72).
Rats showed impaired glucose tolerance during an IVGTT when given a 2‐hour pulse of green light but not by red or blue light. There was no effect of light of any wavelength on plasma insulin levels during the IVGTT or on basal plasma glucose and insulin levels (78). In Arvicanthis, a pulse of blue light elevated basal plasma glucose levels in female HFHS animals but not in male animals. Plasma glucose levels after an OGTT were elevated and basal plasma insulin levels were decreased by blue light in male Arvicanthis when fed a chow or HFHS diet but not in females (97). In rats exposed to chronic red dLAN for 6 weeks, the daily rhythm in basal plasma glucose and insulin levels was maintained, but basal plasma glucose levels were elevated, whereas peak values in basal plasma insulin levels were decreased (96). A 30‐minute pulse of blue‐enriched white light increased plasma glucose levels, whereas blue‐filtered white light did not have an effect in mice. Expression of insulin receptor substrate 2 was increased by blue‐cut white light in mice liver and skeletal muscle, whereas, contrarily, Insulin receptor substrate 2 was decreased in the liver by blue‐enriched white light (72).
Thus, both in the SCN and in the adrenal gland, the impact of blue light was stronger than that of green light. It is therefore likely that light of any wavelength travels through the SCN to the adrenal gland but is initially modulated by the ipRGCs. Yet the wavelength effects seem to be species, duration, and intensity dependent. Surprisingly, an experiment in rats found that 6 weeks of red dLAN had a wide range of effects on metabolism, a finding that is not in line with the effects of red light on the SCN and seems to contrast with other evidence, i.e., nocturnal rodents being unable to respond to red light (51). However, red light was also found to impact metabolism in other experiments, suggesting chronic exposure to red light perhaps impacts clock function or might impact metabolism independent of the SCN. Further research comparing exposure to light of different wavelengths and in different rodent species will be important to elucidate more precisely how LAN impacts metabolism through different pathways. Additionally, research aiming to study the different effects of light should report the spectral sensitivity within each retinal photopigment complement (98), as it facilitates comparisons among studies and because the importance of non‐ipRGCs photoreceptors in circadian entrainment has recently been re‐established by Mouland et al. (33).
The Effects of Exposure to LAN of Different Wavelengths on Human Metabolism
Experimental evidence has pointed out that blue light is most potent in suppressing melatonin release in humans (99, 100). Both red and green light were shown to suppress melatonin as well, albeit to a lesser extent than blue light (101, 102). In an experiment on evening computer use, melatonin levels were decreased in participants that used backlit screens, which emitted high‐intensity blue light, compared with participants that used nonbacklit screens (103). Comparably, participants that used blue light–enhancing goggles during computer use showed decreased melatonin levels compared with participants that used the computer without goggles. Interestingly, participants that used a computer without goggles and participants that used blue light–filtering goggles had comparable melatonin levels, suggesting there is an intensity‐dependent threshold for blue light to affect melatonin levels (104). Similarly, in an experiment on evening smartphone use, participants that used a regular smart phone had melatonin levels comparable to participants that used a smartphone with blue light suppression (105).
Evening exposure to blue light was shown to decrease subjective sleepiness in humans (101, 103, 105), whereas green light was not (101). Concordantly, electroencephalogram (EEG) power spectra were impacted during evening blue light exposure (102, 103, 106, 107), suggesting blue light might have altered alertness. Interestingly, red light was also shown to impact EEG power spectra (102), whereas green light did not (106). Notably, in one experiment, a pulse of evening blue light was shown to impact EEG power spectra the following morning as well as decrease energy expenditure and reduce the thermic effect of breakfast (107). Core body temperature was shown to be altered by evening blue light pulses but not by green light pulses (101, 106). Contrastingly, smartphone use without blue light suppression did not impact body temperature or plasma cortisol levels, nor did smartphone use with blue light suppression (105). Importantly, an experiment that filtered light of short wavelengths in the evening, as well as 70% of all light, found reduced basal plasma glucose and insulin levels, indicating a reduction in blue light before bedtime may improve insulin sensitivity (72).
Although evidence on light of different wavelengths is scarce, it looks as though blue light increases arousal in humans and impacts energy expenditure and glucose metabolism. As screen use is a frequent source of evening blue light, restricting blue light emission from devices could be an important step in preventing the harmful health consequences of exposure to LAN. Further research will have to indicate to what extent light of short wavelengths is responsible for the deleterious effects of LAN and whether filtering only blue light is enough of an intervention, as previous human evidence does not support the contribution of the S cones to the neuroendocrine disruptive effects of light (34), and because evidence in animals implies that all light, not just blue, affects metabolism.
Conclusion
The metabolic implications of exposure to chronic and acute LAN and LAN of different wavelengths are summarized in Figure 2. It seems that chronic LAN exposure gives rise to metabolic inefficiency, especially by disturbing daily behavioral rhythms, through its impact on the SCN. However, other more acute experiments have shown that LAN also directly impacts peripheral tissues, independent of circadian rhythm disturbance, probably via the ventral region of the SCN or via non‐SCN pathways. In the majority of cases, the metabolic consequences of acute LAN most likely will be limited, as homeostasis is very well able to cope with such challenges. On the contrary, it is not clear what the consequences are of prolonged and repeated exposures to acute LAN. However, in the real‐life situation, most organisms (including humans) will be exposed to chronic LAN and experience the metabolic consequences of both the acute and chronic effects of light. The metabolic consequences of chronic LAN are mainly caused by its disruptive effects on the SCN. On the contrary, for the acute effects of LAN, it is not clear whether they involve the SCN or not. In fact, a combination of SCN‐mediated and non‐SCN–mediated effects is also possible. In addition, for now, it is also not clear whether there are fundamental differences between the metabolic consequences of acute and chronic LAN, but it seems reasonable to assume that, if anything, the effects of acute LAN will enhance those of chronic LAN.
Figure 2.
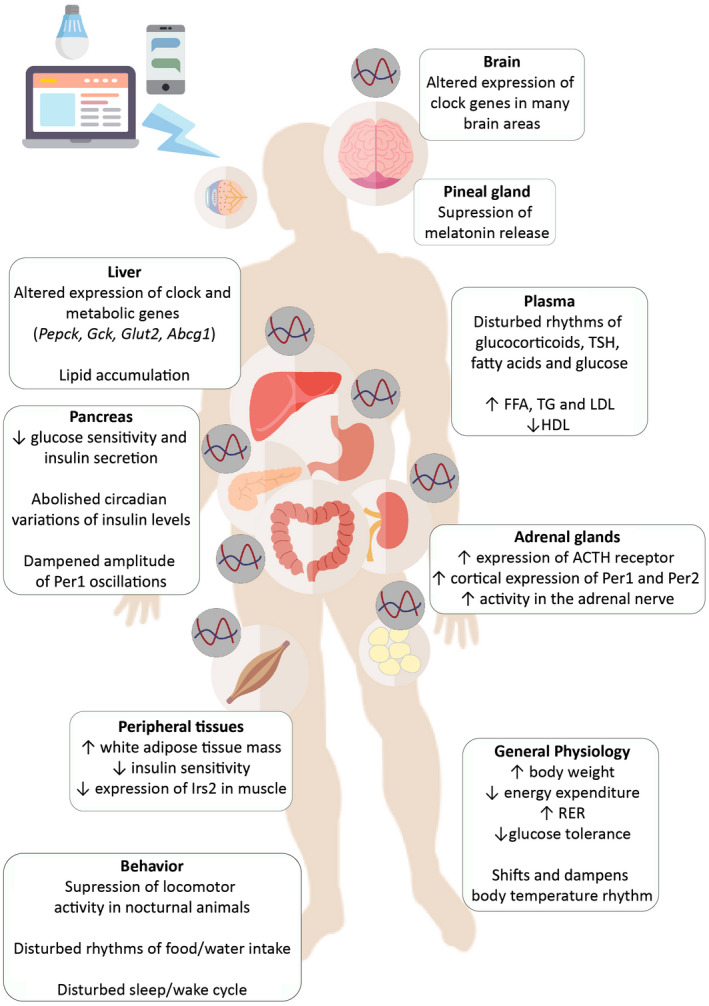
Summary of the metabolic implications of exposure to light at night. TSH, thyroid‐stimulating hormone; FFA, free fatty acids; TG, triglycerides; LDL, low‐density lipoprotein; HDL, high‐density lipoprotein; RER, respiratory exchange ratio.
Moreover, the effects of LAN are wavelength dependent and differ within and among peripheral tissues, raising the questions of to what extent the effects of light are modulated by the SCN and whether light also affects metabolism via non‐SCN pathways (Figure 1). In this regard, several recent observations are interesting. First, using mice with a genetic ablation of all ipRGCs except those that project to the SCN, acute non‐SCN–dependent effects of light were shown on physiology and behavior (13, 108). Second, Koronowsky et al. (45) showed that the autonomous oscillations of the liver clock are independent from all other clocks (including the SCN clock) yet depends on the presence of an LD cycle. Third, subcutaneous WAT adipocytes in both mice and humans have been reported to be directly sensitive to light, as these cells also express melanopsin (and encephalopsin, another photopigment) (109, 110). However, for now, it is not clear whether these photopigments in WAT have any physiological relevance. Further research using LAN of different wavelengths will be necessary in answering these questions but will also help to determine what type of light has the most harmful consequences on health. As it has been shown that both acute and chronic LAN exposure impact glucose metabolism and are associated with the development of overweight, obesity, and cardiovascular disease in humans, it is of great importance that research on this topic continues.
Funding agencies
This work was supported by a doctoral fellowship from the “NeuroTime” Erasmus + Program (AMV) and the University of Amsterdam (AK).
Disclosure
The authors declared no conflict of interest.
Author contributions
GF wrote the first version of the manuscript. GF and AMV wrote the next versions of the manuscript. AMV made the illustrations. AK revised and edited the manuscript.
Supporting information
Table S1
Table S2
Table S3
Contributor Information
Anayanci Masís‐Vargas, Email: a.masisvargas@amsterdamumc.nl.
Andries Kalsbeek, Email: a.masisvargas@amsterdamumc.nl.
References
- 1. World Health Organization . Obesity and overweight. http://www.who.int/en/news‐room/fact‐sheets/detail/obesity‐and‐overweight. Updated April 1, 2020. Accessed November 12, 2019.
- 2. Mure LS, Vinberg F, Hanneken A, Panda S. Functional diversity of human intrinsically photosensitive retinal ganglion cells. Science 2019;366:1251‐1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bailes HJ, Lucas RJ. Human melanopsin forms a pigment maximally sensitive to blue light (λmax ≈ 479 nm) supporting activation of Gq/11 and Gi/o signalling cascades. Proc R Soc B Biol Sci 2013;280:20122987. doi: 10.1098/rspb.2012.2987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Color and Vision Research Labs . Cone fundamentals. http://www.cvrl.org/. Accessed March 9, 2020.
- 5. Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 2002;295:1070‐1073. [DOI] [PubMed] [Google Scholar]
- 6. Hattar S, Kumar M, Park A, et al. Central projections of melanopsin‐ expressing retinal ganglion cells in the mouse. J Comp Neurol 2006;497:326‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ralph RM, Foster RG, Davis CF, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science 1990;247:975‐978. [DOI] [PubMed] [Google Scholar]
- 8. Dunlap JC. Molecular bases for circadian clocks. Cell 1999;96:271‐290. [DOI] [PubMed] [Google Scholar]
- 9. Schwartz WJ, Tavakoli‐Nezhad M, Lambert CM, Weaver DR, De La Iglesia HO. Distinct patterns of period gene expression in the suprachiasmatic nucleus underlie circadian clock photoentrainment by advances or delays. Proc Natl Acad Sci U S A 2011;108:17219‐17224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schwartz MW, Woods SC, Porte D, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 2000;404:661‐671. [DOI] [PubMed] [Google Scholar]
- 11. Buijs FN, Guzmán‐Ruiz M, León‐Mercado L, et al. Suprachiasmatic nucleus interaction with the arcuate nucleus. Essential for organizing physiological rhythms. eNeuro 2017;4:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chou TC, Scammell TE, Gooley JJ, Gaus SE, Saper CB, Lu J. Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. J Neurosci 2003;23:10691‐10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fernandez DC, Fogerson PM, Lazzerini Ospri L, et al. Light affects mood and learning through distinct retina‐brain pathways. Cell 2018;175:71‐84.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stamatakis AM, Van Swieten M, Basiri ML, Blair GA, Kantak P, Stuber GD. Lateral hypothalamic area glutamatergic neurons and their projections to the lateral habenula regulate feeding and reward. J Neurosci 2016;36:302‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perreau‐Lenz S, Kalsbeek A, Garidou ML, et al. Suprachiasmatic control of melatonin synthesis in rats: inhibitory and stimulatory mechanisms. Eur J Neurosci 2003;17:221‐228. [DOI] [PubMed] [Google Scholar]
- 16. Buijs RM. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur J Neurosci 1999;11:1535‐1544. [DOI] [PubMed] [Google Scholar]
- 17. Kalsbeek A, Fliers E, Franke AN, Wortel J, Buijs RM. Functional connections between the suprachiasmatic nucleus and the thyroid gland as revealed by lesioning and viral tracing techniques in the rat. Endocrinology 2000;141:3832‐3841. [DOI] [PubMed] [Google Scholar]
- 18. Buijs RM, Chun SJ, Niijima A, Romijn HJ, Nagai K. Parasympathetic and sympathetic control of the pancreas: a role for the suprachiasmatic nucleus and other hypothalamic centers that are involved in the regulation of food intake. J Comp Neurol 2001;431:405‐423. [DOI] [PubMed] [Google Scholar]
- 19. La Fleur SE, Kalsbeek A, Wortel J, Buijs RM. Polysynaptic neural pathways between the hypothalamus, including the suprachiasmatic nucleus, and the liver. Brain Res 2000;871:50‐56. [DOI] [PubMed] [Google Scholar]
- 20. Kreier F, Fliers E, Voshol PJ, et al. Selective parasympathetic innervation of subcutaneous and intra‐abdominal fat ‐ functional implications. J Clin Invest 2002;110:1243‐1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taylor T, Wondisford FE, Blaine T, Weintraub BD, Taylor T. The paraventricular nucleus of the hypothalamus has a major role in thyroid hormone feedback regulation of thyrotropin synthesis and secretion. Endocrinology 1990;126:317‐324. [DOI] [PubMed] [Google Scholar]
- 22. Herman JP, McKlveen JM, Ghosal S, et al. Regulation of the hypothalamic‐pituitary‐ adrenocortical stress response. Compr Physiol 2016;6:603‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yamazaki S, Numano R, Abe M, et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science 2000;288:682‐685. [DOI] [PubMed] [Google Scholar]
- 24. Balsalobre A, Brown SA, Marcacci L, et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 2000;289:2344‐2347. [DOI] [PubMed] [Google Scholar]
- 25. Hirota T, Okano T, Kokame K, Shirotani‐Ikejima H, Miyata T, Fukada Y. Glucose down‐regulates Per1 and Per2 mRNA levels and induces circadian gene expression in cultured rat‐1 fibroblasts. J Biol Chem 2002;277:44244‐44251. [DOI] [PubMed] [Google Scholar]
- 26. Brown SA, Zumbrunn G, Fleury‐Olela F, Preitner N, Schibler U. Rhythms of mammalian body temperature can sustain peripheral circadian clocks. Curr Biol 2002;12:1574‐1583. [DOI] [PubMed] [Google Scholar]
- 27. Liu L, Wang Z, Cao J, Dong Y, Chen Y. Effect of melatonin on monochromatic light‐induced changes in clock gene circadian expression in the chick liver. J Photochem Photobiol B Biol 2019;197:111537. doi: 10.1016/j.jphotobiol.2019.111537 [DOI] [PubMed] [Google Scholar]
- 28. Wolff G, Esser KA. Scheduled exercise phase shifts the circadian clock in skeletal muscle. Med Sci Sport Exerc 2012;44:1663‐1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Damiola F, Le Minli N, Preitner N, Kornmann B, Fleury‐Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 2000;14:2950‐2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Segers A, Desmet L, Thijs T, Verbeke K, Tack J, Depoortere I. The circadian clock regulates the diurnal levels of microbial short‐chain fatty acids and their rhythmic effects on colon contractility in mice. Acta Physiol 2019;225:e13193. doi: 10.1111/apha.13193 [DOI] [PubMed] [Google Scholar]
- 31. International Commission on Illumination . CIE S 026/E:2018 CIE system for metrology of optical radiation for ipRGC‐influenced responses to light. Color Res Appl 2018;44:316. [Google Scholar]
- 32. Spitschan M, Stefani O, Blattner P, Gronfier C, Lockley S, Lucas R. How to report light exposure in human chronobiology and sleep research experiments. Clocks & Sleep 2019;1:280‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mouland JW, Martial F, Watson A, et al. Cones support alignment to an inconsistent world by suppressing mouse circadian responses to the blue colors associated with twilight report cones support alignment to an inconsistent world by suppressing mouse circadian responses to the blue colors assoc. Curr Biol 2019;29:4260‐4267.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Spitschan M, Lazar R, Yetik E, Cajochen C. No evidence for an S cone contribution to the human circadian response to light. bioRxiv 2019;29:763359. doi: 10.1101/763359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Spitschan M, Woelders T. The method of silent substitution for examining melanopsin contributions to pupil control. Front Neurol 2018;9:941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Coomans CP, van den Berg SAA, Houben T, et al. Detrimental effects of constant light exposure and high‐fat diet on circadian energy metabolism and insulin sensitivity. FASEB J 2013;27:1721‐1732. [DOI] [PubMed] [Google Scholar]
- 37. Ohta H, Yamazaki S, McMahon DG. Constant light desynchronizes mammalian clock neurons. Nat Neurosci 2005;8:267‐269. [DOI] [PubMed] [Google Scholar]
- 38. Fonken LK, Workman JL, Walton JC, et al. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci 2010;107:18664‐18669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Madahi PG, Ivan O, Adriana B, Diana O, Carolina E. Constant light during lactation programs circadian and metabolic systems. Chronobiol Int 2018;35:1153‐1167. [DOI] [PubMed] [Google Scholar]
- 40. Depres‐Brummer P, Levi F, Metzger G, Touitou Y. Light‐induced suppression of the rat circadian system. Am J Physiol 1995;268:R1111‐R1116. [DOI] [PubMed] [Google Scholar]
- 41. Dauchy RT, Dauchy EM, Tirrell RP, et al. Dark‐phase light contamination disrupts circadian rhythms in plasma measures of endocrine physiology and metabolism in rats. Comp Med 2010;60:348‐356. [PMC free article] [PubMed] [Google Scholar]
- 42. Abílio VC, Freitas FM, Dolnikoff MS, Castrucci AML, Filho RF. Effects of continuous exposure to light on behavioral dopaminergic supersensitivity. Biol Psychiatry 1999;45:1622‐1629. [DOI] [PubMed] [Google Scholar]
- 43. Fonken LK, Finy MS, Walton JC, et al. Influence of light at night on murine anxiety‐ and depressive‐like responses. Behav Brain Res 2009;205:349‐354. [DOI] [PubMed] [Google Scholar]
- 44. Qian J, Block GD, Colwell CS, Matveyenko AV. Consequences of exposure to light at night on the pancreatic islet circadian clock and function in rats. Diabetes 2013;62:3469‐3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Koronowski KB, Kinouchi K, Welz PS, et al. Defining the independence of the liver circadian clock. Cell 2019;177:1448‐1462.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fonken LK, Aubrecht TG, Meléndez‐Fernández OH, Weil ZM, Nelson RJ. Dim light at night disrupts molecular circadian rhythms and increases body weight. J Biol Rhythms 2013;28:262‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ikeno T, Weil ZM, Nelson RJ. Dim light at night disrupts the short‐day response in Siberian hamsters. Gen Comp Endocrinol 2014;197:56‐64. [DOI] [PubMed] [Google Scholar]
- 48. Stenvers DJ, Van Dorp R, Foppen E, et al. Dim light at night disturbs the daily sleep‐wake cycle in the rat. Sci Rep 2016;6:4‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Borniger JC, Maurya SK, Periasamy M, Nelson RJ. Acute dim light at night increases body mass, alters metabolism, and shifts core body temperature circadian rhythms. Chronobiol Int 2014;31:917‐925. [DOI] [PubMed] [Google Scholar]
- 50. Bedrosian TA, Galan A, Vaughn CA, Weil ZM, Nelson RJ. Light at night alters daily patterns of cortisol and clock proteins in female siberian hamsters. J Neuroendocrinol 2013;25:590‐596. [DOI] [PubMed] [Google Scholar]
- 51. Bedrosian TA, Vaughn CA, Galan A, Daye G, Weil ZM, Nelson RJ. Nocturnal light exposure impairs affective responses in a wavelength‐dependent manner. J Neurosci 2013;33:13081‐13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Panagiotou M, Meijer JH, de Boer T. Effect of chronic dim‐light‐at‐night exposure on sleep EEG and behavior in young and aged mice. Sleep Med 2017;40:e76. [DOI] [PubMed] [Google Scholar]
- 53. Molcan L, Sutovska H, Okuliarova M, Senko T, Krskova L, Zeman M. Dim light at night attenuates circadian rhythms in the cardiovascular system and suppresses melatonin in rats. Life Sci 2019;231:116568. doi: 10.1016/j.lfs.2019.116568 [DOI] [PubMed] [Google Scholar]
- 54. Rumanova VS, Okuliarova M, Molcan L, Sutovska H, Zeman M. Consequences of low‐intensity light at night on cardiovascular and metabolic parameters in spontaneously hypertensive rats. Can J Physiol Pharmacol 2019;97:863‐871. [DOI] [PubMed] [Google Scholar]
- 55. Fonken LK, Lieberman RA, Weil ZM, Nelson RJ. Dim light at night exaggerates weight gain and inflammation associated with a high‐fat diet in male mice. Endocrinology 2013;154:3817‐3825. [DOI] [PubMed] [Google Scholar]
- 56. Aubrecht TG, Jenkins R, Nelson RJ. Dim light at night increases body mass of female mice. Chronobiol Int 2015;32:557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Russart KLG, Chbeir SA, Nelson RJ, Magalang UJ. Light at night exacerbates metabolic dysfunction in a polygenic mouse model of type 2 diabetes mellitus. Life Sci 2019;231:116574. doi: 10.1016/j.lfs.2019.116574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rohling JHT, vanderLeest HT, Michel S, Vansteensel MJ, Meijer JH. Phase resetting of the mammalian circadian clock relies on a rapid shift of a small population of pacemaker neurons. PLoS One 2011;6:16‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chepesiuk R. Missing the dark: health effects of light pollution. Environ Health Perspect 2009;117:20‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Koo YS, Song JY, Joo EY, et al. Outdoor artificial light at night, obesity, and sleep health: cross‐sectional analysis in the KoGES study. Chronobiol Int 2016;33:301‐314. [DOI] [PubMed] [Google Scholar]
- 61. Xiao Q, Gee G, Jones RR, Jia P, James P, Hale L. Cross‐sectional association between outdoor artificial light at night and sleep duration in middle‐to‐older aged adults: the NIH‐AARP Diet and Health Study. Environ Res 2020;180:108823. doi: 10.1016/j.envres.2019.108823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Obayashi K, Saeki K, Iwamoto J, et al. Exposure to light at night, nocturnal urinary melatonin excretion, and obesity/dyslipidemia in the elderly: a cross‐sectional analysis of the HEIJO‐KYO study. J Clin Endocrinol Metab 2013;98:337‐344. [DOI] [PubMed] [Google Scholar]
- 63. Obayashi K, Saeki K, Kurumatani N. Light exposure at night is associated with subclinical carotid atherosclerosis in the general elderly population: the HEIJO‐KYO cohort. Chronobiol Int 2015;32:310‐317. [DOI] [PubMed] [Google Scholar]
- 64. Park YMM, White AJ, Jackson CL, Weinberg CR, Sandler DP. Association of exposure to artificial light at night while sleeping with risk of obesity in women. JAMA Intern Med 2019;179:1061‐1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. McFadden E, Jones ME, Schoemaker MJ, Ashworth A, Swerdlow AJ. The relationship between obesity and exposure to light at night: cross‐sectional analyses of over 100,000 women in the breakthrough generations study. Am J Epidemiol 2014;180:245‐250. [DOI] [PubMed] [Google Scholar]
- 66. Rybnikova NA, Haim A, Portnov BA. Does artificial light‐at‐night exposure contribute to the worldwide obesity pandemic? Int J Obes 2016;40:815‐823. [DOI] [PubMed] [Google Scholar]
- 67. Abay KA, Amare M. Night light intensity and women’s body weight: evidence from Nigeria. Econ Hum Biol 2018;31:238‐248. [DOI] [PubMed] [Google Scholar]
- 68. Malhotra AS, Pal K, Prasad R, Bajaj AC, Kumar R, Sawhney RC. Plasma insulin and growth hormone during antarctic residence. Jpn J Physiol 1998;48:167‐169. [DOI] [PubMed] [Google Scholar]
- 69. Campbell IT, Jarrett RJ, Rutland P, Stimmler L. The plasma insulin and growth hormone response to oral glucose: diurnal and seasonal observations in the Antarctic. Diabetologia 1975;150:147‐150. [DOI] [PubMed] [Google Scholar]
- 70. Campbell IT, Jarrett RJ, Keen H. Diurnal and seasonal variation in oral glucose tolerance: studies in the Antarctic. Diabetologia 1975;11:139‐145. [DOI] [PubMed] [Google Scholar]
- 71. Sawhney RC, Malhotra AS, Nair CS, et al. Thyroid function during a prolonged stay in Antarctica. Eur J Appl Physiol Occup Physiol 1995;72:127‐133. [DOI] [PubMed] [Google Scholar]
- 72. Nagai N, Ayaki M, Yanagawa T, et al. Suppression of blue light at night ameliorates metabolic abnormalities by controlling circadian rhythms. Investig Opthalmology Vis Sci 2019;60:3786. [DOI] [PubMed] [Google Scholar]
- 73. Masana MI, Benloucif S, Dubocovich ML. Light‐induced c‐fos mRNA expression in the suprachiasmatic nucleus and the retina of C3H/HeN mice. Mol Brain Res 1996;42:193‐201. [DOI] [PubMed] [Google Scholar]
- 74. Aronin N, Sagar SM, Sharp FR, Schwartz WJ. Light regulates expression of a Fos‐related protein in rat suprachiasmatic nuclei. Proc Natl Acad Sci U S A 1990;87:5959‐5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yan L, Okamura H. Gradients in the circadian expression of Per1 and Per2 genes in the rat suprachiasmatic nucleus. Eur J Neurosci 2002;15:1153‐1162. [DOI] [PubMed] [Google Scholar]
- 76. Munch IC, Mller M, Larsen PJ, Vrang N. Light‐induced c‐Fos expression in suprachiasmatic nuclei neurons targeting the paraventricular nucleus of the hamster hypothalamus: phase dependence and immunochemical identification. J Comp Neurol 2002;442:48‐62. [DOI] [PubMed] [Google Scholar]
- 77. Thompson S, Lupi D, Hankins MW, Peirson SN, Foster RG. The effects of rod and cone loss on the photic regulation of locomotor activity and heart rate. Eur J Neurosci 2008;28:724‐729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Opperhuizen A‐L, Stenvers DJ, Jansen RD, Foppen E, Fliers E, Kalsbeek A. Light at night acutely impairs glucose tolerance in a time‐, intensity‐ and wavelength‐dependent manner in rats. Diabetologia 2017;60:1333‐1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cailotto C, Lei J, van der Vliet J, et al. Effects of nocturnal light on (clock) gene expression in peripheral organs: a role for the autonomic innervation of the liver. PLoS One 2009;4:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mohawk JA, Pargament JM, Lee TM. Circadian dependence of corticosterone release to light exposure in the rat. Physiol Behav 2007;92:800‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kiessling S, Sollars PJ, Pickard GE. Light stimulates the mouse adrenal through a retinohypothalamic pathway independent of an effect on the clock in the suprachiasmatic nucleus. PLoS One 2014;9:e92959. doi: 10.1371/journal.pone.0092959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ishida A, Mutoh T, Ueyama T, et al. Light activates the adrenal gland: Timing of gene expression and glucocorticoid release. Cell Metab 2005;2:297‐307. [DOI] [PubMed] [Google Scholar]
- 83. Masís‐Vargas A, Ritsema WIGR, Mendoza J, Kalsbeek A. Metabolic effects of light at night are time‐ and wavelength‐dependent in rats. Obesity (Silver Spring) 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Fan SMY, Chang YT, Chen CL, et al. External light activates hair follicle stem cells through eyes via an ipRGC–SCN–sympathetic neural pathway. Proc Natl Acad Sci U S A 2018;115:E6880‐E6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Aras E, Ramadori G, Kinouchi K, et al. Light entrains diurnal changes in insulin sensitivity of skeletal muscle via ventromedial hypothalamic neurons. Cell Rep 2019;27:2385‐2398.e3. [DOI] [PubMed] [Google Scholar]
- 86. Cho CH, Yoon HK, Kang SG, Kim L, Il Lee E, Lee HJ. Impact of exposure to dim light at night on sleep in female and comparison with male subjects. Psychiatry Investig 2018;15:520‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Scheer FA, Buijs RM. Light affects morning salivary cortisol in humans. J Clin Endocrinol Metab 1999;84:3395‐3398. [DOI] [PubMed] [Google Scholar]
- 88. Scheer FAJL, Van Doornen LJP, Buijs RM. Light and diurnal cycle affect human heart rate: possible role for the circadian pacemaker. J Biol Rhythms 1999;14:202‐212. [DOI] [PubMed] [Google Scholar]
- 89. Versteeg RI, Stenvers DJ, Visintainer D, et al. Acute effects of morning light on plasma glucose and triglycerides in healthy men and men with type 2 diabetes. J Biol Rhythms 2017;32:130‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Scheer FAJL, Van Doornen LJP, Buijs RM. Light and diurnal cycle affect autonomic cardiac balance in human; possible role for the biological clock. Auton Neurosci Basic Clin 2004;110:44‐48. [DOI] [PubMed] [Google Scholar]
- 91. Albreiki MS, Middleton B, Hampton SM. A single night light exposure acutely alters hormonal and metabolic responses in healthy participants. Endocr Connect 2017;6:100‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gil‐Lozano M, Hunter PM, Behan LA, Gladanac B, Casper RF, Brubaker PL. Short‐term sleep deprivation with nocturnal light exposure alters time‐dependent glucagon‐like peptide‐1 and insulin secretion in male volunteers. Am J Physiol Endocrinol Metab 2015;310:E41‐E50. [DOI] [PubMed] [Google Scholar]
- 93. Pilorz V, Tam SKE, Hughes S, et al. Melanopsin regulates both sleep‐promoting and arousal‐promoting responses to light. PLoS Biol 2016;14:1‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Evans JA, Elliott JA, Gorman MR. Circadian effects of light no brighter than moonlight. J Biol Rhythms 2007;22:356‐367. [DOI] [PubMed] [Google Scholar]
- 95. Zubidat AE, Nelson RJ, Haim A. Spectral and duration sensitivity to light‐at‐night in “blind” and sighted rodent species. J Exp Biol 2011;214:3206‐3217. [DOI] [PubMed] [Google Scholar]
- 96. Dauchy RT, Wren MA, Dauchy EM, et al. The influence of red light exposure at night on circadian metabolism and physiology in Sprague‐Dawley rats. J Am Assoc Lab Anim Sci 2015;54:40‐50. [PMC free article] [PubMed] [Google Scholar]
- 97. Masís‐Vargas A, Hicks D, Kalsbeek A, Mendoza J. Blue light at night acutely impairs glucose tolerance and increases sugar intake in the diurnal rodent Arvicanthis ansorgei in a sex‐dependent manner. Physiol Rep 2019;7:1‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lucas RJ, Peirson SN, Berson DM, et al. Measuring and using light in the melanopsin age. Trends Neurosci 2014;37:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Brainard GC, Sliney D, Hanifin JP, et al. Sensitivity of the human circadian system to short‐wavelength (420‐nm) light. J Biol Rhythms 2008;23:379‐386. [DOI] [PubMed] [Google Scholar]
- 100. Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non‐rod, non‐cone photoreceptor system in humans. J Physiol 2001;535:261‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Cajochen C, Münch M, Kobialka S, et al. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab 2005;90:1311‐1316. [DOI] [PubMed] [Google Scholar]
- 102. Figueiro MG, Bierman A, Plitnick B, Rea MS. Preliminary evidence that both blue and red light can induce alertness at night. BMC Neurosci 2009;10:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Cajochen C, Frey S, Anders D, et al. Evening exposure to a light‐emitting diodes (LED)‐backlit computer screen affects circadian physiology and cognitive performance. J Appl Physiol 2011;110:1432‐1438. [DOI] [PubMed] [Google Scholar]
- 104. Figueiro MG, Wood B, Plitnick B, Rea MS. The impact of light from computer monitors on melatonin levels in college students. Neuroendocrinol Lett 2011;32:158‐163. [PubMed] [Google Scholar]
- 105. Heo JY, Kim K, Fava M, et al. Effects of smartphone use with and without blue light at night in healthy adults: a randomized, double‐blind, cross‐over, placebo‐controlled comparison. J Psychiatr Res 2017;87:61‐70. [DOI] [PubMed] [Google Scholar]
- 106. Münch M, Kobialka S, Steiner R, Oelhafen P, Wirz‐Justice A, Cajochen C. Wavelength‐dependent effects of evening light exposure on sleep architecture and sleep EEG power density in men. Am J Physiol Regul Integr Comp Physiol 2006;290:1421‐1428. [DOI] [PubMed] [Google Scholar]
- 107. Kayaba M, Iwayama K, Ogata H, et al. The effect of nocturnal blue light exposure from light‐emitting diodes on wakefulness and energy metabolism the following morning. Environ Health Prev Med 2014;19:354‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Rupp AC, Ren M, Altimus CM, et al. Distinct ipRGC subpopulations mediate light’s acute and circadian effects on body temperature and sleep. Elife 2019;8. doi: 10.7554/eLife.44358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Nayak G, Zhang KX, Vemaraju S, et al. Adaptive thermogenesis in mice is enhanced by opsin 3‐dependent adipocyte light sensing. Cell Rep 2020;30:672‐686.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ondrusova K, Fatehi M, Barr A, et al. Subcutaneous white adipocytes express a light sensitive signaling pathway mediated via a melanopsin/TRPC channel axis. Sci Rep 2017;7:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3


