Abstract
Globally, nearly half of patients with type 2 diabetes (T2D) do not successfully achieve target HbA1c with basal insulin, despite meeting fasting plasma glucose (FPG) targets. In this post hoc analysis of the LixiLan‐L study, we determined whether iGlarLixi, a fixed‐ratio combination of insulin glargine Gla‐100 (iGlar) and the glucagon‐like peptide‐1 receptor agonist lixisenatide (Lixi), addresses the challenge of reducing residual hyperglycaemia in patients with T2D. In LixiLan‐L, a randomized, open‐label study, 1018 patients with T2D on basal insulin for ≥6 months ± oral antidiabetes drugs entered a 6‐week run‐in period, during which they were switched to and/or optimized for a daily dose of iGlar while continuing only metformin. Following the run‐in period, 736 patients were then randomized to receive iGlarLixi or were continued on iGlar for 30 weeks ± metformin. Residual hyperglycaemia was defined as HbA1c ≥ 7.0% despite FPG of <140 mg/dL. The proportion of patients with residual hyperglycaemia was similar in both treatment arms at screening (~~42%), and increased after the run‐in period (~~62%). After 30 weeks, the proportion of patients with residual hyperglycaemia declined to 23.8% in the iGlarLixi versus 47.1% in the iGlar arm (P < .0001). The proportion of patients achieving both HbA1c (<7.0%) and FPG (<140 mg/dL) targets was higher in the iGlarLixi compared with the iGlar arm (50.3% vs. 27.4%, respectively; P < .0001). iGlarLixi effectively reduces residual hyperglycaemia in patients with T2D on basal insulin therapy.
Keywords: basal insulin, GLP‐1 analogue, phase III study, type 2 diabetes
1. INTRODUCTION
With progression of type 2 diabetes (T2D) and the decline of beta‐cell function, oral antidiabetes drugs (OADs) are often not enough to maintain adequate glycaemic control, and many patients eventually require insulin therapy, usually basal insulin. 1 Until recently, basal insulin was the initial conventional injectable therapy. In recent years, the introduction of glucagon‐like peptide‐1 receptor agonists (GLP‐1 RAs) has made the availability of a variety of injectable therapeutic options for use in patients with T2D possible when OADs become inadequate. These injectable therapies include, besides basal insulins alone, GLP‐1 RA alone and a fixed‐ratio combination (FRC) of basal insulin with GLP‐1 RAs. With basal insulin alone, about 24% to 54% of patients globally do not reach HbA1c goals (<7.0% [<53 mmol/mol]), despite achieving fasting plasma glucose (FPG) target levels (<130/140 mg/dL [<7.2/7.8 mmol/L]). 2 This condition, referred to as ‘residual hyperglycaemia’ (i.e. HbA1c ≥ 7.0% and FPG < 140 mg/dL), is associated with elevated postprandial glucose (PPG) excursions and highlights the need for antihyperglycaemic agents targeting PPG. 2
Postprandial hyperglycaemia plays an important role in overall glycaemic control, and the contribution of PPG to HbA1c is more prominent at HbA1c < 8.5% levels. 3 Several studies have shown an association between PPG or postoral glucose load and cardiovascular (CV) risk, including coronary heart disease, stroke, peripheral arterial disease and CV death. 4 , 5 It is also known that glucose metabolism is closely linked with lipid metabolism. 6 Elevated PPG and increased levels of triglyceride containing lipoproteins after meals result in postprandial dysmetabolism, which further increases CV morbidity and mortality. 6 These findings suggest the importance of targeting PPG for managing residual hyperglycaemia.
A position statement by the American Diabetes Association and European Association for the Study of Diabetes recommends that if monotherapy is unlikely to successfully attain therapeutic goals, GLP‐1 RAs can be used as add‐ons to metformin, particularly in patients with a high CV risk or when the intent is also to avoid weight gain or hypoglycaemia. 7 However, not all GLP‐1 RAs have the same mechanism of action and properties. Long‐acting GLP‐1 RAs exert a greater effect on FPG levels, primarily mediated through their insulinotropic and glucagonostatic actions, while short‐acting GLP‐1 RAs predominately cause reduction of the PPG excursions through delayed gastric emptying. 8
iGlarLixi, an FRC of insulin glargine Gla‐100 (iGlar) and a once‐daily, short‐acting GLP‐1 RA (lixisenatide [Lixi]), targets both FPG and PPG and thereby helps to achieve glycaemic goals. The clinical efficacy and safety of iGlarLixi have been established in the LixiLan‐L study, 9 which showed greater reduction in HbA1c levels at week 30 with iGlarLixi than with iGlar in patients with T2D inadequately treated with basal insulin. In this post hoc analysis of the LixiLan‐L trial, we investigated the effect of iGlarLixi on residual hyperglycaemia in patients with T2D.
2. MATERIALS AND METHODS
In LixiLan‐L, a randomized, open‐label study (NCT02058160), 1018 patients with a ≥1‐year history of T2D who were on basal insulin with or without OADs for more than 6 months before screening entered a 6‐week run‐in period. During the run‐in phase, all OADs except metformin were stopped, patients were switched to iGlar if they had previously been receiving another basal insulin, and the daily dose of iGlar was titrated and/or stabilized for all. After the 6‐week run‐in period, 736 patients with HbA1c of 7% to 10%, a mean fasting self‐measured plasma glucose of ≤140 mg/dL, and receiving a dose of iGlar 20 to 50 units (U) daily, were randomized to receive either iGlarLixi or continue on iGlar for 30 weeks, with or without metformin. iGlarLixi was self‐administered once daily within 60 minutes before breakfast. 9
According to the A1C‐Derived Average Glucose (ADAG) 10 study, HbA1c < 7% would correspond to an FPG of 140 mg/dL (specifically, patients with HbA1c 6.5%‐6.99% had a mean FPG of 142 mg/dL in the ADAG study). Thus, patients were grouped into four glycaemic control categories based on their baseline HbA1c and FPG values:
Patients at both HbA1c and FPG targets: HbA1c < 7.0% and FPG < 140 mg/dL.
Patients with overall hyperglycaemia: HbA1c ≥ 7.0% and FPG ≥ 140 mg/dL.
Patients with residual hyperglycaemia: HbA1c ≥ 7.0% and FPG < 140 mg/dL.
Patients with elevated FPG of ≥140 mg/dL but HbA1c at <7.0% target.
Patients in the LixiLan‐L study had an HbA1c of 7% to 10% and a mean fasting self‐measured plasma glucose at week −1 (an average of 7 days prior to randomization) of ≤140 mg/dL, whereas this analysis focuses on patients with residual hyperglycaemia (HbA1c > 7% and FPG [laboratory measured] of <140 mg/dL) at randomization (week 0).
Statistical analysis was based on the modified intention‐to‐treat (mITT) population set, which included patients with baseline and at least one postbaseline measurement. Out of 736 randomized patients, 731 (366 from the iGlarLixi arm and 365 from the iGlar arm) were included in the mITT population. The difference in the proportion of patients with residual hyperglycaemia (category c) between the two treatment arms (iGlarLixi and iGlar) was compared using the Cochran–Mantel–Haenszel test, weighted by each of the following strata: randomization strata of HbA1c (<8.0%, ≥8.0%) at visit 5 (week −1, the last week of the run‐in period); and randomization strata of metformin use at screening (Yes, No).
Changes from baseline in HbA1c, FPG, body weight and insulin dose were analysed using a mixed‐effects model for repeated measurements with treatment arms, randomization strata of HbA1c (<8.0%, ≥8.0%) at visit 5 (week −1, the last week of the run‐in period), randomization strata of metformin use at screening (Yes, No), visit (week 8, 12, 24 and 30), treatment‐by‐visit interaction, and country as fixed effects, and baseline value‐by‐visit interaction as a covariate. Two‐hour PPG was only assessed at baseline and week 30. Therefore, changes from baseline in 2‐hour PPG were analysed by an analysis of covariance model with treatment arms, randomization strata of HbA1c (<8.0%, ≥8.0%) at screening, randomization strata of metformin use at screening (Yes, No), and country as fixed effects, and baseline value as a covariate. Difference in the proportion of patients with symptomatic hypoglycaemia was based on a weighted average of proportion difference between treatment groups (iGlarLixi and iGlar) from each strata (randomization strata of HbA1c [<8.0%, ≥8.0%] at visit 5 [week −1] and randomization strata of metformin use at screening [Yes, No]) using Cochran–Mantel–Haenszel weightings. This post hoc analysis reported nominal P‐values without performing a multiplicity adjustment.
3. RESULTS
At screening, the proportion of patients with residual hyperglycaemia was similar in the two treatment arms (41.7%, i.e. 305 of 731 mITT patients in the LixiLan‐L study). After the 6‐week run‐in period, at baseline, the proportion of patients with residual hyperglycaemia was similar in both treatment arms and increased to ~~62.4% (456 of 731 mITT patients) (Figure 1A). The demographics and baseline characteristics of patients with residual hyperglycaemia at baseline did not differ between the two treatment arms (Table 1).
FIGURE 1.
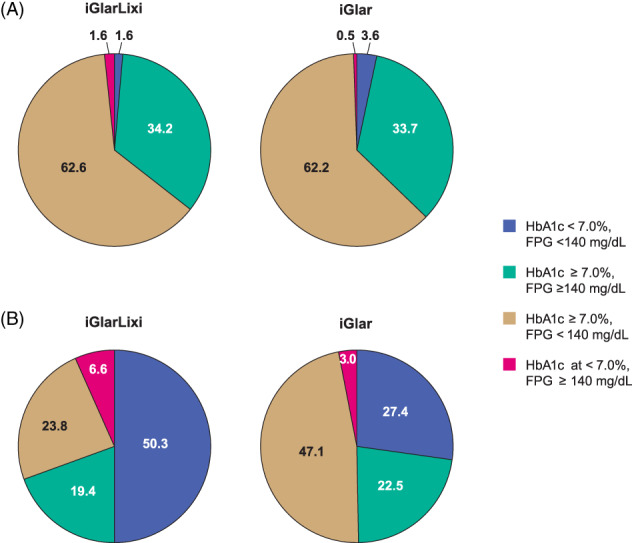
Proportions of patients with HbA1c/FPG categories at baseline (A) and week 30 (B). Note that percentages do not add up to 100% because of missing values at week 30. FPG, fasting plasma glucose; iGlar, insulin glargine Gla‐100; iGlarLixi, fixed‐ratio combination of insulin glargine and lixisenatide
TABLE 1.
Characteristics of patients with residual hyperglycaemia (HbA1c ≥ 7.0% and FPG < 140 mg/dL) in the LixiLan‐L study at baseline (A) and week 30 (B)
| (A) | |||
|---|---|---|---|
| Characteristics at baseline | iGlarLixi (N = 229) | iGlar (N = 227) | P‐value |
| Age | 59.4 (9.4) | 60.0 (8.5) | .4187 |
| Race, n (%) | |||
| White | 205 (89.5) | 203 (89.4) | .9777 |
| Black | 13 (5.7) | 18 (7.9) | |
| Asian | 10 (4.4) | 5 (2.2) | |
| Other | 1 (0.4) | 1 (0.4) | |
| Ethnicity, n (%) | |||
| Hispanic | 56 (24.5) | 41 (18.1) | .0957 |
| Not Hispanic | 173 (75.5) | 186 (81.9) | |
| Body weight, kg | 86.45 (14.05) | 87.19 (14.93) | .5870 |
| BMI, kg/m2 | 30.99 (4.17) | 30.98 (4.28) | .9705 |
| T2D duration, y | 12.46 (6.72) | 12.17 (6.84) | .6551 |
| Duration of baseline insulin treatment, y | 3.22 (3.13) | 3.35 (3.17) | .6704 |
| Insulin dose, U | 35.26 (9.52) | 35.34 (8.70) | .9281 |
| OAD use at screening, n (%) | |||
| None | 16 (7.0) | 11 (4.8) | .6707 |
| Metformin | 201 (87.8) | 206 (90.7) | |
| Others | 12 (5.2) | 10 (4.4) | |
| HbA1c, % | 8.07 (0.65) | 8.06 (0.68) | .9068 |
| FPG, mg/dL | 111.77 (19.46) | 111.56 (19.05) | .9093 |
| 2‐h PPG, mg/dL | 256.80 (67.58) | 265.57 (64.87) | .1602 |
| B | ||||
|---|---|---|---|---|
| Changes from baseline at week 30 | iGlarLixi (N = 229) | iGlar (N = 227) | LS mean difference | P‐value |
| HbA1c, % | 6.9 (0.9) | 7.5 (0.9) | ||
| Change | −1.2 (0.1) | −0.7 (0.1) | −0.6 (0.1) | <.0001 |
| FPG, mg/dL | 115.1 (37.8) | 116.8 (36.3) | ||
| Change | 7.1 (4.2) | 9.5 (4.2) | −2.4 (3.7) | .5091 |
| Body weight, kg | 86.1 (13.9) | 88.1 (15.4) | ||
| Change | −0.7 (0.3) | 0.9 (0.3) | −1.5 (0.3) | <.0001 |
| 2‐h PPG, mg/dL | 167.2 (62.0) | 240.8 (69.0) | ||
| Change | −87.4 (7.3) | −15.8 (7.2) | −71.6 (6.3) | <.0001 |
| Insulin dose, U | 45.3 (12.6) | 45.8 (12.7) | ||
| Change | 8.6 (1.1) | 9.6 (1.1) | −1.0 (1.0) | .3052 |
| % of patients with clinically significant hypoglycaemia (<54 mg/dL) | 17.9 | 12.8 | .1377 | |
| % of patients with nausea/vomiting | 11.4 | 1.8 | <.0001 | |
| % of patients with diarrhoea/nausea/vomiting | 12.7 | 4.4 | .0019 |
Abbreviations: BMI, body mass index; FPG, fasting plasma glucose; iGlar, insulin glargine Gla‐100; iGlarLixi, fixed‐ratio combination of insulin glargine and lixisenatide; LS, least squares; N, number of patients analysed; n, number of patients in each category; OAD, oral antidiabetes drug; PPG, postprandial plasma glucose; SE, standard error; T2D, type 2 diabetes; U, units.
All data are change from baseline, LS mean (SE) unless stated otherwise.
At week 12, the proportion of patients with residual hyperglycaemia was significantly lower in the iGlarLixi arm compared with the iGlar arm (33.1% vs. 51.5%; P < .0001), and this difference was maintained throughout the course of the study (Figure 2). At the end of the study (week 30), the proportion of patients with residual hyperglycaemia declined further to 23.8% in the iGlarLixi arm versus 47.1% in the iGlar arm (P < .0001). Correspondingly, the proportion of patients achieving both HbA1c and FPG targets (HbA1c < 7.0% and FPG < 140 mg/dL) was higher in the iGlarLixi arm (50.3%) compared with the iGlar arm (27.4%) (Figure 1B). A similarly lower proportion of patients with residual hyperglycaemia in the iGlarLixi arm compared with the iGlar arm was also observed in a sensitivity analysis with a more stringent criteria of residual hyperglycaemia of FPG < 126 mg/dL and HbA1c ≥ 6.5% (Figure S1). The proportion of patients achieving both of the more stringent HbA1c and FPG targets (HbA1c < 6.5% and FPG < 126 mg/dL) was similarly higher in the iGlarLixi compared with the iGlar arm (23.8% vs. 11.0%, respectively, at week 30; Table S1).
FIGURE 2.
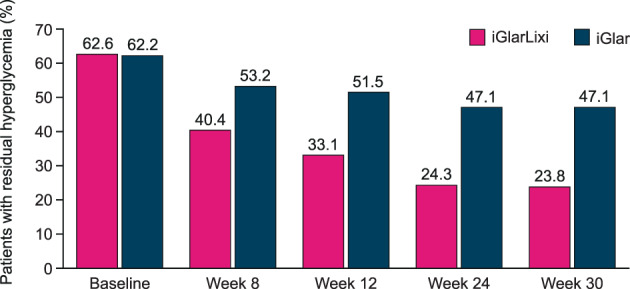
Proportion of patients with residual hyperglycaemia (HbA1c ≥ 7.0%/FPG < 140 mg/dL) throughout the study. P = .0004 for week 8; P < .0001 for weeks 12, 24 and 30. iGlar, insulin glargine Gla‐100; iGlarLixi, fixed‐ratio combination of insulin glargine and lixisenatide
In the sensitivity subgroup analyses, according to duration of T2D (≥10 vs. <10 years), or baseline body mass index (BMI ≥ 30 vs. <30 kg/m2), or baseline HbA1c (≥8.5% vs. <8.5%), the proportion of patients with residual hyperglycaemia remained lower in the iGlarLixi arm than in the iGlar arm (Table 2).
TABLE 2.
Proportion of patients with residual hyperglycaemia (HbA1c ≥ 7.0%/FPG < 140 mg/dL) at baseline and week 30 according to T2D duration, BMI and HbA1c at baseline
| iGlarLixi | iGlar | |||||
|---|---|---|---|---|---|---|
| Baseline, n (%) | Week 30, n (%) | Baseline, n (%) | Week 30, n (%) | Proportion difference between treatments (95% CI) | P‐value | |
| T2D duration, y | ||||||
| ≥10 | 132 (66.3) | 47 (23.6) | 136 (63.6) | 112 (52.3) | 29.1 (20.5‐37.7) | <.0001 |
| <10 | 97 (58.1) | 40 (24.0) | 91 (60.7) | 60 (40.0) | 16.2 (6.4‐26.0) | .0015 |
| BMI, kg/m2 | ||||||
| ≥30 | 126 (57.8) | 50 (22.9) | 127 (60.5) | 95 (45.2) | 22.1 (13.6‐30.5) | <.0001 |
| <30 | 103 (69.6) | 37 (25.0) | 100 (64.5) | 77 (49.7) | 25.5 (15.4‐35.5) | <.0001 |
| HbA1c, % | ||||||
| ≥8.5 | 64 (61.0) | 34 (32.4) | 61 (57.0) | 64 (59.8) | 26.9 (14.3‐39.6) | <.0001 |
| <8.5 | 165 (63.2) | 53 (20.3) | 166 (64.3) | 108 (41.9) | 22.0 (14.5‐29.6) | <.0001 |
Abbreviations: BMI, body mass index; CI, confidence interval; iGlar, insulin glargine Gla‐100; iGlarLixi, fixed‐ratio combination of insulin glargine and lixisenatide; n, number of patients in each category; T2D, type 2 diabetes.
As by definition the subgroup of patients with residual hyperglycaemia were already at FPG target at baseline, there was no noticeable decrease in FPG levels from baseline to week 30 in either of the treatment arms. However, in both treatment arms, PPG and HbA1c decreased, and the decrease was more pronounced in the iGlarLixi arm versus iGlar arm (LS mean [SD] difference: PPG, −71.6 [6.3] mg/dL, P < .0001; HbA1c, −0.6% [0.1%], P < .0001). As the timing of iGlarLixi administration was before breakfast, the reduction of PPG with iGlarLixi was greater after breakfast than after lunch and after dinner (Figure S2). At the end of the study, the average daily dose of insulin was comparable between the two arms: 45.3 and 45.8 U in the iGlarLixi and iGlar arms, respectively. In addition, there was a 0.7 kg weight loss in the iGlarLixi arm and a 0.9 kg weight gain in the iGlar arm (difference: +1.5 kg) (Table 1B).
At week 30, among patients with residual hyperglycaemia at baseline, the proportion of those with level 2 hypoglycaemia (glucose < 54 mg/dL) was low and did not differ between the two treatment arms (P = .1377). However, as expected, the proportion of patients with gastrointestinal adverse events (AEs) was higher in the iGlarLixi arm versus the iGlar arm (12.7% vs. 4.4%, P = .0019, Table 1B).
4. DISCUSSION
At screening and following run‐in, the proportion of patients with T2D with residual hyperglycaemia was similarly high in both treatment arms, but declined progressively to a greater extent in the iGlarLixi arm compared with the iGlar arm throughout the course of the study. Our data show that iGlarLixi achieves glycaemic targets (HbA1c < 7.0%) by targeting both FPG (through the iGlar component) and PPG (through the Lixi component). The results are in accordance with previous studies, showing that the control of PPG contributes to achieving the overall glycaemic targets. 11
In the management of T2D, healthcare providers often assess glycaemic control by measuring the levels of FPG and HbA1c, but not PPG. 12 , 13 However, a recurrent discordance between HbA1c and FPG, which is not because of measurement errors (true ‘residual hyperglycaemia’), points to the likelihood of postprandial hyperglycaemia as the main contributor to persistent hyperglycaemia in these patients. Residual hyperglycaemia may thus indicate a need to consider additional, complementary therapies that more specifically target elevated PPG. Residual hyperglycaemia is common in patients with T2D treated with basal insulin, as basal insulin predominantly targets FPG without a direct impact on PPG levels throughout the day. 2 In some patients with T2D with postprandial hyperglycaemia, basal insulin therapy alone fails to achieve glycaemic targets, 14 , 15 and therapies that target postprandial hyperglycaemia should thus be considered.
The basal insulin component of iGlarLixi acts primarily on FPG by decreasing hepatic glucose production, while the Lixi component enhances beta‐cell function and decreases glucagon secretion in a glucose‐dependent manner while delaying gastric emptying, thereby reducing PPG excursions. 16 In this analysis, iGlarLixi compared with iGlar was able to attain the HbA1c target and also correct residual hyperglycaemia in those with a longer duration of T2D and elevated baseline HbA1c, who presumably have more severe impairment of pancreatic beta‐cell secretory function. This effect could potentially be a result of the correction of elevated PPG by delaying gastric emptying in an insulin‐independent manner by the Lixi component.
An earlier post hoc, time‐to‐control analysis of the LixiLan‐L study 17 estimated that 46% of patients receiving iGlarLixi reached HbA1c < 7% at week 12, and 50% of patients receiving iGlarLixi reached this target during the study in 153 days. Among the patients receiving iGlar, only 24% achieved HbA1c < 7% at week 12, while the target HbA1c was never reached by 50% of them during the study. A parallel post hoc analysis of another study in T2D uncontrolled on OADs (LixiLan‐O) 17 showed that 60% of patients receiving iGlarLixi versus 45% receiving iGlar reached HbA1c < 7% after 12 weeks of treatment, with 50% of patients reaching target HbA1c in approximately half the time with iGlarLixi versus iGlar (85 vs. 166 days).
Other therapies that specifically target PPG include oral acarbose 18 and injectable prandial insulin. However, both of these therapies require administration of multiple daily doses and this may affect adherence and tolerability to therapy. In addition, prandial insulins are associated with weight gain and an increased risk of hypoglycaemia, especially when compared with GLP‐1 RA in combination regimens. 19 The present study shows that the GLP‐1 RA/basal insulin combination is more effective on residual hyperglycaemia than the long‐acting basal insulin alone, with the possibility of promoting less weight gain and without excess hypoglycaemia. Hence, GLP‐1 RA/basal insulin FRC may be a viable alternative to basal insulin in patients with T2D, particularly those with concerns of weight gain and/or hypoglycaemia who require treatment intensification.
In this analysis, patients treated with iGlarLixi were more probable to experience AEs related to the gastrointestinal system compared with iGlar. However, the frequency of these AEs was lower than that reported with Lixi alone in a post hoc analysis, owing to gradual titration of the GLP‐1 RA component and use of a low mean dose. 20
Limitations of this analysis include the open‐label design of the LixiLan‐L study to address the differences in therapy administration between the treatment arms. Because of the short duration of the study, we could not assess the robustness of the glucose‐lowering effects of the drug for more than 30 weeks. In addition, the run‐in period to optimize basal insulin depicts a sequential approach to therapy (basal insulin followed by combination with a GLP‐1 RA), whereas it may be of interest to understand the effects of an initial FRC approach on residual hyperglycaemia. Finally, patients in the LixiLan‐L study were not routinely assessed for the presence of autonomic neuropathy. Indeed, vagal neuropathy may affect the ability of GLP‐1 to delay gastric emptying, thus impairing the control of postprandial hyperglycaemia 21 ; however, the effects of the short‐acting GLP‐1 RA exenatide were apparently preserved in patients with cardiac autonomic neuropathy. 22
In conclusion, residual hyperglycaemia is commonly seen in patients with T2D on basal insulin therapy. By targeting both FPG and PPG, iGlarLixi provides an effective therapeutic option to address residual hyperglycaemia in patients inadequately controlled on basal insulin. The greater efficacy of iGlarLixi in comparison with basal insulin intensification on residual hyperglycaemia is observed regardless of baseline T2D duration, BMI and HbA1c, with less weight gain and no excess risk of hypoglycaemia. Targeting both FPG and PPG through a dual molecule combination approach such as iGlarLixi may help to circumvent the current barriers to treatment intensification and minimize exposure to hyperglycaemia, thus preventing long‐term complications of T2D.
CONFLICT OF INTEREST
F.G. — Consultant: AstraZeneca, Boehringer Ingelheim, Merck Sharp and Dohme, Novo Nordisk, Roche Diabetes Care, Sanofi, and Takeda; research support: Eli Lilly, Lifescan and Takeda. R.R. — Consultant: Sanofi, Novo Nordisk, Eli Lilly, Merck, and Takeda; research support: Boehringer Ingelheim, Novo Nordisk and Merck. J.V. — Speakers’ bureau: Ethicon, Eli Lilly, Medtronic, Merck Sharpe and Dohme, Novo Nordisk and Sanofi; advisory panels: Janssen, Novo Nordisk and Sanofi; consultant: Medtronic. V.R.A. — Consultant: Janssen, Novo Nordisk and Sanofi; employee: MedStar Health Research Institute; research support: Amylin, AstraZeneca, Bristol‐Myers Squibb, Boehringer Ingelheim, Eisai, GI Dynamics, GlaxoSmithKline, Halozyme, Hanmi, Intarcia, Janssen, Novo Nordisk, Sanofi and Takeda. A.S. — Employee: Sanofi (at time of study completion), Intercept Pharmaceuticals (current). M.L. — Consultant: Sanofi. N.M. — nothing to declare.
AUTHOR CONTRIBUTIONS
F.G., R.R., J.V., V.R.A., A.S., M.L. and N.M. contributed to the concept and design of the study. F.G., A.S., M.L. and N.M. contributed to acquisition of data. F.G., R.R., J.V., V.R.A., A.S., M.L. and N.M. participated in data analysis and interpretation of data. F.G., R.R., J.V., V.R.A., A.S., M.L. and N.M. were involved in drafting the manuscript and critical revision. F.G., R.R., J.V., V.R.A., A.S., M.L. and N.M. provided their final approval of the manuscript. All authors have read, reviewed and agreed to the final version of the manuscript.
Supporting information
Table S1. Proportion of patients in the four glycaemic control categories based on their baseline HbA1c and FPG values throughout the study. HbA1c, High ≥6.5%, Low <6.5%; FPG: High ≥126 mg/dL, Low <126 mg/dL.
Figure S1. Proportion of patients with a more stringent criterion for residual hyperglycaemia (HbA1c ≥ 6.5%/FPG < 126 mg/dL) throughout the study. P = .1143 for week 8; P < .05 for week 12; P < .005 for weeks 24 and 30. iGlar, insulin glargine Gla‐100; iGlarLixi, fixed‐ratio combination of insulin glargine and lixisenatide.
Figure S2. 7‐point self‐measured plasma glucose in patients with residual hyperglycaemia (HbA1c ≥ 7.0% and FPG < 140 mg/dL) in LixiLan‐L study at baseline (continuous lines) and week 30 (dotted lines). iGlar, insulin glargine Gla‐100; iGlarLixi, fixed‐ratio combination of insulin glargine and lixisenatide.
ACKNOWLEDGMENTS
The authors thank Tata Consultancy Services, India (paid for by Sanofi) for providing technical editing services. Scientific editorial assistance was provided by Rohan Mitra and Anahita Gouri from Sanofi India. These results were previously presented as Poster 1095‐P at the American Diabetes Association (Orlando, FL, USA) in 2018. Editorial assistance was provided by Fishawack Communications Ltd. and was funded by Sanofi. The LixiLan‐L trial was sponsored by Sanofi.
Morea N, Retnakaran R, Vidal J, et al. iGlarLixi effectively reduces residual hyperglycaemia in patients with type 2 diabetes on basal insulin: A post hoc analysis from the LixiLan‐L study. Diabetes Obes Metab. 2020;22:1683–1689. 10.1111/dom.14077
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14077.
Funding information The LixiLan‐L trial was sponsored by Sanofi
REFERENCES
- 1. Khunti K, Giorgino F, Berard L, Mauricio D, Harris SB. The importance of the initial period of basal insulin titration in people with diabetes. Diabetes Obes Metab. 2020;22:722‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Raccah D, Chou E, Colagiuri S, et al. A global study of the unmet need for glycemic control and predictor factors among patients with type 2 diabetes mellitus who have achieved optimal fasting plasma glucose control on basal insulin. Diabetes Metab Res Rev. 2017;33(3):e2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care. 2003;26(3):881‐885. [DOI] [PubMed] [Google Scholar]
- 4. DECODE Study Group, European Diabetes Epidemiology Group . Is the current definition for diabetes relevant to mortality risk from all causes and cardiovascular and noncardiovascular diseases? Diabetes Care. 2003;26(3):688‐696. [DOI] [PubMed] [Google Scholar]
- 5. Cavalot F, Petrelli A, Traversa M, et al. Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: lessons from the San Luigi Gonzaga Diabetes Study. J Clin Endocrinol Metab. 2006;91(3):813‐819. [DOI] [PubMed] [Google Scholar]
- 6. Tushuizen ME, Diamant M, Heine RJ. Postprandial dysmetabolism and cardiovascular disease in type 2 diabetes. Postgrad Med J. 2005;81(951):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2020;63(2):221‐228. [DOI] [PubMed] [Google Scholar]
- 8. Meier JJ. GLP‐1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8(12):728‐742. [DOI] [PubMed] [Google Scholar]
- 9. Aroda VR, Rosenstock J, Wysham C, et al. Efficacy and safety of LixiLan, a titratable fixed‐ratio combination of insulin glargine plus lixisenatide in type 2 diabetes inadequately controlled on basal insulin and metformin: the LixiLan‐L randomized trial. Diabetes Care. 2016;39(11):1972‐1980. [DOI] [PubMed] [Google Scholar]
- 10. Wei N, Zheng H, Nathan DM. Empirically establishing blood glucose targets to achieve HbA1c goals. Diabetes Care. 2014;37(4):1048‐1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Woerle HJ, Neumann C, Zschau S, et al. Impact of fasting and postprandial glycemia on overall glycemic control in type 2 diabetes. Importance of postprandial glycemia to achieve target HbA1c levels. Diabetes Res Clin Pract. 2007;77(2):280‐285. [DOI] [PubMed] [Google Scholar]
- 12. Gerich JE, Odawara M, Terauchi Y. The rationale for paired pre‐ and postprandial self‐monitoring of blood glucose: the role of glycemic variability in micro‐ and macrovascular risk. Curr Med Res Opin. 2007;23(8):1791‐1798. [DOI] [PubMed] [Google Scholar]
- 13. Monnier L, Colette C, Owens D. Postprandial and basal glucose in type 2 diabetes: assessment and respective impacts. Diabetes Technol Ther. 2011;13((suppl 1)):S25‐S32. [DOI] [PubMed] [Google Scholar]
- 14. Rhinehart AS. Adding GLP‐1 receptor agonist therapy to basal insulin for postprandial glucose control. Clin Diabetes. 2015;33(2):73‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Riddle MC. Basal glucose can be controlled, but the prandial problem persists ‐ it’s the next target! Diabetes Care. 2017;40(3):291‐300. [DOI] [PubMed] [Google Scholar]
- 16. Davidson JA, Desouza C, Fonseca V, et al. Glycaemic target attainment in people with type 2 diabetes treated with insulin glargine/lixisenatide fixed‐ratio combination: a post hoc analysis of the LixiLan‐O and LixiLan‐L trials. Diabet Med. 2020;37(2):256‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frias J, Puig Domingo M, Meneghini L, et al. More patients reach glycaemic control with a fixed‐ratio combination of insulin glargine and lixisenatide (iGlarLixi) than with basal insulin at 12 weeks of treatment: a post hoc time‐to‐control analysis of LixiLan‐O and LixiLan‐L. Diabetes Obes Metab. 2018;20(9):2314‐2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim MK, Suk JH, Kwon MJ, et al. Nateglinide and acarbose for postprandial glucose control after optimizing fasting glucose with insulin glargine in patients with type 2 diabetes. Diabetes Res Clin Pract. 2011;92(3):322‐328. [DOI] [PubMed] [Google Scholar]
- 19. Castellana M, Cignarelli A, Brescia F, Laviola L, Giorgino F. GLP‐1 receptor agonist added to insulin versus basal‐plus or basal‐bolus insulin therapy in type 2 diabetes: a systematic review and meta‐analysis. Diabetes Metab Res Rev. 2019;35(1):e3082. [DOI] [PubMed] [Google Scholar]
- 20. Trujillo JM, Roberts M, Dex T, Chao J, White J, LaSalle J. Low incidence of gastrointestinal adverse events over time with a fixed‐ratio combination of insulin glargine and lixisenatide versus lixisenatide alone. Diabetes Obes Metab. 2018;20(11):2690‐2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Delgado‐Aros S, Vella A, Camilleri M, et al. Effects of glucagon‐like peptide‐1 and feeding on gastric volumes in diabetes mellitus with cardio‐vagal dysfunction. Neurogastroenterol Motil. 2003;15(4):435‐443. [DOI] [PubMed] [Google Scholar]
- 22. Linnebjerg H, Park S, Kothare PA, et al. Effect of exenatide on gastric emptying and relationship to postprandial glycemia in type 2 diabetes. Regul Pept. 2008;151(1–3):123‐129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Proportion of patients in the four glycaemic control categories based on their baseline HbA1c and FPG values throughout the study. HbA1c, High ≥6.5%, Low <6.5%; FPG: High ≥126 mg/dL, Low <126 mg/dL.
Figure S1. Proportion of patients with a more stringent criterion for residual hyperglycaemia (HbA1c ≥ 6.5%/FPG < 126 mg/dL) throughout the study. P = .1143 for week 8; P < .05 for week 12; P < .005 for weeks 24 and 30. iGlar, insulin glargine Gla‐100; iGlarLixi, fixed‐ratio combination of insulin glargine and lixisenatide.
Figure S2. 7‐point self‐measured plasma glucose in patients with residual hyperglycaemia (HbA1c ≥ 7.0% and FPG < 140 mg/dL) in LixiLan‐L study at baseline (continuous lines) and week 30 (dotted lines). iGlar, insulin glargine Gla‐100; iGlarLixi, fixed‐ratio combination of insulin glargine and lixisenatide.


