Abstract
Background and Aim
In the phase 3 CONCUR trial (NCT01584830), regorafenib improved overall survival (OS) versus placebo in Asian patients with treatment‐refractory metastatic colorectal cancer (mCRC). We conducted a post hoc subgroup analysis of Chinese patients in CONCUR.
Methods
Adults with mCRC progressing despite at least two prior treatment regimens and Eastern Cooperative Oncology Group performance status 0–1 were randomized 2:1 to regorafenib 160 mg once daily or placebo for the first 3 weeks of each 4‐week cycle. Dose modifications were permitted. The primary endpoint was OS. Secondary endpoints included progression‐free survival, objective overall response, disease control rate, and safety.
Results
A total of 172 Chinese patients were randomized and treated (regorafenib n = 112, placebo n = 60). OS was significantly improved with regorafenib versus placebo (8.4 vs 6.2 months, respectively; hazard ratio [HR] 0.56, 95% CI 0.39–0.80; one‐sided P = 0.000632), as was progression‐free survival (HR 0.32, 95% CI 0.22–0.47; one‐sided P < 0.000001). The most common drug‐related grade ≥ 3 treatment‐emergent adverse events (TEAEs; regorafenib, placebo) were hand–foot skin reaction (19%, 0%), hypertension (13%, 3%), hypophosphatemia (7%, 0%), increased alanine aminotransferase (6%, 0%), and increased aspartate aminotransferase (5%, 0%). In patients receiving regorafenib and placebo, respectively, TEAEs led to treatment discontinuation in 14% and 7%, dose reduction in 39% and 0%, and dose interruption in 64% and 20%.
Conclusions
This retrospective analysis showed that regorafenib provided an OS benefit over placebo for Chinese patients with previously treated mCRC. TEAEs were consistent with the regorafenib safety profile and manageable with treatment modifications.
Keywords: Chinese, clinical trial, colorectal cancer, regorafenib
Introduction
Colorectal cancer is the fifth most common cancer in China and the fifth leading cause of cancer death, and its incidence in China has increased over the past three decades.1, 2, 3, 4 About 25% of patients with colorectal cancer have metastatic disease at initial diagnosis, and up to 60% will develop metastases.5 Treatment for metastatic colorectal cancer (mCRC) includes chemotherapy with fluoropyrimidine plus oxaliplatin or irinotecan, and biologic agents targeting vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) in patients with RAS wild‐type tumors.5, 6 Some patients experience disease progression after receiving these therapies.
Regorafenib is an oral multikinase inhibitor that improves survival in patients with mCRC progressing on standard therapies.7, 8, 9 In the phase 3 CORRECT trial (NCT01103323), regorafenib was superior to placebo for overall survival (OS) with a hazard ratio (HR) of 0.77 (95% confidence interval [CI] 0.64–0.94; one‐sided P = 0.0052).8 Of the 111 Asian patients in CORRECT, 100 were from Japan.8, 10 The phase 3 CONCUR trial (NCT01584830) evaluated regorafenib in a broader population of Asian patients with treatment‐refractory mCRC and confirmed the OS benefit (HR 0.55, 95% CI 0.40–0.77; one‐sided P = 0.00016).9 We conducted a post hoc subgroup analysis to assess regorafenib in Chinese patients (from mainland China, Taiwan, and Hong Kong) enrolled in CONCUR.
Methods
Study design
The randomized, double‐blind, phase 3 CONCUR trial was carried out in 25 centers in mainland China, Hong Kong, South Korea, Taiwan, and Vietnam, and the results have been previously reported.9 Patients with mCRC and at least two prior lines of treatment were randomized 2:1 to best supportive care and regorafenib 160 mg once daily or matching placebo for the first 3 weeks of each 4‐week cycle. Prior treatment with bevacizumab, cetuximab, or panitumumab was allowed but not required. Treatment continued until disease progression, death, or unacceptable toxicity.
The cut‐off date for the primary analysis was November 29, 2013. At that time, five Chinese patients in the regorafenib group were still receiving treatment. An updated safety analysis was performed after the last patient went off study (cut‐off date: January 14, 2016). Because the number of patients receiving treatment at the time of the primary analysis was small, no additional efficacy analyses were performed. Therefore, efficacy and health‐related quality of life analyses are based on the cut‐off date of November 29, 2013, and safety analyses are based on the cut‐off date of January 14, 2016.
The study protocol and all amendments were approved by each study site's independent ethics committee or institutional review board and complied with Good Clinical Practice guidelines, the Declaration of Helsinki, and applicable local laws. All patients provided written informed consent.
Outcomes and assessments
The primary endpoint was OS, defined as the time from randomization to death from any cause. Secondary efficacy endpoints were progression‐free survival (PFS), defined as the time from randomization to first disease progression or death from any cause; objective overall response, defined as complete or partial response; and disease control, defined as complete or partial response, or stable disease ≥ 6 weeks after randomization. Other endpoints included safety, duration of response, duration of stable disease, and health‐related quality of life.
Tumor response and progression were assessed by investigators radiologically using Response Evaluation in Solid Tumors version 1.1 or clinically if a radiologic assessment was not possible. Treatment‐emergent adverse events (TEAEs) were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
Health‐related quality of life was measured using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire‐Core 30 (EORTC QLQ‐C30) and the EuroQol‐5 dimension (EQ‐5D) questionnaire, which includes a visual analog scale (EQ‐VAS).11, 12 The following differences were considered clinically meaningful: ≥ 10 points on the EORTC QLQ‐C30 scale, ≥ 0.07 points on the EQ‐5D index, and ≥ 7 points on EQ‐VAS.13, 14, 15
Statistical methods
Statistical analyses were performed using sas version 9.1. In this post hoc subgroup analysis, OS and PFS for the full China subgroup were analyzed using a stratified log–rank test, stratified by the same factors used for randomization: single versus multiple organ metastasis and time from diagnosis of metastatic disease (< 18 months vs ≥ 18 months). Kaplan–Meier estimates for OS and Kaplan–Meier survival curves were presented for each treatment group. For the full China subgroup, the HRs (regorafenib/placebo) for OS and PFS and their 95% CIs were calculated using the Cox model, stratified using the same factors stated earlier. Objective response rates and disease control rates in the two treatment groups were compared using a stratified Cochran–Mantel–Haenszel test, adjusting for the same stratification factors as for OS and PFS. Safety analyses were descriptive and by treatment arm. For patient‐reported outcomes, the time‐adjusted area under the curve (AUC) between groups was compared using an analysis of covariance model. For each outcome, least squares mean (LSM) estimates and 95% CIs were estimated for each treatment group and for the treatment group difference.
Results
Of the 204 patients randomized in CONCUR, 172 Chinese patients (84%) were randomized and treated (regorafenib n = 112; placebo n = 60) (Fig. S1). Patients were from mainland China (n = 129), Hong Kong (n = 23), and Taiwan (n = 20). Baseline characteristics were generally balanced between the treatment groups (Table 1). More than one‐third of patients had not received targeted biologic therapy (regorafenib 38%, placebo 35%).
Table 1.
Baseline characteristics
| Regorafenib (n = 112) | Placebo (n = 60) | |
|---|---|---|
| Age group | ||
| Median (range) | 58.0 (31.0–79.0) | 55.0 (33.0–84.0) |
| < 65 years, n (%) | 79 (71) | 52 (87) |
| ≥ 65 years, n (%) | 33 (29) | 8 (13) |
| Sex, n (%) | ||
| Male | 74 (66) | 27 (45) |
| Female | 38 (34) | 33 (55) |
| Median body mass index, kg/m2 | 23.4 | 22.8 |
| ECOG performance status, n (%) | ||
| 0 | 31 (28) | 13 (22) |
| 1 | 81 (72) | 47 (78) |
| Primary site of disease, n (%) | ||
| Colon | 62 (55) | 41 (68) |
| Rectum | 46 (41) | 18 (30) |
| Colon and rectum | 4 (4) | 1 (2) |
| Time from diagnosis of metastatic disease | ||
| Median, months (IQR) | 19.6 (13.6–27.8) | 19.9 (13.4–27.4) |
| < 18 months, n (%) | 46 (41) | 28 (47) |
| ≥ 18 months, n (%) | 66 (59) | 32 (53) |
| Metastatic sites, n (%) | ||
| Single | 19 (17) | 13 (22) |
| Multiple | 93 (83) | 47 (78) |
| KRAS mutation, n (%) | ||
| Yes | 39 (35) | 16 (27) |
| No | 44 (39) | 25 (42) |
| Unknown | 29 (26) | 19 (32) |
| BRAF mutation, n (%) | ||
| Yes | 0 | 1 (2) |
| No | 15 (13) | 8 (13) |
| Unknown | 97 (87) | 51 (85) |
| Histology, n (%) | ||
| Adenocarcinoma | 106 (95) | 58 (97) |
| Mucinous carcinoma | 6 (5) | 2 (3) |
| Previous targeted biologic therapy, n (%) | ||
| None | 42 (38) | 21 (35) |
| Any (anti‐VEGF†, anti‐EGFR‡, or both) | 70 (63) | 39 (65) |
| Anti‐VEGF, but not anti‐EGFR | 26 (23) | 13 (22) |
| Anti‐EGFR, but not anti‐VEGF | 22 (20) | 14 (23) |
| Anti‐VEGF and anti‐EGFR | 22 (20) | 12 (20) |
| Previous systemic anticancer therapy lines, n (%) | ||
| Any intention | ||
| 2 | 28 (25) | 11 (18) |
| 3 | 20 (18) | 15 (25) |
| ≥ 4 | 64 (57) | 34 (57) |
| On or after diagnosis of metastatic disease | ||
| 1–2 | 42 (38) | 21 (35) |
| 3 | 21 (19) | 13 (22) |
| ≥ 4 | 45 (40) | 26 (43) |
Bevacizumab.
Cetuximab or panitumumab.
ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; IQR, interquartile range; VEGF, vascular endothelial growth factor.
Efficacy
The median treatment durations at the time of the primary analysis were 1.7 months (regorafenib) and 1.6 months (placebo); mean (standard deviation [SD]) durations were 3.6 (3.7) and 1.5 (1.0) months, respectively. The mean (SD) daily doses of study medication were 144.3 mg (18.9; regorafenib) and 160.0 mg (0; placebo).
Overall survival was significantly improved with regorafenib compared with placebo (HR 0.56, 95% CI 0.39–0.80; one‐sided P = 0.000632; Fig. 1a). Median OS (95% CI) was 8.4 (6.4–9.7) versus 6.2 months (4.8–7.6). PFS was also significantly improved with regorafenib (median PFS 2.0 vs 1.7 months; HR 0.32, 95% CI 0.22–0.47; one‐sided P < 0.000001; Fig. 1b). There was an OS benefit with regorafenib across all protocol‐defined subgroups except the small subgroup who received only prior anti‐VEGF treatment (HR 0.98; n = 39); a PFS benefit with regorafenib was observed across all protocol‐defined subgroups (Fig. 2). In total, 37% of patients had received systemic anticancer therapy during follow‐up at the time of the primary analysis (regorafenib 33%, placebo 43%).
Figure 1.
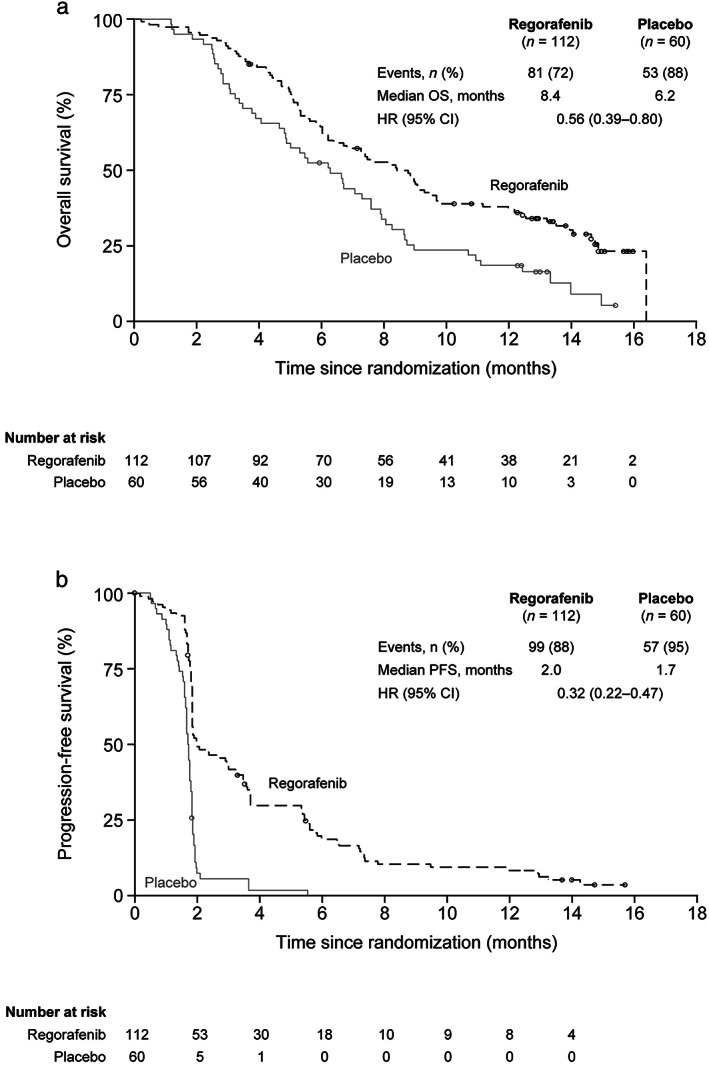
Overall survival and progression‐free survival. (a) Overall survival. (b) Progression‐free survival. CI, confidence interval; HR, hazard ratio; OS, overall survival; PFS, progression‐free survival. (a and b)  , Censored;
, Censored;  , Regorafenib;
, Regorafenib;  , Placebo.
, Placebo.
Figure 2.
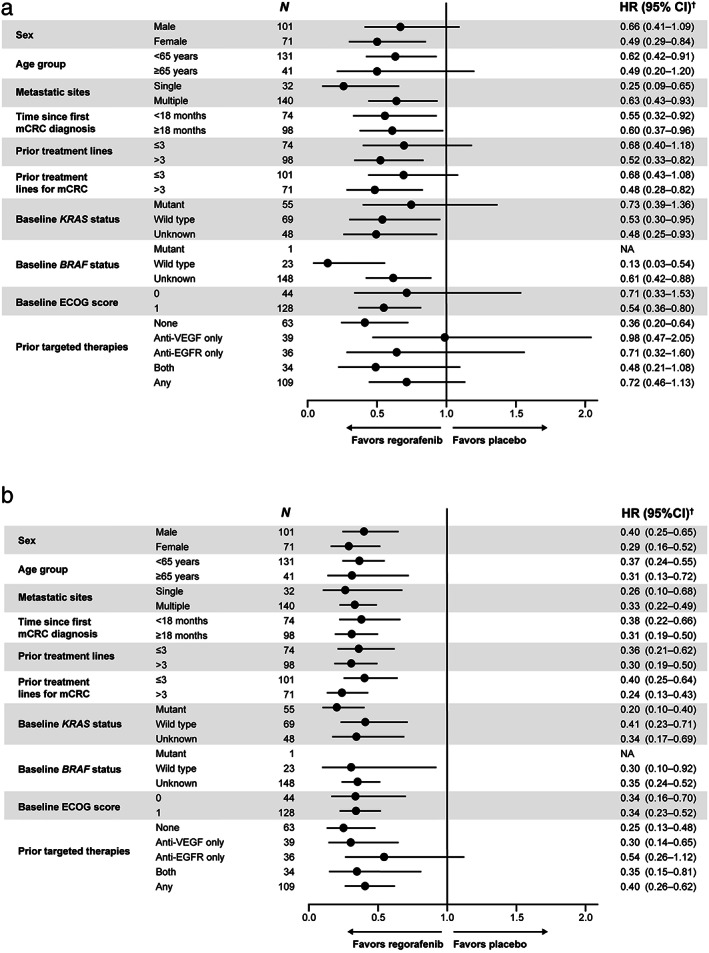
Subgroup analyses of overall survival and progression‐free survival. (a) Overall survival. (b) Progression‐free survival. †Unstratified Cox regression model. CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; HR, hazard ratio; mCRC, metastatic colorectal cancer; NA, not applicable; VEGF, vascular endothelial growth factor.
Exploratory analyses showed that regorafenib improved OS in patients with no prior targeted therapy (n = 63; HR 0.36, 95% CI 0.20–0.64) and in those with any prior targeted therapy (n = 109; HR 0.72, 95% CI 0.46–1.13) (Figs 2a,3). Further breakdown of the subgroup of patients with any prior targeted therapy by the type of prior therapy (anti‐VEGF, anti‐EGFR, or both) suggests that there may be no OS benefit for patients who received only anti‐VEGF treatment (Fig. 2a); however, the HR for PFS for this subgroup was 0.30 (Fig. 2b), and because these are exploratory analyses with small sample sizes and numbers of events, caution should be taken when drawing conclusions. To assess whether these subgroup analyses were confounded by differences in post‐study treatment, an exploratory analysis censoring patients at the start of post‐study treatment was performed and showed a consistent benefit in favor of regorafenib (Table S1).
Figure 3.
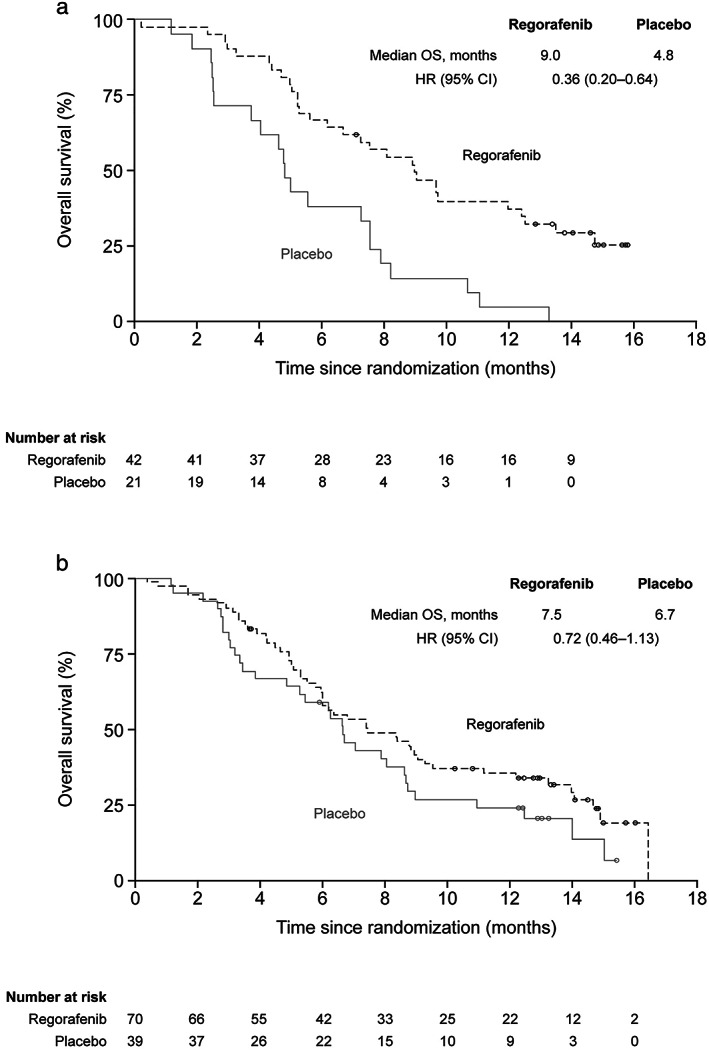
Kaplan–Meier analyses of overall survival by prior targeted therapy. (a) Patients who received no prior targeted therapy. (b) Patients who received any prior targeted therapy. CI, confidence interval; HR, hazard ratio; OS, overall survival. (a and b)  , Censored;
, Censored;  , Regorafenib;
, Regorafenib;  , Placebo.
, Placebo.
The objective overall response was 4% for regorafenib (n = 4; all partial responses) and 0% for placebo (P > 0.05; Table S2), and the disease control rate was higher with regorafenib versus placebo (46% vs 7%; P < 0.000001). The median duration of response for regorafenib was 4.8 months (95% CI 2.0–not estimated). The median duration of stable disease was 2.0 months (95% CI 1.8–3.3) for regorafenib and 1.7 months (1.7–1.8) for placebo.
Of the 112 regorafenib‐treated patients, 27% (n = 30) had PFS > 4 months. The subgroup with PFS > 4 months tended to be slightly older and to have a single metastatic site and no prior targeted therapy, compared with the subgroup with shorter PFS (Table 2). Median OS was 16.4 months for patients with PFS > 4 months and 6.0 months for patients with PFS ≤ 4 months, although this analysis should be interpreted with caution because it is based on classifying patients using post‐baseline information (PFS > or ≤ 4 months).
Table 2.
Baseline characteristics in subgroups of regorafenib‐treated patients with PFS ≤ 4 months and PFS > 4 months
| Regorafenib (n = 112) | ||
|---|---|---|
| PFS ≤ 4 months (n = 82) | PFS > 4 months (n = 30) | |
| Age group | ||
| Median (range) | 57 (34–76) | 60 (31–79) |
| < 65 years, n (%) | 61 (74) | 18 (60) |
| ≥ 65 years, n (%) | 21 (26) | 12 (40) |
| Sex, n (%) | ||
| Male | 54 (66) | 20 (67) |
| Female | 28 (34) | 10 (33) |
| Median body mass index, kg/m2 | 23.7 | 23.1 |
| ECOG performance status, n (%) | ||
| 0 | 21 (26) | 10 (33) |
| 1 | 61 (74) | 20 (67) |
| Primary site of disease, n (%) | ||
| Colon | 48 (59) | 14 (47) |
| Rectum | 31 (38) | 15 (50) |
| Colon and rectum | 3 (4) | 1 (3) |
| Time from diagnosis of metastatic disease | ||
| Median, months (IQR) | 21 (14–28) | 18 (12–27) |
| < 18 months, n (%) | 31 (38) | 15 (50) |
| ≥ 18 months, n (%) | 51 (62) | 15 (50) |
| Metastatic sites, n (%) | ||
| Single | 10 (12) | 9 (30) |
| Multiple | 72 (88) | 21 (70) |
| KRAS mutation, n (%) | ||
| Yes | 29 (35) | 10 (33) |
| No | 34 (41) | 10 (33) |
| Unknown | 19 (23) | 10 (33) |
| BRAF mutation, n (%) | ||
| No | 8 (10) | 7 (23) |
| Unknown | 74 (90) | 23 (77) |
| Histology, n (%) | ||
| Adenocarcinoma | 77 (94) | 29 (97) |
| Mucinous carcinoma | 5 (6) | 1 (3) |
| Previous targeted biologic therapy, n (%) | ||
| None | 26 (32) | 16 (53) |
| Any (anti‐VEGF†, anti‐EGFR‡, or both) | 56 (68) | 14 (47) |
| Anti‐VEGF but not anti‐EGFR | 21 (26) | 5 (17) |
| Anti‐EGFR but not anti‐VEGF | 18 (22) | 4 (13) |
| Anti‐VEGF and anti‐EGFR | 17 (21) | 5 (17) |
Bevacizumab.
Cetuximab or panitumumab.
ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; IQR, interquartile range; PFS, progression‐free survival; VEGF, vascular endothelial growth factor.
Safety
At the updated safety analysis, the median treatment durations were 1.7 months (regorafenib) and 1.6 months (placebo); mean (SD) durations were 4.1 (5.8) and 1.5 (1.0) months, respectively. The mean (SD) daily doses were 144.1 mg (19.3; regorafenib) and 160.0 mg (0; placebo). Treatment interruptions occurred in 69% of patients receiving regorafenib and 25% receiving placebo (Table S3). Dose reductions/re‐challenges at lower‐than‐the‐protocol dose were recorded in 22%/45% of patients receiving regorafenib and in no patients receiving placebo (Table S3). Radiologic disease progression was the most common reason for treatment discontinuation, followed by adverse events (AEs) not associated with disease progression (Fig. S1). A total of 17 patients (regorafenib 11, placebo 6) died during treatment or through 30 days post‐treatment, due to progressive disease (n = 14), AEs not associated with disease progression (n = 2), and an unknown reason (n = 1).
All regorafenib‐treated patients and 88% of placebo recipients experienced a TEAE (Table S4). Grade ≥ 3 TEAEs occurred in 70% of regorafenib‐treated patients and 47% of placebo recipients. The most common TEAEs (all‐grade; regorafenib vs placebo) were palmar‐plantar erythrodysesthesia syndrome (hand–foot skin reaction [HFSR]; 73% vs 7%), increased bilirubin (51% vs 23%), increased aspartate aminotransferase (AST; 31% vs 25%), increased alanine aminotransferase (ALT; 29% vs 18%), and diarrhea (29% vs 7%). Grade 5 TEAEs occurred in 10% of patients in each treatment arm. Drug‐related TEAEs of grades 1–4 occurred in 96% of regorafenib‐treated patients and 45% of placebo recipients and were grade 3 or 4 in 54% and 15%, respectively (Table 3). The most common drug‐related grade 3 or 4 TEAEs (regorafenib vs placebo) were HFSR (19% vs 0%), hypertension (13% vs 3%), hypophosphatemia (7% vs 0%), increased ALT (6% vs 0%), and increased AST (5% vs 0%). Two grade 5 TEAEs in the regorafenib group were deemed drug related by the investigator (one cardiac arrest and one death not otherwise specified) and have been previously described.9
Table 3.
Drug‐related treatment‐emergent adverse events occurring at any grade in ≥ 10% of patients or at grade 3 or 4 in any patients in either group (safety analysis set)
| Adverse events (CTCAE), n (%) | Regorafenib (n = 112) | Placebo (n = 60) | ||||
|---|---|---|---|---|---|---|
| Grades 1 and 2 | Grade 3 | Grade 4 | Grades 1 and 2 | Grade 3 | Grade 4 | |
| Any event† | 47 (42) | 58 (52) | 2 (2) | 18 (30) | 8 (13) | 1 (2) |
| Hand–foot skin reaction‡ | 60 (54) | 21 (19) | NA | 3 (5) | 0 | NA |
| Blood bilirubin increased | 38 (34) | 4 (4) | 1 (1) | 4 (7) | 1 (2) | 0 |
| Hypertension | 12 (11) | 15 (13) | 0 | 1 (2) | 2 (3) | 0 |
| Aspartate aminotransferase increased | 18 (16) | 6 (5) | 0 | 6 (10) | 0 | 0 |
| Alanine aminotransferase increased | 16 (14) | 7 (6) | 0 | 4 (7) | 0 | 0 |
| Diarrhea | 20 (18) | 2 (2) | 0 | 0 | 1 (2) | 0 |
| Fatigue | 15 (13) | 3 (3) | NA | 3 (5) | 1 (2) | NA |
| Hoarseness | 22 (20) | 0 | NA | 0 | 0 | NA |
| Thrombocytopenia | 10 (9) | 2 (2) | 1 (1) | 1 (2) | 0 | 0 |
| Proteinuria | 12 (11) | 2 (2) | NA | 0 | 1 (2) | NA |
| Oral mucositis | 12 (11) | 0 | 0 | 0 | 0 | 0 |
| Lipase increased | 3 (3) | 5 (4) | 0 | 3 (5) | 1 (2) | 0 |
| Hypophosphatemia | 5 (4) | 8 (7) | 0 | 0 | 0 | 0 |
| Leukopenia | 11 (10) | 3 (3) | 0 | 1 (2) | 0 | 0 |
| Maculopapular rash§ | 5 (4) | 5 (4) | NA | 2 (3) | 0 | NA |
| Anorexia | 6 (5) | 1 (1) | 0 | 3 (5) | 0 | 0 |
| Abdominal pain | 5 (4) | 1 (1) | NA | 2 (3) | 0 | NA |
| Neutropenia | 5 (4) | 3 (3) | 0 | 0 | 0 | 0 |
| Anemia | 4 (4) | 1 (1) | 1 (1) | 0 | 0 | 0 |
| Fever | 4 (4) | 1 (1) | 0 | 0 | 0 | 0 |
| Alkaline phosphatase increased | 3 (3) | 1 (1) | 0 | 0 | 1 (2) | 0 |
| Skin and subcutaneous tissue disorders—other | 3 (3) | 1 (1) | 0 | 1 (2) | 0 | 0 |
| Hypokalemia | 2 (2) | 1 (1) | 0 | 0 | 0 | 0 |
| Myalgia | 2 (2) | 1 (1) | NA | 0 | 0 | NA |
| Atrial fibrillation | 1 (1) | 0 | 0 | 0 | 0 | 1 (2) |
| Serum amylase increased | 1 (1) | 0 | 0 | 0 | 1 (2) | 0 |
| Conduction disorder | 0 | 0 | 0 | 0 | 1 (2) | 0 |
| Heart failure | 0 | 0 | 0 | 0 | 0 | 1 (2) |
| γ‐glutamyltransferase increased | 0 | 2 (2) | 0 | 0 | 0 | 0 |
| Wound infection | 0 | 1 (1) | 0 | 0 | 0 | 0 |
| Vaginal fistula | 0 | 1 (1) | 0 | 0 | 0 | 0 |
| Acute kidney injury | 0 | 0 | 0 | 0 | 0 | 1 (2) |
| Visceral arterial ischemia | 0 | 1 (1) | 0 | 0 | 0 | 0 |
For patients with more than one adverse event, only the highest grade of the most severe event is shown.
CTCAE term: palmar‐plantar erythrodysesthesia syndrome.
CTCAE term: rash maculopapular.
CTCAE version v4.0. Number of patients (%) with the specified event starting or worsening between start of treatment and 30 days after end of treatment. Data in each column show the number of patients experiencing that grade as their worst severity of the relevant adverse event.
CTCAE, Common Terminology Criteria for Adverse Events; NA, not applicable.
Treatment‐emergent serious AEs (SAEs) irrespective of relation to study drug occurred in 32% and 30% of patients in the regorafenib and placebo groups, respectively (Table S5); drug‐related SAEs occurred in 11% and 3%, respectively (Table S6). Hepatobiliary SAEs occurred in one patient in each treatment group, and neither was deemed drug related. No treatment‐emergent hepatic failure, hepatic necrosis, or deaths due to hepatobiliary adverse events were noted.
Permanent treatment discontinuation due to TEAEs occurred in 14% and 7% of patients in the regorafenib and placebo groups, respectively. TEAEs leading to discontinuation were broadly distributed across CTCAE terms, and the only TEAEs leading to discontinuation in more than one patient were increased blood bilirubin (regorafenib 4%, placebo 0%) and increased ALT (regorafenib 2%, placebo 0%). HFSR led to discontinuation in one patient in the regorafenib group. Dose reductions due to TEAEs occurred in 39% of regorafenib patients and no placebo patients; dose interruptions due to TEAEs occurred in 64% and 20% of patients, respectively. The most common TEAEs leading to dose reduction (regorafenib vs placebo) were HFSR (24% vs 0%), increased bilirubin (6% vs 0%), increased ALT (3% vs 0%), increased AST (3% vs 0%), and hypertension (3% vs 0%). The most common TEAEs leading to dose interruption (regorafenib vs placebo) were HFSR (24% vs 0%), increased bilirubin (16% vs 3%), increased ALT (8% vs 0%), increased AST (9% vs 0%), maculopapular rash (5% vs 0%), and hypertension (5% vs 0%).
Health‐related quality of life
At baseline, mean (SD) EORTC QLQ‐C30 global health status or quality of life scores (regorafenib, placebo) were 66.7 (18.4) and 57.2 (22.6), and mean scores at the end of treatment were 52.5 (20.3) and 50.2 (25.6), respectively. The LSM difference between treatment groups in time‐adjusted AUC of the EORTC QLQ‐C30 global health status or quality of life score was −0.09 (95% CI −3.3 to 3.1). For the EQ‐5D index, mean (SD) baseline scores (regorafenib, placebo) were 0.84 (0.18) and 0.75 (0.21), and mean scores at the end of treatment were 0.59 (0.38) and 0.57 (0.35), respectively. The LSM difference between treatment groups in time‐adjusted AUC of the EQ‐5D index was −0.04 (95% CI −0.09 to 0.01). Mean (SD) baseline EQ‐VAS scores (regorafenib, placebo) were 74.5 (16.2) and 72.0 (15.9), and mean scores at end of treatment were 63.5 (20.5) and 61.4 (21.2). The LSM difference between treatment groups in time‐adjusted AUC of the EQ‐VAS scores was 0.14 (95% CI −2.58 to 2.86). These results suggest that the quality of life and health status were similar between the treatment groups.
Discussion
Our results show that regorafenib improved survival for Chinese patients in CONCUR, who constituted 84% of the overall CONCUR population.9 Median OS for regorafenib‐treated Chinese patients was 8.4 months versus 6.2 months for placebo (HR 0.56, 95% CI 0.39–0.80; one‐sided P = 0.000632). PFS in Chinese patients was also significantly better with regorafenib than placebo, as was the disease control rate. These results are consistent with the benefit for regorafenib shown in the large, international CORRECT trial, which included mainly non‐Asian patients (Table 4).8
Table 4.
| CORRECT (N = 760) | CONCUR (N = 204) | CONCUR China subgroup (n = 172) | |
|---|---|---|---|
| Patient population (region/country) | 83%: North America, Western Europe, Israel, and Australia | 84%: Mainland China, Taiwan, and Hong Kong | 100%: Mainland China, Taiwan, and Hong Kong |
| 14%: Asia | 16%: Asia other than China | ||
| 3%: Eastern Europe | |||
| Median OS (regorafenib vs placebo) | 6.4 vs 5.0 months | 8.8 vs 6.3 months | 8.4 vs 6.2 months |
| HR (95% CI) | 0.77 (0.64, 0.94) | 0.55 (0.40, 0.77) | 0.56 (0.39, 0.80) |
| P‐value (one‐sided) | = 0.0052 | = 0.00016 | = 0.000632 |
| Median PFS (regorafenib vs placebo) | 1.9 vs 1.7 months | 3.2 vs 1.7 months | 2.0 vs 1.7 months |
| HR (95% CI) | 0.49 (0.42, 0.58) | 0.31 (0.22, 0.44) | 0.32 (0.22, 0.47) |
| P‐value (one‐sided) | < 0.0001 | < 0.0001 | < 0.000001 |
| Most common drug‐related grade ≥ 3 TEAEs (regorafenib group) | HFSR† (17%) | HFSR† (16%) | HFSR† (19%) |
| Fatigue† (10%) | Hypertension (11%) | Hypertension (13%) | |
| Diarrhea (7%) | Hyperbilirubinemia (7%) | Hypophosphatemia (7%) | |
| Hypertension (7%) | Hypophosphatemia (7%) | ALT increased (6%) | |
| Rash or desquamation (6%) | ALT increased (7%) | AST increased (5%) |
Grade 3 is the highest defined severity.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; HFSR, hand–foot skin reaction; HR, hazard ratio; OS, overall survival; PFS, progression‐free survival; TEAE, treatment‐emergent adverse event.
Analyses of OS and PFS in Chinese patients showed a benefit for regorafenib in patients who received any prior targeted therapy. The OS benefit (HR 0.72; median OS 7.5 vs 6.7 months) was similar to that observed in the CORRECT study (HR 0.77), in which all patients had received prior bevacizumab and 51% had received prior anti‐EGFR treatment.8 Exploratory analyses in the current study showed that the benefit with regorafenib was larger in the subset of patients who received no prior targeted therapy (HR 0.36; median OS 9.0 vs 4.8 months) than in those who received any prior targeted therapy. This suggests that the magnitude of benefit with regorafenib may be influenced by prior treatments. However, these exploratory analyses of OS by prior targeted treatment require confirmation in a prospective trial.
A large proportion of patients in our analysis had an unknown KRAS or BRAF status because testing for KRAS or BRAF mutations was not common clinical practice in China at the time the CONCUR study was conducted. Our results show that regorafenib provided benefit in all the subgroups tested, including patients with unknown KRAS or BRAF status (Fig. 2).
The regorafenib safety profile in this analysis is generally consistent with earlier studies.8, 9, 16, 17, 18, 19, 20 Studies of regorafenib and other multikinase inhibitors show that drug‐related HFSR is more frequent among Asian than non‐Asian patients.9, 10, 21, 22 The rate of regorafenib‐related HFSR in the current analysis was 72%, higher than the corresponding rate in the largely non‐Asian population of CORRECT (47%) and closer to the rate in the CORRECT Japanese subpopulation (80%).8, 10 Of note, grade 3 regorafenib‐related HFSR occurred in 19% of patients, similar to the rate in the overall CORRECT population (17%) and lower than that in the CORRECT Japanese subpopulation (28%).8, 10 Although HFSR was common in Chinese patients in CONCUR, it was manageable, leading to treatment discontinuation in only one patient.
Hepatotoxicity with regorafenib has also been reported to be more common in Asian than non‐Asian populations.10 Consistent with these findings, our results showed that the rates of drug‐related grade ≥ 3 increased bilirubin, increased ALT, and increased AST with regorafenib were higher than in the CORRECT study.8 The reason for higher rates of liver enzyme elevations and HFSR events in Asian compared with non‐Asian populations is not known. In an analysis of CORRECT, no correlation was found between the incidence of regorafenib‐related TEAEs and body mass index or body surface area.10 An analysis of regorafenib pharmacokinetics in the CORRECT and CONCUR studies found no notable differences in the exposure of regorafenib or its pharmacologically active metabolites between Asian and non‐Asian patients or between patients from mainland China and other geographic regions.23
The rates of discontinuation due to TEAEs in our analysis were low (14% regorafenib, 7% placebo), similar to the rates in the overall CONCUR population.9 In the Chinese subgroup, the only two TEAEs leading to discontinuation in more than one patient were increased bilirubin (n = 5) and increased ALT (n = 2), suggesting that most TEAEs were managed with dose modifications, allowing patients to remain on treatment. Research suggests that the most common regorafenib‐related TEAEs first occur during the initial treatment cycles.24, 25 This highlights the importance of using recommended treatment modifications early in the course of therapy to customize the dose to the patient's tolerability. In addition, the results of a recent randomized, phase 2 study (ReDOS) show that initiating regorafenib at a dose lower than the approved 160‐mg dose followed by dose escalation is a viable strategy for individualizing dosing that can be used in clinical practice and may allow patients to stay on treatment longer.26 Our results for patient‐reported outcomes show that the quality of life and health status were similar between the regorafenib and placebo groups.
In conclusion, this post hoc subgroup analysis shows that regorafenib confers benefit over placebo for OS, PFS, and disease control among Chinese patients with mCRC who progressed on prior therapies. The safety profile of regorafenib in the Chinese subgroup of CONCUR was consistent with the known safety profile of regorafenib in Asian patients.
Supporting information
Table S1. Exploratory subgroup analysis of overall survival by prior targeted therapy† (patients censored at start of post‐study treatment; primary analysis; data cut‐off November 29, 2013).
Table S2. Tumor response in Chinese patients (primary analysis; data cut‐off November 29, 2013).
Table S3. Treatment modifications (data cut‐off January 14, 2016).
Table S4. Treatment‐emergent adverse events, regardless of relationship to study drug, reported in at least 5% of patients in either group (data cut‐off January 14, 2016).
Table S5. Treatment‐emergent serious adverse events, regardless of relationship to study drug (data cut‐off January 14, 2016).
Table S6. Drug‐related treatment‐emergent serious adverse events (data cut‐off January 14, 2016).
Figure S1. Chinese patients randomized and treated in CONCUR (primary analysis; data cut‐off November 29, 2013).
Acknowledgments
Editorial assistance in the preparation of this manuscript was provided by Jennifer Tobin of OPEN Health Medical Communications (Choice) with financial support from Bayer.
Xu, J. , Xu, R.‐H. , Qin, S. , Pan, H. , Bai, Y. , Chi, Y. , Wang, L. , Bi, F. , Cheng, Y. , Liu, T. , Ma, D. , Shen, L. , Ba, Y. , Liang, J. , Wang, X. , Yau, T. C. C. , Ma, B. B. , Yeh, K.‐H. , Lin, J.‐K. , Kappeler, C. , Shapiro, J. , Kalmus, J. , and Li, J. (2020) Regorafenib in Chinese patients with metastatic colorectal cancer: Subgroup analysis of the phase 3 CONCUR trial. Journal of Gastroenterology and Hepatology, 35: 1307–1316. 10.1111/jgh.14974.
Declaration of conflict of interest: T. C. C. Y. is an advisory board member for Bayer Schering Pharma AG. K.‐H. Y. has received personal fees from Amgen, Boehringer Ingelheim, Bayer, Bristol‐Myers Squibb, Merck Sharp & Dohme, Merck Serono, Eli Lilly and Company, Ono Pharmaceutical, and Takeda. J. K. is an employee of Bayer and owns stock in Bayer. C. K. is an employee of Bayer AG. J. S. is an employee of Bayer. J. X., R.‐H. X., S. Q., H. P., Y. Bai, Y. Chi, L. W., F. B., Y. Cheng, T. L., D. M., L. S., Y. Ba, J. Liang, X. W., B. B. M., J.‐K. L., and J. Li have no conflicts of interest to declare.
Financial support: The study was funded by Bayer.
References
- 1. Center MM, Jemal A, Ward E. International trends in colorectal cancer incidence rates. Cancer Epidemiol. Biomarkers Prev. 2009; 18: 1688–1694. [DOI] [PubMed] [Google Scholar]
- 2. Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J. Clin. 2009; 59: 366–378. [DOI] [PubMed] [Google Scholar]
- 3. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017; 66: 683–691. [DOI] [PubMed] [Google Scholar]
- 4. Chen W, Zheng R, Baade PD et al Cancer statistics in China, 2015. CA Cancer J. Clin. 2016; 66: 115–132. [DOI] [PubMed] [Google Scholar]
- 5. National Comprehensive Cancer Network . Clinical practice guidelines in oncology (NCCN guidelines): colon cancer. Version 4.2018. 2018. [DOI] [PMC free article] [PubMed]
- 6. Van Cutsem E, Cervantes A, Adam R et al ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016; 27: 1386–1422. [DOI] [PubMed] [Google Scholar]
- 7. Wilhelm SM, Dumas J, Adnane L et al Regorafenib (BAY 73‐4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int. J. Cancer 2011; 129: 245–255. [DOI] [PubMed] [Google Scholar]
- 8. Grothey A, Van Cutsem E, Sobrero A et al Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo‐controlled, phase 3 trial. Lancet 2013; 381: 303–312. [DOI] [PubMed] [Google Scholar]
- 9. Li J, Qin S, Xu R et al Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Oncol. 2015; 16: 619–629. [DOI] [PubMed] [Google Scholar]
- 10. Yoshino T, Komatsu Y, Yamada Y et al Randomized phase III trial of regorafenib in metastatic colorectal cancer: analysis of the CORRECT Japanese and non‐Japanese subpopulations. Invest. New Drugs 2015; 33: 740–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. European Organisation for Research and Treatment of Cancer . EORTC QLQ‐C30 scoring manual, 3rd edn. 2001. [DOI] [PubMed]
- 12. Oppe M, Rabin R, de Charro F. EuroQol EQ‐5D user guide, version 1. 2007.
- 13. Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ‐5D utility and VAS scores in cancer. Health Qual. Life Outcomes 2007; 5: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ‐5D utility and VAS scores in cancer [erratum]. Health Qual. Life Outcomes 2010; 8: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health‐related quality‐of‐life scores. J. Clin. Oncol. 1998; 16: 139–144. [DOI] [PubMed] [Google Scholar]
- 16. Demetri GD, Reichardt P, Kang YK et al Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo‐controlled, phase 3 trial. Lancet 2013; 381: 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mross K, Frost A, Steinbild S et al A phase I dose‐escalation study of regorafenib (BAY 73‐4506), an inhibitor of oncogenic, angiogenic, and stromal kinases, in patients with advanced solid tumors. Clin. Cancer Res. 2012; 18: 2658–2667. [DOI] [PubMed] [Google Scholar]
- 18. Strumberg D, Scheulen ME, Schultheis B et al Regorafenib (BAY 73‐4506) in advanced colorectal cancer: a phase I study. Br. J. Cancer 2012; 106: 1722–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bruix J, Tak WY, Gasbarrini A et al Regorafenib as second‐line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open‐label, phase II safety study. Eur. J. Cancer 2013; 49: 3412–3419. [DOI] [PubMed] [Google Scholar]
- 20. Bruix J, Qin S, Merle P et al Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet 2017; 389: 56–66. [DOI] [PubMed] [Google Scholar]
- 21. Ueda T, Uemura H, Tomita Y et al Efficacy and safety of axitinib versus sorafenib in metastatic renal cell carcinoma: subgroup analysis of Japanese patients from the global randomized phase 3 AXIS trial. Jpn. J. Clin. Oncol. 2013; 43: 616–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yoo C, Kim JE, Lee JL et al The efficacy and safety of sunitinib in Korean patients with advanced renal cell carcinoma: high incidence of toxicity leads to frequent dose reduction. Jpn. J. Clin. Oncol. 2010; 40: 980–985. [DOI] [PubMed] [Google Scholar]
- 23. Cleton A, Sturm I, Trnkova J, Grevel J, Fiala‐Buskies S, Lettieri J. Pharmacokinetics of regorafenib in the phase 3 CONCUR and CORRECT trials in patients with metastatic colorectal cancer (mCRC) [Abstract]. Ann Oncol 2015; 26: 261. [Google Scholar]
- 24. Van Cutsem E, Martinelli E, Cascinu S et al Regorafenib for patients with metastatic colorectal cancer who progressed after standard therapy: results of the large, single‐arm, open‐label phase IIIb CONSIGN study. Oncologist 2019; 24: 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Merle P, Granito A, Huang YH et al Time course of treatment‐emergent adverse events (TEAEs) in the randomized, controlled phase 3 RESORCE trial of regorafenib for patients with hepatocellular carcinoma progressing on sorafenib treatment [Abstract]. Hepatology 2017; 66: 726A. [Google Scholar]
- 26. Bekaii‐Saab TS, Ou FS, Ahn DH et al Regorafenib dose‐optimisation in patients with refractory metastatic colorectal cancer (ReDOS): a randomised, multicentre, open‐label, phase 2 study. Lancet Oncol. 2019; 20: 1070–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Exploratory subgroup analysis of overall survival by prior targeted therapy† (patients censored at start of post‐study treatment; primary analysis; data cut‐off November 29, 2013).
Table S2. Tumor response in Chinese patients (primary analysis; data cut‐off November 29, 2013).
Table S3. Treatment modifications (data cut‐off January 14, 2016).
Table S4. Treatment‐emergent adverse events, regardless of relationship to study drug, reported in at least 5% of patients in either group (data cut‐off January 14, 2016).
Table S5. Treatment‐emergent serious adverse events, regardless of relationship to study drug (data cut‐off January 14, 2016).
Table S6. Drug‐related treatment‐emergent serious adverse events (data cut‐off January 14, 2016).
Figure S1. Chinese patients randomized and treated in CONCUR (primary analysis; data cut‐off November 29, 2013).


