Abstract
Objectives
To describe the current knowledge and practice of sarcopenia diagnosis and treatment among health‐care professionals before, directly after and 6 months after a professional development event on sarcopenia.
Methods
This longitudinal study included Australian and New Zealand health‐care professionals who completed questionnaires on knowledge, practice and barriers regarding sarcopenia before, directly after and 6 months after attending a professional development event on sarcopenia.
Results
A total of 250 professionals participated; 84 completed the 6‐month questionnaires. Before, directly after and at 6 months, respectively, 14.7%, 93.4% and 59.5% identified sarcopenia as a disease; 2.0%, 79.6% and 38.1% correctly answered the sex‐specific cut‐offs for low handgrip strength. Respectively, 12.0% and 14.3% reported to make sarcopenia diagnoses as part of their practice before and at 6 months.
Conclusions
Knowledge about sarcopenia is limited among health‐care professionals who attended a professional development event. Retention of knowledge remains a challenge to be addressed.
Keywords: diagnosis, health personnel, knowledge, sarcopenia, therapy
Policy Impact
Intention to diagnose sarcopenia contrasts the practice in diagnosing sarcopenia. There is an opportunity to facilitate changes in practice regarding sarcopenia diagnosis and treatment among health‐care professionals. This may include the provision of diagnostic tools, development of treatment protocols and increase in awareness among health‐care professionals.
Practice Impact
Health‐care professionals’ knowledge improved after a single lecture on sarcopenia, but retention of knowledge remains a challenge to be addressed. At the individual level, health‐care professionals should actively engage in continuous professional development to acquire up‐to‐date knowledge.
1. INTRODUCTION
The presence of low muscle mass and function1 is termed sarcopenia, which is present in 10% of community‐dwelling older adults.2 Adverse health outcomes of sarcopenia include functional decline, falls, fractures, hospitalisation and mortality.3, 4 Sarcopenia is estimated to increase hospitalisation costs by 34% for older adults5 and to contribute 1.5% of total health‐care costs in the United States.6 With the increase in life expectancy, sarcopenia becomes a global public health problem.7 Like many other diseases, sarcopenia is asymptomatic in its initial stage.8 Therefore, early diagnosis and subsequent intervention are essential. For this, awareness among health‐care professionals is a prerequisite.
Most Dutch health‐care professionals reported to know what sarcopenia is.9 Among 683 members from the Japanese Association of Rehabilitation Nutrition, including mainly dietitians and physiotherapists working in acute general wards and convalescent rehabilitation wards, less than half (42%) measured all items necessary for the diagnosis of sarcopenia, including muscle mass, muscle strength and physical performance.10 Because of the difference in health‐care systems,11 findings from the aforementioned studies cannot directly be translated to other countries. In addition, no study has reported the retention of actual knowledge after a professional development event on sarcopenia. There is also a need to further address knowledge gaps in relation to possible barriers to diagnosing and treating sarcopenia in daily clinical practice.
The primary aim of this study was to describe the current knowledge and practice regarding sarcopenia. Secondary aims were to assess the changes in knowledge and practice 6 months after attending a professional development event on sarcopenia and to identify barriers in diagnosing and treating sarcopenia in a cohort of Australian and New Zealand health‐care professionals.
2. METHODS
2.1. Study design
This longitudinal study included allied health professionals and physicians who attended a professional development event (“Sarcopenia Roadshow”). It was held at six locations in Australia and New Zealand (ie Sydney, Melbourne, Auckland, Tauranga, Palmerston and Christchurch) between September 2017 and October 2017. Sarcopenia Roadshow was advertised via local hospitals and community health services, and attendance was free of charge. Immediately before and directly after the events, attending health‐care professionals were invited to complete the questionnaires. Six months after attendance, a follow‐up questionnaire was sent by email to health‐care professionals who agreed to be contacted for follow‐up. Reminders were sent 2 weeks after the initial request. Ethical guidelines were followed in accordance with the Declaration of Helsinki. No medical ethical approval was required.
2.2. Sarcopenia Roadshow
The Sarcopenia Roadshow was a 2‐hour lecture series delivered by a geriatrician and a senior dietitian to increase the awareness of sarcopenia among health‐care professionals. The lecture encompassed the presentation of the following topics: ageing trajectory of muscle mass, the European Working Group on Sarcopenia in Older People (EWGSOP) 2010 definition12 and diagnostic tools commonly used to measure muscle mass, muscle strength and gait speed in clinical practice. Treatment of sarcopenia focused on non‐pharmacological interventions including resistance exercise and adequate protein intake. The rationale for a purely didactic delivery is based on the awareness‐to‐adherence model.13 According to this model, to comply with a new practice, health‐care professionals have to first become aware of the practice, then move to a process of agreement with it and then decide to adopt it in the care they provide, followed by adhering to the practice.14 Since sarcopenia has been recently recognised as a disease,15, 16, 17 raising awareness by means of traditional modalities, such as lectures, may be an effective initial step in predisposing health‐care professionals to change their practices.
2.3. Questionnaires
A structured questionnaire was developed and modified from a previous study.9 Face validity was tested among five allied health professionals and four physicians to ensure the questionnaire was easily understood. The questions included demographics (age, sex, profession, years of practice and working affiliation), knowledge about sarcopenia diagnostic strategy and treatment, and practices in diagnosing and treating sarcopenia. The first part of the questionnaire aimed to describe the current knowledge and practice of sarcopenia before attendance. The second part aimed to examine the intention to implement diagnostic strategies and treat sarcopenia in clinical practice directly after attendance. The third part aimed to assess whether the knowledge was retained and whether there was any change in practice regarding diagnosing and treating sarcopenia 6 months after attendance. The questionnaires are presented in Appendix S1.
2.4. Statistical analyses
Data were analysed using Statistical Package for the Social Sciences, version 24.0 (SPSS Inc). Continuous variables were checked for normality and presented as mean (standard deviation [SD]) if normally distributed and as median (interquartile range [IQR]) if skewed. Categorical variables were presented as number (n) and percentage (%). t tests and chi‐square tests were used to compare the characteristics of health‐care professionals who did and did not complete the follow‐up questionnaires and between health‐care professionals dependent on their knowledge of sarcopenia. Visualisation of results was performed using GraphPad Prism 5.01.
3. RESULTS
3.1. Characteristics of attending health‐care professionals
A total of 287 health‐care professionals attended the lectures, and 250 (87%) responded to the questionnaires. Six months after attendance, questionnaires were sent out to 194 health‐care professionals who agreed to be contacted for follow‐up, of whom 16 could not be contacted. Of the 178 health‐care professionals whom we successfully contacted, 84 (47.2%) completed the follow‐up questionnaires. Table 1 shows the characteristics of health‐care professionals. The median [IQR] age was 40 [28‐55] years, and 83.8% were female, 59.8% were dietitians, 22.0% physicians, 14.6% nurses and 3.7% other disciplines. The median [IQR] years of practice was 10 [3‐30] years. The characteristics of those who completed the follow‐up questionnaires were not significantly different from those who did not, except for having received sarcopenia‐related education in the last 6 months.
Table 1.
Characteristics of attending health‐care professionals (n = 250), stratified by those who did and did not complete the follow‐up questionnaires
| N | Total (n = 250) | FU | ||
|---|---|---|---|---|
| Completed (n = 84) | Did not complete (n = 166) | |||
| Age, y, median [IQR] | 230 | 40 [28‐55] | 40 [28‐54] | 41 [27‐56] |
| Female | 241 | 202 (83.8) | 71 (86.6) | 131 (82.4) |
| Profession | 246 | |||
| Dietitian | 147 (59.8) | 54 (65.1) | 93 (57.1) | |
| Physician | 54 (22.0) | 16 (19.3) | 38 (23.3) | |
| Nurses | 36 (14.6) | 11 (13.3) | 25 (15.3) | |
| Others | 9 (3.7) | 2 (2.4) | 7 (4.3) | |
| Years of practice, median [IQR] | 245 | 10 [3‐30] | 10 [3‐26] | 11 [3‐30] |
| Setting | 242 | |||
| Community service | 36 (14.9) | 15 (18.3) | 21 (13.1) | |
| General practice | 65 (26.9) | 17 (20.7) | 48 (30.0) | |
| Outpatient clinic | 23 (9.5) | 8 (9.8) | 15 (9.4) | |
| Nursing home | 20 (8.3) | 6 (7.3) | 14 (8.8) | |
| Hospital | 132 (54.5) | 46 (56.1) | 86 (53.8) | |
| Other settings | 29 (12.0) | 7 (8.5) | 22 (13.8) | |
| Work with patients aged ≥65 y, yes | 241 | 235 (97.5) | 77 (96.3) | 158 (98.1) |
| Received sarcopenia‐related education in the last 6 mo | 242 | 41 (16.9) | 20 (24.7) | 21 (13.0) |
Variables are presented as n (%) unless indicated otherwise.
Abbreviations: FU, follow‐up; IQR, interquartile range; y, years; mo, months.
3.2. Knowledge about sarcopenia
Table 2 shows the current knowledge about sarcopenia among health‐care professionals. Before, directly after and 6 months after attendance, respectively, 14.7%, 93.4% and 59.5% of the professionals correctly stated that sarcopenia is a disease. Respectively, 73.6%, 84.5% and 83.3% correctly disagreed with the statement “Sarcopenia cannot be prevented,” and 73.3%, 92.1% and 88.1% correctly disagreed with the statement “Overweight or obese individuals have a lower risk of sarcopenia compared to individuals with normal weight.”
Table 2.
Knowledge about sarcopenia, its diagnostic strategy and treatment among health‐care professionals before, directly after and 6 months after attendance
| Before | Directly after | 6 mo after | |||
|---|---|---|---|---|---|
| Total (n = 250) | Completed FUa (n = 84) | Total (n = 250) | Completed FUa (n = 84) | Completed FUa (n = 84) | |
| Knowledge about the concept | |||||
| Sarcopenia is recognised as a… | |||||
| Disease | 34 (14.7) | 12 (15.6) | 228 (93.4) | 80 (95.2) | 50 (59.5) |
| Syndrome | 33 (14.2) | 12 (15.6) | 1 (0.4) | 0 | 13 (15.5) |
| Condition | 138 (59.5) | 45 (58.4) | 15 (6.1) | 4 (4.8) | 21 (25.0) |
| Don't know | 27 (11.6) | 8 (10.4) | 0 | 0 | 0 |
| Sarcopenia cannot be prevented. | |||||
| Agree | 41 (17.2) | 12 (14.8) | 36 (15.1) | 13 (15.7) | 12 (14.3) |
| Disagree | 176 (73.6) | 60 (71.4) | 202 (84.5) | 70 (84.3) | 70 (83.3) |
| Don't know | 22 (9.2) | 9 (11.1) | 1 (0.4) | 0 | 2 (2.4) |
| Overweight or obese individuals have a lower risk of sarcopenia compared to individuals with normal weight. | |||||
| Agree | 21 (8.9) | 5 (6.3) | 5 (2.1) | 1 (1.2) | 7 (8.3) |
| Disagree | 173 (73.3) | 58 (73.4) | 222 (92.1) | 81 (96.4) | 74 (88.1) |
| Don't know | 42 (17.8) | 16 (20.3) | 14 (5.8) | 2 (2.4) | 3 (3.6) |
| Diagnostic strategy | |||||
| Which criteria should be used to diagnose sarcopenia? | |||||
| Clinical impression | 112 (47.3) | 32 (40.0) | 22 (8.9) | 6 (7.1) | 24 (28.6) |
| Muscle mass | 213 (89.9) | 70 (87.5) | 231 (93.9) | 80 (95.2) | 66 (78.6) |
| Muscle strength | 193 (81.4) | 67 (83.8) | 241 (96.4) | 82 (97.6) | 77 (91.7) |
| Physical performance | 142 (59.9) | 60 (75.0) | 218 (88.6) | 75 (89.3) | 75 (89.3) |
| Nutritional status | 147 (62.0) | 44 (55.0) | 77 (31.3) | 20 (23.8) | 28 (33.3) |
| Body mass index | 77 (32.5) | 23 (28.7) | 13 (5.3) | 4 (4.8) | 14 (16.7) |
| Frailty index | 149 (62.9) | 46 (57.5) | 34 (13.8) | 13 (15.5) | 30 (35.7) |
| Others | 8 (3.4) | 3 (3.8) | 15 (6.1) | 5 (6.0) | 1 (1.2) |
| At what age do muscle mass and muscle strength start to decline? y, median [IQR] | 50 [35‐60] | 50 [30‐60] | 25 [25‐30] | 25 [25‐30] | 30 [26‐35] |
| What is the cut‐off for low handgrip strength? | |||||
| For men, kg, median [IQR] | 20 [10‐30] | 20 [8‐30] | 30 [30‐30] | 30 [30‐30] | 30 [27‐30] |
| Correct answer | 11 (4.4) | 3 (3.6) | 206 (82.4) | 71 (88.8) | 33 (39.2) |
| For women, kg, median [IQR] | 14 [6‐25] | 12 [6‐23] | 20 [20‐20] | 20 [20‐20] | 20 [20‐20] |
| Correct answer | 6 (2.4) | 1 (1.2) | 202 (80.8) | 69 (86.3) | 33 (39.2) |
| Both answers correct | 5 (2.0) | 1 (1.2) | 199 (79.6) | 69 (82.1) | 32 (38.1) |
| Treatment of sarcopenia | |||||
| Sarcopenia should be treated with… | |||||
| Physical exercise | 225 (94.9) | 76 (93.8) | 244 (99.6) | 84 (100) | 84 (100) |
| Aerobic exercise | 69 (31.5) | 23 (31.1) | 88 (35.2) | 27 (32.1) | 19 (22.6) |
| Resistance exercise | 194 (88.6) | 68 (91.9) | 240 (99.6) | 84 (100) | 83 (98.8) |
| Balance exercise | 106 (48.4) | 36 (48.6) | 116 (48.1) | 38 (45.2) | 39 (46.4) |
| Nutritional intervention | 228 (95.8) | 77 (95.1) | 239 (97.6) | 82 (97.6) | 82 (97.6) |
| Protein | 209 (95.4) | 72 (97.3) | 235 (97.5) | 82 (97.6) | 82 (97.6) |
| Vitamin D | 123 (56.2) | 49 (66.2) | 219 (90.9) | 75 (89.3) | 62 (73.8) |
| Calcium | 93 (42.5) | 34 (45.9) | 199 (82.6) | 70 (83.3) | 52 (61.9) |
| Pharmacological intervention | 51 (21.4) | 18 (22.2) | 28 (11.4) | 11 (13.1) | 1 (1.2) |
| Don't know | 8 (3.4) | 2 (2.5) | 0 | 0 | 0 |
Variables are presented as n (%) unless indicated otherwise.
Abbreviations: FU, follow‐up; IQR, interquartile range; y, years; mo, months.
Among health‐care professionals who completed the follow‐up questionnaires 6 mo after attendance.
Muscle mass, muscle strength and physical performance were correctly identified as diagnostic criteria of sarcopenia by 89.9%, 81.4% and 59.9% of the health‐care professionals, respectively. Health‐care professionals overestimated the age at which muscle mass and muscle strength start to decline, from a median age of 50 years [35‐60] before, to an estimation closer to the correct answer of 30 years directly after (25 years [25‐30]) and 6 months after attendance (30 years [26‐35]). Sex‐specific cut‐off points for low handgrip strength were correctly answered by 2.0%, 79.6% and 38.1% of the health‐care professionals before, directly after and 6 months after attendance, respectively.
Resistance exercise and adequate protein intake were correctly identified as sarcopenia treatment by about 90% of health‐care professionals. There was a substantial decrease in the percentage of health‐care professionals who thought that sarcopenia should be treated with pharmacological intervention, from 21.4% before to 11.4% directly after and 1.2% 6 months after attendance.
Professionals who work in community services and received previous sarcopenia‐related education had significantly better knowledge about sarcopenia before attendance of the Sarcopenia Roadshow (Appendix S2).
The knowledge about sarcopenia among health‐care professionals who completed the follow‐up questionnaires was similar to those who had no follow‐up.
3.3. Sarcopenia diagnosis in clinical practice
Figure 1 shows the sarcopenia diagnosis in clinical practice before, directly after and 6 months after attendance. Appendix S3 presents the number of health‐care professionals responding to different questions regarding sarcopenia diagnosis in clinical practice. Twelve per cent of the health‐care professionals reported to make sarcopenia diagnoses as part of their practice before attendance. Although 62.8% intended to diagnose sarcopenia, only 14.3% reported to make sarcopenia diagnoses as part of their practice at 6 months of follow‐up. Lack of diagnostic tools was reported to be the main reason for not diagnosing sarcopenia (55.3% and 59.4% before and 6 months after attendance, respectively). Another frequently reported reason both before and 6 months after attendance (30.0% and 30.4%, respectively) was that professionals thought it was not their role to diagnose sarcopenia. Nurses were the main professional group (52.0%) who thought that it was not their role to diagnose sarcopenia, followed by dietitians (28.2%) and physicians (9.3%). Appendix S4 shows the diagnostic criteria and definition used by health‐care professionals who diagnosed sarcopenia. Muscle mass, muscle strength and physical performance were the least frequently used diagnostic criteria before attendance. Although over half of the health‐care professionals intended to use these diagnostic criteria directly after attendance, the number of health‐care professionals who used these diagnostic criteria remained low at 6 months of follow‐up. Of the health‐care professionals reporting the use of muscle mass as a diagnostic criterion at follow‐up, more than half used inappropriate methods such as calf circumference and skinfold thickness. Less than half of the health‐care professionals applied the diagnostic criteria to all older adults. Among health‐care professionals who diagnosed sarcopenia, the use of the main operational definitions of sarcopenia (ie EWGSOP 2010,12 International Working Group on Sarcopenia [IWGS]18 and Janssen 200419) was low, and instead, inappropriate definitions (ie European Society for Parenteral and Enteral Nutrition malnutrition definition, frailty by Fried and frailty by Rockwood) were applied before and 6 months after attendance.
Figure 1.
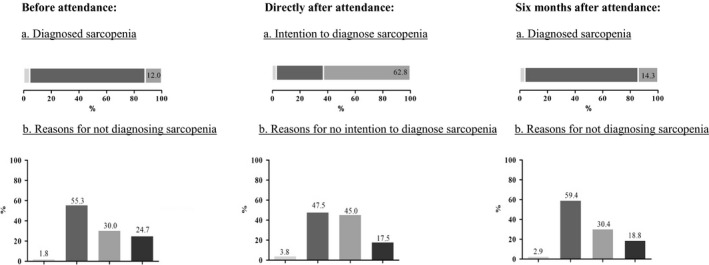
Sarcopenia diagnosis in clinical practice among health‐care professionals before (n = 250), directly after (n = 250) and 6 months after attendance (n = 84). A, ( ) didn’t respond; (
) didn’t respond; ( ) no; (
) no; ( ) yes. B, (
) yes. B, ( ) do not work with older adults; (
) do not work with older adults; ( ) do not have the tools; (
) do not have the tools; ( ) not responsible for diagnosing; (
) not responsible for diagnosing; ( ) other reasons
) other reasons
3.4. Sarcopenia treatment in clinical practice
Table 3 shows the sarcopenia treatment in clinical practice before and 6 months after attendance. For those who were responsible to provide sarcopenia treatment, there was an increase in providing resistance exercise from 12.4% before attendance to 28.6% at 6 months of follow‐up, while adequate protein intake was provided to patients diagnosed with sarcopenia by most of the health‐care professionals. When diagnosed, 51.7% stated to consult other disciplines before attendance, with a slight increase to 62.9% at follow‐up. Overall, the reported rate of consultation with physicians including general practitioners and specialists increased from 58.7% before attendance to 70.6% at 6 months of follow‐up. Responses of health‐care professionals who completed the follow‐up questionnaires were similar to all the health‐care professionals.
Table 3.
Sarcopenia treatment in clinical practice among health‐care professionals before and 6 mo after attendance
| Before | 6 mo after | ||
|---|---|---|---|
| Total (n = 250) | Completed FUa (n = 84) | Completed FUa (n = 84) | |
| Responsible for providing sarcopenia treatment, yes | 141/222 (63.5) | 48/75 (64.0) | 42/82 (50.0) |
| Physical exercise | 25 (18.5) | 6 (12.8) | 13 (31.0) |
| Aerobic | 9 (7.0) | 1 (2.2) | 5 (11.9) |
| Resistance | 16 (12.4) | 5 (10.9) | 12 (28.6) |
| Balance | 11 (8.5) | 3 (6.5) | 9 (21.4) |
| Nutritional intervention | 129 (96.3) | 45 (95.7) | 42 (100) |
| Protein | 89 (91.8) | 35 (94.6) | 42 (100) |
| Vitamin D | 53 (54.6) | 21 (56.8) | 27 (64.3) |
| Calcium | 47 (48.5) | 21 (56.8) | 29 (69.0) |
| Consult with other disciplines when there is a patient diagnosed with sarcopenia, yes | 109/211 (51.7) | 34/68 (50.0) | 17/27 (62.9) |
| Dietitian | 59 (54.1) | 16 (48.5) | 9 (52.9) |
| Exercise physiologist | 40 (36.7) | 16 (48.5) | 3 (17.6) |
| Physician | 64 (58.7) | 21 (63.6) | 12 (70.6) |
| Nurse | 22 (20.2) | 7 (21.2) | 2 (11.8) |
| Occupational therapist | 29 (26.6) | 8 (24.2) | 1 (5.9) |
| Physiotherapist | 79 (72.5) | 22 (66.7) | 11 (64.7) |
| Podiatrist | 5 (4.6) | 2 (6.1) | 0 |
| Others | 2 (1.8) | 0 | 1 (5.9) |
Variables are presented as n (%).
Abbreviation: FU, follow‐up; mo, months.
Among health‐care professionals who completed the follow‐up questionnaires 6 mo after attendance.
3.5. Barriers in sarcopenia diagnosis and treatment
Table 4 shows the barriers reported by health‐care professionals. Five out of the 12 health‐care professionals, who diagnosed sarcopenia at 6 months of follow‐up, perceived to experience barriers during diagnosis. Lack of diagnostic tools (n = 3) and time constraints (n = 3) were reported to be the main barriers.
Table 4.
Barriers reported by health‐care professionals 6 months after attendance
| N (%) | |
|---|---|
| Barriers during diagnosis of sarcopenia (n = 12), yes | 5 (41.7) |
| Acquisition of a device to measure muscle mass | 3 (60.0) |
| Not trained to measure muscle mass | 2 (40.0) |
| Acquisition of handgrip strength device | 2 (40.0) |
| Do not have the skill in measuring handgrip strength | 1 (20.0) |
| Time constraints to perform the diagnostic tests | 3 (60.0) |
| No specific funding source for sarcopenia | 1 (20.0) |
| Barriers during implementation of treatment plan (n = 42), yes | 25 (59.5) |
| Restructuring of routine care | 5 (20.0) |
| Lack of awareness among health‐care professionals | 14 (56.0) |
| Lack of collaboration among health‐care professionals | 4 (16.0) |
| No treatment protocol | 19 (76.0) |
| Not a priority | 9 (36.0) |
| Patient refused to be treated | 3 (12.0) |
| Patient not aware of the treatment importance | 7 (28.0) |
| Others | 7 (28.0) |
| Barriers in treating patients (n = 42), yes | 17 (40.5) |
| No access to other experienced health‐care professionals | 4 (23.5) |
| Lack of awareness among health‐care professionals | 8 (47.1) |
| Lack of motivation among health‐care professionals | 6 (35.3) |
| Patients not motivated to be treated | 4 (23.5) |
| Patients not compliant to treatment plan | 5 (29.4) |
| Financial implications of treatment for patient | 4 (23.5) |
| Not enough manpower to treat | 4 (23.5) |
| Others | 4 (23.5) |
Of the 42 professionals who treated sarcopenia at 6 months of follow‐up, n = 25 perceived to experience barriers during implementing the treatment plan. Lack of treatment protocol (n = 19) and awareness among health‐care professionals (n = 14) were the most frequently perceived barriers. Seventeen health‐care professionals perceived to experience barriers during the actual treatment, of which lack of awareness (n = 8) and lack of motivation (n = 6) were the most common ones.
4. DISCUSSION
Knowledge about sarcopenia and its diagnostic strategy is limited among Australian and New Zealand health‐care professionals attending a professional development event. Knowledge was not retained 6 months after lecture attendance. Although over half of the professionals intended to diagnose sarcopenia directly after attendance, the practice of diagnosing sarcopenia remained low at 6 months of follow‐up. Lack of diagnostic tools and time constraints were reported as the main barriers.
4.1. Knowledge about sarcopenia
Previous studies from Australia20 and European countries21 found that respectively, 46% and 74% of dietitians correctly recorded sarcopenia as a diagnosis in a case study. Given that sarcopenia has been recognised as a disease by the International Classification of Disease, Tenth Revision, Clinical Modification (ICD‐10‐CM) since October 201615 and in Australia since July 2019,16, 17 an increase in knowledge and diagnosis of sarcopenia among health‐care professionals may be expected. However, the current study shows that only a small percentage of health‐care professionals correctly identified sarcopenia as a disease and that the knowledge about the diagnostic strategy is limited. This is in contrast to a study among Dutch health‐care professionals attending a professional development event on sarcopenia, in which 70% stated to know what sarcopenia is and 21% reported to know how to formally diagnose sarcopenia.9 However, conclusions that can be drawn from these results are limited as no further questions were asked to assess the actual knowledge.
The current study is the first to report the retention of knowledge after a professional development event on sarcopenia. The fact that knowledge was not retained over 6 months after a single educational event reinforces the need for continuous education to guarantee sufficient knowledge about sarcopenia and to provide evidence‐based practice for optimal patient care. A review about the retention of basic science knowledge in medical school showed that approximately two‐thirds to three‐fourths of knowledge gained via formal education in schools is retained after 1 year.22 Systematic reviews examining the effectiveness of professional development events showed that multiple exposures to professional development events were associated with a greater effect on the professionals’ knowledge and performance compared to a single exposure.23, 24 To promote professional behaviour change in health care and raise the awareness of sarcopenia among health‐care professionals, interventions should combine audits, feedback and reminders in addition to education.25 Education should be continuous, implemented more frequently and combined with regular auditing and practice follow‐up in an attempt to sustain knowledge and professional behaviour change.
4.2. Sarcopenia diagnosis in clinical practice
Sarcopenia diagnosis in clinical practice was low before attendance. Although an algorithm for sarcopenia diagnosis based on measurements of muscle mass, handgrip strength and gait speed has been suggested by the EWGSOP,12 the current study found that these criteria were used the least. Only a few health‐care professionals who diagnosed sarcopenia applied an appropriate sarcopenia definition such as EWGSOP 2010,12 IWGS18 and Janssen 2004.19
In a previous survey among Australian dietitians, 19.0% reported diagnosing sarcopenia and the top three criteria used to diagnose sarcopenia were loss of muscle mass (31%), loss of muscle strength (28%) and weight loss (16%).20 The practice of sarcopenia diagnosis differs between countries. Sarcopenia diagnosis was reported by 65.9% of dietitians in Europe.21 Among Japanese health‐care professionals (including mainly dietitians and physiotherapists), 41.6% measured all items required for sarcopenia diagnosis, including muscle mass (51.5%), muscle strength (69.1%) and physical function (67.9%).10 Although health‐care professionals in the current study had a strong intention to diagnose sarcopenia directly after attendance, the percentage of health‐care professionals who diagnosed sarcopenia remained low 6 months after attendance. This is in contrast to 53.8% of Dutch health‐care professionals who indicated to have implemented the diagnostic strategy in clinical practice.9 The difference in the practice of sarcopenia diagnosis may be due to the different health‐care system and awareness among health‐care professionals. Furthermore, clarity between health‐care professionals around responsibilities in diagnosing sarcopenia is warranted. Expectations of who should be diagnosing sarcopenia differ widely among health‐care professionals.
4.3. Experienced barriers
The current study found that a lack of diagnostic tools was the main reason for not diagnosing sarcopenia. This is in line with our previous study; the availability of diagnostic tools was the most often‐reported barrier during the implementation of diagnostic strategy among Dutch health‐care professionals.9 Lack of awareness and lack of motivation among health‐care professionals were common perceived barriers during sarcopenia treatment in the current study. However, it is known that collaboration between health‐care professionals supports high‐quality and safe care for patients.26 Therefore, increasing awareness and motivation among other health‐care professionals within a team is essential. Previously, it was shown that institutes with multidisciplinary teams had a higher proportion of measurements of muscle mass, muscle strength and physical performance for diagnosing sarcopenia.27 This highlights the importance of collaboration between health‐care professionals.
Considering the impact of sarcopenia on public health such as high rates of physical disability, nursing home admissions, depression, hospitalisation and mortality, and the associated health‐care costs,7 funding and resources from the government and health organisations are required to provide diagnostic tools, manpower and education for effective sarcopenia diagnosis and treatment. For this, a collaboration between health‐care professionals, as well as advocacy from health professional associations, is crucial so that information from those working on the front lines can be delivered to policymakers.28, 29
4.4. Implications
In addition to placing an emphasis on education, a supportive work environment may further enable health‐care professionals to diagnose and treat sarcopenia.30 At the individual level, health‐care professionals should actively engage in continuous professional development to acquire up‐to‐date knowledge and collaborate with other health‐care professionals. At the organisation level, funding and resources should be allocated to allow for professional development and the manpower required for sarcopenia diagnosis and treatment.
4.5. Strengths and limitations
This is the first study assessing the knowledge and practice of sarcopenia among health‐care professionals after sarcopenia was recognised as a disease in October 2016. In addition, this is the first study to evaluate the retention of knowledge 6 months after a professional development event in sarcopenia. Findings from this study may not be generalisable to the general population of health‐care professionals, as the current population addressed interested professionals who voluntarily signed up for an educational event. Another limitation was the attrition in the response rate 6 months after attending the Sarcopenia Roadshow, and those who responded may have over‐ or underestimated the results. Furthermore, a newly developed custom self‐report questionnaire was used which may have induced socially desirable responses.
5. CONCLUSIONS
There is limited knowledge about sarcopenia and its diagnostic strategy among Australian and New Zealand health‐care professionals attending a professional development event on sarcopenia. A single educational event resulted in an improvement in health‐care professionals’ knowledge in this topic, but retention of knowledge remains a challenge to be addressed. Intention to diagnose sarcopenia contrasts the practice in diagnosing sarcopenia. Next to required educational strategies, practical issues have to be resolved to overcome barriers in diagnosing and treating sarcopenia.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Supporting information
ACKNOWLEDGEMENTS
This project was supported by the European Union's Horizon 2020 research and innovation program under the Marie Sklodowska‐Curie grant agreement No. 675003 (PANINI program) and No. 689238 (PreventIT). The funders had no role in the design and conduct of the study, data collection and analysis, interpretation of data or preparation of the manuscript. We thank Nutricia Australia for the contribution in recruitment of participants.
Yeung SSY, Reijnierse EM, Trappenburg MC, Meskers CGM, Maier AB. Current knowledge and practice of Australian and New Zealand health‐care professionals in sarcopenia diagnosis and treatment: Time to move forward! Australas J Ageing.2020;39:e185–e193. 10.1111/ajag.12730
REFERENCES
- 1. Cruz‐Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing. 2014;43:748‐759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R. Prevalence of sarcopenia in the world: a systematic review and meta‐ analysis of general population studies. J Diabetes Metab Disord. 2017;16:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beaudart C, Zaaria M, Pasleau F, Reginster JY, Bruyere O. Health outcomes of sarcopenia: a systematic review and meta‐analysis. PLoS ONE. 2017;12:e0169548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yeung S, Reijnierse EM, Pham VK, et al. Sarcopenia and its association with falls and fractures in older adults: a systematic review and meta‐analysis. J Cachexia Sarcopenia Muscle. 2019;10:485‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sousa AS, Guerra RS, Fonseca I, Pichel F, Ferreira S, Amaral TF. Financial impact of sarcopenia on hospitalization costs. Eur J Clin Nutr. 2016;70:1046‐1051. [DOI] [PubMed] [Google Scholar]
- 6. Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80‐85. [DOI] [PubMed] [Google Scholar]
- 7. Beaudart C, Rizzoli R, Bruyere O, Reginster JY, Biver E. Sarcopenia: burden and challenges for public health. Arch Public Health. 2014;72:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hertz K, Santy‐Tomlinson J. Fragility Fracture Nursing: Holistic Care and Management of the Orthogeriatric Patient. Cham, Switzerland: Springer Open, 2018. [PubMed] [Google Scholar]
- 9. Reijnierse EM, de van der Schueren MAE, Trappenburg MC, Doves M, Meskers CGM, Maier AB. Lack of knowledge and availability of diagnostic equipment could hinder the diagnosis of sarcopenia and its management. PLoS ONE. 2017;12:e0185837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nakahara S, Wakabayashi H, Maeda K, Nishioka S, Kokura Y. Sarcopenia and cachexia evaluation in different healthcare settings: a questionnaire survey of health professionals. Asia Pac J Clin Nutr. 2018;27:167‐175. [DOI] [PubMed] [Google Scholar]
- 11. Mossialos E, Djordjevic A, Osborn R, Sarnak D. International Profiles of Health Care Systems. New York, USA: The Commonwealth Fund; 2017. [Google Scholar]
- 12. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pathman DE, Konrad TR, Freed GL, Freeman VA, Koch GG. The awareness‐to‐adherence model of the steps to clinical guideline compliance. The case of pediatric vaccine recommendations. Med Care. 1996;34:873‐889. [DOI] [PubMed] [Google Scholar]
- 14. Davis D, Davis N. Selecting educational interventions for knowledge translation. CMAJ. 2010;182:E89‐E93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anker SD, Morley JE, von Haehling S. Welcome to the ICD‐10 code for sarcopenia. J Cachexia Sarcopenia Muscle. 2016;7:512‐514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Australia and New Zealand Society for Sarcopenia and Frailty Research . ANZSSFR Task Force on Diagnostic Criteria for Sarcopenia – statement on announcement of ICD‐10‐AM code for sarcopenia. https://static1.squarespace.com/static/5a71028fa803bb54cb6b5bad/t/5d1980e513050b000173484e/1561952487142/ANZSSFR+Taskforce+Sarcopenia+Statement+July+2019.pdf. Accessed August 9, 2019.
- 17. Zanker J, Scott D, Reijnierse EM, et al. Establishing an operational definition of sarcopenia in Australia and New Zealand: Delphi method based consensus statement. J Nutr Health Aging. 2019;23:105‐110. [DOI] [PubMed] [Google Scholar]
- 18. Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International Working Group on Sarcopenia. J Am Med Dir Assoc. 2011;12:249‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159:413‐421. [DOI] [PubMed] [Google Scholar]
- 20. Yaxley A, Miller MD. The challenge of appropriate identification and treatment of starvation, sarcopenia, and cachexia: a survey of Australian dietitians. J Nutr Metab. 2011;2011:603161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ter Beek L, Vanhauwaert E, Slinde F, et al. Unsatisfactory knowledge and use of terminology regarding malnutrition, starvation, cachexia and sarcopenia among dietitians. Clin Nutr. 2016;35:1450‐1456. [DOI] [PubMed] [Google Scholar]
- 22. Custers E. Long‐term retention of basic science knowledge: a review study. Adv Health Sci Educ Theory Pract. 2010;15:109‐128. [DOI] [PubMed] [Google Scholar]
- 23. Mansouri M, Lockyer J. A meta‐analysis of continuing medical education effectiveness. J Contin Educ Health Prof. 2007;27:6‐15. [DOI] [PubMed] [Google Scholar]
- 24. Cervero RM, Gaines JK. The impact of CME on physician performance and patient health outcomes: an updated synthesis of systematic reviews. J Contin Educ Health Prof. 2015;35:131‐138. [DOI] [PubMed] [Google Scholar]
- 25. Johnson MJ, May CR. Promoting professional behaviour change in healthcare: what interventions work, and why? A theory‐led overview of systematic reviews. Br Med J Open. 2015;5:e008592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morley L, Cashell A. Collaboration in health care. J Med Imaging Radiat Sci. 2017;48:207‐216. [DOI] [PubMed] [Google Scholar]
- 27. Kokura Y, Wakabayashi H, Maeda K, Nishioka S, Nakahara S. Impact of a multidisciplinary rehabilitation nutrition team on evaluating sarcopenia, cachexia and practice of rehabilitation nutrition. J Med Invest. 2017;64:140‐145. [DOI] [PubMed] [Google Scholar]
- 28. Shaw D. Advocacy: the role of health professional associations. Int J Gynaecol Obstet. 2014;127(Suppl 1):S43‐S48. [DOI] [PubMed] [Google Scholar]
- 29. Rieger PT, Moore P. Professional organizations and their role in advocacy. Semin Oncol Nurs. 2002;18:276‐289. [DOI] [PubMed] [Google Scholar]
- 30. Chauhan BF, Jeyaraman MM, Mann AS, et al. Behavior change interventions and policies influencing primary healthcare professionals' practice‐an overview of reviews. Implement Sci. 2017;12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


