Abstract
Reduction in the expression or function of α5-subunit-containing GABAA receptors (α5GABAARs) leads to improvement in several hippocampus-dependent memory domains. However, studies thus far mostly lack anatomical specificity in terms of neuronal circuits and populations. We demonstrate that mice with a selective knockdown of α5GABAARs in CA1 pyramidal neurons (α5CA1KO mice) show improved spatial and trace fear-conditioning memory. Unexpectedly, α5CA1KO mice were comparable to controls in contextual fear-conditioning but showed an impairment in context discrimination, suggesting fine-tuning of activity in CA1 pyramidal cell dendrites through α5-mediated inhibition might be necessary for distinguishing highly similar contexts.
Gamma-aminobutyric acid type A receptors (GABAARs) containing the α5 subunit (α5GABAARs) received recent attention due to their therapeutic potential in disorders of brain excitation/inhibition imbalance and cognitive impairment (e.g., Alzheimer's disease, autism spectrum disorders, Down syndrome; Rudolph and Mohler 2014). The interest in α5GABAARs as a therapeutic target stems partially from their unique anatomical expression pattern: While the α5 subunit is found in only 5% of the GABAARs in the brain, they are highly concentrated in the hippocampus, where α5GABAARs make up almost 25% of the total GABAAR population (Fritschy et al. 1997).
The expression and neurophysiological profile of α5GABAARs has been best studied in the hippocampal CA1 subregion. In CA1, α5GABAARs are expressed at both synaptic and extrasynaptic locations, and their cell surface location is dynamically regulated: During times of reduced neuronal activity, α5GABAARs form extrasynaptic clusters, while increased excitation leads to increased synaptic recruitment (Hausrat et al. 2015). The activation of high-affinity extrasynaptic α5GABAARs by ambient GABA leads to tonic inhibition, which regulates overall neuronal excitability. Synaptic α5GABAARs, on the other hand, mediate inhibitory postsynaptic currents (IPSCs) in dendritic synapses on CA1 pyramidal neurons. These dendritic α5GABAARs are outwardly rectifying, with increased rectification above membrane potentials of −50 mV, and have slow kinetics, providing a perfect match to the voltage- and time-dependent activation of synaptic NMDARs (Shulz et al. 2018).
In addition to their ideal positioning to regulate NMDA-mediated plasticity of CA1 pyramidal neurons, recent work suggests that α5GABAARs are also expressed in CA1 interneurons. In CA1 interneurons, α5GABAARs play a vital role in the recruitment of specific interneuron types into network function and in disinhibition of CA1 principal neurons (Magnin et al. 2019). There is some evidence that the involvement of α5GABAARs in long-term potentiation of Shaffer collaterals, a form of neuronal plasticity thought to underlie memory encoding, also depends on α5GABAARs in nonpyramidal neurons of the CA1 (Rodgers et al. 2015).
Reduced α5GABAAR expression or activity leads to improvements in hippocampus-dependent memory (Collinson et al. 2002; Crestani et al. 2002; Yee et al. 2004; Martin et al. 2009; Milic et al. 2013). However, it is not clear which of the behavioral changes observed in studies with global genetic or systemic pharmacological manipulations are attributable to the α5GABAARs in CA1, where the physiological functions of α5GABAARs are well-studied (Engin et al. 2018). Additionally, interneuronal and pyramidal expression of α5GABAARs may serve distinct functions in CA1 neuronal plasticity. However, it is not known which α5GABAARs (i.e., interneuronal or pyramidal) mediate α5GABAAR involvement in CA1-dependent mnemonic processes.
In an effort to answer these questions, we used mice where α5GABAARs were selectively knocked down in the pyramidal neurons of the CA1 (α5CA1KO; Rodgers et al. 2015), by crossing mice where exons 4/5 of the Gabra5 gene were flanked by lox P sites (α5F/F mice; Engin et al. 2015) with a line of cre mice (T29-1 mice; Tsien et al. 1996) with cre expression limited to CA1 pyramidal neurons.
Based on the qualitative observations of immunohistochemically stained sections (Fig. 1A), all experiments in this study were limited to mice between 8 and 12 wk of age, where the reduction in α5GABAARs is specific to CA1. To ascertain comparability of results with earlier studies, the experiments were limited to male mice. Global α5 knockout mice (α5GlobalKO; Rodgers et al. 2015) were used as a positive control to replicate previously observed effects (with knockout or knockdown mice or with α5-selective negative allosteric modulators (α5-NAMs)) and as such, support validity of the behavioral tests. We aimed to answer the question of anatomical localization of effects to the CA1 pyramidal cell population by testing global and CA1 KO mice together and probing whether an effect observed in the α5GlobalKO mice under the current breeding and testing conditions is present in α5CA1KO mice.
Figure 1.
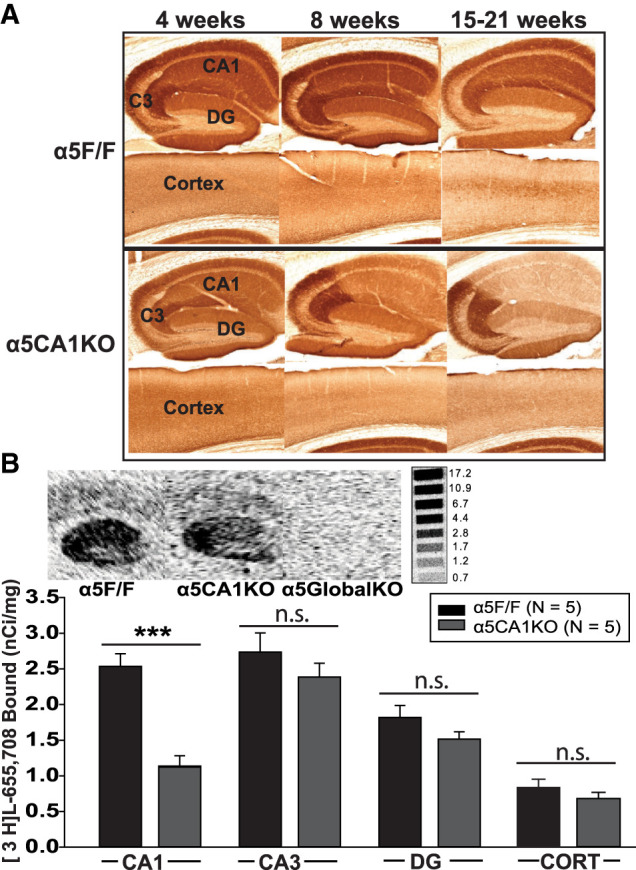
Qualitative and quantitative validation of CA1-selective knockout of α5 subunits. (A) Brain sections from α5F/F (top) and α5CA1KO (bottom) mice immunohistochemically stained for α5 subunit (antibodies and methods same as described in Rodgers et al. 2015 and Engin et al. 2015). As seen, the α5 knockout in CA1 is progressive, starting after 4 wk and becoming more pronounced by 8 wk. While the knockout seems most extensive after 15 wk, a reduction in α5 expression in the cortex is also visible at this time point. Thus, experiments were restricted to 8–12 wk-old animals. (B) (Top) Autoradiographs showing the distribution of [3H]L-655,708 binding sites in the hippocampi of 9–12 wk-old α5F/F, α5CA1KO, and α5GlobalKO mice (Methods same as Engin et al. 2015). (Bottom) Density of [3H]L-655,708 binding sites (nanocuries per milligrams) in α5F/F and α5CA1KO. Binding sites are significantly reduced specifically in the CA1 of α5CA1KO mice compared to controls, with comparable binding site density in CA3, DG, and cortex (CORT) of α5CA1KO and α5F/F control animals. (***) P < 0.001.
Mice were bred on a C57BL/6J background for at least five generations. All experiments were approved by the McLean Hospital Institutional Animal Care and Use Committee. The data were analyzed using the appropriate ANOVA for the experimental design, followed, where significant, by Holm-Sidak post hoc tests comparing α5CA1KO and α5GlobalKO groups to the α5F/F controls.
[3H]L-655,708 (83 Ci/mmol, GE Healthcare) binding was used as a quantitative proxy for the abundance of α5GABAARs in CA1, CA3, dentate gyrus (DG) and cortex of α5CA1KO and α5F/F mice between 9–12 wk of age. We observed a ∼40% decrease in α5GABAARs in the CA1 of α5CA1KO mice compared to α5F/F controls (Fig. 1B), with no significant change in CA3, DG, or cortex, confirming specificity of the knockdown to CA1 within this age range (Two-Way ANOVA, Genotype: F(1,32) = 19.13, P < 0.001; Region: F(3,32) = 40.60, P < 0.001; Genotype × Region: F(3,32) = 4.47, P = 0.01. Post hoc comparison, α5CA1KO vs. α5F/F: CA1: t = 5.31, P < 0.001; not significant for CA3, DG, or cortex).
Hippocampus-dependent memory was evaluated using spatial association, trace and contextual fear conditioning tasks, based on evidence that reduction of α5GABAAR activity can improve performance in these tasks. All behavioral tasks were described previously (Engin et al. 2015).
In the Morris water maze (MWM), the animals received four 1-min training trials per day. The submerged platform remained at the same position for the first 10 d. On days 3, 6, and 9, a 2-min probe trial was conducted, with the platform removed, before proceeding with the training trials. Both α5CA1KOs and α5GlobalKOs showed significantly shorter latencies to reach the platform location during probe trials on days 3 and 6 (Fig. 2A), in line with reports of improved MWM performance in α5GlobalKO and α5NAM-injected mice (Collinson et al. 2002; Martin et al. 2009). (Two-Way ANOVA; Probe Day (within-subjects): (F(5,104) = 5.59, P < 0.001); Day × Genotype: (F(10,104) = 2.10, P = 0.03); Post hoc comparisons to α5F/F: Day 3 (α5CA1KO: t = 2.98, P = 0.01; α5GlobalKO: t = 2.15, P = 0.03); Day 6 (α5CA1KO: t = 2.39, P = 0.02; α5GlobalKO: t = 2.85, P = 0.01); not significant for other probe days). A similar pattern was observed when the amount of time spent in the platform quadrant during probe tests was compared between groups, with α5CA1KO and α5GlobalKO mice spending more time in this quadrant, presumably searching for the platform, on probe days 3 and 6 (Fig. 2B; Probe Day: (F(5,104) = 9.03, P < 0.001); Genotype: (F(2,104) = 4.87, P = 0.02)). Figure 2C depicts the average distance to platform on the first trial of each training day. As mice were released into different quadrants in random order during training trials, part of the variability in the first trial data is due to the effects of the distance between release quadrant and the platform location. α5CA1KO and α5GlobalKO mice seemed to dwell slightly closer to the platform overall during the first training trials of days 2, 4, and 5 (significant only on Day 2), in line with better performance on probe days 3 and 6. There was no difference between groups on any of the reported measures during the reversal phase of the task.
Figure 2.
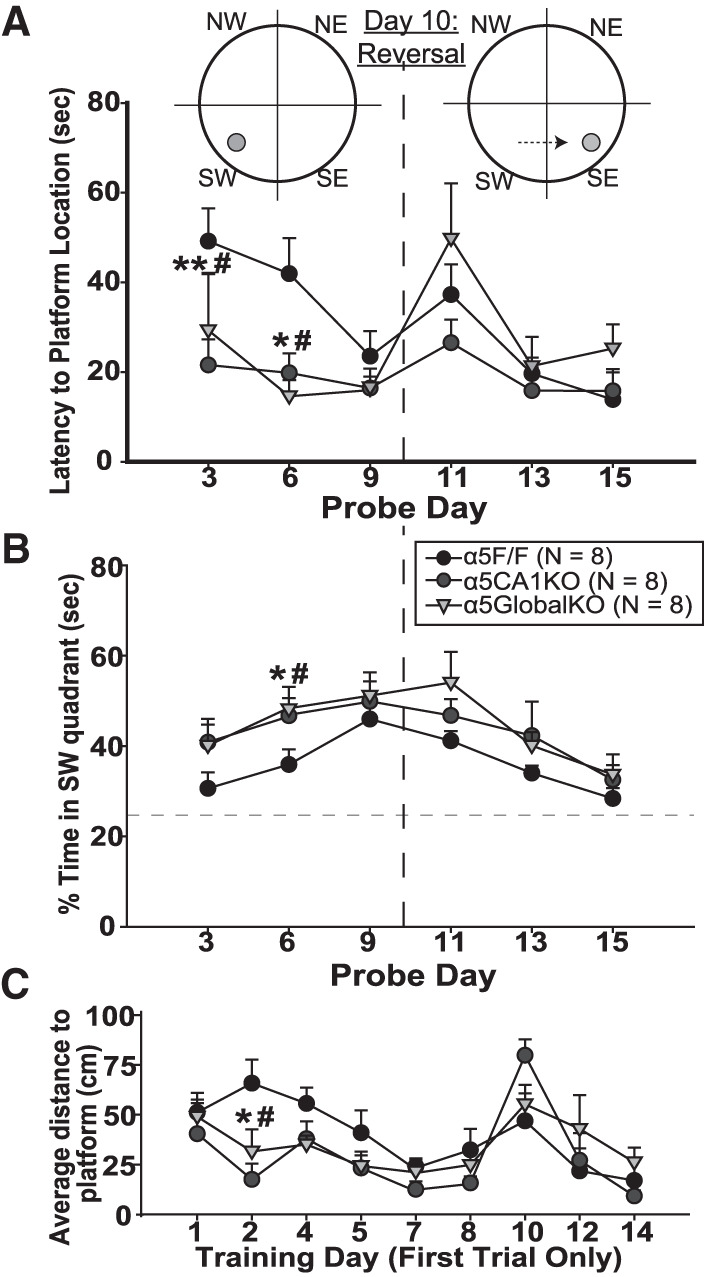
Improved spatial memory in α5CA1KO mice. (A) Latency to reach the platform location on probe days of the Morris water maze (MWM). α5CA1KO and α5GlobalKO mice reached the platform location faster during the probe trials on days 3 and 6; by day 9, the α5F/F mice were performing similarly to α5CA1KO and α5GlobalKO mice. During the reversal phase, which started after the achievement of equal performance to avoid a confound, all genotypes performed similarly. (B) Percentage of time spent in the platform quadrant (SW) of the MWM on probe days. (C) Average distance to platform during the first trial of training days. (*) P < 0.05, (**) P < 0.01 for comparisons between α5CA1KO and α5F/F mice; (#) P < 0.05 for comparisons between α5GlobalKO and α5F/F mice.
Thus, selective knockout of α5GABAARs in CA1 pyramidal neurons improves spatial learning but does not affect reversal learning in MWM. Increased synaptic confinement of α5GABAARs in radixin knockout mice was reported to cause an opposite profile, with no effect on the initial learning of the MWM but impairments in reversal learning (Hausrat et al. 2015). This finding suggests that reversal learning relies on extrasynaptic α5GABAAR-mediated tonic inhibition outside of CA1. Indeed, we previously reported that selective knockout of α5GABAARs in DG granule cells impairs reversal learning in MWM without affecting initial MWM learning (Engin et al. 2015), suggesting MWM reversal learning may depend on extrasynaptic α5GABAARs in DG granule cells. On the other hand, synaptic α5GABAARs might form a brake on NMDA-mediated synaptic plasticity in CA1, the release of which improves initial learning of the MWM task. If so, blocking specifically the synaptic α5GABAARs in CA1 or confining CA1 pyramidal α5GABAARs to extrasynaptic locations might improve MWM learning.
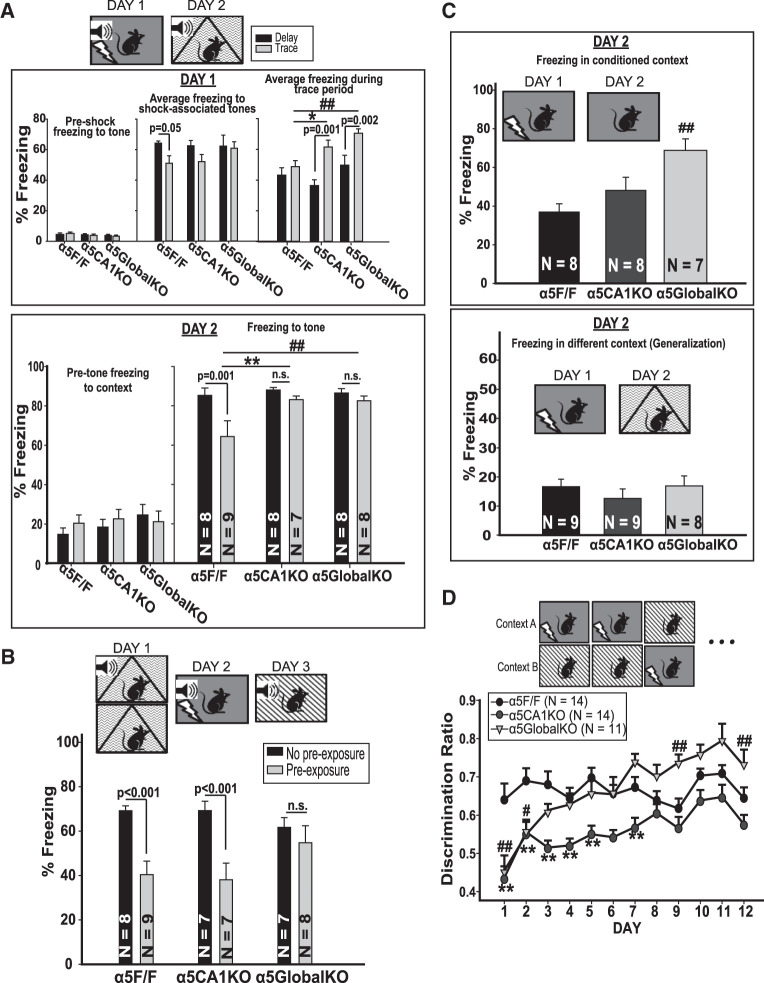
Global α5GABAAR knockdown or knockout has been shown to improve a hippocampus-dependent form of auditory fear-conditioning where a trace period is introduced between the conditioned (i.e., the tone) and unconditioned (i.e., shock) stimulus (Crestani et al. 2002; Yee et al. 2004; Martin et al. 2009). We conducted auditory fear-conditioning using a trace (i.e., 20 sec trace period) or delay (i.e., shock and tone coterminating; hippocampus-independent) protocol. On Day 1, all mice were subjected to five tone (20 sec, 70 dB, 2800 Hz)—shock (2 sec, 0.7 mA) pairings in conditioning boxes (Med-Associates). During conditioning sessions (Fig. 3A, top panel), all groups of mice showed similar levels of freezing to the tone, with the exception of α5F/F control mice in trace condition showing less freezing compared to those in delay condition. α5CA1KO mice seemed to have a similar trend of reduced freezing for trace condition, but it failed to reach significance (P = 0.136). Interestingly, when freezing during the trace period (which corresponds to the 20 sec following shock in delay-conditioned mice) was compared, α5GlobalKO and α5CA1KO mice in trace conditioning showed increased freezing compared to controls and compared to delay-conditioned animals of the same genotype. Twenty-four hours later, the mice were placed in a different context and freezing to the tone was scored automatically (Fig. 3A, lower panel). Pretone freezing (i.e., nonspecific freezing) was similar in all groups. As expected, control mice trained in a trace protocol showed less freezing compared to those in a delay protocol (i.e., the “trace effect,” suggesting weaker conditioning). α5GlobalKO mice did not show the “trace effect,” showing equal freezing when trained in trace or delay protocols. Trace-conditioned α5GlobalKO mice showed significantly more freezing compared to trace-conditioned controls, with no difference between delay-conditioned groups. This was also true for the α5CA1KO mice (Fig. 2A; Two-Way ANOVA; Genotype: F(2,41) = 3.73, P = 0.03; Condition (delay/trace): F(1,41) = 7.53, P = 0.01. Post hoc comparison for within-genotype trace effect, α5F/F: (t = 3.49, P = 0.001), not significant for α5CA1KO and α5Global KO; Between-genotype comparisons in trace condition; α5GlobalKO vs. F/F: t = 2.93, P = 0.001; α5CA1KO vs. α5F/F: t = 3.01, P = 0.001).
Figure 3.
Task-dependent changes in fear-conditioning in α5CA1KO mice. (A) Delay and trace auditory fear-conditioning. (Top panel) (Left) Behavior during conditioning session on Day 1. All animals showed similarly low freezing prior to shocks. (Middle) The average freezing during tone presentation (starting from the second tone presentation as the first tone is neutral). α5F/F mice in trace condition showed less shock associated freezing compared to their delay-conditioned conspecifics. There were no significant differences between genotypes. (Right) Average freezing during trace period (i.e., period of 20 sec postshock in delay-conditioned mice). Trace-conditioned α5CA1KO and α5GlobalKO mice showed more freezing during the trace period compared to trace-conditioned α5F/F control mice, as well as their delay-conditioned conspecifics. (Lower panel) (Left) All groups were similar in terms of pretone nonspecific freezing in a novel context. Right: α5F/F control mice showed a “trace effect” with lower freezing in trace compared to delay condition during a recall task 24 h after conditioning (P < 0.001). The trace effect was abolished in α5CA1KO and α5GlobalKO mice. Trace-conditioned α5CA1KO and α5GlobalKO mice showed more freezing compared to trace-conditioned controls. (B) Latent inhibition to fear-conditioned cue. Data from Day 3 of the latent inhibition task is presented. As seen, α5F/F (P < 0.001) and α5CA1KO (P < 0.001) mice that were preexposed to the tone on Day 1 of the experiment showed lower freezing to the tone compared to the non-pre-exposed animals of the same genotype; that is, latent inhibition effect. Preexposed α5GlobalKO mice did not show latent inhibition. There were no significant genotype effects. (C) Contextual fear-conditioning. (Top) α5GlobalKO mice showed increased freezing to the conditioning context 24 h following the conditioning session. α5CA1KO mice were comparable to controls. (Bottom) α5F/F/, α5CA1KO, and α5GlobalKO mice showed similar freezing when placed in a different context than the conditioning context 24 h after conditioning. The 10%–15% time spent freezing in this different context represents some level of generalization in all animals, as preconditioning freezing is usually in the 5%–10% range (data not shown). (D) Context discrimination. α5CA1KO mice showed lower discrimination between the shock-associated and safe context compared to α5F/F controls until Day 8 of the discrimination task, after which their discrimination ratio was comparable to controls. α5GlobalKO mice were similar to α5CA1KO mice and showed lower discrimination than α5F/F controls on the initial days of the experiment. However, unlike α5CA1KO mice, α5GlobalKO mice reached similar discrimination as controls by Day 3. Interestingly, α5GlobalKO mice seem to discriminate the two contexts better than controls toward the end of the test, with a significant difference on days 9 and 12. (*) P < 0.05, (**) P < 0.01 for comparisons between α5CA1KO and α5F/F mice; (#) P < 0.05 for comparisons between α5GlobalKO and α5F/F mice.
According to a recent study, there is a small pool (∼10%) of CA1 pyramidal neurons that are inherently active under resting/free exploration conditions (named “primed neurons” by the authors), while 70% of pyramidal neurons remain silent regardless of behavioral state, with the rest falling into an intermediate zone between the two extremes (Zhou et al. 2020). Trace fear-conditioning did not alter the overall activity architecture of CA1 pyramidal neurons, however, increased coherence and signal-to-noise ratio (SNR) among the primed neurons, without increasing activity of the silent neurons. The primed neurons returned to random activity following training but resumed synchronized activity during recall. Moreover, SNR and synchronization of the primed neurons positively correlated with freezing during both training and recall. The knockdown of α5GABAARs in CA1 pyramidal neurons may improve trace fear-conditioning through increasing the size of the primed neuron pool by reducing tonic inhibition or through increasing synchronized burst firing of primed neurons by increasing their excitability. Both processes would be primarily related to a reduction in extrasynaptic α5GABAARs. Considering increased neuronal activity, as during fear-conditioning, leads to preferential anchoring of α5GABAARs in synapses (Hausrat et al. 2015), reduction of extrasynaptic α5GABAAR activity may be a natural part of memory encoding in trace fear-conditioning.
We next trained animals in an auditory fear-conditioning protocol aimed at generating latent inhibition of conditioned fear response, based on earlier evidence for the involvement of α5GABAARs in latent inhibition (Gerdjikov et al. 2008). On Day 1, the mice in the “preexposure” group were presented with 30 tones (20 sec, 70 dB, 2800 Hz), while the “no preexposure” group mice were placed in the same context without tone presentation. On Day 2, all mice were fear-conditioned to the tone in a different context. On Day 3, freezing to the tone was assessed in a third distinct context. α5F/F mice showed the expected latent inhibition effect, with preexposed groups showing less freezing to the tone. This effect was abolished in α5GlobalKO mice, but was present in α5CA1KO mice (Fig. 3B), suggesting α5GABAARs on different neurons than the CA1 pyramidal cells are responsible for the involvement of α5GABAARs in latent inhibition (Two-Way ANOVA; Genotype: F(2,40) = 0.33, P = 0.72; Condition: F(1,40) = 22.28, P < 0.001; Genotype × Condition: F(2,40) = 2.63, P = 0.08; Post hoc comparisons of condition within genotypes: α5F/F: t = 3.72, P < 0.001; α5CA1KO: t = 3.65, P < 0.001; α5GlobalKO: not significant; Post hoc comparisons of genotype within exposure conditions: not significant).
The involvement of the hippocampus in the latent inhibition phenomenon has been controversial, with studies suggesting that the involvement depends on the specifics of the behavioral paradigm used (Reilly et al. 1993; Sotty et al. 1996; Grecksch et al. 1999; Holt and Maren 1999; Pouzet et al. 2004). The concentrated expression of α5GABAARs in the hippocampus combined with the abolishment of the latent inhibition effect in α5GlobalKO mice seem to suggest the involvement of the hippocampus in the specific behavioral paradigm used here. Indeed, in previous work, we demonstrated that the specific knockout of α5GABAARs in DG (but not CA3) principal neurons abolishes latent inhibition (Engin et al. 2015), lending further support to the sensitivity of the current behavioral paradigm to the effects of hippocampal manipulations and the lack of involvement of the CA1 α5GABAARs in the latent inhibition phenomenon.
Next, contextual memory and context discrimination were evaluated in three sets of animals. The first group of animals was given two shocks (2 sec, 1.5 mA) and were returned to the same context 24 h later to measure freezing. While α5GlobalKOs showed enhanced freezing to the context, the α5CA1KOs were comparable to controls (Fig. 3C, top; one-way ANOVA; (F(2,20) = 7.62, P = 0.003; Post hoc comparison: α5GlobalKO: t = 3.87, P = 0.002). The second group was placed in a different context than the conditioning context 24 h postconditioning. The three genotypes showed comparable levels of freezing, suggesting equal ability to distinguish between the conditioning context and this highly distinct context (Fig. 2C, bottom). The third group was subjected to multiday training where the mice were exposed to two very similar contexts each day in random order. One context was always associated with a single foot-shock (2 sec, 0.4 mA), while the other was safe. α5GlobalKO animals showed significantly worse discrimination compared to controls for the first 2 d of the test, performing similarly to controls after this, and showing even better discrimination than controls at later stages. α5CA1KO mice performed similarly to α5GlobalKOs for the first 2 d, but their performance remained low, showing worse discrimination than controls until day 8 of the task (Fig. 3D; two-way ANOVA; Day (within subjects): F(11,379) = 1.669, P = 0.08; genotype: F(2,379) = 1.70, P = 0.20; Day × Genotype: F(22,379) = 1.72, P = 0.02). The finding that both α5GlobalKO and α5CA1KO mice eventually reach control levels with repeated training is in line with earlier work suggesting that the hippocampus is required for fast, efficient acquisition of context discrimination (e.g., Wiltgen et al. 2006; McHugh et al. 2007). In contrast to rapid learning in the hippocampus, neocortex has been suggested as a slow-learning system that acquires regularities over multiple learning experiences (Wiltgen et al. 2006; O'Reilly and Rudy 2001). Global or CA1-specific (or DG-specific; Engin et al. 2015) knockout of α5GABAARs seems to impair this hippocampus-dependent, initial phase of discrimination learning. As the CA1- and DG-specific knockouts only affect parts of the hippocampus, we see no differences in the later, presumably hippocampus-independent part of the task. In α5GlobalKO's, on the other hand, we see improved performance over controls on the final days of the task. This suggests that the knockout of α5 in cortical regions (α5 is expressed at moderate levels in deep layers of cortex; Fritschy and Mohler 1995) improves neocortical slow-learning of statistical regularities.
The findings from the contextual fear-conditioning tasks are unexpected. First, lesions of dorsal CA1 impair both the encoding and retrieval of contextual fear memories (Hunsaker and Kesner 2008). However, reducing α5GABAAR-mediated inhibition of CA1 pyramidal neurons does not seem to improve contextual fear memory, suggesting that intact functioning of CA1 might be necessary for the formation of contextual fear memories, but increasing CA1 excitability does not lead to stronger context learning. On the other hand, we showed in earlier experiments that selective knockout of α5GABAARs in either DG or CA3 principal neurons improves contextual fear memory (Engin et al. 2015), similar to our report here with α5GlobalKO mice.
Second, discrimination between two similar contexts is often used as a behavioral proxy for pattern separation; a concept that has been linked to DG (McHugh et al. 2007; Santoro 2013; van Dijk and Fenton 2018). In line with this, DG granule cell selective knockout of α5GABAARs leads to impaired context discrimination (Engin et al. 2015). However, until recently CA1 has been thought to be involved in temporal, but not spatial/contextual pattern separation (Kesner 2013). As the two contexts in our discrimination task are presented in random order, temporal pattern separation is not expected to contribute to performance.
Similar to our findings with α5CA1KO mice, overexpressing activated CaMKII in CA1 has been demonstrated to impair context discrimination without affecting overall contextual fear-conditioning or long-term context memory (Ye et al. 2019). CaMKII is activated specifically in spines that are stimulated during memory formation and increases maturation of these specific spines. The stimulation and consequent activation of specific spines in CA1 during memory encoding seems to be necessary for context discrimination, as nonspecific expression of CaMKII in all spines impairs context discrimination. With elevated neuronal excitation increasing synaptic clustering of α5GABAARs (Hausrat et al. 2015), α5GABAARs may play an important role in limiting the number of active spines in dendrites and preventing nonspecific activation. This kind of fine-tuning may not be necessary for overall learning of contextual information but is likely to be essential for complex mnemonic processes such as discrimination of overlapping stimuli. Thus, one way that the CA1-selective knockout of α5GABAARs may disrupt context discrimination is to distribute mnemonic molecular processes equally across dendritic spines of pyramidal neurons. This hypothesis needs to be studied further in future work to identify the mechanisms of CA1 α5GABAAR involvement in context discrimination.
Overall, our findings indicate that reducing α5GABAAR-mediated inhibition in CA1 pyramidal neurons improves the preservation of a memory trace in auditory fear-conditioning and associative spatial learning in MWM. Latent inhibition to conditioned fear and contextual fear-conditioning were unaffected. While performance in all other memory domains was improved or unaltered, context discrimination was impaired in α5CA1KO mice. This finding corroborates earlier evidence (Prut et al. 2010; Engin et al. 2015) that procognitive effects of α5NAMs may come at a cost in specific cognitive domains; a finding that has implications for drug development targeting cognitive improvement, for example, in Alzheimer's disease. Despite evidence that α5GABAARs are expressed and perform important physiological functions in CA1 interneurons, our findings suggest that most of the cognition-related functions of CA1 α5GABAARs are mediated through α5GABAARs in pyramidal neurons, in line with findings of other groups (Magnin et al. 2019).
Acknowledgments
This work was supported by award numbers R01GM086448 (U.R.), R01GM128183 (U.R.), and K01MH107787 (E.E.). In the last 2 years, U.R. has served as a consultant for Concert Pharmaceuticals. We thank Dr. Susumu Tonegawa (HHMI, Massachusetts Institute of Technology) for providing the T29-1 mice.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.052084.120.
References
- Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, Smith A, Otu FM, Howell O, Atack JR, et al. 2002. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the α 5 subunit of the GABAA receptor. J Neurosci 22: 5572–5580. 10.1523/JNEUROSCI.22-13-05572.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Bluthmann H, Mohler H, Rudolph U. 2002. Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors. Proc Natl Acad Sci 99: 8980–8985. 10.1073/pnas.142288699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin E, Zarnowska ED, Benke D, Tsvetkov E, Sigal M, Keist R, Bolshakov VY, Pearce RA, Rudolph U. 2015. Tonic inhibitory control of dentate gyrus granule cells by alpha5-containing GABAA receptors reduces memory interference. J Neurosci 35: 13698–13712. 10.1523/JNEUROSCI.1370-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin E, Benham RS, Rudolph U. 2018. An emerging circuit pharmacology of GABAA receptors. Trends Pharmacol Sci 39: 710–732. 10.1016/j.tips.2018.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Mohler H. 1995. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol 359: 154–194. 10.1002/cne.903590111 [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Benke D, Johnson DK, Mohler H, Rudolph U. 1997. GABA(A)-receptor α subunit is an essential prerequisite for receptor formation in vivo. Neuroscience 81: 1043–1053. 10.1016/S0306-4522(97)00244-3 [DOI] [PubMed] [Google Scholar]
- Gerdjikov TV, Rudolph U, Keist R, Mohler H, Feldon J, Yee BK. 2008. Hippocampal α 5 subunit-containing GABA A receptors are involved in the development of the latent inhibition effect. Neurobiol Learn Mem 89: 87–94. 10.1016/j.nlm.2007.06.003 [DOI] [PubMed] [Google Scholar]
- Grecksch G, Bernstein HG, Becker A, Hollt V, Bogerts B. 1999. Disruption of latent inhibition in rats with postnatal hippocampal lesions. Neuropsychopharmacology 20: 525–532. 10.1016/S0893-133X(98)00081-5 [DOI] [PubMed] [Google Scholar]
- Hausrat TJ, Muhia M, Gerrow K, Thomas P, Hirdes W, Tsukita S, Heisler FF, Herich L, Dubroqua S, Breiden P, et al. 2015. Radixin regulates synaptic GABAA receptor density and is essential for reversal learning and short-term memory. Nat Commun 6: 6872 10.1038/ncomms7872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt M, Maren S. 1999. Muscimol inactivation of the dorsal hippocampus impairs contextual retrieval of fear memory. J Neurosci 19: 9054–9062. 10.1523/JNEUROSCI.19-20-09054.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, Kesner RP. 2008. Dissociations across the dorsal-ventral axis of CA3 and CA1 for encoding and retrieval of contextual and auditory-cued fear. Neurobiol Learn Mem 89: 61–69. 10.1016/j.nlm.2007.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP. 2013. Role of the hippocampus in mediating interference as measured by pattern separation processes. Behav Process 93: 148–154. 10.1016/j.beproc.2012.09.018 [DOI] [PubMed] [Google Scholar]
- Magnin E, Francavilla R, Amalyan S, Gervais E, David LS, Luo X, Topolnik L. 2019. Input-specific synaptic location and function of the α5 GABAA receptor subunit in the mouse CA1 hippocampal neurons. J Neurosci 39: 788–801. 10.1523/JNEUROSCI.0567-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Oh GHT, Orser BA. 2009. Etomidate targets α(5) gamma-aminobutyric acid subtype a receptors to regulate synaptic plasticity and memory blockade. Anesthesiology 111: 1025–1035. 10.1097/ALN.0b013e3181bbc961 [DOI] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. 2007. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science 317: 94–99. 10.1126/science.1140263 [DOI] [PubMed] [Google Scholar]
- Milic M, Timic T, Joksimovic S, Biawat P, Rallapalli S, Divljakovic J, Radulovic T, Cook JM, Savic MM. 2013. PWZ-029, an inverse agonist selective for α(5) GABA(A) receptors, improves object recognition, but not water-maze memory in normal and scopolamine-treated rats. Behav Brain Res 241: 206–213. 10.1016/j.bbr.2012.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly RC, Rudy JW. 2001. Conjunctive representations in learning and memory: principles of cortical and hippocampal function. Psychol Rev 108: 311–345. 10.1037/0033-295X.108.2.311 [DOI] [PubMed] [Google Scholar]
- Pouzet B, Zhang WN, Weiner I, Feldon J, Yee BK. 2004. Latent inhibition is spared by N-methyl-D-aspartate (NMDA)-induced ventral hippocampal lesions, but is attenuated following local activation of the ventral hippocampus by intracerebral NMDA infusion. Neuroscience 124: 183–194. 10.1016/j.neuroscience.2003.11.007 [DOI] [PubMed] [Google Scholar]
- Prut L, Prenosil G, Willadt S, Vogt K, Fritschy JM, Crestani F. 2010. A reduction in hippocampal GABAA receptor alpha5 subunits disrupts the memory for location of objects in mice. Gene Brain Behav 9: 478–488. [DOI] [PubMed] [Google Scholar]
- Reilly SC, Harley W, Revusky S. 1993. Ibotenate lesions of the hippocampus enhance latent inhibition in conditioned taste aversion and increase resistance to extinction in conditioned taste preference. Behav Neurosci 107: 996–1004. 10.1037/0735-7044.107.6.996 [DOI] [PubMed] [Google Scholar]
- Rodgers FC, Zarnowska ED, Laha KT, Engin E, Zeller A, Keist R, Rudolph U, Pearce RA. 2015. Etomidate impairs long-term potentiation in vitro by targeting alpha5-subunit containing gabaa receptors on nonpyramidal cells. J Neurosci 35: 9707–9716. 10.1523/JNEUROSCI.0315-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Mohler H. 2014. GABAA receptor subtypes: therapeutic potential in Down syndrome, affective disorders, schizophrenia, and autism. Annu Rev Pharmacol Toxicol 54: 483–507. 10.1146/annurev-pharmtox-011613-135947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro A. 2013. Reassessing pattern separation in the dentate gyrus. Front Behav Neurosci 7: 96 10.3389/fnbeh.2013.00096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulz JM, Knoflach F, Hernandez M-C, Bischofberger J. 2018. Dendrite-targeting interneurons control synaptic NMDA-receptor activation via nonlinear α5-GABAA receptors. Nat Commun 9: 3576 10.1038/s41467-018-06004-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotty F, Sandner G, Gosselin O. 1996. Latent inhibition in conditioned emotional response: c-fos immunolabelling evidence for brain areas involved in the rat. Brain Res 737: 243–254. 10.1016/0006-8993(96)00737-8 [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S. 1996. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell 87: 1327–1338. 10.1016/S0092-8674(00)81827-9 [DOI] [PubMed] [Google Scholar]
- van Dijk MT, Fenton AA. 2018. On how the dentate gyrus contributes to memory discrimination. Neuron 98: 832 10.1016/j.neuron.2018.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Anagnostaras SG, Sage JR, Fanselow MS. 2006. Context fear learning in the absence of the hippocampus. J Neurosci 26: 5484–5491. 10.1523/JNEUROSCI.2685-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S, Kim JI, Kim J, Kaang BK. 2019. Overexpression of activated CaMKII in the CA1 hippocampus impairs context discrimination, but not contextual conditioning. Mol Brain 12: 32 10.1186/s13041-019-0454-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee BK, Hauser J, Dolgov VV, Keist R, Mohler H, Rudolph U, Feldon J. 2004. GABA receptors containing the alpha5 subunit mediate the trace effect in aversive and appetitive conditioning and extinction of conditioned fear. Eur J Neurosci 20: 1928–1936. 10.1111/j.1460-9568.2004.03642.x [DOI] [PubMed] [Google Scholar]
- Zhou YX, Qiu LY, Wang HY, Chen XM. 2020. Induction of activity synchronization among primed hippocampal neurons out of random dynamics is key for trace memory formation and retrieval. FASEB J 34: 3658–3676. 10.1096/fj.201902274R [DOI] [PMC free article] [PubMed] [Google Scholar]