Abstract
Objectives
The treatment paradigm in newly diagnosed multiple myeloma (NDMM) is evolving toward individualized, risk‐directed, and longer duration of therapy (DOT). The objective of this study was to describe treatment patterns and outcomes in non‐transplant NDMM in four European countries.
Methods
This retrospective chart review included adults with NDMM diagnosed between January 1, 2012, and December 31, 2013 (early cohort), or April 1, 2016, and March 31, 2017 (recent cohort).
Results
Among 836 patients, molecular testing was performed in 21% and 35% patients of early vs recent cohorts; proteasome inhibitor (PI)/alkylator combinations were the principal first‐line (1 L) therapy (39% vs 43%). Use of immunomodulatory drug (IMID)/alkylator combinations declined from early to recent cohort (26% vs 13%) but IMID (7% vs 16%) use increased. Few patients (5%) received 1 L maintenance therapy. Two‐thirds of patients were treated with a fixed duration intent, with a median 7‐month 1 L DOT and progression‐free survival (PFS) of 32.8 months in the early cohort. Both 1 L DOT and PFS were longer with oral compared to injectable regimens.
Conclusions
Although frontline treatment patterns changed significantly, 1 L DOT is short. The uptake of molecular testing and 1 L maintenance is low. These results highlight areas of unmet need in NDMM.
Keywords: clinical practice patterns, medical records, multiple myeloma, retrospective studies, treatment outcomes
1. INTRODUCTION
Multiple myeloma (MM) is the second most common hematologic malignancy in Europe. In 2018, 48,300 adults were estimated to be newly diagnosed with MM (NDMM), and 30 900 have died from the disease. 1 , 2 , 3 Emerging treatment options as standard of care have resulted in considerably improved outcomes in NDMM. 4 Since 2007, following the approvals of bortezomib and lenalidomide, the median overall survival (OS) has doubled in the NDMM population. 5 Despite the rapidly evolving treatment landscape, myeloma remains incurable. Individualized treatment based on patient characteristics such as age and concomitant comorbidities, disease factors (including genetic changes), and prior treatment history is often a guideline‐recommended approach. 6 , 7 This is particularly true for elderly patients (75 years and above), those with comorbidities and/or poor performance status who do not undergo a stem cell transplant (non‐SCT). Existing therapies are associated with significant toxicities and may lead to early treatment discontinuation when given to non‐SCT patients. 8 The shortened duration of therapy (DOT) in 1 L may adversely impact clinical outcomes, as evidence suggests that extended treatment leads to better outcomes in NDMM. 9 , 10 Therefore, a tailored approach to treatment based on individual patient profile is necessary to achieve optimal treatment effectiveness and reduce the risk of toxicity in non‐SCT patients.
Patients recruited to clinical trials investigating novel treatments are largely not representative of the wider non‐SCT NDMM patient populations due to strict eligibility criteria. Analyses of real‐world patient data indicate that 40%‐75% of patients were ineligible to enter clinical trials. 11 , 12 , 13 As a result, wide discrepancies have been noted between the reported clinical efficacy of novel therapies from trials and benefits of treatments for patients in the real world. 14 However, trial ineligible patients (eg, due to advanced age or comorbidities) constitute a large percentage of the MM population. 13 For example, in the United Kingdom (UK) from 2014 to 2016, 44% of MM cases were diagnosed in persons aged 75 years and above, and 30.3% were 75 years or older in Germany in 2017. 15 , 16 In France, the median age of patients newly diagnosed with MM was 74 years, with 57.5% reporting comorbidities that impacted myeloma treatment. 17 Moreover, the percentage of elderly and comorbid NDMM patients is expected to increase further due to the aging demographic in Europe. 18 Overall, real‐world evidence on contemporary treatment patterns among non‐SCT NDMM patients and their treatment outcomes in Europe is limited. Adequate evaluation of these data will help understand the efficacy and effectiveness gap between clinical trial and real‐world patient populations.
The objective of this study was to characterize real‐world patient characteristics, treatment patterns, and outcomes among NDMM non‐SCT patients in France, Germany, Italy, and the UK. The study was also designed to assess treatment pattern changes over time, describing treatment of patients diagnosed in 2012‐2013 and patients diagnosed in 2016‐2017.
2. METHODS
2.1. Study design
This was a retrospective, observational medical chart review study wherein site physicians or their designees abstracted data for non‐SCT NDMM patients at their practice using an electronic case report form. In France, Germany, and the UK, study sites and investigators remained anonymous to the sponsor, and the sponsor was not disclosed to physicians; in Italy, the investigators could not be blinded to the sponsor due to the requirement for site‐level ethics approval that required disclosure of the sponsor. In the UK and Germany, physicians were recruited from a national database. In France, proprietary panels were used to identify study investigators. In Italy, sites were recruited from a list of potential investigators and sites provided by local affiliates of the sponsor of this study. In France, Germany, and the UK, reviews by a central institutional review board and/or an ethics waiver were granted as required in each country. In Italy, protocols and study material were submitted to central and/or local ethics committees as required by the participating sites. Medical charts were reviewed by treating physicians, and MM treatment data from diagnosis to most recent visit or death were collected. Investigators were prompted to include up to six non‐SCT NDMM patients randomly from their files. Soft quotas based on the population distribution were applied to facilitate regional representation in Germany, France, and the UK. In France, soft quotas were also applied in the practice setting (office‐ vs hospital‐based). Geographic and regional distributions of the study sites can be found in Appendix Table A1.
2.2. Selection criteria
To account for evolving treatment patterns due to recently approved treatments in NDMM, as well as to enable assessment of clinical outcomes with sufficient follow‐up, patients were sampled from two diagnostic periods defined as the “early cohort” (patients diagnosed between January 1, 2012, and December 31, 2013) and the “recent cohort” (patients diagnosed between April 1, 2016, and March 31, 2017).
Patient inclusion criteria were as follows: ≥18 years of age at diagnosis; newly diagnosed with active symptomatic MM (defined as having ≥10% abnormal plasma cells in the bone marrow or a plasmacytoma confirmed by biopsy); and one or more of the following myeloma defining events: ≥60% abnormal plasma cells in the bone marrow; an increased level of calcium in the blood; kidney damage; anemia; one or more sites of osteolytic bone lesions found on imaging tests; an abnormal serum‐free light chain ratio (≥100 involved kappa, or ≤0.01 involved lambda 19 ). Additional inclusion criteria were as follows: Patients did not undergo frontline SCT due to age, comorbidities, impaired fitness, preference, or any other reason; received systemic therapy as first line of therapy postinitial diagnosis; had a minimum of 4 months of follow‐up since start of 1 L systemic therapy treatment unless patient died in this period; and investigator was able to report on complete MM diagnosis and treatment details up to most recent visit or death.
Patients who were enrolled in a clinical trial for 1 L systemic therapy postinitial diagnosis of MM and those with any prior diagnoses of another malignancy within 5 years of diagnosis of MM and evidence of residual disease, except for adequately treated non‐melanoma skin cancer, or in situ neoplasm (eg, neoplastic bowel polyp, in situ breast cancer, localized prostate cancer), were excluded from the study.
2.3. Study variables
Patient medical chart data extraction, from the time of MM diagnosis to most recent visit or death, included the following: demographics (sex, age, ethnicity) and baseline clinical characteristics. Baseline characteristics included CRAB symptoms (hypercalcemia, renal insufficiency, anemia, bone lesions), comorbidities, MM international staging system (ISS) stage, MM type (secretory, non‐secretory), immunoglobulin class, Eastern Cooperative Oncology Group performance status (ECOG PS), frailty status (fit, intermediate fitness, or frail, as assessed by the investigator), and cytogenetic risk at diagnosis. Treatment characteristics (including treatment agents, dates, reasons for discontinuation), evidence of response per International Myeloma Working Group (IMWG) criteria, 20 and disease progression per IMWG criteria were also extracted from the patient medical charts.
Cytogenetic risk was defined as high if del(17p), t(4;14), and/or t(14;16) were present. The Charlson comorbidity index (CCI) was calculated as a summation of assigned weights of selected conditions without considering MM diagnosis in the summation. 21
Initiation of a new line of therapy was defined as interruption of a planned period of observation of therapy by a need for additional treatment for the disease (eg, retreatment with the same or subset of a prior regimen if treatment‐free period is at least 6 months), or when a planned course of therapy was modified to include other treatment agents, alone, or in combination (eg, switch in at least one agent or add‐on of an agent, other than steroids, irrespective of treatment‐free interval) as a result of progressive disease, relapse, or toxicity.
First‐line medication regimens were categorized based on the number of medications (monotherapy/doublet vs triplet/quadruplet), drug class (defined below), and route of administration (oral or injectable) included in the regimens. Medications were categorized by the following classes: (a) IMID‐based: lenalidomide, pomalidomide, or thalidomide; (b) PI‐based: bortezomib, carfilzomib, or ixazomib; (c) alkylator‐based: melphalan, cyclophosphamide or bendamustine; and (d) steroids: dexamethasone, prednisone, methylprednisolone. Based on this categorization, first‐line regimens were described as: PI (no IMID or alkylator as part of the regimen), IMID (no PI or alkylator), alkylator (no PI or IMID), PI/IMID (no alkylator), PI/alkylator (no IMID), and IMID/alkylator (no PI).
IMID‐based regimens that did not contain a PI were classified as oral regimens. Since none of the study patients were treated with the orally administered ixazomib, PI or PI combinations such as PI/IMID‐ or PI/alkylator‐based regimens were classified as injectables. Due to lack of information on administration route, alkylator‐based regimens without an IMID or PI could not be categorized as oral or injectable. All regimens were assumed to be given in combination with a steroid even when steroids were not listed as part of the combination. Data on maintenance therapy (received after the induction therapy, but prior to progression) as reported by the physician were also extracted.
Additionally, for each treatment, the treatment plan (treatment until progression, fixed DOT, or treatment to best response/plateau) and reason for discontinuation were extracted from patient medical record.
To address the study objective, several time‐dependent clinical outcomes were derived using the data extracted from the patient medical charts: DOT, time to best response, and progression‐free survival (PFS). Duration of therapy was defined as the time from the start of regimen (the first component of the regimen) to the end of all drug components of the regimen, including reported therapy end date or death, whichever occurred earlier within each line of therapy. For DOT within a line of therapy, the DOT started from the first administered regimen (eg, induction) to the last administered regimen (eg, maintenance). A regimen which ended because of end of study/follow‐up was considered incomplete and, therefore, was censored at date of last follow‐up. Time to best response was defined as the start of induction therapy to the day of best response, categorized as stringent complete response, complete response, very good partial response, or partial response. 20 Patients with missing response dates were censored at start of a new line of therapy, last follow‐up, or death, whichever occurred first. Progression‐free survival was defined as the time from the start of each line of a therapy to the progression date or death (whichever occurred first) before start of next line of therapy. If no progression date was reported and a patient started another line of therapy, the start day of the next line of therapy was assumed as the date of progression. Patients who did not receive another line of therapy and who did not experience a progression or death were censored at last follow‐up. Due to limited follow‐up time, the clinical outcomes were only analyzed among patients in the early cohort.
2.4. Statistical analysis
Categorical outcomes were summarized using the percentage and count in each category. Continuous endpoints were summarized using the summary statistics of mean, standard deviation, median, and range. Group differences were tested using Pearson's chi‐square test or Fisher's exact test for categorical variables and Student's t test for continuous variables. All statistical tests were performed to test for differences in distribution of baseline characteristics between the four countries (France, Germany, Italy, and the UK) and two cohorts (early vs recent) in this study. All inferences were made assuming a two‐sided test with an alpha level of 0.05. Time‐dependent endpoints, including DOT, time to best response, and PFS, were analyzed in the early cohort in terms of total number of events observed and the proportion of patients experiencing events, after accounting for censoring using Kaplan‐Meier (KM) curves that were adjusted for key baseline patient demographic and clinical characteristics (demographics, CRAB symptoms, comorbidities, ISS stage, MM type, immunoglobulin class, ECOG PS, frailty status, and cytogenetic risk at diagnosis). All analyses were conducted using SAS® software, version 9.4.
3. RESULTS
3.1. Patient demographic and clinical characteristics
A total of 171 investigators/sites (France: 51; Germany: 57; Italy: 10; UK: 53) participated in the study, of which 124 (72.5%) were hospital‐based (France: 47; Germany: 34; Italy: 7; UK: 36), 36 (21.1%) were based at specialist cancer centers, and 11 (6.4%) were based in other settings. The sites were regionally distributed along the predefined soft quota (Appendix Table A1). Most investigators had a specialty in hematology‐oncology (59%) or hematology (38%).
The sites enrolled 836 patients (early cohort: 592, recent cohort: 244 (Table 1 and Appendix Table A2). Median age (overall) was 73.0 years (early cohort: 72.5; recent cohort: 73.0), with 36% being ≥75 years old (early cohort: 34%; recent cohort: 39%). Patients in France were significantly younger than in the other countries examined in this study (mean age: 69.1 years; P < .001). Most patients (overall) were male (61%), with the proportion of male patients varying between 51% in Italy and 67% in Germany. The majority of patients had an ECOG PS of 0 or 1 (52%), fit (16%) or intermediate frailty status (51%), and ISS stage III (56%). In France, more patients presented with a low ECOG PS and fit frailty status than in the other countries. Most of the patients presented with a CCI > 0, with 28% of patients having a CCI of 0. Of all countries, France had the highest proportion of patients (40%) with a CCI of 0.
Table 1.
Baseline patient characteristics
| All (N = 836) | France (N = 269) | Germany (N = 213) | Italy (N = 136) | UK (N = 218) | P value | |
|---|---|---|---|---|---|---|
| Age, n (%) | ||||||
| Median | 73.0 | 70.0 | 74.0 | 73.0 | 73.0 | <.001 |
| <65 y | 84 (10) | 56 (21) | 2 (1) | 2 (1) | 24 (11) | <.001 |
| 65‐74 y | 453 (54) | 152 (57) | 120 (56) | 80 (59) | 101 (46) | |
| 75+ y | 299 (36) | 61 (23) | 91 (43) | 54 (40) | 93 (43) | |
| Gender, n (%) | ||||||
| Male | 511 (61) | 163 (61) | 142 (67) | 70 (51) | 136 (62) | .041 |
| Female | 325 (39) | 106 (39) | 71 (33) | 66 (49) | 82 (38) | |
| ECOG PS, n (%) | ||||||
| 0 | 46 (6) | 10 (4) | 4 (2) | 16 (12) | 16 (7) | <.001 |
| 1 | 383 (46) | 153 (57) | 103 (48) | 19 (14) | 108 (50) | |
| 2 | 263 (31) | 78 (29) | 90 (42) | 19 (14) | 76 (35) | |
| 3 | 57 (7) | 22 (8) | 12 (6) | 5 (4) | 18 (8) | |
| 4 | 9 (1) | 2 (1) | 4 (2) | 3 (2) | 0 (0) | |
| Unknown | 78 (9) | 4 (1) | 0 (0) | 74 (54) | 0 (0) | |
| Frailty status, n (%) | ||||||
| Fit | 130 (16) | 93 (35) | 10 (5) | 7 (5) | 20 (9) | <.001 |
| Intermediate fitness | 427 (51) | 123 (46) | 163 (77) | 25 (18) | 116 (53) | |
| Frail | 218 (26) | 53 (20) | 39 (18) | 51 (38) | 75 (34) | |
| Unknown | 61 (7) | 0 (0) | 1 (0) | 53 (39) | 7 (3) | |
| CCI, n (%) | ||||||
| 0 | 231 (28) | 108 (40) | 32 (15) | 50 (37) | 41 (19) | <.001 |
| 1 | 204 (24) | 68 (25) | 63 (30) | 23 (17) | 50 (23) | |
| 2+ | 368 (44) | 81 (30) | 114 (54) | 53 (39) | 120 (55) | |
| Unknown | 33 (4) | 12 (4) | 4 (2) | 10 (7) | 7 (3) | |
| ISS Stage, n (%) | ||||||
| Stage I | 85 (10) | 36 (13) | 15 (7) | 13 (10) | 21 (10) | <.001 |
| Stage II | 170 (20) | 78 (29) | 24 (11) | 22 (16) | 46 (21) | |
| Stage III | 469 (56) | 128 (48) | 170 (80) | 43 (32) | 128 (59) | |
| Unknown | 112 (13) | 27 (10) | 4 (2) | 58 (43) | 23 (11) | |
| Immunoglobulin class, n (%) | ||||||
| IgG | 432 (52) | 139 (52) | 134 (63) | 68 (50) | 91 (42) | <.001 |
| IgA | 153 (18) | 42 (16) | 36 (17) | 31 (23) | 44 (20) | |
| Light chain only | 106 (13) | 23 (9) | 27 (13) | 19 (14) | 37 (17) | |
| IgM | 14 (2) | 8 (3) | 6 (3) | 0 (0) | 0 (0) | |
| IgE | 10 (1) | 5 (2) | 3 (1) | 0 (0) | 2 (1) | |
| IgD | 6 (1) | 4 (1) | 2 (1) | 0 (0) | 0 (0) | |
| Other a | 2 (0) | 0 (0) | 0 (0) | 2 (1) | 0 (0) | |
| Biclonal | 1 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0) | |
| Unknown/not documented | 112 (13) | 48 (18) | 5 (2) | 16 (12) | 43 (20) | |
| Extramedullary disease, n (%) | 90 (11) | 35 (13) | 19 (9) | 10 (7) | 26 (12) | .249 |
| CRAB symptoms, n (%) | 750 (90) | 249 (93) | 208 (98) | 113 (83) | 180 (83) | <.001 |
| Renal insufficiency | 133 (16) | 46 (17) | 40 (19) | 17 (13) | 30 (14) | .317 |
| Hypercalcemia | 119 (14) | 55 (20) | 24 (11) | 4 (3) | 36 (17) | <.001 |
| Anemia | 362 (43) | 120 (45) | 71 (33) | 50 (37) | 121 (56) | <.001 |
| Bone lesions | 643 (77) | 216 (80) | 197 (92) | 90 (66) | 140 (64) | <.001 |
| Cytogenetic testing done, n (%) | 210 (25) | 113 (42) | 25 (12) | 31 (23) | 41 (19) | <.001 |
| High risk | 51 (6) | 27 (10) | 6 (3) | 6 (4) | 12 (6) | <.001 |
| Standard risk | 159 (19) | 86 (32) | 19 (9) | 25 (18) | 29 (13) | |
| Unknown risk | 626 (75) | 156 (58) | 188 (88) | 105 (77) | 177 (81) | |
| Follow‐up duration from NDMM diagnosis in months, Median (IQR) | 20.9 (14.0 to 49.9) | 20.9 (8.0 to 44.3) | 18.9 (18.9 to 18.9) | 21.2 (15.2 to 53.8) | 56.5 (10.1 to 62.0) | |
Other Ig class includes Bence Jones/lambda, multi‐molecular MM. Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; ISS, international staging system; UK, United Kingdom
3.2. Cytogenetic testing
The overall use of cytogenetic testing was low (25%), although the rate has significantly increased over time when comparing results from the early vs recent cohorts (21% vs 35%; P < .05, Table 2). The rates of testing also differed significantly across countries (both cohorts combined), with the highest rate of testing (42%) reported in France and the lowest (12%) reported in Germany (P < .001). The percentage of patients with high cytogenetic risk (among those tested) was 24% overall (with 26% and 22% in the early and recent cohorts, respectively).
Table 2.
Use of cytogenetic testing by country and cohort
| All | France | Germany | Italy | UK | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Early (N = 592) | Recent (N = 244) | Early (N = 176) | Recent (N = 93) | Early (N = 156) | Recent (N = 57) | Early (N = 95) | Recent (N = 41) | Early (N = 165) | Recent (N = 53) | |
| Cytogenetic testing done, n (%) | 125 (21) a , a | 85 (35) a , a | 69 (39) | 44 (47) | 15 (10) | 10 (18) | 19 (20) | 12 (29) | 22 (13) a , a | 19 (36) a , a |
| High risk | 32 (5) a , a | 19 (8) a , a | 17 (10) | 10 (11) | 4 (3) | 2 (4) | 4 (4) | 2 (5) | 7 (4) a , a | 5 (9) a , a |
| Standard risk | 93 (16) | 66 (27) | 52 (30) | 34 (37) | 11 (7) | 8 (14) | 15 (16) | 10 (24) | 15 (9) | 14 (26) |
| Unknown risk | 467 (79) | 159 (65) | 107 (61) | 49 (53) | 141 (90) | 47 (82) | 76 (80) | 29 (71) | 143 (87) | 34 (64) |
Denoting significant differences between early and recent cohort (P < .05). Abbreviations: CCI, Charlson comorbidity index; ECOG PS, Eastern Cooperative Oncology Group performance status; ISS, international staging system; UK, United Kingdom.
3.3. First‐line treatment patterns
The most common 1 L regimens were PI/alkylator‐based (40%), followed by IMID/alkylator‐based (22%) (Table 3). The use of PI/alkylator‐based regimens was driven mostly by bortezomib/melphalan (VM) ± steroid treatment (31%; Appendix Table A3). Cyclophosphamide/thalidomide ± steroid (CT ± steroid) was the most common among those on IMID/alkylator‐based regimens (15%). The PI/alkylator‐based regimens were used most frequently in all countries, except in the UK where the use of IMID/alkylators constituted 50% of all 1 L regimens used, the majority of which was CT ± steroid. In Italy, PI/alkylators were especially predominant, with over 77% utilization for 1 L therapy.
Table 3.
Overview of first‐line regimens by country and cohort
| Category, N (%) | All | France | Germany | Italy | UK | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All (N = 836) | Early (N = 592) | Recent (N = 244) | All (N = 269) | Early (N = 176) | Recent (N = 93) | All (N = 213) | Early (N = 156) | Recent (N = 57) | All (N = 136) | Early (N = 95) | Recent (N = 41) | All (N = 218) | Early (N = 165) | Recent (N = 53) | |
| Induction by number ofaAgents | |||||||||||||||
| Doublet or less | 281 (34) | 189 (32) | 92 (38) | 106 (39) | 62 (35) | 44 (47) | 86 (40) | 58 (37) | 28 (49) | 23 (17) | 13 (14) | 10 (24) | 66 (30) | 56 (34) | 10 (19) |
| Triplet or more | 554 (66) | 402 (68) | 152 (62) | 163 (61) | 114 (65) | 49 (53) | 127 (60) | 98 (63) | 29 (51) | 112 (83) | 81 (86) | 31 (76) | 152 (70) | 109 (66) | 43 (81) |
| Induction by route ofaAdministration a | |||||||||||||||
| Oral | 80 (10) | 40 (7) | 40 (16) | 37 (14) | 14 (8) | 23 (25) | 20 (9) | 12 (8) | 8 (14) | 6 (4) | 0 (0) | 6 (15) | 17 (8) | 14 (8) | 3 (6) |
| Injectables | 489 (58) | 336 (57) | 153 (63) | 179 (67) | 120 (68) | 59 (63) | 113 (53) | 80 (51) | 33 (58) | 118 (87) | 85 (89) | 33 (80) | 79 (36) | 51 (31) | 28 (53) |
| Other | 267 (32) | 216 (36) | 51 (21) | 53 (20) | 42 (24) | 11 (12) | 80 (38) | 64 (41) | 16 (28) | 12 (9) | 10 (11) | 2 (5) | 122 (56) | 100 (61) | 22 (42) |
| Maintenance n (%) | 43 (5) | 32 (5) | 11 (5) | 13 (5) | 9 (5) | 4 (4) | 1 (0) | 1 (1) | 0 (0) | 26 (19) | 19 (20) | 7 (17) | 3 (1) | 3 (2) | 0 (0) |
Abbreviations: UK, United Kingdom.
IMID‐based regimens that did not contain a PI were classified as oral regimens, and PI or PI combinations such as PI/IMID‐ or PI/alkylator‐based regimens were classified as injectables.
More than half of the prescribed 1 L regimens were injectable regimens (8%) with the proportion ranging from 36% in the UK to 87% in Italy (Table 3). About 10% of the 1 L regimens were oral (France: 14%; Italy: 4%; Germany: 9%; UK: 8%). The majority of patients received triplet vs doublet therapy (67% vs 29%, respectively).
A small proportion of patients (5% of each cohort) received maintenance as part of frontline therapy, with 58% of those undergoing maintenance therapy receiving a PI‐based (V ± steroid) treatment, and 40% receiving an IMID‐based treatment.
3.4. Early vs recent cohort
In France, the use of IMID/alkylator‐based and PI/alkylator‐based regimens decreased from early to recent cohort, while the use of IMID‐based regimens (Figure 1), primarily lenalidomide (R) ± steroid treatment (6% to 23%), increased in the recent cohort. In Germany, the use of IMID/alkylator‐based and alkylator‐based regimens decreased, while the use of PI‐based regimens increased. In Italy, the least variability between early and recent cohorts was observed, where PI/alkylator‐based combinations, primarily VM ± steroid, were the mainstay of treatment in both cohorts (76% and 71%, respectively). The uptake of R ± steroid treatment was 15% in the recent cohort, with decreased use of PI‐based and IMID/alkylator‐based regimens. In the UK, IMID/alkylator‐based combinations (primarily CT ± steroid; 44%) were the most common regimens (53%) and PI/alkylator‐based combinations were less common (12%) in the early cohort. In the recent cohort, the use of CT ± steroid (from 44% to 34%) decreased, while the use of PI/alkylator‐based regimens increased (from 12% to 34%). In addition, bortezomib, the most commonly prescribed 1 L injectable agent, was initiated as a subcutaneous injection, rather than an intravenous infusion, in 69.7% of patients in the early cohort and 85.0% in the recent cohort.
Figure 1.
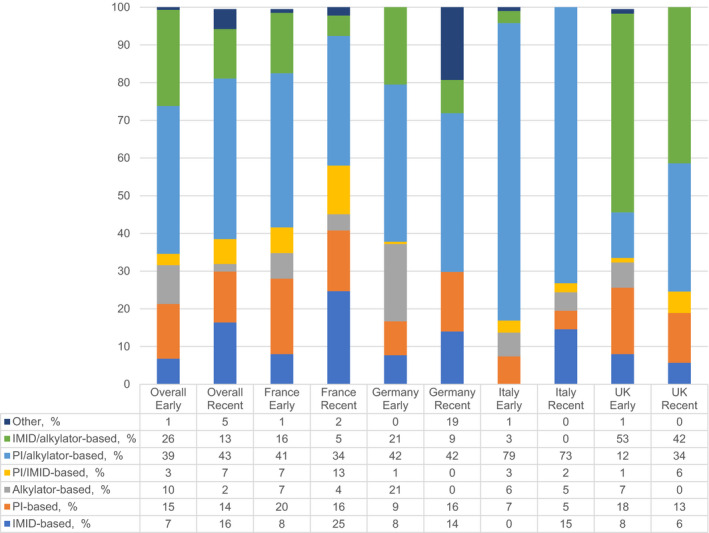
First‐line regimens (Drug Class Categories) by Country and Cohort. Abbreviations: IMID, immunomodulatory drug; PI, protease inhibitor; UK, United Kingdom [Colour figure can be viewed at wileyonlinelibrary.com]
Triplet therapy use was similar in both cohorts (67% vs 66%), although country‐specific differences were observed. In France, the use of single/doublet regimens increased from 35% to 47% from the early to the recent cohort, and the use of triplet/quadruplet regimens decreased from 65% to 53%, respectively. In Italy, the use of singe/doublet regimens increased from 14% to 24%, and the use of triplet/quadruplet regimens decreased from 86% to 76%. In the UK, the use of singe/doublet regimens decreased from 34% to 19%, and the use of triplet/quadruplet regimens increased from 66% to 81%. In Germany, the use of single/doublet regimens increased from 37% to 49%, and the use of triplet/quadruplet regimens decreased from 63% to 51%.
3.5. Treatment plan
Most regimens were prescribed to treat patients for a fixed duration of time (66% overall), ranging from 62% in the UK to 78% in Italy (Table 4). Half of PI‐based regimens were prescribed for a fixed duration, whereas IMID‐based regimens were mostly prescribed until progression (46%) or best response (39%). Among patients receiving IMID‐based regimens, the proportion of those who were being treated until progression in the recent cohort almost doubled from 33% to 60%. Most injectable regimens were planned for a fixed DOT (76%, with the proportion ranging from 67% in the UK to 83% in Italy), whereas oral regimens were planned to treat either until progression (46%, ranging from 18% in the UK to 62% in France) or best response (39%, ranging from 24% in France to 59% in the UK).
Table 4.
First‐line treatment plans by country and cohort
| Overall | France | Germany | Italy | UK | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Early | Recent | All | Early | Recent | All | Early | Recent | All | Early | Recent | All | Early | Recent | |
| All regimens, N | 836 | 592 | 244 | 269 | 176 | 93 | 213 | 156 | 57 | 136 | 95 | 41 | 218 | 165 | 53 |
| To progression, % | 13 | 12 | 17 | 23 | 18 | 32 | 11 | 12 | 7 | 10 | 10 | 10 | 6 | 6 | 8 |
| Fixed duration, % | 66 | 66 | 65 | 64 | 68 | 55 | 63 | 58 | 77 | 78 | 77 | 81 | 62 | 64 | 59 |
| To best response, % | 21 | 23 | 18 | 14 | 14 | 13 | 26 | 30 | 16 | 13 | 14 | 10 | 31 | 30 | 34 |
| IMID‐based, N | 80 | 40 | 40 | 37 | 14 | 23 | 20 | 12 | 8 | 6 | 0 | 6 | 17 | 14 | 3 |
| To progression, % | 46 | 33 | 60 | 62 | 43 | 74 | 45 | 42 | 50 | 33 | 0 | 33 | 18 | 14 | 33 |
| Fixed duration, % | 15 | 23 | 8 | 14 | 21 | 9 | 10 | 17 | 0 | 17 | 0 | 17 | 24 | 29 | 0 |
| To best response, % | 39 | 45 | 33 | 24 | 36 | 17 | 45 | 42 | 50 | 50 | 0 | 50 | 59 | 57 | 67 |
| PI‐based, N | 119 | 86 | 33 | 51 | 36 | 15 | 23 | 14 | 9 | 9 | 7 | 2 | 36 | 29 | 7 |
| To progression, % | 21 | 23 | 15 | 29 | 31 | 27 | 17 | 29 | 0 | 22 | 29 | 0 | 11 | 10 | 14 |
| Fixed duration, % | 50 | 50 | 52 | 29 | 31 | 27 | 78 | 64 | 100 | 67 | 57 | 100 | 58 | 66 | 29 |
| To best response, % | 29 | 27 | 33 | 41 | 39 | 47 | 4 | 7 | 0 | 11 | 14 | 0 | 31 | 24 | 57 |
| Alkylator, N | 67 | 61 | 6 | 16 | 12 | 4 | 32 | 32 | 0 | 8 | 6 | 2 | 11 | 11 | 0 |
| To progression, % | 28 | 23 | 83 | 75 | 67 | 100 | 16 | 16 | 0 | 25 | 17 | 50 | 0 | 0 | 0 |
| Fixed duration, % | 60 | 64 | 17 | 25 | 33 | 0 | 72 | 72 | 0 | 63 | 67 | 50 | 73 | 73 | 0 |
| To best response, % | 12 | 13 | 0 | 0 | 0 | 0 | 13 | 13 | 0 | 13 | 17 | 0 | 27 | 27 | 0 |
| PI/alkylator‐based, N | 336 | 232 | 104 | 104 | 72 | 32 | 89 | 65 | 24 | 105 | 75 | 30 | 38 | 20 | 18 |
| To progression, % | 4 | 5 | 3 | 2 | 1 | 3 | 5 | 6 | 0 | 6 | 7 | 3 | 5 | 5 | 6 |
| Fixed duration, % | 85 | 82 | 91 | 95 | 96 | 94 | 75 | 68 | 96 | 85 | 81 | 93 | 76 | 75 | 78 |
| To best response, % | 11 | 14 | 6 | 3 | 3 | 3 | 20 | 26 | 4 | 10 | 12 | 3 | 18 | 20 | 17 |
| IMID/alkylator‐based, N | 183 | 151 | 32 | 34 | 29 | 5 | 37 | 32 | 5 | 3 | 3 | 0 | 109 | 87 | 22 |
| To progression, % | 5 | 5 | 6 | 9 | 7 | 20 | 3 | 3 | 0 | 0 | 0 | 0 | 5 | 5 | 5 |
| Fixed duration, % | 62 | 63 | 56 | 79 | 79 | 80 | 35 | 38 | 20 | 67 | 67 | 0 | 65 | 67 | 59 |
| To best response, % | 33 | 33 | 38 | 12 | 14 | 0 | 62 | 59 | 80 | 33 | 33 | 0 | 30 | 29 | 36 |
| Oral, N | 80 | 40 | 40 | 37 | 14 | 23 | 20 | 12 | 8 | 6 | 0 | 6 | 17 | 14 | 3 |
| To progression, % | 46 | 33 | 60 | 62 | 43 | 74 | 45 | 42 | 50 | 33 | 0 | 33 | 18 | 14 | 33 |
| Fixed duration, % | 15 | 23 | 8 | 14 | 21 | 9 | 10 | 17 | 0 | 17 | 0 | 17 | 24 | 29 | 0 |
| To best response, % | 39 | 45 | 33 | 24 | 36 | 17 | 45 | 42 | 50 | 50 | 0 | 50 | 59 | 57 | 67 |
| Injectable, N | 489 | 336 | 153 | 179 | 120 | 59 | 113 | 80 | 33 | 118 | 85 | 33 | 79 | 51 | 28 |
| To progression, % | 9 | 10 | 6 | 11 | 12 | 10 | 7 | 10 | 0 | 7 | 8 | 3 | 8 | 8 | 7 |
| Fixed duration, % | 76 | 73 | 82 | 75 | 75 | 76 | 76 | 68 | 97 | 83 | 79 | 94 | 67 | 69 | 64 |
| To best response, % | 15 | 17 | 12 | 13 | 13 | 14 | 17 | 23 | 3 | 10 | 13 | 3 | 25 | 24 | 29 |
Treatment plan of PI/IMID‐based treatments (N = 34) and other regimens (N = 17) not listed due to space and low sample size.
Abbreviations: IMID, immunomodulatory drug; PI, protease inhibitor; UK, United Kingdom.
3.6. Reasons for discontinuation
Nearly all patients (95%) discontinued 1 L therapy during the study period (Table 5 ). The most common reason for discontinuation was planned treatment completion (61%) and remission or maximum clinical benefits achieved (21%, Figure 2; Table 5). Oral regimens were mostly discontinued due to remission (46%), whereas injectable regimens were mostly discontinued because of planned therapy completion (67%). About 12% and 15% of injectable regimens were discontinued due to treatment failure and remission, respectively.
Table 5.
First‐line regimen discontinuation rates and reasons for discontinuation by country and cohort
| All | France | Germany | Italy | UK | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Early | Recent | All | Early | Recent | All | Early | Recent | All | Early | Recent | All | Early | Recent | |
| N | 836 | 592 | 244 | 269 | 176 | 93 | 213 | 156 | 57 | 136 | 95 | 41 | 218 | 165 | 53 |
| All regimens, N | 95 | 99 | 86 | 88 | 97 | 72 | 98 | 98 | 98 | 98 | 100 | 93 | 99 | 100 | 94 |
| Treatment failure, % | 13 | 12 | 13 | 12 | 11 | 13 | 10 | 11 | 7 | 20 | 18 | 26 | 11 | 12 | 10 |
| AE/death, % | 4 | 4 | 5 | 4 | 4 | 4 | 0 | 0 | 0 | 13 | 11 | 18 | 2 | 3 | 0 |
| Remission/maximum clinical benefit achieved, % | 21 | 21 | 22 | 9 | 9 | 10 | 25 | 22 | 36 | 6 | 7 | 3 | 39 | 40 | 36 |
| Planned treatment completed, % | 61 | 61 | 59 | 74 | 75 | 72 | 62 | 64 | 57 | 56 | 60 | 47 | 47 | 45 | 52 |
| Other, % | 2 | 2 | 1 | 0 | 1 | 0 | 2 | 3 | 0 | 5 | 4 | 5 | 0 | 0 | 2 |
| IMID, N | 70 | 88 | 53 | 57 | 86 | 39 | 80 | 75 | 88 | 50 | 0 | 50 | 94 | 100 | 67 |
| Treatment failure, % | 21 | 9 | 43 | 24 | 8 | 44 | 19 | 0 | 43 | 67 | 0 | 67 | 13 | 14 | 0 |
| AE/death, % | 5 | 3 | 10 | 10 | 8 | 11 | 0 | 0 | 0 | 33 | 0 | 33 | 0 | 0 | 0 |
| Remission/maximum clinical benefit achieved, % | 46 | 49 | 43 | 33 | 25 | 44 | 63 | 67 | 57 | 0 | 0 | 0 | 56 | 57 | 50 |
| Planned treatment completed, % | 27 | 40 | 5 | 33 | 58 | 0 | 19 | 33 | 0 | 0 | 0 | 0 | 31 | 29 | 50 |
| PI, N | 97 | 100 | 88 | 94 | 100 | 80 | 100 | 100 | 100 | 100 | 100 | 100 | 97 | 100 | 86 |
| Treatment failure, % | 16 | 16 | 14 | 10 | 8 | 17 | 22 | 36 | 0 | 44 | 29 | 100 | 11 | 14 | 0 |
| AE/death, % | 3 | 3 | 3 | 4 | 3 | 8 | 0 | 0 | 0 | 11 | 14 | 0 | 3 | 3 | 0 |
| Remission/maximum clinical benefit achieved, % | 26 | 24 | 31 | 4 | 6 | 0 | 39 | 21 | 67 | 0 | 0 | 0 | 54 | 55 | 50 |
| Planned treatment completed, % | 54 | 55 | 52 | 81 | 83 | 75 | 39 | 43 | 33 | 33 | 43 | 0 | 31 | 28 | 50 |
| Other, % | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11 | 14 | 0 | 0 | 0 | 0 |
| Alkylator, N | 93 | 98 | 33 | 69 | 92 | 0 | 100 | 100 | 0 | 100 | 100 | 100 | 100 | 100 | 0 |
| Treatment failure, % | 24 | 25 | 0 | 73 | 73 | 0 | 13 | 13 | 0 | 25 | 33 | 0 | 9 | 9 | 0 |
| AE/death, % | 5 | 3 | 50 | 9 | 9 | 0 | 0 | 0 | 0 | 25 | 17 | 50 | 0 | 0 | 0 |
| Remission/maximum clinical benefit achieved, % | 11 | 12 | 0 | 9 | 9 | 0 | 13 | 13 | 0 | 0 | 0 | 0 | 18 | 18 | 0 |
| Planned treatment completed, % | 55 | 55 | 50 | 9 | 9 | 0 | 66 | 66 | 0 | 50 | 50 | 50 | 73 | 73 | 0 |
| Other, % | 5 | 5 | 0 | 0 | 0 | 0 | 9 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PI/alkylator, N | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Treatment failure, % | 11 | 12 | 11 | 4 | 3 | 6 | 10 | 12 | 4 | 18 | 17 | 20 | 16 | 20 | 11 |
| AE/death, % | 4 | 4 | 5 | 3 | 3 | 3 | 0 | 0 | 0 | 10 | 9 | 13 | 3 | 5 | 0 |
| Remission/maximum clinical benefit achieved, % | 11 | 11 | 10 | 5 | 4 | 6 | 13 | 17 | 4 | 6 | 7 | 3 | 34 | 35 | 33 |
| Planned treatment completed, % | 71 | 70 | 72 | 88 | 89 | 84 | 74 | 68 | 92 | 61 | 63 | 57 | 45 | 40 | 50 |
| Other, % | 3 | 3 | 3 | 1 | 1 | 0 | 2 | 3 | 0 | 5 | 4 | 7 | 3 | 0 | 6 |
| IMID/alkylator, N | 98 | 99 | 91 | 91 | 97 | 60 | 100 | 100 | 100 | 100 | 100 | 0 | 99 | 100 | 95 |
| Treatment failure, % | 7 | 7 | 10 | 13 | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 7 | 14 |
| AE/death, % | 2 | 2 | 0 | 3 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 |
| Remission/maximum clinical benefit achieved, % | 30 | 31 | 24 | 13 | 14 | 0 | 24 | 28 | 0 | 33 | 33 | 0 | 37 | 38 | 33 |
| Planned treatment completed, % | 61 | 60 | 66 | 71 | 68 | 100 | 76 | 72 | 100 | 67 | 67 | 0 | 53 | 53 | 52 |
PI/IMID‐based treatments (N = 34) and other regimens (N = 17) not listed due to space and low sample size.
Abbreviations: AE, adverse event; IMID, immunomodulatory drug; PI, protease inhibitor; UK, United Kingdom.
Figure 2.
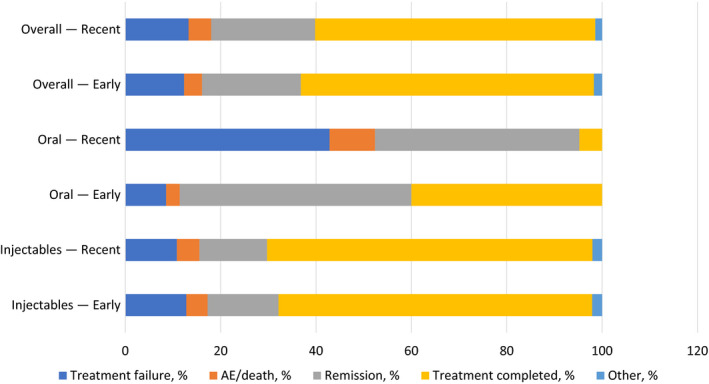
Reason for discontinuation [Colour figure can be viewed at wileyonlinelibrary.com]
3.7. Clinical outcomes (Early Cohort)
Among patients in the early cohort, median 1 L DOT was 7 months (95% CI: 6.5 to 7.3 months; Figure 3). For patients receiving oral treatment, median DOT was significantly longer than for those with injectable treatment (11.7 vs 7.3 months; P < .0001).
Figure 3.
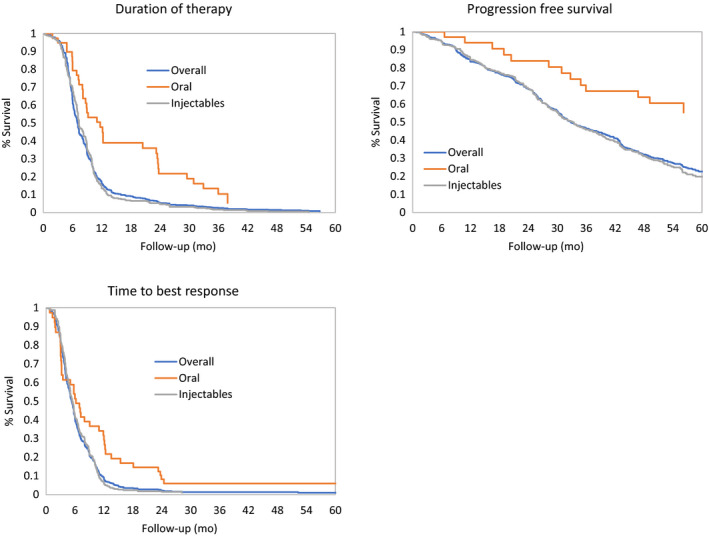
First‐line Duration of Therapy, Progression‐Free Survival, Time to Best Response by Regimen Type in Patients in the Early Cohort. All analyses were adjusted for age, CRAB symptoms, comorbidities, ethnicity, ISS stage, MM type, immunoglobulin class, ECOG PS, frailty status, and cytogenetic risk at diagnosis. Unadjusted patient numbers were as follows: overall (N = 592), oral (N = 40), injectables (N = 336). Abbreviations: CI, confidence interval; NE, not estimated; PFS, progression‐free survival [Colour figure can be viewed at wileyonlinelibrary.com]
Best 1 L response was achieved at a median of 5.2 months (95% CI: 5.0 to 5.6 months; Figure 3). Median time to best response was significantly longer in patients with oral treatment compared to patients with injectable treatment (6.9 months vs 5.5 months; P < .0001).
PFS from start of 1 L therapy was 32.8 months (95% CI: 30.9 to 36.7 months). Patients with oral regimens did not reach the median PFS at the end of the follow‐up. Among patients treated with injectables, the median PFS was 33.4 months. At 36 months, the rate of progression‐free patients was of 67.1% (95% CI: 52.7% to 85.5%) with orals compared to 45.8% (95% CI: 40.9% to 51.3%) with injectables. This comparison should be carefully interpreted according to the nature of treatments.
4. DISCUSSION
In line with other observational studies, we found a large variation of treatment patterns in clinical practice. 22 , 23 Similar to the results of a large registry study across Europe, the Middle East, and Africa where 58% of non‐SCT patients diagnosed between 2010 and 2012 received a bortezomib‐based regimen with or without thalidomide/lenalidomide, 59% of patients in our study received a 1 L regimen containing bortezomib. 23 Nordic registry studies report that clinical outcomes of elderly non‐SCT NDMM patients benefit from the use of novel agents in 1 L therapy, and randomized controlled trial (RCT) results support this practice. The frequency of bortezomib‐based regimens in our study reflects a common treatment selection rationale in clinical trials and real‐world clinical practice alike 22 , 24 , 25 ; The majority of bortezomib use was subcutaneous rather than IV, which increases convenience by allowing for shorter chair time, reducing the frequency of side effects, and increasing physician and patient convenience. 26 Increasing use of lenalidomide and dexamethasone (Rd) likely reflects RCT evidence published between the diagnosis periods for the early and recent cohorts, 9 which drove the addition of Rd as a first‐line option in ESMO guidelines. 27 However, the choice of a suitable treatment in clinical practice depends on patient‐ and disease‐related factors such as comorbidities and individual cytogenetic risk, as well as local reimbursement. 6
As expected, treatment patterns differed across the 4 European countries studied. For example, in the UK uptake of lenalidomide has been limited by reimbursement only in patients who are unable to tolerate or have contraindications to thalidomide. 28 In Germany, national guidelines in place during the recent study period (2013) recommend thalidomide, lenalidomide, and bortezomib combinations, consistent with the observed treatment patterns. 29 In France, the use of IMID/alkylator‐based and PI/alkylator‐based regimens was initially predominant, because the reimbursement of lenalidomide was approved at a later period. Therefore, the use of lenalidomide and steroids increased in the more recent era. Treatment patterns in Italy had the least variability, strongly favoring VMP and reflecting local guidelines recommending melphalan and prednisone in combination with either bortezomib or thalidomide. 30
The frequency of cytogenetic testing increased significantly from 21% to 35% in non‐SCT MM patients diagnosed in 2012‐2013 compared to those diagnosed in 2016‐2017 (P < .001). However, the rate of cytogenetic risk testing remains low, although del(17p), t(4;14), and t(14;16) testing is essential for risk‐directed therapy. 7 , 27 , 31 Median progression‐free survival was 16 months for Rd and 38 months for VRd in high‐risk patients, but due to the small sample size (N = 44), the difference did not reach statistical significance. 32 IMID‐based and PI/IMID regimens have also shown favorable outcomes in high‐risk NDMM patients, although supporting data are limited compared to those in patients with relapsed or refractory disease. 7 , 33 , 34 , 35
The use of maintenance therapy in our study was low, without significant differences between cohorts over time. There are currently no treatments approved for maintenance therapy in non‐SCT NDMM by the European Medicines Agency (EMA). However, the latest evidence supports the use of IMID‐ or PI‐based maintenance therapy to improve PFS and OS in patients who achieve complete response or very good partial response on induction therapy. In an RCT enrolling non‐SCT NDMM patients, bortezomib‐melphalan‐prednisone‐thalidomide (VMPT) induction followed by VT maintenance (VMPT‐VT) was compared with VMP followed by no maintenance. 36 Median PFS was significantly longer with VMPT‐VT (35.3 months) than with VMP (24.8 months, P < .001). 36 The 5‐year OS was also greater with VMPT‐VT (61%) than with VMP (51%, P = .01). The FIRST trial also showed that median progression‐free survival was longer in non‐SCT patients receiving Rd continuously until disease progression (26.0 months) compared to Rd for 18 cycles (Rd18; 21.0 months) and continuous melphalan plus prednisone and thalidomide (MPT; 21.9 months; P < .00001). 37 Non‐SCT NDMM patients 65 years of age or older receiving induction therapy with melphalan‐prednisone‐lenalidomide and lenalidomide maintenance (MPR‐R) also had significantly longer median PFS (31 months) compared to patients receiving only induction with MPR (14 months) or MP (14 months). 38 Orally administered maintenance therapy options are particularly suited for prolonged use due to convenience of administration. 39 Barriers to the implementation of maintenance therapy such as a lack of approval, toxicity, including secondary primary malignancies, cost, and impact on the quality of life need to be further investigated. 40
Most 1 L regimens in our study were discontinued due to achieving their planned fixed duration. This is particularly true for injectable regimens versus oral regimens that were associated with a longer DOT. With the availability of well‐tolerated oral treatments, the majority of non‐SCT NDMM patients may benefit from continuous therapy since continuous therapy results in improved outcomes. 10 , 41 , 42 In a pooled analysis of three phase III trials with 1,218 mostly non‐SCT NDMM patients, 604 patients were randomly assigned to continuous therapy with novel agents (thalidomide, lenalidomide, or bortezomib) defined as an up‐front therapy (induction/consolidation) followed by maintenance therapy, and 614 patients were randomly assigned to treatment (induction/consolidation) with a fixed duration of up to 1 year. Continuous treatment improved median PFS (32 months in patients receiving maintenance vs 16 months not receiving maintenance, P < .001) and 4‐year OS (69% in patients receiving maintenance vs 60% in those not receiving maintenance, P = .003). 10 Others have likewise reported that the majority of non‐SCT NDMM patients benefited from continuous therapy in terms of prolonged PFS, and to this end recommend oral therapy. 41 How far the results from any of these studies can be extrapolated to the non‐SCT population in the real‐world setting needs to be explored, since the interpretation of data across and between clinical and real‐world study populations is impacted by a multitude of confounding factors. 14 For instance, the ranges of median PFS values for treatment of relapsed/refractory MM patients were generally shorter in the real‐world setting compared to clinical studies, but the gap was especially heightened for injectable PI therapies. 14 In the present study, the median DOT of oral regimens and PFS were significantly longer than those of injectable regimens, consistent with other real‐world studies indicating favorable PFS (ranging from 11.1‐27.6 months) for oral treatments. 43 , 44 , 45 Real‐world studies comparing oral versus injectable regimens for NDMM patients are rare, but there is a need for the analysis of these treatments in real‐world settings.
4.1. Strengths and Limitations
The strengths of this study include the depth of real‐world information on treatment characteristics, treatment patterns, and clinical outcomes of non‐SCT patients with NDMM. The validity of our data is supported by the comparison of baseline demographic data and clinical characteristics of our study population with other chart review studies across Europe, as well as by clinician expert opinion. In addition to existing chart review studies, our study provides detailed information about demographic and clinical characteristics, evolving treatment patterns, and clinical outcomes of non‐SCT NDMM population in European countries. The limitations of our study mostly pertain to sample selection criteria, due to the nature of the study. Though efforts were made to ensure random selection, selection bias toward younger, healthier patients with complete data may have impacted clinical outcomes such as time to response and PFS. Treatment patterns and clinical outcomes in this study represent the practices of participating study physicians/sites and may vary from non‐participating physicians and their patients, although efforts were made to approximate a geographically representative sample. Assessments of disease progression and treatment response may be different in the routine clinical setting as compared to the monitoring that would be expected in a controlled trial setting, given stringent per‐protocol frequency of follow‐up, disease management, and clinical outcome assessment criteria. 46 Similarly, comorbidities and toxicities may be underreported in real‐world patient charts.
5. CONCLUSIONS
The adoption of new treatment guidelines for induction therapy in Europe has outpaced the uptake of cytogenetic testing in patients with NDMM. The low rate of cytogenetic testing and short DOT in first‐line therapy observed in non‐SCT NDMM patients in France, Germany, Italy, and the UK highlights a key area of MM care in need of improvement to optimize patient care. Further research on how best to optimize treatment duration and personalize therapy (based on frailty status and genetic features) is necessary to positively impact on quality of life and clinical outcomes.
Appendix 1.
Table A1.
Study Site Quotas by Geographic Region
| France | Soft quota |
|---|---|
| Northwestern [Normandie, Centre‐Val‐de‐Loire, Pays de la Loire, Bretagne] | 14% |
| Northeastern [Champagne‐Ardennes, Nord‐Pas‐de‐Calais, Picardie, Lorraine, Alsace, Franche‐Comte, Bourgogne, Rhone‐Alpes] | 21% |
| Southeastern [Languedoc‐Roussillon, Provence‐Alpes‐Cote‐d'Azur, Auvergne, Rhone‐Alpes] | 24% |
| Paris | 35% |
| Southwestern [Poitou‐Charente, Limousin, Aquitaine, Midi‐Pyenees] | 6% |
| Germany | |
| Schleswig‐Holstein; Hamburg; Niedersachsen; Bremen | 15% |
| Nordrhein‐Westfalen | 20% |
| Hessen; Rheinland‐Pfalz; Baden‐Württemberg; Saarland | 25% |
| Bayern | 20% |
| Berlin; Brandenburg; Mecklenburg‐Vorpommern; Thüringen; Sachsen; Sachsen‐Anhalt | 20% |
| UK | |
| North | 19% |
| Midlands and East | 13% |
| Greater London and South East | 35% |
| South West | 11% |
| South Central | 12% |
| Scotland | 5% |
| Wales and Northern Ireland | 5% |
In Italy, hospitals from the following regions were to be included (without soft quota): Nord Ovest (Milano, Bergamo, Pavia, Parma, Torino, Genova); Nord Est (Venezia, Udine, Trieste, Bologna, Verona, Bolzano, Brescia); Centro (Roma, Perugia, Ancona, Firenze); Sud e isole (Napoli, Avellino, Bari, Reggio Calabria, Palermo, Catania, Cagliari).
Table A2.
Baseline patient characteristics by country and cohort
| All | France | Germany | Italy | UK | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Early (N = 592) | Recent (N = 244) | Early (N = 176) | Recent (N = 93) | Early (N = 156) | Recent (N = 57) | Early (N = 95) | Recent (N = 41) | Early (N = 165) | Recent (N = 53) | |
| Age, n (%) | ||||||||||
| Median | 72.5 | 73.0 | 70.0 | 70.0 | 74.0 | 74.0 | 72.0 | 74.0 | 73.0 | 74.0 |
| <65 y | 60 (10) | 24 (10) | 38 (22) | 18 (19) | 2 (1) | 0 (0) | 2 (2) | 0 (0) | 18 (11) | 6 (11) |
| 65‐74 y | 329 (56) | 124 (51) | 104 (59) | 48 (52) | 90 (58) | 30 (53) | 55 (58) | 25 (61) | 80 (48) | 21 (40) |
| 75+ y | 203 (34) | 96 (39) | 34 (19) | 27 (29) | 64 (41) | 27 (47) | 38 (40) | 16 (39) | 67 (41) | 26 (49) |
| Gender, n (%) | ||||||||||
| Male | 365 (62) | 146 (60) | 114 (65) | 49 (53) | 101 (65) | 41 (72) | 53 (56) | 17 (41) | 97 (59) | 39 (74) |
| Female | 227 (38) | 98 (40) | 62 (35) | 44 (47) | 55 (35) | 16 (28) | 42 (44) | 24 (59) | 68 (41) | 14 (26) |
| ECOG PS, n (%) | ||||||||||
| 0 | 33 (6) | 13 (5) | 7 (4) | 3 (3) | 3 (2) | 1 (2) | 10 (11) | 6 (15) | 13 (8) | 3 (6) |
| 1 | 270 (46) | 113 (46) | 97 (55) | 56 (60) | 79 (51) | 24 (42) | 14 (15) | 5 (12) | 80 (48) | 28 (53) |
| 2 | 182 (31) | 81 (33) | 54 (31) | 24 (26) | 59 (38) | 31 (54) | 13 (14) | 6 (15) | 56 (34) | 20 (38) |
| 3 | 47 (8) | 10 (4) | 15 (9) | 7 (8) | 11 (7) | 1 (2) | 5 (5) | 0 (0) | 16 (10) | 2 (4) |
| 4 | 7 (1) | 2 (1) | 1 (1) | 1 (1) | 4 (3) | 0 (0) | 2 (2) | 1 (2) | 0 (0) | 0 (0) |
| Unknown | 53 (9) | 25 (10) | 2 (1) | 2 (2) | 0 (0) | 0 (0) | 51 (54) | 23 (56) | 0 (0) | 0 (0) |
| Frailty status, n (%) | ||||||||||
| Fit | 90 (15) | 40 (16) | 62 (35) | 31 (33) | 9 (6)† | 1 (2)† | 5 (5) | 2 (5) | 14 (8) | 6 (11) |
| Intermediate fitness | 290 (49) | 137 (56) | 75 (43) | 48 (52) | 114 (73) | 49 (86) | 18 (19) | 7 (17) | 83 (50) | 33 (62) |
| Frail | 168 (28) | 50 (20) | 39 (22) | 14 (15) | 33 (21) | 6 (11) | 34 (36) | 17 (41) | 62 (38) | 13 (25) |
| Unknown | 44 (7) | 17 (7) | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 38 (40) | 15 (37) | 6 (4) | 1 (2) |
| CCI, n (%) | ||||||||||
| 0 | 156 (26) | 75 (31) | 68 (39) | 40 (43) | 22 (14) | 10 (18) | 33 (35) | 17 (41) | 33 (20) | 8 (15) |
| 1 | 138 (23) | 66 (27) | 46 (26) | 22 (24) | 42 (27) | 21 (37) | 14 (15) | 9 (22) | 36 (22) | 14 (26) |
| 2+ | 274 (46) | 94 (39) | 55 (31) | 26 (28) | 88 (56) | 26 (46) | 41 (43) | 12 (29) | 90 (55) | 30 (57) |
| Unknown | 24 (4) | 9 (4) | 7 (4) | 5 (5) | 4 (3) | 0 (0) | 7 (7) | 3 (7) | 6 (4) | 1 (2) |
| ISS Stage, n (%) | ||||||||||
| Stage I | 60 (10) | 25 (10) | 23 (13) | 13 (14) | 12 (8) | 3 (5) | 7 (7) | 6 (15) | 18 (11) | 3 (6) |
| Stage II | 116 (20) | 54 (22) | 50 (28) | 28 (30) | 20 (13) | 4 (7) | 16 (17) | 6 (15) | 30 (18) | 16 (30) |
| Stage III | 337 (57) | 132 (54) | 87 (49) | 41 (44) | 120 (77) | 50 (88) | 34 (36) | 9 (22) | 96 (58) | 32 (60) |
| Unknown | 79 (13) | 33 (14) | 16 (9) | 11 (12) | 4 (3) | 0 (0) | 38 (40) | 20 (49) | 21 (13) | 2 (4) |
| Immunoglobulin class, n (%) | ||||||||||
| IgG | 298 (50) | 134 (55) | 89 (51) | 50 (54) | 101 (65) | 33 (58) | 45 (47) | 23 (56) | 63 (38) | 28 (53) |
| IgA | 101 (17) | 52 (21) | 29 (16) | 13 (14) | 17 (11)† | 19 (33)† | 22 (23) | 9 (22) | 33 (20) | 11 (21) |
| Light chain only | 82 (14) | 24 (10) | 16 (9) | 7 (8) | 23 (15) | 4 (7) | 12 (13) | 7 (17) | 31 (19) | 6 (11) |
| IgM | 14 (2) | 0 (0) | 8 (5) | 0 (0) | 6 (4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| IgE | 8 (1) | 2 (1) | 4 (2) | 1 (1) | 3 (2) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 1 (2) |
| IgD | 6 (1) | 0 (0) | 4 (2) | 0 (0) | 2 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Other | 2 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (2) | 0 (0) | 0 (0) | 0 (0) |
| Biclonal | 1 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 0 (0) |
| Unknown | 80 (14) | 32 (13) | 26 (15) | 22 (24) | 4 (3) | 1 (2) | 14 (15) | 2 (5) | 36 (22) | 7 (13) |
| Extramedullary disease, n (%) | 70 (12) | 20 (8) | 24 (14) | 11 (12) | 18 (12)† | 1 (2)† | 8 (8) | 2 (5) | 20 (12) | 6 (11) |
| CRAB symptoms, n (%) | 527 (89) | 223 (91) | 162 (92) | 87 (94) | 151 (97) | 57 (100) | 81 (85) | 32 (78) | 133 (81) | 47 (89) |
| Renal insufficiency | 105 (18)† | 28 (11)† | 33 (19) | 13 (14) | 34 (22)† | 6 (11)† | 14 (15) | 3 (7) | 24 (15) | 6 (11) |
| Hypercalcemia | 94 (16)† | 25 (10)† | 39 (22) | 16 (17) | 23 (15)† | 1 (2)† | 2 (2) | 2 (5) | 30 (18) | 6 (11) |
| Anemia | 258 (44) | 104 (43) | 78 (44) | 42 (45) | 51 (33) | 20 (35) | 40 (42)† | 10 (24)† | 89 (54) | 32 (60) |
| Bone lesions | 446 (75) | 197 (81) | 143 (81) | 73 (78) | 140 (90)† | 57 (100)† | 63 (66) | 27 (66) | 100 (61) | 40 (75) |
Table A3.
First‐line Regimens (Specific Agents) by Country and Cohort
| Category, N (%) | All | France | Germany | Italy | UK | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All (N = 836) | Early (N = 592) | Recent (N = 244) | All (N = 269) | Early (N = 176) | Recent (N = 93) | All (N = 213) | Early (N = 156) | Recent (N = 57) | All (N = 136) | Early (N = 95) | Recent (N = 41) | All (N = 218) | Early (N = 165) | Recent (N = 53) | |
| Induction by mechanism of action | |||||||||||||||
| PI/alkylator | 336 (40) | 232 (39) | 104 (43) | 104 (39) | 72 (41) | 32 (34) | 89 (42) | 65 (42) | 24 (42) | 105 (77) | 75 (79) | 30 (73) | 38 (17) | 20 (12) | 18 (34) |
| VC ± steroid | 80 (10) | 61 (10) | 19 (8) | 15 (6) | 9 (5) | 6 (6) | 39 (18) | 37 (24) | 2 (4) | 4 (3) | 3 (3) | 1 (2) | 22 (10) | 12 (7) | 10 (19) |
| VM ± steroid | 256 (31) | 171 (29) | 85 (35) | 89 (33) | 63 (36) | 26 (28) | 50 (23) | 28 (18) | 22 (39) | 101 (74) | 72 (76) | 29 (71) | 16 (7) | 8 (5) | 8 (15) |
| IMID/alkylator | 183 (22) | 151 (26) | 32 (13) | 34 (13) | 29 (16) | 5 (5) | 37 (17) | 32 (21) | 5 (9) | 3 (2) | 3 (3) | 0 (0) | 109 (50) | 87 (53) | 22 (42) |
| CR ± steroid | 1 (0) | 1 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0) | 1 (1) | 0 (0) |
| CT ± steroid | 123 (15) | 100 (17) | 23 (9) | 0 (0) | 0 (0) | 0 (0) | 32 (15) | 27 (17) | 5 (9) | 0 (0) | 0 (0) | 0 (0) | 91 (42) | 73 (44) | 18 (34) |
| MR ± steroid | 1 (0) | 0 (0) | 1 (0) | 1 (0) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| MT ± steroid | 58 (7) | 50 (8) | 8 (3) | 33 (12) | 29 (16) | 4 (4) | 5 (2) | 5 (3) | 0 (0) | 3 (2) | 3 (3) | 0 (0) | 17 (8) | 13 (8) | 4 (8) |
| PI | 119 (14) | 86 (15) | 33 (14) | 51 (19) | 36 (20) | 15 (16) | 23 (11) | 14 (9) | 9 (16) | 9 (7) | 7 (7) | 2 (5) | 36 (17) | 29 (18) | 7 (13) |
| V ± steroid | 119 (14) | 86 (15) | 33 (14) | 51 (19) | 36 (20) | 15 (16) | 23 (11) | 14 (9) | 9 (16) | 9 (7) | 7 (7) | 2 (5) | 36 (17) | 29 (18) | 7 (13) |
| IMID | 80 (10) | 40 (7) | 40 (16) | 37 (14) | 14 (8) | 23 (25) | 20 (9) | 12 (8) | 8 (14) | 6 (4) | 0 (0) | 6 (15) | 17 (8) | 14 (8) | 3 (6) |
| R ± steroid | 60 (7) | 24 (4) | 36 (15) | 32 (12) | 11 (6) | 21 (23) | 14 (7) | 7 (4) | 7 (12) | 6 (4) | 0 (0) | 6 (15) | 8 (4) | 6 (4) | 2 (4) |
| T ± steroid | 20 (2) | 16 (3) | 4 (2) | 5 (2) | 3 (2) | 2 (2) | 6 (3) | 5 (3) | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 9 (4) | 8 (5) | 1 (2) |
| Alkylator | 67 (8) | 61 (10) | 6 (2) | 16 (6) | 12 (7) | 4 (4) | 32 (15) | 32 (21) | 0 (0) | 8 (6) | 6 (6) | 2 (5) | 11 (5) | 11 (7) | 0 (0) |
| C ± steroid | 6 (1) | 6 (1) | 0 (0) | 3 (1) | 3 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (1) | 3 (2) | 0 (0) |
| M + Other ±steroid | 1 (0) | 1 (0) | 0 (0) | 1 (0) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| M ± steroid | 43 (5) | 37 (6) | 6 (2) | 12 (4) | 8 (5) | 4 (4) | 15 (7) | 15 (10) | 0 (0) | 8 (6) | 6 (6) | 2 (5) | 8 (4) | 8 (5) | 0 (0) |
| Other | 17 (2) | 17 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 17 (8) | 17 (11) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| PI/IMID | 34 (4) | 18 (3) | 16 (7) | 24 (9) | 12 (7) | 12 (13) | 1 (0) | 1 (1) | 0 (0) | 4 (3) | 3 (3) | 1 (2) | 5 (2) | 2 (1) | 3 (6) |
| VR ± steroid | 10 (1) | 4 (1) | 6 (2) | 9 (3) | 4 (2) | 5 (5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0) | 0 (0) | 1 (2) |
| VT ± steroid | 24 (3) | 14 (2) | 10 (4) | 15 (6) | 8 (5) | 7 (8) | 1 (0) | 1 (1) | 0 (0) | 4 (3) | 3 (3) | 1 (2) | 4 (2) | 2 (1) | 2 (4) |
| Other | 17 (2) | 4 (1) | 13 (5) | 3 (1) | 1 (1) | 2 (2) | 11 (5) | 0 (0) | 11 (19) | 1 (1) | 1 (1) | 0 (0) | 2 (1) | 2 (1) | 0 (0) |
| VMT ± steroid | 1 (0) | 1 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Other | 16 (2) | 3 (1) | 13 (5) | 3 (1) | 1 (1) | 2 (2) | 11 (5) | 0 (0) | 11 (19) | 0 (0) | 0 (0) | 0 (0) | 2 (1) | 2 (1) | 0 (0) |
| Maintenance | |||||||||||||||
| Maintenance n (%) | 43 (5) | 32 (5) | 11 (5) | 13 (5) | 9 (5) | 4 (4) | 1 (0) | 1 (1) | 0 (0) | 26 (19) | 19 (20) | 7 (17) | 3 (1) | 3 (2) | 0 (0) |
| V ± steroid | 25 (58) | 17 (53) | 8 (73) | 5 (38) | 4 (44) | 1 (25) | 0 (0) | 0 (0) | 0 (0) | 19 (73) | 12 (63) | 7 (100) | 1 (33) | 1 (33) | 0 (0) |
| VT ± steroid | 1 (2) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (4) | 1 (5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| R ± steroid | 10 (23) | 8 (25) | 2 (18) | 4 (31) | 2 (22) | 2 (50) | 1 (100) | 1 (100) | 0 (0) | 5 (19) | 5 (26) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| T ± steroid | 4 (9) | 4 (13) | 0 (0) | 2 (15) | 2 (22) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (67) | 2 (67) | 0 (0) |
| Other | 3 (7) | 2 (6) | 1 (9) | 2 (15) | 1 (11) | 1 (25) | 0 (0) | 0 (0) | 0 (0) | 1 (4) | 1 (5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Abbreviations: C, cyclophosphamide; M, melphalan; R, lenalidomide; T, thalidomide; UK, United Kingdom; V, bortezomib.
Mohty M, Knauf W, Romanus D, et al. Real‐world treatment patterns and outcomes in non‐transplant newly diagnosed multiple Myeloma in France, Germany, Italy, and the United Kingdom. Eur J Haematol. 2020;105:308–325. 10.1111/ejh.13439
REFERENCES
- 1. Engelhardt M, Terpos E, Kleber M, et al. European Myeloma Network recommendations on the evaluation and treatment of newly diagnosed patients with multiple myeloma. Haematologica. 2014;99:232‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Airtum Working Group , Busco S, Buzzoni C, et al. Italian cancer figures–Report 2015: The burden of rare cancers in Italy. Epidemiol Prev. 2016;40:1‐120. [DOI] [PubMed] [Google Scholar]
- 3. Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356‐387. [DOI] [PubMed] [Google Scholar]
- 4. Larocca A, Mina R, Gay F, et al. Emerging drugs and combinations to treat multiple myeloma. Oncotarget. 2017;8:60656‐60672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516‐2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cook G, Zweegman S, Mateos MV, et al. A question of class: treatment options for patients with relapsed and/or refractory multiple myeloma. Crit Rev Oncol Hematol. 2018;121:74‐89. [DOI] [PubMed] [Google Scholar]
- 7. Sonneveld P, Avet‐Loiseau H, Lonial S, et al. Treatment of multiple myeloma with high‐risk cytogenetics: a consensus of the International Myeloma Working Group. Blood. 2016;127:2955‐2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Palumbo A, Bringhen S, Mateos M‐V, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood. 2015;125:2068‐2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant‐ineligible patients with myeloma. N Engl J Med. 2014;371:906‐917. [DOI] [PubMed] [Google Scholar]
- 10. Palumbo A, Gay F, Cavallo F, et al. Continuous therapy versus fixed duration of therapy in patients with newly diagnosed multiple myeloma. J Clin Oncol. 2015;33:3459‐3466. [DOI] [PubMed] [Google Scholar]
- 11. Shah JJ, Abonour R, Gasparetto C, et al. Analysis of common eligibility criteria of randomized controlled trials in newly diagnosed multiple myeloma patients and extrapolating outcomes. Clin Lymphoma Myeloma Leuk. 2017;17):575‐583.e2. [DOI] [PubMed] [Google Scholar]
- 12. Klausen TW, Gregersen H, Abildgaard N, et al. The majority of newly diagnosed myeloma patients do not fulfill the inclusion criteria in clinical phase III trials. Leukemia. 2019;33:546‐549. [DOI] [PubMed] [Google Scholar]
- 13. Chari A, Romanus D, Palumbo A, et al. Randomized clinical trial representativeness and outcomes in real‐world patients: comparison of 6 hallmark randomized clinical trials of relapsed/refractory multiple myeloma. Clin Lymphoma Myeloma Leuk. 2020;20):8‐17.e16. [DOI] [PubMed] [Google Scholar]
- 14. Richardson PG, San Miguel JF, Moreau P, et al. Interpreting clinical trial data in multiple myeloma: translating findings to the real‐world setting. Blood Cancer J. 2018;8:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cancer Research UK . Myeloma incidence statistics. https://www.cancerresearchuk.org/health‐professional/cancer‐statistics/statistics‐by‐cancer‐type/myeloma/incidence#ref‐. Accessed 28‐06‐2019
- 16. Robert Koch Institut . Inzidenz Plasmozytom (C90). https://www.krebsdaten.de/Krebs. Accessed 28‐06‐2019
- 17. Dumontet C, Couray‐Targe S, Teisseire M, et al. Real life management of patients hospitalized with multiple myeloma in France. PLoS One. 2018;13:e0196596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Turesson I, Velez R, Kristinsson SY, et al. Patterns of multiple myeloma during the past 5 decades: stable incidence rates for all age groups in the population but rapidly changing age distribution in the clinic. Mayo Clin Proc. 2010;85:225‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. National Comprehensive Cancer Network (NCCN) . NCCN Guidelines Version 1.2019: Multiple Myeloma. https://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf. Accessed September 20 2018
- 20. Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328‐e346. [DOI] [PubMed] [Google Scholar]
- 21. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373‐383. [DOI] [PubMed] [Google Scholar]
- 22. Remes K, Anttila P, Silvennoinen R, et al. Real‐world treatment outcomes in multiple myeloma: Multicenter registry results from Finland 2009–2013. PLoS One. 2018;13:e0208507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mohty M, Terpos E, Mateos MV, et al. Multiple myeloma treatment in real‐world clinical practice: results of a prospective, multinational. Noninterventional Study. Clin Lymphoma Myeloma Leuk. 2018;18:e401‐e419. [DOI] [PubMed] [Google Scholar]
- 24. Liwing J, Uttervall K, Lund J, et al. Improved survival in myeloma patients: starting to close in on the gap between elderly patients and a matched normal population. Br J Haematol. 2014;164:684‐693. [DOI] [PubMed] [Google Scholar]
- 25. Kouroukis TC, Baldassarre FG, Haynes AE, et al. Bortezomib in multiple myeloma: systematic review and clinical considerations. Curr Oncol. 2014;21:e573‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous bortezomib in patients with relapsed multiple myeloma: subanalysis of patients with renal impairment in the phase III MMY‐3021 study. Haematologica. 2015;100:e207‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moreau P, San Miguel J, Sonneveld P, et al. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2017;28:iv52‐iv61. [DOI] [PubMed] [Google Scholar]
- 28. National Institute for Health and Clinical Excellence (NICE) . Final appraisal determination: Bortezomib and thalidomide for the first‐line treatment of multiple myeloma. https://www.nice.org.uk/guidance/ta228/documents/multiple‐myeloma‐first‐line‐bortezomib‐and‐thalidomide‐final‐appraisal‐determination3. Accessed January 21 2020
- 29. Kortum M, Driessen C, Einsele H, et al.Multiple myeloma: 2013 German guidelines. https://www.onkopedia.com/de/onkopedia/archive/guidelines/multiples‐myelom/version‐10042018T124640/@@guideline/html/index.html. Accessed March 12 2020
- 30. Barosi G, Merlini G, Billio A, et al. SIE, SIES, GITMO evidence‐based guidelines on novel agents (thalidomide, bortezomib, and lenalidomide) in the treatment of multiple myeloma. Ann Hematol. 2012;91:875‐888. [DOI] [PubMed] [Google Scholar]
- 31. Stella F, Pedrazzini E, Agazzoni M, et al. Cytogenetic alterations in multiple myeloma: prognostic significance and the choice of frontline therapy. Cancer Invest. 2015;33:496‐504. [DOI] [PubMed] [Google Scholar]
- 32. Durie BGM, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem‐cell transplant (SWOG S0777): a randomised, open‐label, phase 3 trial. Lancet. 2017;389:519‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kumar S, Flinn I, Richardson PG, et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood. 2012;119:4375‐4382. [DOI] [PubMed] [Google Scholar]
- 34. Mateos MV, Dimopoulos MA, Cavo M, et al. Daratumumab plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma. N Engl J Med. 2018;378:518‐528. [DOI] [PubMed] [Google Scholar]
- 35. Facon T, Kumar S, Plesner T, et al. Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. N Engl J Med. 2019;380:2104‐2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Palumbo A, Rajkumar SV, San Miguel JF, et al. International Myeloma Working Group consensus statement for the management, treatment, and supportive care of patients with myeloma not eligible for standard autologous stem‐cell transplantation. J Clin Oncol. 2014;32:587‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Facon T, Dimopoulos MA, Dispenzieri A, et al. Final analysis of survival outcomes in the phase 3 FIRST trial of up‐front treatment for multiple myeloma. Blood. 2018;131:301‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Palumbo A, Hajek R, Delforge M, et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med. 2012;366:1759‐1769. [DOI] [PubMed] [Google Scholar]
- 39. Rifkin RM, Bell JA, DasMahapatra P, et al. Treatment satisfaction and burden of illness in patients with newly diagnosed multiple myeloma. Pharmacoecon Open. 2019. 10.1007/s41669-019-00184-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee HS, Min CK. Optimal maintenance and consolidation therapy for multiple myeloma in actual clinical practice. Korean J Intern Med. 2016;31:809‐819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ludwig H, Zojer N. Fixed duration vs continuous therapy in multiple myeloma. Hematology Am Soc Hematol Educ Program. 2017;2017:212‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Merola D, Yong C, Noga SJ, et al. Costs associated with productivity loss among U.S. patients newly diagnosed with multiple myeloma receiving oral versus injectable. Chemotherapy. J Manag Care Spec Pharm. 2018;24:1019‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Terpos E, Maouche N, Minarik J, et al. "Real World" Data on the Efficacy and Safety of Ixazomib in Combination with Lenalidomide and Dexamethasone in Relapsed/Refractory Multiple Myeloma: A Combined Study from the Greek. Czech and UK Databases. Blood. 2017;130:3087. [Google Scholar]
- 44. American Thrombosis & Hemostasis Network . ATHN Research Report Brief ‐ September 30, 2017. https://athn.org/documents/document_file/302. Accessed August 28 2018
- 45. Davies F, Rifkin R, Costello C, et al.Comparative effectiveness of triplets containing bortezomib (B), Carfilzomib (C), Daratumumab (D), or Ixazomib (I) in relapsed/refractory multiple myeloma (RRMM) in routine care in the US. Poster presented at: European Hematology Association (EHA) 24th Congress 2019; Amsterdam, The Netheralands.
- 46. Gaultney JG, Franken MG, Uyl‐de Groot CA, et al. Experience with outcomes research into the real‐world effectiveness of novel therapies in Dutch daily practice from the context of conditional reimbursement. Health Policy. 2015;119:186‐194. [DOI] [PubMed] [Google Scholar]


