Abstract
Background
Aging signs can be corrected through volume restoration in multiple soft tissue layers and in the supraperiosteal plane using hyaluronic acid (HA) or nonhyaluronic acid (non‐HA) fillers. The non‐HA bioresorbable polycaprolactone (PCL)‐based filler with collagen‐stimulating properties has a proven safety profile, but rare potential complications such as nodules and granuloma can occur. Furthermore, PCL‐based fillers cannot be immediately removed by injection of an enzyme. These potential drawbacks have yet to be described in the literature.
Aims
The author performed 1111 treatments between 2015 and 2018. This study aims to review and analyze these treatments to ascertain the complication rates of the PCL‐based filler. Suggestions for complication prevention and management are also discussed.
Methods
780 patients treated with the PCL‐based filler were reviewed by the physician between April 2015 and May 2018. During this period, 5595 syringes were used in 1111 treatments. All complication data were acquired by phone interviews, reports by patients, or observation at follow‐up visits. Complications were subdivided into early‐onset (occurring up to 2 weeks after treatment) and late‐onset events (occurring more than 2 weeks to years after treatment).
Results
Among the 1111 treatments, there were 50 cases (4.5%) of edema that lasted longer than 2 weeks, 30 cases (2.7%) of bruising, 8 cases (0.72%) of malar edema, 5 cases (0.45%) of temporarily palpable lumps and 2 cases (0.18%) of discoloration. There were no cases of intravascular injection, nodules/granulomas, or infection.
Conclusion
The complication rate of the PCL‐based filler was found to be low, and there were no cases of intravascular injection, nodules, and/or granulomas during the 3‐year observation. Longer‐lasting edema was associated with a higher injection volume and malar edema was related to lymphatic compression.
Keywords: collagen stimulator, complications, dermal filler, neocollagenesis, PCL, polycaprolactone, safety, volume restoration
1. INTRODUCTION
Aging leads to volume loss occurring in all layers of the facial anatomical structures due to bone remodeling and deflation of fat pads. These processes begin in the late second or the third decade of life 1 , 2 and are associated with the appearance of various aging signs. Using hyaluronic acid (HA) or non‐HA fillers, aging signs can be corrected through volume restoration in multiple soft tissue layers and at the supraperiosteal level. The non‐HA bioresorbable polycaprolactone (PCL)‐based filler (Ellansé®; Sinclair Pharmaceuticals) has a proven safety profile, but rare potential complications such as nodules (in cases of technical errors) or granuloma (in case of a change in the host's immune status following a triggering factor) can occur. Furthermore, PCL‐based fillers cannot be immediately removed by injection of an enzyme. These potential drawbacks have not been described in the literature. One article reported a foreign body reaction 3 years after injection of the PCL‐based filler that regressed 1 month after treatment using oral doxycycline, 3 indicating an inflammatory/infectious cause. Nodule formation is a minor complication frequently associated with fillers, including HAs.
This retrospective study reviewed complications from the treatments performed using Ellansé by a single clinician (the author) since the product's market approval in Taiwan in 2015. The complication rates of the injection techniques employed were then analyzed. Possible causes, prevention, and management of these complications are then discussed.
The PCL‐based filler is a novel collagen stimulator composed of PCL microspheres (30%) suspended in an aqueous carboxymethyl cellulose (CMC) gel carrier (70%), 4 which provides an immediate but temporary filling effect. The PCL microspheres contribute to long‐term volume by stimulating new collagen production. 5 As the CMC gel is absorbed in the first 6‐8 weeks, the loss in volume from the carrier gel is gradually replaced by the newly formed collagen because of the PCL‐induced neocollagenesis. 5
The PCL microspheres are 25‐50 µm in size and are therefore protected from phagocytosis. 6 They degrade into nontoxic, bioresorbable products that are metabolized into CO2 and H2O and excreted through normal pathways. The total bioresorption time of the product depends on the length of the PCL‐polymer chain; in this regard, four product versions are available: S, M, L and E, which have bioresorption times of at least 1, 2, 3 and 4 years, respectively. In the present study, the S version was used, 7 as it was the only product approved in Taiwan at the time of the treatment. Among the 780 patients, only 127 received more than one treatment in the 3‐year study period.
2. MATERIALS AND METHODS
The research followed the tenets of the Declaration of Helsinki. All the treatments reviewed in the present article were carried out by a single board‐certified dermatologist in his private practice. Each syringe (1 mL) of the material was premixed with 0.2 mL of 2% lidocaine prior to use. 8 The product was then placed into the supraperiosteal plane, the superficial fat plane, and the deep fat plane, depending on the depth level at which the physician intended to restore volume. The supraperiosteal placement was carried out by vertical puncture using 27‐G, 1‐in needles. The amount injected was determined by the surface area to be treated (0.2 mL/cm2). For the superficial and deep fat placements, a retrograde fanning technique was performed using 25‐G, 40‐mm cannulas. The amount injected also depended on the surface area to treat, with each linear thread of 0.1 mL/cm2. 9 The injection locations covered all the facial areas detailed in the publication of recommendations, 7 without placement of the product in any contraindicated areas, such as the periorbital area, lips, and labella. The endpoint was defined as full correction of the treated defect, without any need for overcorrection. A post‐treatment telephone interview was routinely carried out 1 day after the procedure, and the complete downtime information of each patient was successfully acquired. A post‐treatment appointment was routinely scheduled 2‐3 weeks after the procedure to allow follow‐up and documentation. If any patient was unable to return, phone calls were made to confirm information regarding adverse events. Therefore, of all the 1111 treatments performed in 780 patients, all post‐treatment events that occurred from day 1 after treatment to the time‐point of the routine follow‐up were recorded. If any doubt and/or unusual findings occurred at any time point after treatment, the patients were free to report to the staff by phone, email, or communication app; if necessary, a further follow‐up visit or additional management would be scheduled.
The present study reviewed all 780 patients treated between April 2015 and May 2018. A total of 5595 syringes were used in the 1111 treatments. Up to May 2019, the patients in this group had been followed for at least 1 year. All complication data were acquired through phone interviews, reporting by patients, or observation at follow‐up visits. Complications were subdivided into early‐onset (occurring up to 2 weeks after treatment) and late‐onset events (occurring >2 weeks to years after treatment). Complication rates were calculated and discussed.
3. RESULTS
Between April 1, 2015 and May 31, 2018, a total of 1111 PCL‐based filler (Ellansé‐S) treatments were performed using a total of 5595 syringes (1 mL/syringe). The average syringe number per patient was 5.04. There were 50 cases (4.50%) of swelling/edema lasting longer than 2 weeks (prolonged swelling), 30 (2.70%) of bruising, 8 (0.72%) of malar edema, 5 (0.45%) of temporary palpable but nonvisible lumps, and 2 cases (0.18%) of discoloration. No cases of intravascular injection, nodules/granuloma, or infection were reported. The complication rate of each type of event is summarized in Table 1.
TABLE 1.
Complication rate per type of event
| Complication type | Bruising/hematoma | Swelling/edema (duration >2 wk) | Temporary palpable but nonvisible lumps | Malar edema | Nodules | Discoloration |
|---|---|---|---|---|---|---|
| Number of events | 30 | 50 | 5 | 8 | 0 | 2 |
| Complication rate (%) | 2.70 | 4.50 | 0.45 | 0.72 | 0.00 | 0.18 |
The injection amount in the nonprolonged swelling and prolonged swelling groups were compared with the absolute average amount (Table 2). In the prolonged swelling group, the average amount was 8.36 mL per patient per treatment: 66% more than the overall average injection amount; in the nonprolonged swelling group, the average injection amount was 4.88 mL per patient per treatment: slightly lower than the overall injection amount.
TABLE 2.
Comparison of amounts used in the nonprolonged swelling group and the prolonged swelling group
| Number of patients | Average amount (mL) per patient per treatment | |
|---|---|---|
| All patients | 1111 | 5.04 |
| Nonprolonged swelling group (duration <2 wk) | 1061 | 4.88 |
| Prolonged swelling group (duration >2 wk) | 50 | 8.36 |
The complications of injectable fillers are frequently classified by time of onset to assist in management and have most commonly been classified as early and late, as these time frames correlate well with the potential underlying etiology. Rohrich et al proposed that complications should be classified as early, late, and delayed, and roughly defined as less than 14 days, 14 days to 1 year, and more than 1 year, respectively. 10 Early complications generally consisted of acute inflammation, infection, or ischemia‐related problems. Late and delayed complications may be secondary to granuloma and biofilm formation, respectively. 11 In the present article, the complications were classified as early (occurring up to 14 days after treatment) and late (occurring weeks to years after treatment) (Table 3).
TABLE 3.
Classification of the complications by the author
| Early events (occurring up to 14 d after treatment) | Late events (occurring from weeks to years after treatment) |
|---|---|
| Bruising/hematoma | Malar edema |
| Swelling/edema (duration >2 wk) | Nodules |
| Temporary palpable but nonvisible lumps | Discoloration |
According to the postmarket survey (PMS) 7 conducted by the manufacturer, more than 490 000 syringes were used from the launch of the product in 2009 to December 2016, with a low complication rate of 0.049% (one event per 2055 syringes). Most of these complications were swelling (0.0195%), lumps/nodules (0.0177%), inflammation/infection (0.0031%), bruising/hematoma (0.0006%), and induration (0.0004%). The complication rates from the PMS were calculated per number of syringes, while those described in the present article were calculated per number of treatments. In the current retrospective analysis, no cases of infection or nodules were reported, but a few cases of delayed‐onset edema of the midface and discoloration at the injection site were reported. Because nodules have particular clinical significance, and because their incidence differed between the PMS and the present study, their cause and prevention are discussed below, along with other complications.
4. DISCUSSION
In the decade since the PCL‐based dermal filler was launched, it has shown advantages over the poly‐l‐lactic acid (PLLA)‐based injectable because its results are immediately visible, as well as over HA‐ and CaHA‐based fillers because it confers stable and durable results. 12 , 13 Use of the PCL‐based dermal filler has not been limited to the subcutaneous and supraperiosteal levels of the face; increase in skin thickness was shown in a study using a particular not recommended dermal injection technique. 14 Subcutaneous placement in the hand dorsa to restore volume has also been documented in the literature. 15 , 16 In the present retrospective study, no hand treatments were performed, so the complications discussed below are associated with placement in the subcutaneous or supraperiosteal level of the facial areas.
4.1. Bruising/hematoma
All injections can potentially cause bruising, but the complication is observed more frequently after injection into the subdermal planes using fanning and threading techniques. Less bruising is seen when products are placed in a bolus at the supraperiosteal level. Although common, bruising of large areas can be disturbing to patients because it entails longer perceived downtimes. This can make patients hesitate before undergoing future treatments (Figure 1). Therefore, prevention of bruising is far to be preferable to management of the complication.
FIGURE 1.
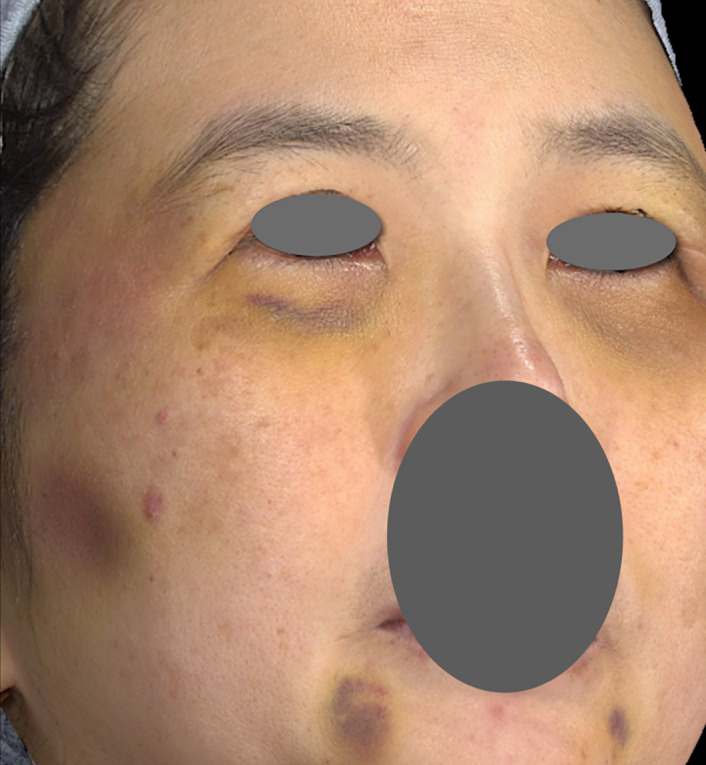
Bruising of large areas after treatment
In this regard, it is important to review the patient's medication history, particularly their use of blood‐thinning medications (aspirin, warfarin, dipyridamole, clopidogrel, nonsteroidal anti‐inflammatory drugs, fish oil, vitamin E supplements, St John's Wort, garlic tablets, Gingko biloba, and ginseng). A slow injection technique prevents vascular injury, especially when the needle passes through layers of soft tissue to reach the supra‐periosteal plane and thereby reduce the bruising rate; whereas in soft tissue, the use of blunt cannula is helpful for avoiding bruising. Blunt cannulas, which are commonly used in the treatment of the forehead, 17 dorsal hands, 15 , 16 and subcutaneous level of other facial areas, also prevent bruising.
In my own experience, bruising can be prevented or reduced by oral Arnica montana tablets 20‐30 minutes prior to injection. Furthermore, immediate compression applied by an assistant at the injection site while the other parts are still being treated greatly helps to prevent and reduce bruising.
4.2. Prolonged swelling/edema (duration >2 weeks)
Soon after treatment using the PCL‐based filler, swelling and edema are the most common reactions. Based on the author's experience, swelling and erythema coupled with induration limited at the injection site are usually observed within the first few minutes to approximately 24‐48 hours after the procedure (Figure 2). From 48 hours to a few days after injection, diffuse facial edema of varying severity is the most common persistent reaction (Figure 3), with reduced induration and usually without erythema. Full resolution can be expected within several days to 1 week, without any intervention required. However, the current retrospective analysis suggested that edema lasting longer than 2 weeks was associated with higher‐volume injection (8.36 mL per treatment). Such edema, even resolving by itself over time, is likely related to the initiation of the host's immune reaction to the product and may cause inconvenience and insecurity to the patients if it lasts longer than 2 weeks. In the 50 patients who showed prolonged edema in the present study, the complication resolved spontaneously without any intervention. Nonetheless, to prevent or shorten the duration of edema, the author now routinely prescribes oral prednisolone (10 mg/d for 3 days) to patients requiring higher injection volumes (more than 5 mL per treatment).
FIGURE 2.
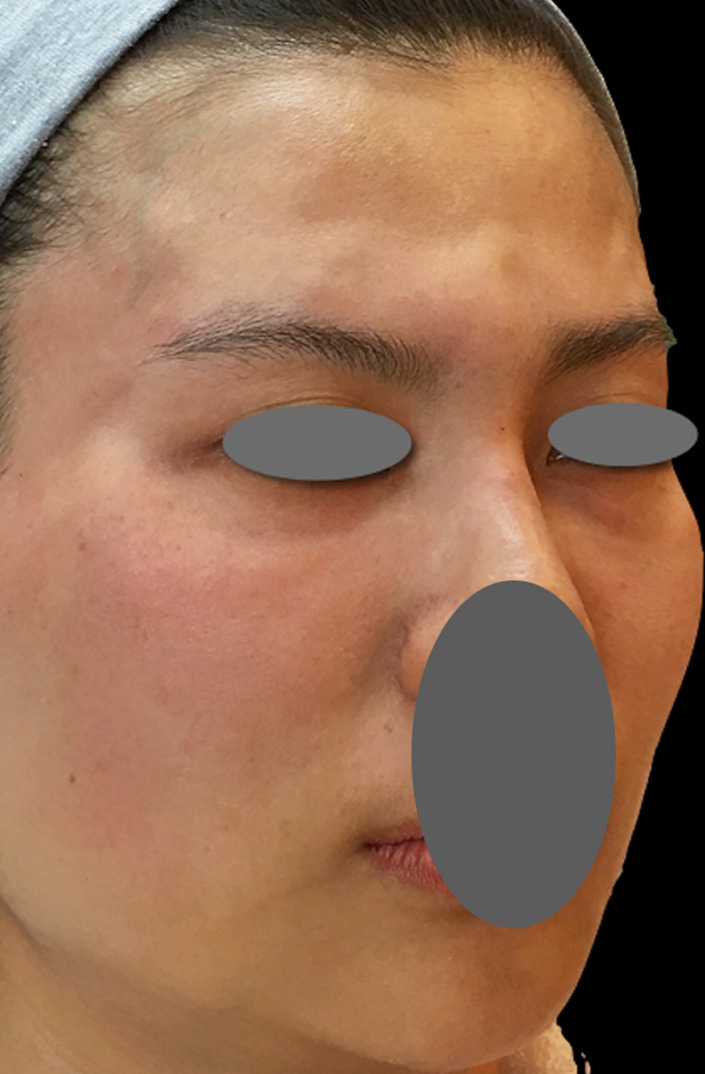
Swelling, erythema, and induration limited to the injection site immediately after injection
FIGURE 3.
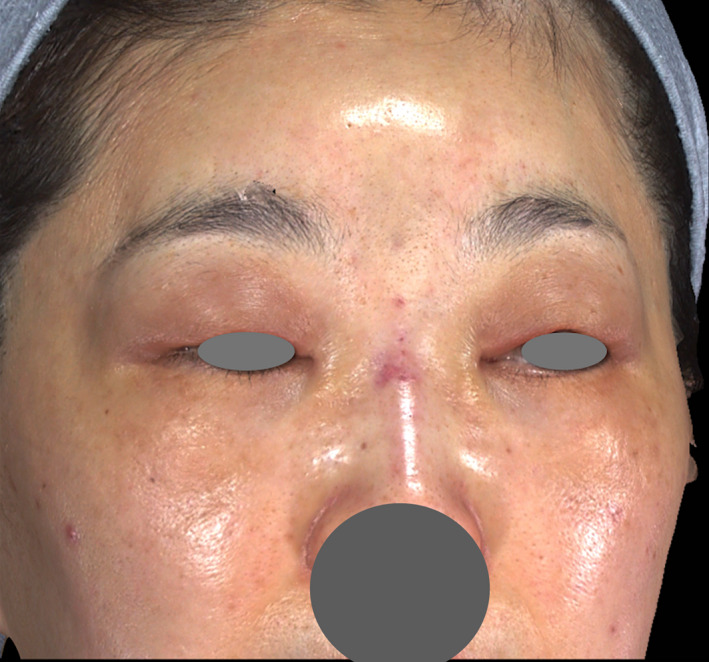
72 h after brow lift and nasal bridge, mild swelling, and edema were seen at the injection site. More evident edema was seen in the regions neighboring the injection site
4.3. Temporary palpable but nonvisible lumps
The injection technique used in the present study involved volume placement in multiple anatomical layers. The deposits placed on the supraperiosteal plane did not exceed 0.2 mL/injection point; in the subcutaneous plane, the amount for each linear thread placed by cannula did not exceed 0.1 mL/cm. In the current analysis, five cases of temporary palpable but nonvisible lumps were observed in regions where the product was placed in multiple anatomical layers and were all found to last less than 4 weeks after injection. The mode of action of all currently commercially available collagen‐stimulating products in the field of tissue augmentation begins with a subclinical inflammatory tissue response after implantation, followed by encapsulation and fibroplasia. 18 These lumps may be associated with the tissue reaction to product implantation and the stacking effect of volumes in multiple tissue layers. All five patients were instructed to wait another 2 weeks before seeking any further intervention, and all of the palpable but nonvisible lumps had resolved without any intervention 2 weeks after the time of the report.
4.4. Late‐onset edema of the mid‐face
Late‐onset edema of the mid‐face (malar edema), which differs from the acute edematous reaction after filler injection, is a late reaction that takes place weeks to months after injection. Malar edema has been reported with all fillers when injected into the infraorbital hollow and tear troughs; it is defined as a clinically significant degree of swelling that lasts >1 month after injection. Malar edema can result from lymphatic compression 19 caused by the filler and can occur both superficially and deeply in the malar septum: a band of connective tissue that divides the superficial suborbicularis oculi fat into superficial and deep compartments. Due to its relative impermeability, the septum allows the occurrence of tissue edema and hemoglobin accumulation superior to its cutaneous insertion. If the compression occurs superficial to the malar septum, malar edema occurs at the lid‐cheek junction, whereas if the compression occurs deep to the malar septum, cheek edema predominates.
With high‐viscosity products like the PCL‐based filler, the incidence of malar edema can be reduced by exercising proper patient selection, limiting injection volume, avoiding placing the product at or beyond the lids‐cheek junction, and injecting the filler deep to the malar septum at the immediate preperiosteal level.
In the present review, eight patients had cheek edema, indicating compression of a deeper plane. The mean total facial injection volume in these eight patients was 22 mL, with a mean of 11.7 mL in the medial part of the mid‐face. Of all 1111 patients, 25 were injected using more than 20 syringes per subject. Of these, 17 did not develop malar edema and had a mean injection amount in the medial mid‐face of 9.3 mL. Furthermore, the mean amount delivered by needle in this nonmalar edema group was 3.2 mL, while a mean of 6.1 mL was delivered by cannula. On the other hand, the mean amount delivered by needle in the malar edema group (eight patients) was 3.3 mL, with a mean of 8.4 mL delivered by cannula. This indicates that an increased filler amount delivered in the deep fat plane by cannula could confer a higher risk of compromised lymphatic drainage (Figure 4). Malar edema was managed in two to four sessions of intralesional injection, with triamcinolone 1 mg/cm2 covering the whole involved area. Specifically, 1 mL of 10 mg/mL triamcinolone was prepared by mixing 0.5 mL of 40 mg/mL triamcinolone with 0.5 mL of 2% lidocaine and 1 mL of normal saline solution. At each point, 0.1 mL was injected, associated with mechanical disruption using a 22‐G cannula. Injections were carried out 1 month apart, until the complete resolution was reached.
FIGURE 4.
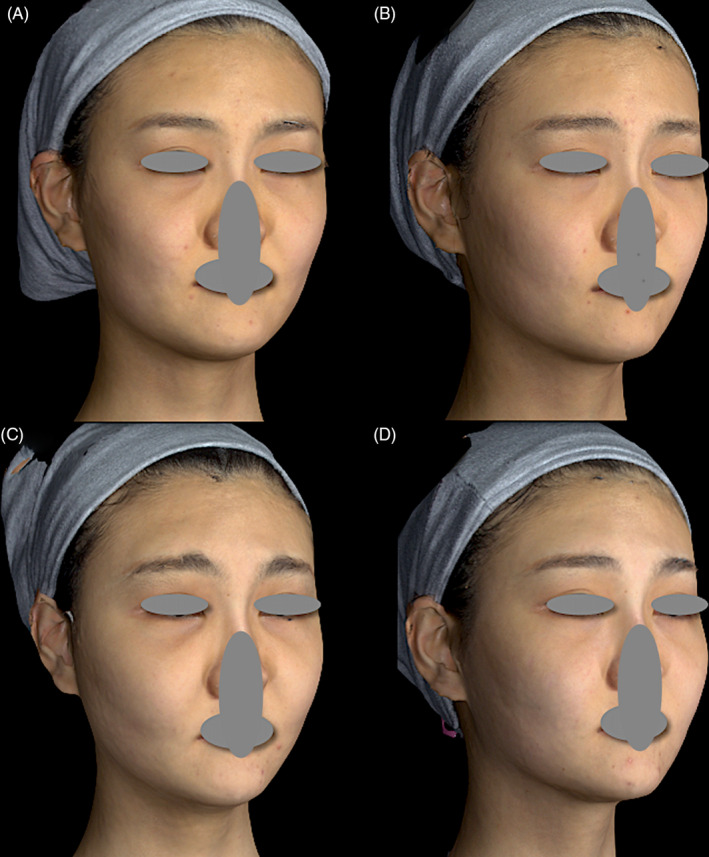
A, A 23‐year‐old female with a history of allergic rhinitis asked to beautify her profile. B, Her forehead, nose, medial maxilla, and medial cheek fat pads were treated using 5 mL Ellansé‐S uneventfully. Favorable improvement was observed 2 mo after treatment. C, Gradual‐onset and lasting edema mainly involving her cheeks and right forehead began 10 mo after injection. D, Malar edema resolved after two sessions of intralesional injection, with 1 mg/cm2 triamcinolone covering the whole involved area—0.1 mL at each injection point (1 mL of 10 mg/mL triamcinolone was prepared by mixing 0.5 mL of 40 mg/mL triamcinolone with 0.5 mL of 2% lidocaine and 1 mL of normal saline). This was combined with mechanical disruption using a 22‐G cannula. Injections were carried out 1 mo apart
4.5. Nodules
Nodules caused by collagen‐stimulating products have long been a concern for patients and doctors. Nodule formation is always associated with the injection technique of the practitioner; in most cases too much material has been injected at a single point, or product has been placed in a hypermobile depth level. Histologically, optimal filler implantation reveals collagen deposition around the PCL spheres, with the presence of some histiocytes over time 5 (Figure 5). In contrast, histopathology of a nodule reveals an overabundance of product; this is distinct from a granuloma, which shows an overabundance of host tissue reaction to a small amount of product, indicating an immune origin that depends on the host's immune status or incidents that cause changes in immune status. Granuloma has been reported with all currently available commercial products, including collagen, HA, PLLA, silicone, calcium hydroxyapatite (CaHA), polymethylmethacrylate, polyacrylamide gel 20 and PCL‐based filler. 21 Most nodules disappear spontaneously within a year.
FIGURE 5.
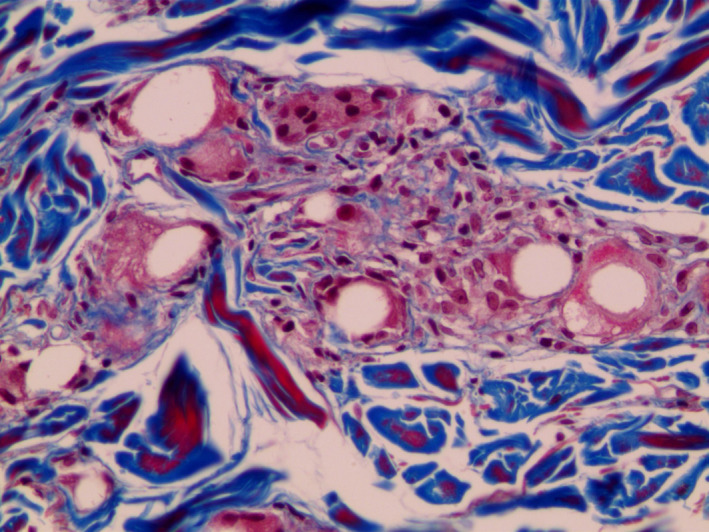
Microscopic image 13 mo after PCL treatment showing PCL microspheres with collagen deposition and a mild fibroblastic and histiocytic tissue response. Martin's Trichrome (MT) staining (×200 magnification). With permission from J Cosmet Laser Ther 2015;17:99‐101 Taylor & Francis
To prevent nodules (in addition to avoiding injection of the product in the lips and the periorbital area), the recommended techniques to be used are linear threading, fanning, and cross‐hatching in the subcutaneous plane and injection of only small bolus (no more than 0.2 mL) supraperiosteally. Overcorrection should be avoided. In the present retrospective review, by following these recommendations the author found no cases of nodules.
4.6. Discoloration
Yellowish discoloration on one or both sides of the lid‐cheek junctions (Figure 6) was found in two patients. In these cases, the product was likely placed too superficially. When the product is inappropriately implanted too superficially into the subdermal or muscular layers, a yellowish hue may occur, mimicking xanthelasma. This yellowish discoloration, recently referred as xanthelasma‐like reaction, 22 usually occurs in the periorbital area. Cases have been reported using PLLA, HA, CaHA, and PCL. Xanthelasma‐like reaction is a late complication reported to develop an average of 12 months after injection, in a few studies found in literature. The clinical presentation involves swelling, festoons, and yellow deposits in the lower eyelids. In the current analysis, xanthelasma‐like reaction developed 10 months after injection in one case and 11 months after injection in the other case. Although the diagnosis of xanthelasma‐like reaction was not confirmed microscopically in either case, it was very likely according to the typical clinical presentation and patient history.
FIGURE 6.
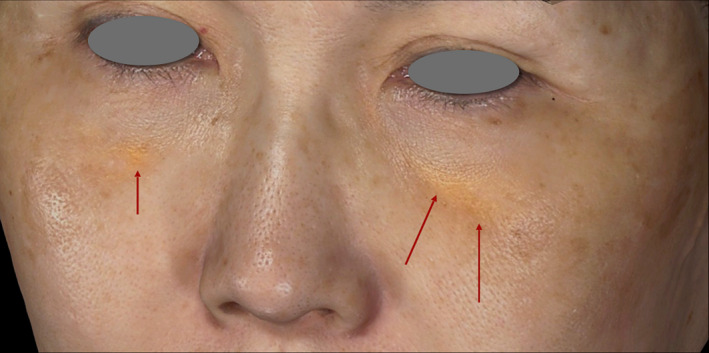
A yellowish hue was observed in a 64‐y‐old female on each side of the lid‐cheek junction 10 mo after <0.05 mL of the material was placed too superficially
The precise mechanism of xanthelasma‐like reaction is unclear; it may involve the binding and internalization of low‐density lipoprotein by macrophages because it shares common microscopic features with that process. To date, xanthelasma‐like reaction cannot be eliminated effectively; hence, prevention is the best solution. In the case of PCL‐based filler, clinicians should avoid superficial injection and injection into the peri‐orbital area to prevent xanthelasma‐like reactions. In the malar area, the filler should be placed supraperiosteally or in the sub‐muscular level in small amounts. Aging‐related volume loss in this region mainly occurs in the bony structure and in deep fat compartments, so volume should be restored in these regions.
4.7. Summary
Dermal fillers are generally considered as safe; immediate injection‐related reactions such as bruising, swelling, tenderness and lumps are common, but disappear rapidly and spontaneously. However, some serious adverse events have been reported, such as granuloma or vascular complications, but they are rare. Safety is particularly important in aesthetic treatments or procedures, and information must continuously be gathered regarding long‐term safety to allow better management.
Clinical trials have shown that the PCL‐based filler has a good safety profile, as has daily clinical practice and postmarket surveillance worldwide over 10 years. Clinical studies have evaluated both its efficacy and safety in different facial areas; the present study focused on safety in particular.
In randomized, prospective studies on nasolabial folds, 12 , 13 the treatment was well tolerated by patients and showed no serious adverse events at any time points, such as nodules, granuloma, or vascular complications. As with any dermal fillers, minor injection‐related reactions were noted that resolved rapidly without intervention. Wrinkle severity and global aesthetic improvement of the nasolabial folds were shown, and patient satisfaction was very high. Interestingly, global rejuvenation was successfully obtained after a multilayer treatment, with the filler placed in three different levels of the face in the same session. 9 One study on the effect on forehead augmentation involving 58 subjects also concluded that no vascular complications occurred using this filler. 17 A pilot clinical study 15 showed similar results in hands, and one recent study using a cannula showed that PCL filler improved dorsal hand volume loss, with minimal side effects. 16
Efficacy and safety follow‐ups using this filler have been carried out for up to 24 months. 12 , 17 Safety was followed up for 30 months in a recent European multicenter clinical trial involving 90 subjects. These studies were detailed in a recent review. 4 Several publications dealing specifically with PCL filler complications have also indicated that the product shows high safety. In one study, a foreign body reaction was observed in one patient 3 years after treatment. The reaction regressed after 1 month of treatment using oral doxycycline. 3 A granuloma complication was diagnosed in one patient who visited the physician 2 years after injection reporting that tiny nodules had appeared after 1 year. On histology examination, numerous colonies of Streptococcus parasanguinis were identified; infection might be suspected as the cause of the reported complication. The patient subsequently withdrew consent for treatment and was lost to follow‐up. 21 In one analysis of cases induced by dermal fillers, one case of xanthelasma‐like reaction using PCL‐based filler was cited, showing that this is an extremely rare reaction. 22 Physicians must be able to properly manage adverse events, should they occur; in this regard, treatment guidelines for PCL‐based filler have been recommended by experts. 4 To date, there is no effective method of product removal, so prevention is the best solution to avoid adverse events, as with any dermal fillers. Tumescent anesthesia has been proposed to reduce pain, swelling, and ecchymosis during facial injection with the PCL‐based filler. 23 Interestingly, ultrasonographic characteristics have been defined, allowing clinicians to identify and localize the PCL filler during injection and in case of complications. 24
The present article provided additional safety information in a large population of 780 patients and 1111 treatments that were followed up for 3 years. As expected, swelling/edema and bruising were the most frequent minor reactions reported. No intravascular complications, nodules/granuloma, or infection was observed during the long‐term study. Given that prevention is of utmost importance, we reported ways to avoid the side effects of filler injection. When considering injection modalities, the area to be injected, volume of injection, and depth of injection are key factors. In the malar area, the material should be injected supraperiosteally or in the sub‐muscular level in small amounts. During the process of aging, volume loss in this region mainly occurs in the bony structure and in the deep fat compartments, so volume should be restored in these regions. Clinicians should avoid placing PCL‐based filler too superficially or in the peri‐orbital area to prevent xanthelasma‐like reactions: an extremely rare side effect.
The present study was limited by its retrospective design. Further, only one clinician carried out the injections, although this may have improved the consistency and reproducibility of the technique and may be a good control variable. In addition, although all early adverse events were recorded, some of the late events may not have been, as not every patient reported to the clinic in the case of any suspected late event. The shortest follow‐up time among the patients was 1 year, so that some late complications cannot completely be ruled out. Furthermore, the present study lacked a histological analysis, so no objective diagnosis was made of late but extremely rare events such as xanthelasma‐like reaction. Taken together, the study confirmed the low rate of adverse events and the absence of unexpected events using the PCL‐based filler.
5. CONCLUSION
The complication rate of the PCL‐based filler was found to be low, and complications were mild in nature. There were no incidences of intravascular injection, nodules, and/or granuloma during the 3‐year observation.
Furthermore, longer‐lasting edema was found to be associated with a higher injection volume and malar edema was related to lymphatic compression, which can be avoided by controlling the injection amount and injection location via anatomical considerations. The study confirms the long‐term safety profile of the PCL‐based filler.
CONFLICTS OF INTEREST
The author declares no conflicts of interest.
ACKNOWLEDGMENTS
The consent and the permission of the provided figures have been fully granted by the patients.
Lin S‐L, Christen M‐O. Polycaprolactone‐based dermal filler complications: A retrospective study of 1111 treatments. J Cosmet Dermatol. 2020;19:1907–1914. 10.1111/jocd.13518
REFERENCES
- 1. Wan D, Amirlak B, Rohrich R, Davis K. The clinical importance of the fat compartments in midfacial aging. Plast Reconstr Surg Glob Open. 2013;1:e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mendelson B, Wong CH. Changes in the facial skeleton with aging: implications and clinical applications in facial rejuvenation. Aesthetic Plast Surg. 2012;36:753‐760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moon SY, Eun DH, Park JH, et al. Foreign body reaction three years after injection with polycaprolactone (Ellanse®). EJD. 2017;27:549‐551. [DOI] [PubMed] [Google Scholar]
- 4. Christen MO, Vercesi F. Polycaprolactone: or how a well‐known and futuristic polymer has become an innovative collagen‐stimulator in esthetics. Clin Cosmet Investig Dermatol. 2020;13:31‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim JA, Van Abel D. Neocollagenesis in human tissue injected with a polycaprolactone‐based dermal filler. J Cosmet Laser Ther. 2015;17:99‐101. [DOI] [PubMed] [Google Scholar]
- 6. Morhenn VB, Lemperle G, Gallo RL. Phagocytosis of different particulate dermal filler substances by human macrophages and skin cells. Dermatol Surg. 2002;28:484‐490. [DOI] [PubMed] [Google Scholar]
- 7. de Melo F, Nicolau P, Piovano L, et al. Recommendations for volume augmentation and rejuvenation of the face and hands with the new generation polycaprolactone based collagen stimulator (Ellansé®). Clin Cosmet Investig Dermatol. 2017;10:431‐440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Melo F, Marijnissen‐Hofsté J. Investigation of physical properties of a polycaprolactone dermal filler when mixed with lidocaine and lidocaine/epinephrine. Dermatol Ther. 2012;2:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lin SL. Polycaprolactone facial volume restoration of a 46‐year‐old Asian women: a case report. J Cosmet Dermatol. 2018;17:328‐332. [DOI] [PubMed] [Google Scholar]
- 10. Rohrich RJ, Nguyen AT, Kenkel JM. Lexicon for soft tissue implants. Dermatol Surg. 2009;35(Suppl 2):1605‐1611. [DOI] [PubMed] [Google Scholar]
- 11. Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6:295‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moers‐Carpi MM, Sherwood S. Polycaprolactone for the correction of nasolabial folds: a 24‐month, prospective, randomized, controlled clinical trial. Dermatol Surg. 2013;39(3 Pt 1):457‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Galadari H, van Abel D, Al Nuami K, Al Faresi F, Galadari I. A randomized, prospective, blinded, split‐face, single‐center study comparing polycaprolactone to hyaluronic acid for treatment of nasolabial folds. J Cosmet Dermatol. 2015;14(1):27‐32. [DOI] [PubMed] [Google Scholar]
- 14. Kim JS. Changes in dermal thickness in biopsy study of histologic findings after a single injection of polycaprolactone‐based filler into the dermis. Aesthet Surg J. 2019;39(12):NP484‐NP494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Figueiredo VM. A five‐patient prospective pilot study of a polycaprolactone based dermal filler for hand rejuvenation. J Cosmet Dermatol. 2013;12(1):73‐77. [DOI] [PubMed] [Google Scholar]
- 16. Lowe NJ, Ghanem AM. Volume restoration of hands with polycaprolactone by cannula delivery: a prospective single center consecutive case series evaluation. J Cosmet Laser Ther. 2020;22(2):55‐59. [DOI] [PubMed] [Google Scholar]
- 17. Bae B, Lee G, Oh S, Hong K. Safety and long‐term efficacy of forehead contouring with a polycaprolactone‐based dermal filler. Dermatol Surg. 2016;42(11):1256‐1260. [DOI] [PubMed] [Google Scholar]
- 18. Fitzgerald R, Vleggaar D. Facial volume restoration of the aging face with poly‐l‐lactic acid. Dermatol Ther. 2011;24:2‐27. [DOI] [PubMed] [Google Scholar]
- 19. Funt DK. Avoiding malar edema during midface/cheek augmentation with dermal fillers. J Clin Aesthet Dermatol. 2011;4:32‐36. [PMC free article] [PubMed] [Google Scholar]
- 20. Bertucci V, Sykes J, Duplechain J, Fitzgerald R. Adverse reactions to injectable fillers. Facial Plast Surg. 2016;32:532‐555. [DOI] [PubMed] [Google Scholar]
- 21. Skrzypek E, Górnicka B, Skrzypek DM, Krzysztof MR. Granuloma as a complication of polycaprolactone‐based dermal filler injection: ultrasound and histopathology studies. J Cosmet Laser Ther. 2019;21(2):65‐68. [DOI] [PubMed] [Google Scholar]
- 22. Or L, Eviatar JA, Massry GG, Bernardini FP, Hartstein ME. Xanthelasma‐like reaction to filler injection. Ophthalmic Plast Reconstr Surg. 2017;33:244‐247. [DOI] [PubMed] [Google Scholar]
- 23. Kim J. Tumescent anesthesia for reducing pain, swelling, and ecchymosis during polycaprolactone filler injections in the face. J Cosmet Laser Ther. 2017;19(7):434‐438. [DOI] [PubMed] [Google Scholar]
- 24. Wortsman X, Quezada N. Ultrasound morphology of polycaprolactone filler. J Ultrasound Med. 2017;36(12):2611‐2615. [DOI] [PubMed] [Google Scholar]


