Abstract
Background and Aims
The Model for End‐Stage Liver Disease (MELD) is used for clinical decision‐making and organ allocation for orthotopic liver transplantation (OLT) and was previously upgraded through inclusion of serum sodium (Na) concentrations (MELD‐Na). However, MELD‐Na may underestimate complications arising from portal hypertension or infection. The von Willebrand factor (vWF) antigen (vWF‐Ag) correlates with portal pressure and seems capable of predicting complications in patients with cirrhosis. Accordingly, this study aimed to evaluate vWF‐Ag as an adjunct surrogate marker for risk stratification on the waiting list for OLT.
Approach and Results
Hence, WF‐Ag at time of listing was assessed in patients listed for OLT. Clinical characteristics, MELD‐Na, and mortality on the waiting list were recorded. Prediction of 3‐month waiting‐list survival was assessed by receiver operating characteristics and net reclassification improvement. Interestingly, patients dying within 3 months on the waiting list displayed elevated levels of vWF‐Ag (P < 0.001). MELD‐Na and vWF‐Ag were comparable and independent in their predictive potential for 3‐month mortality on the waiting list (area under the curve [AUC], vWF‐Ag = 0.739; MELD‐Na = 0.764). Importantly, a vWF‐Ag cutoff at 413% identified patients at risk for death within 3 months of listing with a higher odds ratio (OR) than the previously published cutoff at a MELD‐Na of 20 points (vWF‐Ag, OR = 10.873, 95% confidence interval [CI], 3.160, 36.084; MELD‐Na, OR = 7.594, 95% CI, 2.578, 22.372; P < 0.001, respectively). Ultimately, inclusion of vWF‐Ag into the MELD‐Na equation significantly improved prediction of 3‐month waiting‐list mortality (AUC, MELD‐Na–vWF = 0.804).
Conclusions
A single measurement of vWF‐Ag at listing for OLT predicts early mortality. Combining vWF‐Ag levels with MELD‐Na improves risk stratification and may help to prioritize organ allocation to decrease waiting‐list mortality.
Abbreviations
- ALT
alanine aminotransferase
- AP
alkaline phosphatase
- aPTT
activated partial thromboplastin time
- AST
aspartate aminotransferase
- AUC
area under the curve
- CI
confidence interval
- GGT
gamma‐glutamyltranspeptidase
- HR
hazard ratio
- MELD
Model for End‐Stage Liver Disease
- MELD‐Na
MELD with Na concentration
- MELD‐Na–vWF
vWF‐Ag incorporated into the MELD‐Na system
- MVA
multivariable analysis
- NA
sodium
- NRI
net reclassification improvement
- OLT
orthotopic liver transplantation
- OR
odds ratio
- PT
prothrombin time
- ROC
receiver operating characteristic
- SBP
spontaneous bacterial peritonitis
- UNOS
United Network for Organ Sharing
- vWF
von Willebrand factor
- vWF‐Ag
vWF antigen
To this date, orthotopic liver transplantation (OLT) is the gold standard in treatment of patients suffering from end‐stage liver disease.1 Although OLT does improve the outcome of these patients in terms of overall survival and quality of life,2 shortage of donor organs and the resulting high mortality on the waiting list for OLT remain a critical problem.3, 4, 5 In fact, the mortality rate in Eurotransplant and the United Network for Organ Sharing is approximately 25% and might even be subject to underreporting,6, 7, 8 which clearly illustrates the need for improved risk stratification in this patient cohort.
The Model for End‐Stage Liver Disease (MELD) was introduced to standardize assessment of waiting‐list mortality nearly 2 decades ago and has become a central part of liver allocation throughout the United States and Europe.7, 9 Subsequently, the original MELD was adapted through inclusion of serum sodium (NA) concentrations (MELD‐Na), which led to improved prediction of early mortality on the waiting list for OLT.10, 11 Still, MELD‐Na is prone to well‐known limitations that comprise the lack of assessment of subclinical infection while on the waiting list and an underestimation of complications resulting from portal hypertension.9, 12, 13, 14, 15 Accordingly, further optimization of the organ allocation process seems inevitable to reduce mortality before OLT.
The von Willebrand factor (vWF) antigen (vWF‐Ag) represents a key component of primary hemostasis that is stored and released by thrombocytes and endothelial cells.16, 17 Interestingly, previous studies have shown that vWF‐Ag can predict long‐term outcomes of patients with chronic liver disease.18, 19 Indeed, vWF‐Ag is a surrogate marker for endothelial dysfunction, a key pathophysiologic driver for complications associated with portal hypertension and/or infections.20 Although the first studies showed a strong correlation of vWF‐Ag with portal hypertension,21 recent data underline a more universal role of vWF‐Ag in predicting outcomes within this patient cohort.22 In particular, vWF‐Ag was shown to be even more accurate in predicting variceal bleeding than hepatic venous pressure gradient (HVPG). Further, an association of vWF‐Ag with markers of bacterial translocation and, even more interestingly, with spontaneous bacterial peritonitis (SBP) was found and shown to be independent of portal pressure.22 As both variceal bleeding and SBP are predominant causes of mortality in patients listed for transplantation,23, 24 and because of the multifactorial involvement of vWF‐Ag in liver pathophysiology, we aimed to evaluate whether vWF‐Ag could improve the apparent shortcomings of the MELD‐Na score in risk assessment of patients listed for transplantation.
Accordingly, we aimed to explore differences in waiting‐list survival regarding levels of vWF‐Ag and to assess whether addition of vWF‐Ag improves mortality risk prediction on the waiting list on top of MELD‐Na.
Patients and Methods
Study Population
All consecutive patients listed for OLT at our center between 2003 and 2016 were included in this study. Routine blood samples at the time of listing included baseline coagulation parameters (prothrombin time [PT], activated partial thromboplastin time [aPTT]), liver function tests (aspartate aminotransferase [AST] alanine aminotransferase [ALT], alkaline phosphatase [AP], gamma‐glutamyltranspeptidase [GGT, and bilirubin), and vWF‐Ag levels. Patients undergoing transplantation for acute hepatic failure or undergoing retransplantation were excluded from this study. As the primary goal of this study was to evaluate a potential benefit of vWF‐Ag in comparison with MELD‐Na alone, standard exception points on the waiting list (e.g., patients with hepatocellular carcinoma) were not taken into consideration, and patients were analyzed according to their calculated MELD‐Na score. Thereby, we aimed to reflect the individual patient risk for early waiting‐list mortality based on the MELD‐Na system without any inherent bias. The study protocol was approved by the institutional review board (Ethical Committee of the Medical University of Vienna, Vienna, Austria, EK‐1196/2018). All clinical research has been performed in adherence to the Declarations of Helsinki and Istanbul.
Calculation of MELD‐Na and Evaluation of vWF‐Ag
MELD was calculated based on the formula given by Malinchoc et al., which includes serum bilirubin, creatinine, and international normalized ratio.25 In addition, MELD‐Na was calculated based on the standard formula under inclusion of serum Na.10 After comparison with vWF‐Ag, MELD‐Na was used for incorporation of vWF‐Ag as reported in the following.
To render vWF‐Ag a standardized and broadly applicable factor, it was evaluated within our clinical routine laboratory. Citrated blood was taken at listing, and concentration of vWF‐Ag was assessed in the subsequently prepared plasma.
Statistical Analyses
Statistical analyses were performed using SPSS software (version 24; SPSS, Inc., Chicago, IL) as well as by using the R statistical software environment (R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org, version 3.5.3, packages survival, mgcv, cmprsk) and were based on nonparametric tests for either paired or independent samples (Mann‐Whitney U test, Wilcoxon test, chi‐squared test). Baseline (i.e., at time of listing) vWF‐Ag and MELD‐Na were obtained and compared between patients who underwent transplantation and patients who died on the waiting list before they reached transplantation. Furthermore, receiver operating characteristic (ROC) analysis was applied to assess the discriminatory potential of the mentioned variables for 3‐month mortality. In addition, this statistical approach was used to identify the optimal cut‐off level with the greatest accuracy of distinguishing high‐risk and low‐risk groups through the use of the Youden J statistic. To determine whether the vWF‐Ag as a continuous parameter had a nonlinear effect on the risk of 3‐month mortality, generalized additive models with smoothing splines were used. Multivariable analysis (MVA) was used to investigate independence of predictive markers for 3‐month waiting‐list survival. Initially, all baseline characteristics were evaluated in a univariate logistic regression model. Subsequently, all markers shown to be significant on univariate analysis were included in the final model, which was computed using stepwise backward elimination. Parameters shown to be significant on MVA were considered independent predictors. To further provide a useful tool for clinical applicability of the presented results, incorporation of vWF‐Ag into the MELD‐Na scoring system was sought. Of note, the aim was to maximize the predictive potential for identification of patients who would die within 3 months while awaiting OLT. Thus, Cox regression analysis was computed to evaluate a potential additional value of vWF‐Ag for MELD‐Na based on predicted 3‐month survival. Finally, net reclassification improvement (NRI) analysis was performed to validate the results regarding the predictive potential and to explore a possible improvement of MELD‐Na after addition of vWF‐Ag. P values <0.05 were considered statistically significant.
Results
Patient Demographics
A total of 1,096 patients were listed for OLT at our center between 2003 and 2016 and were included in our prospective database. Of these 1,096 patients, 1,058 were listed for their first transplantation. Further, 19 patients were excluded from the analysis, as they were listed for fulminant liver failure. Ultimately, data on vWF‐Ag were available in 269 patients listed for OLT who were included in the final analysis. Supporting Fig. S1 illustrates the process of patient exclusion. Of note, vWF‐Ag evaluation occurred haphazardly and without clinical consequence in these patients. Notably, levels of vWF‐Ag were not available for patients with clinical deterioration and subsequent exclusion. Hence, the statistical analysis relied on comparing patients who did reach the endpoint of transplantation within 3 months and patients who survived on the list until this time point with patients who died within 3 months on the waiting list.
Supporting Table S1 shows the comparison between patients included in the analysis and patients excluded because of missing vWF‐Ag levels. Patients’ ages at listing differed significantly between the two cohorts, explained by a significantly higher frequency of patients younger than 15 years in the population of patients without available data on vWF‐Ag. However, all remaining parameters showed an equal distribution. Characteristics of patients included in the final analysis are displayed in Table 1. In total, 31 patients (11.5%) died within the first 3 months after listing.
Table 1.
Patient Demographics
| Parameters | Entire Cohort (n = 269) | M↓ W↓* (n = 94) | M↑ W↓* (n = 36) | M↓ W↑* (n = 49) | M↑ W↑* (n = 90) |
|---|---|---|---|---|---|
| Sex, n (%) | |||||
| Male | 203 (75.5) | 71 (75.5) | 29 (80.6) | 38 (77.6) | 65 (72.2) |
| Female | 66 (24.5) | 23 (24.5) | 7 (19.4) | 11 (22.4) | 25 (27.8) |
| Age at listing (years), median (range) | 56 (15‐73) | 56 (22‐72) | 53 (19‐68) | 59 (40‐72) | 56 (15‐73) |
| Parameters at listing, median (range) | |||||
| vWF‐Ag (%) | 419 (76‐1,270) | 297 (76‐410) | 342 (224‐411) | 420 (415‐830) | 420 (417‐1,270) |
| MELD‐Na | 19 (7‐40) | 14 (7‐19) | 23 (20‐40) | 17 (10‐19) | 24 (20‐38) |
| PT (%) | 51 (14‐142) | 64 (37‐136) | 45 (14‐142) | 58 (40‐112) | 36 (15‐76) |
| aPTT (seconds) | 41.6 (31.0‐67.0) | 40.6 (31.2‐51.2) | 44.2 (31.5‐61.8) | 40.6 (34.2‐49.5) | 45.0 (31.0‐67.0) |
| AST (U/L) | 62 (17‐2,187) | 63 (17‐2,187) | 59 (23‐270) | 56 (24‐202) | 65 (22‐453) |
| ALT (U/L) | 40 (10‐1,663) | 44 (10‐1,663) | 33 (12‐161) | 35 (15‐126) | 38 (10‐256) |
| Bilirubin (mg/dL) | 2.95 (0.42‐52.02) | 1.69 (0.42‐6.71) | 5.80 (0.65‐52.02) | 2.32 (0.68‐10.33) | 5.69 (0.87‐48.81) |
| AP (U/L) | 128 (45‐2,552) | 111 (45‐927) | 129 (62‐2,552) | 134 (47‐377) | 150 (51‐1,000) |
| GGT (U/L) | 88 (12‐2,733) | 98 (12‐695) | 95 (15‐2,733) | 102 (14‐481) | 72 (17‐689) |
| Creatinine (mg/dL) | 0.90 (0.48‐3.56) | 0.84 (0.54‐1.95) | 1.07 (0.53‐2.74) | 0.89 (0.58‐2.04) | 1.02 (0.48‐3.56) |
| Na (mmol/L) | 136 (117‐145) | 138 (130‐144) | 135 (123‐144) | 138 (129‐145) | 132 (117‐143) |
| Indication for LTx, n (%) | |||||
| Alcohol‐associated cirrhosis | 89 (33.1) | 14 (14.9) | 14 (38.9) | 19 (38.8) | 42 (46.7) |
| Tumor | 62 (23.0) | 46 (48.9) | 4 (11.1) | 8 (16.3) | 4 (4.4) |
| Viral hepatitis | 42 (15.6) | 14 (14.9) | 4 (11.1) | 10 (20.4) | 14 (15.6) |
| Biliary disorders | 23 (8.6) | 11 (11.7) | 7 (19.4) | 2 (4.1) | 3 (3.3) |
| AI hepatitis | 14 (5.2) | 2 (2.1) | 2 (5.6) | 1 (2.0) | 9 (10.0) |
| Cryptogenic cirrhosis | 13 (4.8) | 2 (2.1) | 2 (5.6) | 2 (4.1) | 7 (7.8) |
| Other indications | 26 (9.7) | 5 (5.3) | 3 (8.3) | 47(14.3) | 11 (12.2) |
M = MELD‐Na (cutoff at 20 points), W = vWF‐Ag (cutoff at 413%).
Abbreviations: AI, autoimmune; LTx, liver transplant.
Patients Dying Within 3 Months on the Waiting List Have Significantly Elevated Levels of vWF‐Ag and MELD‐Na at Time of Listing
Initially, evaluation of differences regarding MELD‐Na and vWF‐Ag between patients with an on‐list survival of more or less than 3 months was sought. Indeed, patients dying within 3 months on the waiting list displayed elevated levels of MELD‐Na (median MELD‐Na, no mortality = 19, median MELD‐Na, mortality = 24, P < 0.001). Similar results were observed for vWF‐Ag (median vWF‐Ag, no mortality = 405%, median vWF‐Ag, mortality = 420%, P < 0.001). Strikingly, vWF‐Ag and MELD‐Na seemed to be comparable in their predictive potential for 3‐month survival on the waiting list (area under the curve [AUC], vWF‐Ag = 0.739, AUC, MELD‐Na = 0.764).
MELD‐Na and vWF‐Ag are Independent Predictors for 3‐Month Mortality on the Waiting List
Comparative analysis of vWF‐Ag and serum Na revealed that there was only a little redundancy for both parameters (Supporting Fig. S2). Interestingly, the majority of patients (i.e., 77.32%) showed high levels of serum Na accompanied by low levels of vWF‐Ag, whereas only 3.72% of patients displayed severe hyponatremia (<130 mmol/L) while having concomitantly increased vWF‐Ag levels. Intriguingly, the incidence of 3‐month mortality showed a significantly different distribution in the specific cohorts according to vWF‐Ag and Na (P = 0.019, Supporting Fig. S2). Of note, besides the cohort defined by high vWF‐Ag and low levels of serum Na, a specifically high frequency of early waiting‐list mortality was observed in patients with Na levels above 130 mmol/L and vWF‐Ag levels above 500% (18.2%).
In contrast, comparative analysis for vWF‐Ag and MELD‐Na showed a more linear relationship. Most patients were found to have both low levels of vWF‐Ag and low MELD‐Na scores (Fig. 1A,B). Further, a clear difference between high and low MELD‐Na scores was observed in regard to 3‐month mortality (Fig. 1D). Nevertheless, the additional stratification of patients with specifically high vWF‐Ag levels at listing (i.e., >500%) revealed a pronounced incidence of 3‐month mortality in this cohort, which was independent of MELD‐Na points.
Figure 1.
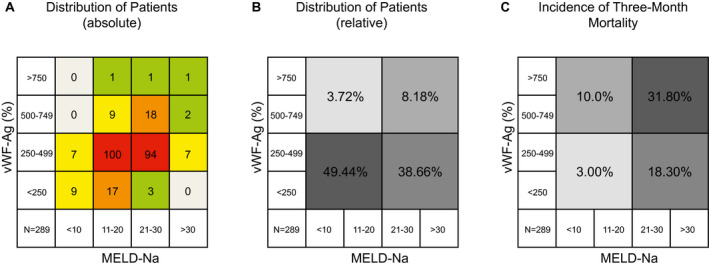
Association of vWF‐Ag with Na and MELD‐Na in patients awaiting OLT. To evaluate a potential redundancy of vWF‐Ag and MELD‐Na, correlative analysis was performed. Absolute distribution of patients in regard to their (A) vWF‐Ag levels and MELD‐Na is visualized. Similarly, the (B) relative distribution is shown. Ultimately, the (C) incidence of 3‐month waiting‐list mortality is shown in accordance to the respective parameters.
As a consequence, the independence of vWF‐Ag in relation to MELD‐Na was assessed in the MVA, including PT and bilirubin, as well as the presence of autoimmune hepatitis, tumor, or cryptogenic cirrhosis. Ultimately, MELD‐Na, vWF‐Ag, and cryptogenic cirrhosis remained significant and hence independent, as visualized in Table 2.
Table 2.
MVA for 3‐Month Survival on Waiting List
| Parameter | Univariate Analysis | MVA | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| vWF‐Ag (%) | 1.005 | 1.002, 1.007 | 0.001 | 1.003 | 1.001, 1.006 | 0.036 |
| MELD‐Na | 1.180 | 1.098, 1.268 | <0.001 | 1.154 | 1.068, 1.246 | <0.001 |
| CLIF‐C AD | 1.082 | 0.974, 1.203 | 0.141 | — | — | — |
| Sex | 0.770 | 0.335, 1.767 | 0.537 | — | — | — |
| Age at listing | 1.021 | 0.981, 1.062 | 0.310 | — | — | — |
| Circulating parameters at listing | ||||||
| PT (%) | 0.970 | 0.945, 0.995 | 0.021 | 1.015 | 0.987, 1.044 | 0.284 |
| aPTT (seconds) | 1.046 | 0.984, 1.113 | 0.149 | — | — | — |
| AST (U/L) | 0.999 | 0.994, 1.004 | 0.683 | — | — | — |
| ALT (U/L) | 0.997 | 0.988, 1.007 | 0.586 | — | — | — |
| Bilirubin (mg/dL) | 1.058 | 1.011, 1.108 | 0.016 | 0.974 | 0.910, 1.043 | 0.450 |
| AP (U/L) | 1.000 | 0.998, 1.002 | 0.832 | — | — | — |
| GGT (U/L) | 0.997 | 0.992, 1.003 | 0.331 | — | — | — |
| Indication for LTx | ||||||
| Alcohol‐associated cirrhosis | 1.790 | 0.838, 3.821 | 0.132 | — | — | |
| Tumor | 0.097 | 0.013, 0.724 | 0.023 | 0.000 | NA | 0.998 |
| Viral hepatitis (B+C) | 0.547 | 0.158, 1.888 | 0.340 | — | — | — |
| Biliary disorders | 0.327 | 0.043, 2.517 | 0.283 | — | — | — |
| Autoimmune hepatitis | 4.893 | 1.525, 15.703 | 0.008 | 1.527 | 0.295, 7.900 | 0.613 |
| Cryptogenic cirrhosis | 3.770 | 1.087, 13.072 | 0.036 | 3.851 | 1.031, 14.384 | 0.045 |
| Other indications | 1.002 | 0.282, 3.552 | 0.998 | — | — | — |
Abbreviations: CLIF‐C AD, chronic liver failure acute decompensation score; LTx, liver transplant; NA, not available.
A Cutoff at 413% vWF‐Ag is Vital for Identification of Patients Dying Early on the Waiting List
The cutoff, maximizing the Youden index with a sensitivity of 90.3% and a specificity of 53.4%, was found to be 413% of vWF‐Ag at listing. Of note, the optimal cutoff for MELD‐Na in this cohort was 20 points (sensitivity = 87.1%, specificity = 64.7%). Subsequently, the cohort was subdivided into two risk groups: vWF‐AgHIGH (>413%) and vWF‐AgLOW (≤413%). Indeed, patients within the high‐risk group were found to have a significantly higher incidence of mortality within 3 months on the waiting list (28 of 139 [20.1%] in vWF‐AgHIGH vs. 3 of 130 [2.3%] in vWF‐AgLOW, P < 0.001; Supporting Fig. S3A), which was comparable with the high‐risk group according to MELD‐Na (27 of 139 [19.4%] in MELD‐NaHIGH vs. 4 of 130 [3.1%] in MELD‐NaLOW, P < 0.001; Supporting Fig. S3A). Strikingly, the cutoff at 413% of vWF‐Ag at listing was found to have a higher odds ratio (OR) for mortality within 3 months on the list when compared with a MELD‐Na cutoff at 20 points (vWF‐Ag, OR = 10.679, 95% confidence interval [CI] = 3.160, 36.084, P < 0.001; MELD‐Na, OR = 7.594, 95% CI = 2.578, 22.372, P < 0.001). The subsequently computed subgroup analysis for 3‐month mortality in patients with low MELD‐Na (≤20) and patients with high MELD‐Na (>20) showed an independent prediction of vWF‐Ag (MELD‐Na ≤ 20, 4 of 44 [9.1%] in vWF‐AgHIGH vs. 0 of 86 [0.0%] in vWF‐AgLOW, P = 0.005; MELD‐Na > 20, 24 of 95 [25.3%] in vWF‐AgHIGH vs. 3 of 44 [6.8%] in vWF‐AgLOW, P = 0.005; Supporting Fig. S3B), whereas no patient in the low‐risk group according to vWF‐Ag and having less than 20 MELD‐Na points died within 3 months on the waiting list (Supporting Fig. S3B).
VWF‐Ag Increases Predictive Potential for 3‐Month Survival of MELD‐Na Alone in Patients Awaiting OLT
To evaluate a continuous nonlinear relationship between vWF‐Ag and risk for 3‐month mortality, a generalized additive model with smoothing splines was applied and visualized in Fig. 2A. An increased risk (OR ≥ 1) was observed for values of vWF‐Ag between 369.8% and 1,106.7%. Further, a linear increase in risk for 3‐month mortality was observed for levels of vWF‐Ag under 369.4%. As a consequence, extreme values for vWF‐Ag were defined at the upper limit of normal (185%) and at 1,100% as the upper limit. These limits included 95.9% of all values for vWF‐Ag in this cohort. In case of patients with higher or lower levels for vWF‐Ag, the value was reduced to the defined limits, respectively. Next, a multivariable Cox regression analysis including MELD‐Na and vWF‐Ag at listing was fitted. Indeed, both factors were significant for survival on the waiting list on univariate analysis (MELD‐Na, hazard ratio [HR] = 1.154, 95% CI = 1.103, 1.208, P < 0.001; vWF‐Ag, HR = 1.004, 95% CI = 1.002, 1.006, P < 0.001) and MVA (MELD‐Na, HR = 1.135, 95% CI = 1.082, 1.192, P < 0.001; vWF‐Ag, HR = 1.002, 95% CI = 1.000, 1.004, P = 0.031). To incorporate vWF‐Ag into the MELD‐Na system, the following equation including the regression coefficients for MELD‐Na and vWF was fitted:
Figure 2.
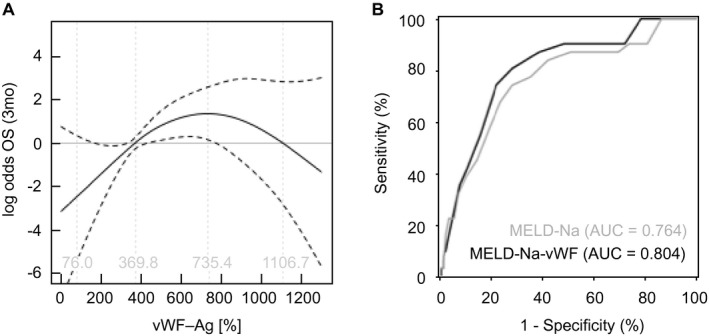
vWF‐Ag allows additional risk stratification on the waiting list for OLT. (A) Continuous risk for 3‐month mortality (OR) was calculated for vWF‐Ag and is visualized. VWF‐Ag was incorporated as described in the main text. (B) Indeed, incorporation of vWF‐Ag was able to increase the AUC of MELD‐Na alone for prediction of 3‐month mortality on the waiting list. (*P < 0.05, **P < 0.005).
Of note, the multiplicator 5.5 was introduced to render the scale comparable to the original MELD‐Na score, while automatically leading to a range from 6 to 40 points. Further, values for MELD‐Na–vWF were rounded to the next integer. Thereby, a bidirectional reclassification was achieved allowing patients to both gain or lose MELD points in accordance to vWF‐Ag. Indeed, the introduction of vWF‐Ag leads to a potential change of 11 points in both directions, as visualized in Fig. 3. Further, Table 3 was computed to visualize the reclassification pattern in the studied cohort.
Figure 3.
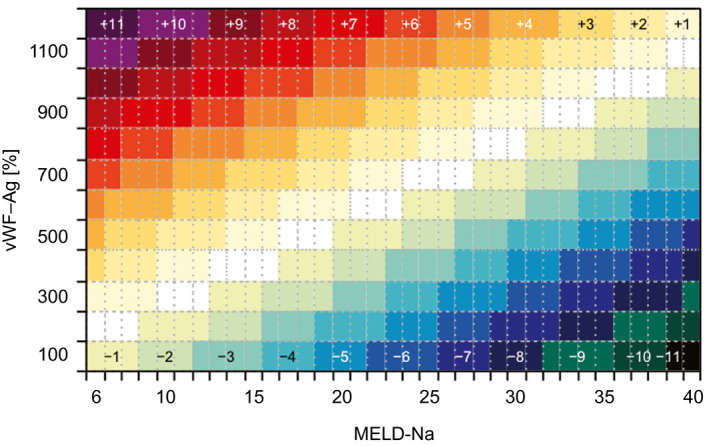
Computation of MELD‐Na–vWF on the Basis of the MELD‐Na after incorporation of vWF‐Ag. This figure illustrates the individual change of MELD‐Na when including vWF‐Ag as an additional variable.
Table 3.
Visualization of Reclassification
| MELD‐Na–vWF | Total | |||||
|---|---|---|---|---|---|---|
| <10 | 10‐19 | 20‐29 | 30‐40 | |||
| MELD‐Na | <10 | 13 | 3 | — | — | 16 |
| 10‐19 | 3 | 120 | 4 | — | 127 | |
| 20‐29 | — | 38 | 78 | — | 116 | |
| 30‐40 | — | — | 6 | 4 | 4 | |
| Total | 16 | 161 | 88 | 4 | 269 | |
ROC analysis was performed and revealed an improved discriminatory potential of MELD‐Na–vWF for 3‐month survival on the waiting list (AUC, MELD‐Na = 0.764; AUC, MELD‐Na–vWF = 0.804, P < 0.001 respectively; Fig. 2B). Based on this ROC analysis, the optimal cutoff for MELD‐Na–vWF was identified at 20 points with a sensitivity of 74.2% and a specificity of 78.2%. This cutoff was found to specifically identify patients who died within 3 months on the waiting list (6 of 177 [3.4%] in MELD‐Na–vWFLOW vs. 25 of 92 [27.2%] in MELD‐Na–vWFHIGH, P < 0.001; Supporting Fig. S3C). Of note, the use of MELD‐Na–vWF lead to a higher incidence of early waiting‐list mortality in the high‐risk group, when compared with the high‐risk group assessed by MELD‐Na alone using the Youden index for MELD‐Na at 20 points (4 of 130 [3.1%] in MELDNaLOW vs. 27 of 139 [19.4%] in MELDNaHIGH, P < 0.001), whereas the incidences of death within 3 months after listing in the low‐risk groups were comparable. This improvement was further characterized by NRI (Supporting Fig. S3D). In total, 47 patients were reclassified correctly when using MELD‐Na–vWF, whereas only 4 patients were reclassified inappropriately. This is further illustrated by a total NRI of 0.125, meaning that the introduction of MELD‐Na–vWF leads to an improvement in accurate prediction of 12.5% in this cohort.
Discussion
Although MELD was initially established for prediction of survival in patients receiving transjugular intrahepatic portosystemic shunts,26, 27 it has become a central part of liver allocation in many countries throughout the world.28, 29 In 2008, Kim et al. documented that the incorporation of Na in the MELD scoring system was able to improve prediction of 3‐month waiting‐list survival. Still, recent data show major shortcomings of MELD‐Na, mainly associated with an overestimation of creatinine within its calculation, leading to underestimation of certain subpopulations awaiting OLT.30, 31, 32 Further, MELD was shown to vary in its prediction of survival according to the underlying cause of cirrhosis, giving patients with viral hepatitis a special disadvantage on the waiting list.33 Hence, adaptation of the MELD‐Na score is a central task for further improvement of allocation policy and for reducing waiting‐list mortality. In particular, the identification of high‐risk patients with a low MELD score at listing and further stratification of patients with a very high MELD score pose difficult challenges.34, 35, 36, 37
In this study, we were able to evaluate the potential of vWF‐Ag to substratify patients, irrespective of their MELD‐Na score. Indeed, patients with vWF‐Ag above 413% at the time of listing were found to have a diminished survival on the waiting list independent of their MELD‐Na score at listing. Of note, these results held through after exclusion of patients suffering from tumors, who were previously shown to have an advantage in terms of waiting‐list survival (Supporting Fig. S4). As a key protein for hemostasis and coagulation, vWF‐Ag is secreted by endothelial cells and platelets as a response to alternations and augmentation of shear stress.17, 38, 39, 40 Hence, vWF‐Ag could be established as a noninvasive marker for portal hypertension and as a predictor of long‐term outcomes in patients suffering from this clinically important condition.18, 19, 21, 41, 42 Although the predictive potential of vWF‐Ag in these patients was thought to be due to its accurate ability to stratify the individual severity of portal hypertension, a recent analysis found an independent predictive potential of vWF‐Ag.22 Interestingly, vWF‐Ag was found to predict bleeding from esophageal varices and SBP better than HVPG as a measure of portal venous pressure/hypertension. A potential explanation of this finding was that vWF‐Ag might serve as a pleiotropic marker including endothelial cell dysfunction and bacterial translocation, as shown through correlation with lipopolysaccharide‐binding protein in circulation. Thus, vWF‐Ag can be seen as a multifactorial marker in patients with end‐stage liver disease, which unifies portal hypertension and associated comorbidities within one single parameter.
The presented data suggest that the incorporation of vWF‐Ag in the MELD‐Na score provides a relevant improvement in terms of risk stratification for patients awaiting OLT. In this context, it was of interest to note that very high vWF‐Ag levels were predominantly found in patients with normal or even high Na levels, suggesting that vWF‐Ag adds additional insight to the MELD score as compared with Na. However, when both criteria were met (low Na and high vWF‐Ag), 3‐month mortality was excessively high at 40%, again suggesting that the dual addition of Na and vWF‐Ag to MELD might be particularly specific as different aspects of advanced liver disease are considered. In addition, an independent predictive potential of vWF‐Ag assessed through MVA was observed in this study. Hence, a model based on Cox regression was fitted and the newly developed MELD‐Na–vWF model resulted. Indeed, this incorporation of vWF‐Ag into the MELD‐Na system was found to increase the potential of predicting 3‐month mortality on the waiting list to a clinically relevant extent, as seen in ROC and NRI analyses. Interestingly, after incorporation of vWF‐Ag and reclassification of patients into the newly developed MELD‐Na–vWF system, an overestimation of MELD‐Na was observed. Although vWF‐Ag is a sensitive marker for portal hypertension and complications associated with cirrhosis, low levels of vWF‐Ag might identify patients who are not prone to disease progression and potentially life‐threatening complications. Indeed, 21 patients of 31 patients dying within 3 months after listing (68%) died because of complications that were reported to be predicted by high levels of vWF‐Ag. Further, vWF‐Ag was recently found to play a direct role in the progression of fibrosis and cirrhosis.43, 44, 45 In particular, experimental data suggest not only a deleterious effect of vWF‐Ag in acute liver injury but also a beneficial effect of vWF‐Ag deficiency in a rodent model of liver fibrosis.43, 45 Thus, a reduction of risk for early waiting‐list mortality in patients with low levels of vWF‐Ag as found in this study might be explained both by reduced risk for severe complications and preservation from rapid progression of chronic liver diseases. Nevertheless, the findings regarding the cutoff for MELD‐Na–vWF at 20 points should be considered exploratory. Although these data clearly support the importance of vWF‐Ag assessment for patients on the waiting list for OLT, the sample size as well as the study type are not suitable for definition of a precise cutoff. In addition, it has to be acknowledged that this study relies on a single‐center data bank without external or internal validation. Yet, MELD‐Na–vWF clearly shows an improvement when compared with MELD‐Na alone, mostly through identification of patients with the highest risk for death while on the waiting list, which obtains a potential for renovation of the currently applied allocation system.
In conclusion, we found that vWF‐Ag, a cheap and easily accessible plasma protein, is able to independently predict 3‐month mortality in patients listed for OLT. Importantly, incorporation of vWF‐Ag in the MELD‐Na score was vital to significantly improve risk assessment for on‐list mortality. Ultimately, the data provided within this analysis suggest that the adoption of vWF‐Ag for the process of organ allocation could eventually result in a higher number of patients reaching the endpoint of transplantation, hence improving survival.
Author Contributions
G.P.G., D.P. substantial contributions to conception and design, data interpretation and writing the original draft; B.R., C.K., G.O. substantial contributions to acquisition of data and data analysis; H.H. statistical analysis; T.R., M.T. substantial contribution to conception and reviewing the manuscript; G.B., P.S. substantial contributions to conception and design, data interpretation, reviewing and editing the manuscript, project administration.
Supporting information
Potential conflict of interest: Dr. Trauner consults, is on the speakers’ bureau of, and received grants from Falk, Gilead, and Merck, Sharp, and Dohme. He consults for and received grants from Albireo and Intercept. He is on the speakers’ bureau of and received grants from Roche. He consults for Phenex, Novartis, Bristol‐Myers Squibb, and Regulus. He received grants from Takeda. All other authors have nothing to report.
REFERENCES
- 1. Dienstag JL, Cosimi AB. Liver transplantation—a vision realized. N Engl J Med 2012;367:1483‐1485. [DOI] [PubMed] [Google Scholar]
- 2. Lee SG. Twenty‐year survival post‐liver transplant: challenges and lessons. Hepatol Int 2015;9:342‐345. [DOI] [PubMed] [Google Scholar]
- 3. Brown RS, Jr . Expanding the bounds of liver transplantation. Liver Transpl 2014;20(Suppl. 2):S14‐S15. [DOI] [PubMed] [Google Scholar]
- 4. Lauterio A, Di Sandro S, Giacomoni A, De Carlis L. The role of adult living donor liver transplantation and recent advances. Expert Rev Gastroenterol Hepatol 2015;9:431‐445. [DOI] [PubMed] [Google Scholar]
- 5. Nemes B, Gámán G, Polak WG, Gelley F, Hara T, Ono S, et al. Extended criteria donors in liver transplantation Part I: reviewing the impact of determining factors. Expert Rev Gastroenterol Hepatol 2016;10:827‐839. [DOI] [PubMed] [Google Scholar]
- 6. Jochmans I, van Rosmalen M, Pirenne J, Samuel U. Adult liver allocation in Eurotransplant. Transplantation 2017;101:1542‐1550. [DOI] [PubMed] [Google Scholar]
- 7. Györi GP, Silberhumer GR, Rahmel A, de Vries E, Soliman T, Zehetmayer S, et al. Impact of dynamic changes in MELD score on survival after liver transplantation—a Eurotransplant registry analysis. Liver Int 2016;36:1011‐1017. [DOI] [PubMed] [Google Scholar]
- 8. Goldberg D, French B, Trotter J, Shetty K, Schiano T, Reddy KR, et al. Underreporting of liver transplant waitlist removals due to death or clinical deterioration: results at four major centers. Transplantation 2013;96:211‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, et al. Model for End‐Stage Liver Disease (MELD) and allocation of donor livers. Gastroenterology 2003;124:91‐96. [DOI] [PubMed] [Google Scholar]
- 10. Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, et al. Hyponatremia and mortality among patients on the liver‐transplant waiting list. N Engl J Med 2008;359:1018‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Umbro I, Tinti F, Fiacco F, Zavatto A, Piselli P, Di Natale V, et al. Resistive index and MELD‐Na: nephrologic monitoring in cirrhotic patients awaiting liver transplantation. Transplant Proc 2013;45:2676‐2679. [DOI] [PubMed] [Google Scholar]
- 12. Merion RM, Wolfe RA, Dykstra DM, Leichtman AB, Gillespie B, Held PJ. Longitudinal assessment of mortality risk among candidates for liver transplantation. Liver Transpl 2003;9:12‐18. [DOI] [PubMed] [Google Scholar]
- 13. Györi GP, Silberhumer GR, Zehetmayer S, Kern B, Hetz H, Soliman T, et al. Dynamic changes in MELD score not only predict survival on the waiting list but also overall survival after liver transplantation. Transpl Int 2012;25:935‐940. [DOI] [PubMed] [Google Scholar]
- 14. Onaca NN, Levy MF, Sanchez EQ, Chinnakotla S, Fasola CG, Thomas MJ, et al. A correlation between the pretransplantation MELD score and mortality in the first two years after liver transplantation. Liver Transpl 2003;9:117‐123. [DOI] [PubMed] [Google Scholar]
- 15. Huo TI, Lin HC, Huo SC, Lee PC, Wu JC, Lee FY, et al. Comparison of four model for end‐stage liver disease‐based prognostic systems for cirrhosis. Liver Transpl 2008;14:837‐844. [DOI] [PubMed] [Google Scholar]
- 16. Randi AM, Laffan MA. Von Willebrand factor and angiogenesis: basic and applied issues. J Thromb Haemost 2017;15:13‐20. [DOI] [PubMed] [Google Scholar]
- 17. Kawecki C, Lenting PJ, Denis CV. von Willebrand factor and inflammation. J Thromb Haemost 2017;15:1285‐1294. [DOI] [PubMed] [Google Scholar]
- 18. La Mura V, Reverter JC, Flores‐Arroyo A, Raffa S, Reverter E, Seijo S, et al. Von Willebrand factor levels predict clinical outcome in patients with cirrhosis and portal hypertension. Gut 2011;60:1133‐1138. [DOI] [PubMed] [Google Scholar]
- 19. Maieron A, Salzl P, Peck‐Radosavljevic M, Trauner M, Hametner S, Schöfl R, et al. Von Willebrand Factor as a new marker for non‐invasive assessment of liver fibrosis and cirrhosis in patients with chronic hepatitis C. Aliment Pharmacol Ther 2014;39:331‐338. [DOI] [PubMed] [Google Scholar]
- 20. Albornoz L, Alvarez D, Otaso JC, Gadano A, Salviú J, Gerona S, et al. Von Willebrand factor could be an index of endothelial dysfunction in patients with cirrhosis: relationship to degree of liver failure and nitric oxide levels. J Hepatol 1999;30:451‐455. [DOI] [PubMed] [Google Scholar]
- 21. Ferlitsch M, Reiberger T, Hoke M, Salzl P, Schwengerer B, Ulbrich G, et al. Von Willebrand factor as new noninvasive predictor of portal hypertension, decompensation and mortality in patients with liver cirrhosis. Hepatology 2012;56:1439‐1447. [DOI] [PubMed] [Google Scholar]
- 22. Mandorfer M, Schwabl P, Paternostro R, Pomej K, Bauer D, Thaler J, et al. Von Willebrand factor indicates bacterial translocation, inflammation, and procoagulant imbalance and predicts complications independently of portal hypertension severity. Aliment Pharmacol Ther 2018;47:980‐988. [DOI] [PubMed] [Google Scholar]
- 23. Huo TI, Lin HC, Lee FY, Hou MC, Lee PC, Wu JC, et al. Occurrence of cirrhosis‐related complications is a time‐dependent prognostic predictor independent of baseline model for end‐stage liver disease score. Liver Int 2006;26:55‐61. [DOI] [PubMed] [Google Scholar]
- 24. La Mura V, Nicolini A, Tosetti G, Primignani M. Cirrhosis and portal hypertension: the importance of risk stratification, the role of hepatic venous pressure gradient measurement. World J Hepatol 2015;7:688‐695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 2000;31:864‐871. [DOI] [PubMed] [Google Scholar]
- 26. Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end‐stage liver disease. Hepatology 2001;33:464‐470. [DOI] [PubMed] [Google Scholar]
- 27. Casadaban LC, Parvinian A, Zivin SP, Lakhoo J, Minocha J, Knuttinen MG, et al. MELD score for prediction of survival after emergent TIPS for acute variceal hemorrhage: derivation and validation in a 101‐patient cohort. Ann Hepatol 2015;14:380‐388. [PubMed] [Google Scholar]
- 28. Sharma P, Schaubel DE, Gong Q, Guidinger M, Merion RM. End‐stage liver disease candidates at the highest model for end‐stage liver disease scores have higher wait‐list mortality than status‐1A candidates. Hepatology 2012;55:192‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim WR, Therneau TM, Benson JT, Kremers WK, Rosen CB, Gores GJ, et al. Deaths on the liver transplant waiting list: an analysis of competing risks. Hepatology 2006;43:345‐351. [DOI] [PubMed] [Google Scholar]
- 30. O'Leary JG, Wong F, Reddy KR, Garcia‐Tsao G, Kamath PS, Biggins SW, et al. Gender‐specific differences in baseline, peak, and delta serum creatinine: the NACSELD experience. Dig Dis Sci 2017;62:768‐776. [DOI] [PubMed] [Google Scholar]
- 31. Somsouk M, Kornfield R, Vittinghoff E, Inadomi JM, Biggins SW. Moderate ascites identifies patients with low model for end‐stage liver disease scores awaiting liver transplantation who have a high mortality risk. Liver Transpl 2011;17:129‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heuman DM, Abou‐Assi SG, Habib A, Williams LM, Stravitz RT, Sanyal AJ, et al. Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death. Hepatology 2004;40:802‐810. [DOI] [PubMed] [Google Scholar]
- 33. Angermayr B, Luca A, König F, Bertolini G, Ploner M, Gridelli B, et al. Aetiology of cirrhosis of the liver has an impact on survival predicted by the Model of End‐stage Liver Disease score. Eur J Clin Invest 2009;39:65‐71. [DOI] [PubMed] [Google Scholar]
- 34. Kwong AJ, Goel A, Mannalithara A, Kim WR. Improved posttransplant mortality after share 35 for liver transplantation. Hepatology 2018;67:273‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nicolas CT, Nyberg SL, Heimbach JK, Watt K, Chen HS, Hathcock MA, et al. Liver transplantation after share 35: impact on pretransplant and posttransplant costs and mortality. Liver Transpl 2017;23:11‐18. [DOI] [PubMed] [Google Scholar]
- 36. Petrowsky H, Rana A, Kaldas FM, Sharma A, Hong JC, Agopian VG, et al. Liver transplantation in highest acuity recipients: identifying factors to avoid futility. Ann Surg 2014;259:1186‐1194. [DOI] [PubMed] [Google Scholar]
- 37. Atiemo K, Skaro A, Maddur H, Zhao L, Montag S, VanWagner L, et al. Mortality risk factors among patients with cirrhosis and a low Model for End‐Stage Liver Disease sodium score (≤15): an analysis of liver transplant allocation policy using aggregated electronic health record data. Am J Transplant 2017;17:2410‐2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kanaji S, Fahs SA, Shi Q, Haberichter SL, Montgomery RR. Contribution of platelet vs. endothelial VWF to platelet adhesion and hemostasis. J Thromb Haemost 2012;10:1646‐1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Herbig BA, Diamond SL. Pathological von Willebrand factor fibers resist tissue plasminogen activator and ADAMTS13 while promoting the contact pathway and shear‐induced platelet activation. J Thromb Haemost 2015;13:1699‐1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Razdan K, Hellums JD, Kroll MH. Shear‐stress‐induced von Willebrand factor binding to platelets causes the activation of tyrosine kinase(s). Biochem J 1994;302(Pt. 3):681‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bosch J, Groszmann RJ, Shah VH. Evolution in the understanding of the pathophysiological basis of portal hypertension: how changes in paradigm are leading to successful new treatments. J Hepatol 2015;62:S121‐S130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ferro D, Quintarelli C, Lattuada A, Leo R, Alessandroni M, Mannucci PM, et al. High plasma levels of von Willebrand factor as a marker of endothelial perturbation in cirrhosis: relationship to endotoxemia. Hepatology 1996;23:1377‐1383. [DOI] [PubMed] [Google Scholar]
- 43. Joshi N, Kopec AK, Ray JL, Cline‐Fedewa H, Groeneveld DJ, Lisman T, et al. Von Willebrand factor deficiency reduces liver fibrosis in mice. Toxicol Appl Pharmacol 2017;328:54‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Matsuyama T, Uemura M, Ishikawa M, Matsumoto M, Ishizashi H, Kato S, et al. Increased von Willebrand factor over decreased ADAMTS13 activity may contribute to the development of liver disturbance and multiorgan failure in patients with alcoholic hepatitis. Alcohol Clin Exp Res 2007;31:S27‐S35. [DOI] [PubMed] [Google Scholar]
- 45. Sun HJ, Chen J, Zhang H, Ni B, van Velkinburgh JC, Liu Y, et al. Von Willebrand factor protects against acute CCl4‐induced hepatotoxicity through phospho‐p38 MAPK signaling pathway inhibition. Immunol Res 2017;65:1046‐1058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


