Abstract
Background
Testicular ultrasound (US) is routinely employed in the evaluation of reproductive and sexual function. However, its use for characteristics other than testicular volume is hampered by a lack of information on the prognostic value of its findings, which to date have only been incorporated in a score proposed by Lenz et al in 1993.
Objectives
We sought to explore whether testicular US examination can predict the quality of spermatogenesis and provide information on testicular endocrine function.
Materials and methods
We retrospectively reviewed 6210 testicular US examinations, finally selecting examinations from 2230 unique men. The following variables were considered: bitesticular volume and testicular asymmetry, parenchymal echotexture, echogenicity and presence of microlithiasis, solid lesions and varicocoele. Concurrent fasting hormonal data were available for 1160 men, while 979 had a semen sample available from the same day as the US examination.
Results
We derived a new US score, termed TU score, that can predict both impaired spermatogenesis (AUC 0.73, sensitivity 72%, specificity 61%, P < .001) and hypogonadism (AUC 0.71, sensitivity 71%, specificity 53%, P < .001) more accurately than the Lenz's score. In a multivariate analysis, a reduced sperm composite index (defined as total spermatozoa × total motility × normal forms) was independently predicted by bitesticular volume and by inhomogeneous echotexture, while hypogonadism was independently predicted also by reduced echogenicity and presence of microlithiasis.
Discussion and conclusions
We describe the testicular US characteristics that are independently associated with impaired spermatogenesis and hypogonadism and propose the TU score as a simple screening method for use in subjects referred for testicular US.
Keywords: hypogonadism, infertility, spermatogenesis, testicular endocrine function, testicular ultrasound, testosterone
1. INTRODUCTION
Testicular US is routinely used in the assessment of male sexual and reproductive function, with both gray‐scale and color‐Doppler US imaging providing useful information in the assessment of testicular morphology and function. 1 It is the gold standard in assessing testicular volume (TV), 2 which tends to be overestimated by Prader's orchidometer. 3 A reduced TV, defined by most as being < 12 mL, 4 is associated with worse sperm parameters, 5 reduced fertility, 6 and hypogonadism. 7 , 8 Normal testicular echotexture comprises homogenously distributed medium‐level echoes, termed homogenous echotexture. 4 Conversely, the presence of areas of altered echogenicity, mostly hypoechoic, which can be subtle, ill‐defined focal or diffuse, has been termed testicular inhomogeneity (or inhomogenous echotexture). 9 Inhomogeneity has been associated with histological evidence of fibrosis, tubular sclerosis, and spermatogenic arrest, 10 , 11 and more recently, it has been proposed as an effective marker of male fertility. 12 Testicular echogenicity resembles the echogenicity of the normal thyroid gland and as a whole depends mostly on seminiferous tubule maturation and the testicular germ cell content, with the prepubertal testes being typically hypoechoic compared to adult. 4 As such, reduced echogenicity has been associated with reduced spermatogenesis and aberrant interstitial proliferation. 9
Testicular microlithiasis (TML) is an incidental US finding of multiple 1‐3 mm bright echogenic foci without acoustic shadowing 4 and is significant when at least 5 such foci are present in a single US scan. According to one classification, these foci can be described as limited, clusters or presenting a diffuse “starry‐sky” appearance. 13 TML is caused by calcium deposits in the seminiferous tubules 14 and has been associated with testicular germ cell neoplasia in situ (GCNIS), especially when other risk factors are also present. 15 , 16 However, not all studies agree on its malignant significance. 17 , 18 TML has also been suggested to be associated with infertility 19 and the testicular dysgenesis syndrome. 20 , 21
The volumetric predominance of one testis over the other, termed testicular asymmetry, has been proposed for the assessment of the functional ability of small testes, especially in patients with a history of cryptorchidism, and as a prognostic factor for varicocoele (VC) repair, with a cutoff for significance of ≥20%. 22 On US examination, non‐palpable testicular solid lesions are usually associated with benign conditions such as focal Leydig cell hyperplasia or Leydig cell tumors, 23 , 24 , 25 whereas palpable solid lesions show a high probability of malignancy. Both types appear to be associated with cryptorchidism and Leydig cell tumors also with impaired spermatogenesis. 23 Lastly, color‐Doppler imaging has been extensively employed in the detection and evaluation of testicular VC, which has been associated with ipsilateral hypotrophy and infertility, although this association is still under debate. 26 , 27
The investigation and description of these testicular characteristics on US is hampered by the relative lack of literature evidence supporting the prognostic significance of its findings in relation to spermatogenesis and testicular endocrine function: to date, they have only been incorporated in a US score proposed by Lenz in 1993. 28 The aim of this study was to explore whether the information derived from testicular US in a large retrospective study could reliably predict sperm parameters and testicular endocrine function.
2. MATERIALS AND METHODS
2.1. Patients
We retrospectively studied 6210 testicular B‐mode and color‐Doppler US examinations performed in our unit between March 2005 and December 2013, corresponding to 3150 unique subjects referred to a tertiary‐care andrological center (Section of Medical Pathophysiology, Food Science and Endocrinology, Department of Experimental Medicine, Rome, Italy). We excluded adolescents younger than 17 years [the age when maximum TV is reached 29 ], subjects with genetic conditions known to be associated with testicular damage (eg, Klinefelter syndrome and congenital hypogonadotropic hypogonadism), subjects who had undergone testicular or pituitary surgery, and individuals treated with drugs active on the hypothalamic‐pituitary‐testicular axis. The final study cohort was composed of 2230 patients (Figure 1). Reasons for referral for testicular US were available for 1531 subjects (68.7% of the total) and are summarized in Table 1. Concurrent hormonal data from the Department's Endocrine Laboratory were available for 1160 subjects, while 979 had undergone a semen analysis, carried out by the local Seminology Unit, on the same day as the testicular US. The Local Review Board approved the study, and all patients provided written informed consent.
Figure 1.
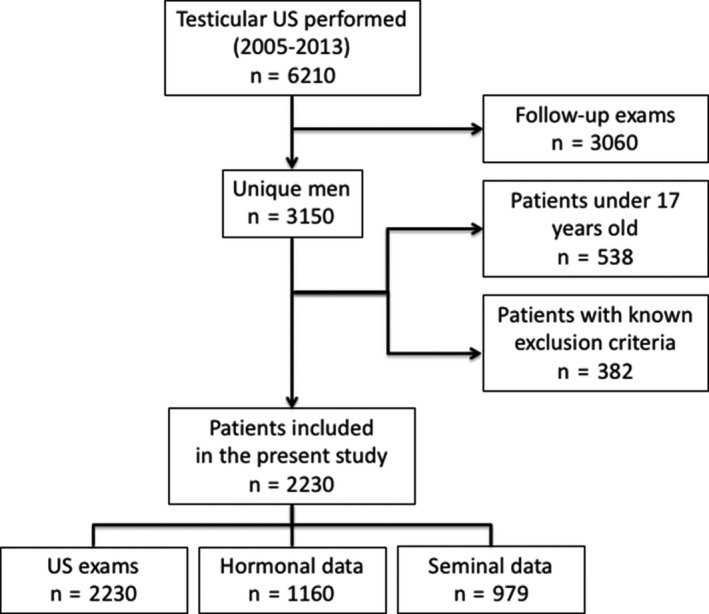
Flow diagram of patients included in the present study
Table 1.
Reason for testicular US referral (available for n = 1531)
| Reason for referral | N (%) |
|---|---|
| Varicocoele | 472 (30.8) |
| Infertility/Seminal alterations | 348 (22.7) |
| Screening and prevention campaign | 124 (8.1) |
| Scrotal pain | 109 (7.1) |
| Suspected testicular lesions | 101 (6.6) |
| Sexual dysfunctions | 69 (4.5) |
| Sexually transmitted diseases | 31 (2.0) |
| Hypogonadism | 28 (1.8) |
| Cryptorchidism | 26 (1.7) |
| Diabetes mellitus and obesity | 23 (1.5) |
| Gynecomastia | 12 (0.8) |
| Other | 190 (12.4) |
2.2. Hormonal evaluation
Morning baseline blood samples (07:45‐09:30 hours) were obtained from all subjects by antecubital venous puncture after an overnight fast. Serum follicle‐stimulating hormone (FSH), luteinizing hormone (LH), sex hormone–binding globulin (SHBG), 17β‐estradiol (E2) and total testosterone (TTe) were double‐measured with chemiluminescent microparticle immunoassay (CMIA, Architect System; Abbott Laboratories). Serum concentrations of inhibin B (INHB) were measured by enzyme‐linked immunosorbent assay (ELISA) (GEN II; Beckman Coulter Laboratories). The laboratory reference ranges for adult men were as follows: 1.38‐9.58 mIU/mL for FSH; 1.79‐8.17 mIU/mL for LH; 12.0‐38.20 nmol/L for TTe; 24‐108 pg/mL for E2; 80‐380 pg/mL for INHB and 11.1‐78.2 nmol/L for SHBG. We calculated absolute and percentage values of free testosterone (cfTe) by using the Vermeulen equation, 30 based on the SHBG levels and assuming a fixed albumin concentration of 4.3 g/dL. We defined eugonadism as TTe > 12 nmol/L and LH < 8.17 mIU/mL (thus excluding subclinical hypogonadism). We also calculated the total testosterone/LH ratio (TTe/LH), 31 markers of Leydig cell function, and the inhibin B/FSH ratio (INHB/FSH), 32 marker of Sertoli cell function.
2.3. Semen analysis
Semen samples were collected by masturbation directly into a sterile plastic container after 3‐5 days of sexual abstinence and examined by optical microscopy (Leica DM5000B; Leica Microsystems, Wetzlar, Germany) according to World Health Organization (WHO) criteria. 33 , 34 The following variables were assessed: sperm concentration (n × 106/mL), total sperm number (n × 106/ejaculate), total motility (%), and morphology (% normal forms). Total motility was selected instead of progressive motility in order to circumvent the differences in motility assessment between semen analyses conducted in accordance with WHO 1999 semen analysis criteria and those conducted from 2010 onward (and hence in accordance with the current WHO 2010 recommendations). 35 This assumption was cross‐validated by linear and logistic regression analyses employing data‐splitting for the pre‐2010 and post‐2010 cohorts, estimating the models in each and comparing the resulting models in terms of respective bs and R 2 values. This comparison led to similar results in both groups in terms of predictors and their coefficients; therefore, the analyses were reconducted on the cohort as a whole and these results are reported herein. We employed a composite index based on the Functioning Sperm Fraction (FSF) in the existing literature, 36 , 37 , 38 defined as the total number of motile spermatozoa in the ejaculate with a normal morphology using a cutoff of 0.625 × 106/ejaculate, derived from the 5th centiles of the WHO 2010 reference parameters (39 × 106 spermatozoa × 40% motility × 4% normal forms). 35
2.4. Ultrasound
US examinations were performed using a Philips IU22 unit (Philips, Bothell, WA, USA) with a 7‐15 MHz wideband linear transducer. The standardized protocol included axial and transverse examinations. 39 Two experienced sonographers performed all US examinations, stored the images and loops for subsequent analysis in our local image archiving system (eFilm Merge, Milwaukee, Wisconsin) and performed the revision in concert, blind to the written reports, while any dispute was adjudicated by a third author. All US examinations comprised the evaluation of the following parameters: left and right testicular volume, echotexture, echogenicity and presence of TML, solid lesions and left and/or right VC. TV was evaluated using the ellipsoid formula (height × width × length [cm] × 0.523) and expressed in mL as both left or right TV and bitesticular volume (BTV). After exploratory data analysis, testicular echotexture was categorized as either homogeneous or inhomogeneous and the results were compared to those obtained using Lenz's 5‐category classification. 28 Overall testicular echogenicity was graded as normal or reduced.
TML was initially graded as absent or isolate, mild (at least five microcalcifications per US scan), moderate (more than 10 per US scan), or starry sky (homogenous presence of high‐density microcalcifications in the testis). 40 However, exploratory data analysis revealed no significant differences between these groups in relation to the prediction of seminal and hormonal parameters, so only two groups were used for statistical analysis: absence or presence of TML.
We then evaluated the presence or absence of solid testicular lesions. Testicular asymmetry was evaluated according to the testicular atrophy index 41 and expressed as a percentage. A cutoff of 20% was adopted as distinctive of significant asymmetry, in accordance with the literature. 22 The presence of VC was assessed according to the Dubin‐Solbiati scale 4 and is reported on 5‐grades. Figure 2 shows typical US images of the evaluated features.
Figure 2.
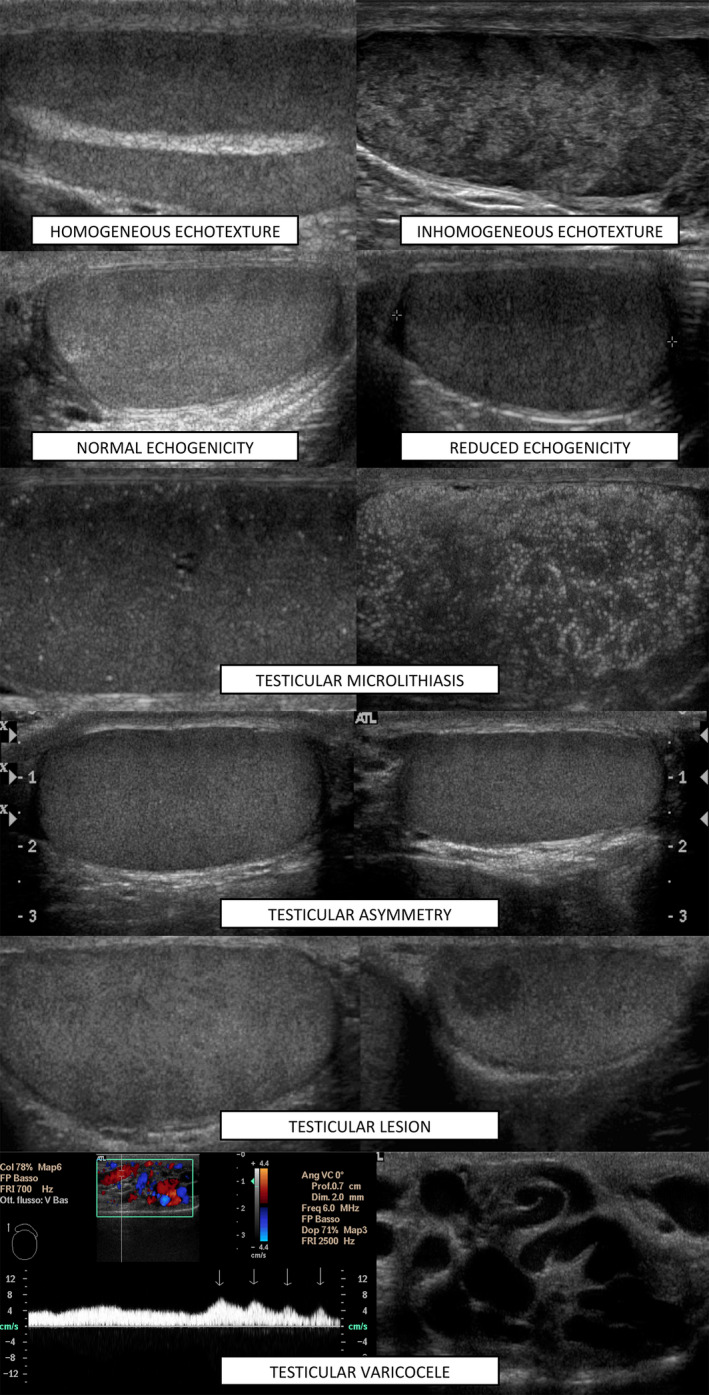
US features considered in developing the in‐house score (TU score)
2.5. Statistical analysis
Data are expressed as mean (M) and/or medians (m), as appropriate, and as standard deviations (SD), 95% confidence intervals (CI), and 25%‐75% interquartile ranges (IQR). Data distribution was visually inspected by analysis of the respective histograms and normality plots. Student's t test, linear regression, and logistic regression were used for normally distributed data. Effect sizes are expressed as odds ratios and as Cohen's ds with descriptors, as originally suggested by Cohen and later expanded. 42 The linear regression analyses used Stein's formula for adjusted R squared (Adj R 2) to evaluate how well the model cross‐validates across a different sample of data from the same population, 43 while for logistic regression analyses, we reported R 2 values according to Nagelkerke ( ). A semi‐quantitative scoring system called the TU score was created using the US characteristics deemed significant by logistic regression analyses. The scores were tuned through analysis of the receiver operating characteristic (ROC) curves for hormonal and semen parameters as follows: BTV (0 points if BTV ≥ 30 mL, +1 point if 24‐29.9 mL, +2 points if 20‐23.9 mL, +3 points if 14‐19.9 mL, +4 points if < 14 mL), inhomogeneous echotexture (+1 point), reduced echogenicity (+1 point), presence of TML (+1 point) (Table 2). The score ranged from 0 to 7, with higher values indicating a more compromised condition. A cutoff of ≥ 2 was derived as a compromise between sensitivity and specificity, with preference given to the former. ROC curves’ AUC were compared according to DeLong. 44 In the correlation analyses, we used Pearson's correlation coefficient (r) and Spearman's correlation coefficient (rS) for normally and non‐normally distributed data, respectively. The level of statistical significance was set to .05. All statistical computations were conducted with IBM SPSS Statistics for Windows, version 25 (IBM Corp.), and MedCalc, version 19.2.1 (MedCalc Software Ltd).
Table 2.
TU score created from the different testicular US characteristics through analysis of the ROC curves for hormonal and semen parameters; the score ranges from 0 to 7
| TU score | 0 | +1 | +2 | +3 | +4 |
|---|---|---|---|---|---|
| BTV (mL) | >30 | 24‐29.9 | 20.0‐23.9 | 14.0‐19.9 | <14.0 |
| Echotexture | Normal | Inhomogeneous | |||
| Microlithiasis | Absent | Present | |||
| Echogenicity | Normal | Reduced | |||
| Lenz's score (1993) | |||||
| 1 | “uniform pattern” | ||||
| 2 | “slightly irregular pattern” | ||||
| 3 | “irregular pattern with small echogenic points” | ||||
| 4 | “very irregular pattern” or “with coarse echogenic points” | ||||
| 5 | “irregular with demarcated areas raising suspicion of a tumor” | ||||
Lenz's original score is reported below; the score ranges from 1 to 5. For both scores, higher values indicate a more compromised condition.
Abbreviations: BTV, bitesticular volume; ROC, receiver operator characteristic; TU, testicular ultrasound; US, ultrasound.
3. RESULTS
Semen parameters, hormonal data, TVs, the Lenz's and the TU scoring systems, both applied on our cohort, are shown in Table 3, while the US findings are summarized in Table 4. The mean age of the participants was 32.5 ± 10.3 years.
Table 3.
Semen parameters, hormonal data and testicular volumes for all patients; Lenz's and TU scores values
| Median [25%‐75% IQ ranges] | |
|---|---|
| Age (years)* | 32.5 ± 10.3 |
| Spermatozoa concentration (×106/mL) | 25.0 [3.0‐62.0] |
| Spermatozoa in ejaculate (×106) | 67.5 [8.0‐180.0] |
| Semen total motility (%) | 40.0 [20.0‐50.0] |
| Semen typical morphology (%) | 20.0 [12.0‐26.0] |
| Composite index (×106) | 4.0 [0.04‐20.1] |
| FSH (mIU/mL) | 4.0 [2.6‐7.3] |
| LH (mIU/mL) | 3.1 [2.3‐4.5] |
| Total testosterone (nmol/L) | 18.8 [14.7‐23.5] |
| SHBG (nmol/L) | 34.7 [25.0‐44.5] |
| Calculated free testosterone (ng/dL) | 10.9 [8.3‐14.1] |
| 17β‐estradiol (pg/mL) | 26.0 [20.0‐32.0] |
| Inhibin B (pg/mL) | 81.0 [34.5‐129.5] |
| TTe/LH ratio | 6.0 [3.9‐8.8] |
| INHB/FSH ratio | 21.5 [4.8‐45.5] |
| Right testicular volume (mL)* | 15.0 ± 5.4 |
| Left testicular volume (mL)* | 14.0 ± 5.0 |
| Bitesticular volume (mL)* | 29.0 ± 9.7 |
| Lenz's score | 1.0 [1.0‐2.0] |
| TU score | 1 [0‐3] |
Parameters marked by * show normal distribution and are presented as mean ± SD.
Abbreviations: FSH, follicle‐stimulating hormone; INHB/FSH, inhibin B/follicle‐stimulating hormone (ratio); LH, luteinizing hormone; SHBG, sex hormone–binding globulin; TTe/LH, total testosterone/luteinizing hormone (ratio); TU, testicular ultrasound.
Table 4.
Testicular ultrasound characteristics of the entire study cohort
|
N 2230 |
% | N 2230 | % | ||
|---|---|---|---|---|---|
| BTV (mL) | Solid lesions | ||||
| Normal (≥24 mL) | 1570 | 70.4 | Absent | 2152 | 96.5 |
| Reduced (<24 mL) | 660 | 29.6 | Present | 78 | 3.5 |
| Asymmetry | Left Varicocoele a | 1266 | 56.8 | ||
| <20% (not significant) | 1648 | 73.9 | 1 | 232 | 10.4 |
| ≥20% (significant) | 582 | 26.1 | 2 | 476 | 21.3 |
| Echotexture | 3 | 402 | 18 | ||
| Homogeneous | 1365 | 61.2 | 4 | 149 | 6.7 |
| Inhomogeneous | 865 | 38.8 | 5 | 7 | 0.3 |
| Microlithiasis | 85 | 3.9 | Right Varicocoele a | 237 | 10.6 |
| Absent | 2143 | 96.1 | 1 | 136 | 6.1 |
| Mild | 86 | 3.9 | 2 | 85 | 3.8 |
| Echogenicity | 3 | 14 | 0.6 | ||
| Normoechoic | 1969 | 88.3 | 4 | 1 | 0 |
| Hypoechoic | 261 | 11.7 | 5 | 1 | 0 |
Abbreviation: BTV, bitesticular volume.
Varicocoele was graded according to the Dubin‐Solbiati scale. 4
3.1. Ultrasound characteristics and testicular volumes
There was a significant correlation between the volumes of the two testicles (r = .763; P < .001), although the mean volume of the right testis (15.0 ± 5.4 mL) was significantly larger than the left (14.0 ± 5.0 mL) (P < .001). No correlation was found between age and BTV (P = .584). Patients with significant testicular asymmetry (26.1%) had a lower BTV than those without asymmetry (mean difference [MD] −2.6 mL; P < .001). Homogeneous testicular echotexture was found in 61.2% of patients and was associated with a larger BTV (MD 5.7 mL; P < .001). Accordingly, most of the patients had Lenz's score 1 or 2 (Tables 1 and 3), and the grade was inversely and significantly correlated with BTV (P < .001). With regard to testicular echogenicity, 88.3% presented a normoechoic testicular parenchyma, while the remainder showed reduced echogenicity (Table 4). Patients with normal echogenicity had a significantly larger BTV (MD 10.4 mL; P < .001).
TML was found in 87 patients, in 74% of whom it was mild. Patients with TML had a lower BTV than those without (MD −5.6 mL; P < .001). There was no significant correlation between the grade of TML and BTV. Solid testicular lesions were found in 3.5% of patients (Table 4), and these patients exhibited a lower BTV (MD −5.4 mL; P < .001) compared to those with absence of lesions.
A variable grade of VC was detected in 58.5% of patients, with the vast majority (97%) affecting the left side. Grade 2 VC was the most prevalent, followed by grade 3 (Table 4), with 8.9% of patients showing bilateral involvement. The patients with VC were younger than those unaffected (MD −2.5 years; P < .001), and the grade of VC was negatively correlated with age (P < .001).
3.2. Relationship between ultrasound characteristics and semen parameters
The composite index (>0.625 × 106/ejaculate as an expression of normal spermatogenesis) was found to be significantly lower in patients with reduced BTV (P < .001), TML (P = .018), inhomogenous echotexture (P < .001), reduced echogenicity (P < .001), presence of solid lesions (P < .001), and significant asymmetry (P = .012); these differences reflected small‐to‐medium effects. Table 5 describes the significant mean differences in semen parameters between groups with different US characteristics. We conducted a linear regression analysis to ascertain which US parameters were able to predict independently semen parameters; the significant results are summarized in Table 6 and reported below. BTV and echotexture were predictors of sperm concentration (×106/mL), total sperm number (×106), total sperm motility (%), percentage of normal forms (%), and the composite index (×106/ejaculate) (P < .001 in all cases). The findings were maintained even when adjusted for the presence of VC. A logistic regression analysis (Table 7) revealed that the only testicular US parameters able independently to predict a normal semen sample, and hence spermatogenesis, were the BTV and the presence of a normal echotexture. For every unit rise in BTV, the odds ratio (OR) for normal composite index was 1.089 (P < .001), while the presence of an inhomogeneous echotexture was associated with an OR of 0.454 (P < .001) (Table 7). The model was found to be a good fit of the data, with an overall predicted correct percentage of 74%. The ROC curve analysis of the TU score's ability to predict a reduced sperm composite index (Figure 3A) showed an area under the curve (AUC) of 0.73 (95% CI, 0.70‐0.76, P < .001), significantly greater than the ROC obtained by applying Lenz's score (AUC = 0.66, 95% CI 0.63‐0.69, P < .001 for the comparison), with a cutoff ≥ 2 points associated with a sensitivity of 72% and a specificity of 61%.
Table 5.
Semen parameters and hormonal data showing a significant mean difference with testicular US characteristics
| Mean difference (95% CI) | P | d a | ||
|---|---|---|---|---|
| Spermatozoa/mL ± SD (×106) | ||||
| BTV | 58.6 ± 65.1 vs 18.2 ± 28.5 | 40.42 (34.3, 46.54) | <.001 | 0.62 |
| TML | 28.1 ± 33.8 vs 44.3 ± 58.7 | −16.18 (5.1, 27.25) | .005 | 0.28 |
| Echotexture | 55.94 ± 58.39 vs 29.85 ± 54.07 | 26.09 (18.81, 33.37) | <.001 | 0.45 |
| Echogenicity | 46.71 ± 57.54 vs 26.18 ± 56.6 | 20.54 (10.29, 30.78) | <.001 | 0.36 |
| Solid lesions | 21.52 ± 33.85 vs 44.8 ± 58.65 | −23.28 (12.64, 33.92) | <.001 | 0.40 |
| Asymmetry | 33 ± 35.83 vs 47.4 ± 63.58 | −14.39 (−20.97, −7.81) | <.001 | 0.23 |
| Total motility ± SD (%) | ||||
| BTV | 39.7 ± 16.5 vs 25.7 ± 17.6 | 13.96 (11.4, 16.51) | <.001 | 0.84 |
| Echotexture | 39.6 ± 16.5 vs 29.7 ± 18.5 | 9.92 (7.41, 12.42) | <.001 | 0.60 |
| Echogenicity | 36.7 ± 17.5 vs 26.1 ± 19.1 | 10.61 (6.7, 14.53) | <.001 | 0.61 |
| Solid lesions | 27.9 ± 19.1 vs 35.7 ± 17.9 | −7.72 (0.94, 14.5) | .027 | 0.43 |
| Normal forms ± SD (%) | ||||
| BTV | 21 ± 9.9 vs 15.5 ± 10.9 | 5.51 (3.91, 7.12) | <0.001 | 0.56 |
| Echotexture | 21.2 ± 9.9 vs 16.9 ± 10.9 | 4.27 (2.79, 5.75) | <0.001 | 0.43 |
| Echogenicity | 19.9 ± 10.3 vs 15.5 ± 11.4 | 4.39 (2.06, 6.72) | <0.001 | 0.43 |
| Composite Index ± SD (×106/ejaculate) | ||||
| BTV | 22.2 ± 34 vs 5.86 ± 13.4 | 16.35 (13.23, 19.47) | <.001 | 0.48 |
| TML | 10 ± 16.2 vs 16.4 ± 29.7 | −6.48 (1.13, 11.83) | .018 | 0.22 |
| Echotexture | 21.7 ± 33.9 vs 9.9 ± 21.2 | 11.81 (8.18, 15.45) | <.001 | 0.35 |
| Echogenicity | 17.7 ± 30.7 vs 7.7 ± 16.5 | 9.95 (6.46, 13.44) | <.001 | 0.35 |
| Solid lesions | 6.5 ± 12.9 vs 16.7 ± 29.8 | −10.13 (5.88, 14.38) | <.001 | 0.34 |
| Asymmetry | 12.7 ± 21.5 vs 17.4 vs 31.5 | −4.63 (−8.23, −1.03) | .012 | 0.15 |
| FSH ± SD (mIU/mL) | ||||
| BTV | 4.05 ± 3.28 vs 10.87 ± 11.47 | −6.81 (−7.96, −5.67) | <.001 | 2.07 |
| TML | 13.05 ± 16.63 vs 6.31 ± 7.38 | 6.74 (−11.3, −2.18) | .005 | 0.91 |
| Echotexture | 4.15 ± 3.53 vs 9.3 ± 10.62 | −5.15 (−6.11, −4.19) | <.001 | 1.46 |
| Echogenicity | 5.55 ± 6.46 vs 12.35 ± 12.82 | −6.8 (−8.77, −4.83) | <.001 | 1.05 |
| Solid lesions | 14.04 ± 13.52 vs 6.23 ± 7.62 | 7.82 (−11.5, −4.13) | <.001 | 1.03 |
| Asymmetry | 8.19 ± 9.94 vs 6.07 ± 7.41 | 2.13 (0.87, 3.38) | .001 | 0.29 |
| LH ± SD (mIU/mL) | ||||
| BTV | 3.14 ± 1.67 vs 5.23 ± 4.9 | −2.08 (−2.57, −1.59) | <.001 | 1.25 |
| TML | 6.06 ± 6.64 vs 3.82 ± 3.16 | 2.23 (−4.06, −0.41) | .017 | 0.71 |
| Echotexture | 3.2 ± 1.63 vs 4.71 ± 4.52 | −1.51 (−1.92, −1.1) | <.001 | 0.93 |
| Echogenicity | 3.53 ± 2.52 vs 6.04 ± 5.95 | −2.51 (−3.4, −1.61) | <.001 | 1.54 |
| Solid lesions | 6.2 ± 5.54 vs 3.81 ± 3.25 | 2.39 (−3.88, −0.89) | .002 | 0.74 |
| Asymmetry | 4.53 ± 4.79 vs 3.71 ± 2.72 | 0.82 (0.25, 1.39) | .005 | 0.30 |
| Total Testosterone ± SD (ng/dL) | ||||
| BTV | 579.9 ± 192.8 vs 527.4 ± 191.9 | 52.5 (28.9, 76.2) | <.001 | 0.27 |
| TML | 454.1 ± 210.1 vs 565.2 ± 191.8 | −111.1 (50.32, 171.87) | .001 | 0.58 |
| Echotexture | 592 ± 188.6 vs 525.6 ± 194.1 | 66.4 (43.6, 89.27) | <.001 | 0.35 |
| Echogenicity | 572 ± 189.7 vs 497.9 ± 204.6 | 74.11 (41.13, 107.08) | <.001 | 0.39 |
| Inhibin B ± SD (pg/mL) | ||||
| BTV | 116.8 ± 74.5 vs 45 ± 49.5 | 71.9 (62.4, 81.3) | <.001 | 0.97 |
| TML | 56 ± 51.1 vs 91.6 ± 75.4 | −35.57 (14.97, 56.16) | .001 | 0.47 |
| Echotexture | 114.4 ± 78.2 vs 63 ± 60.3 | 51.44 (40.83, 62.1) | <.001 | 0.66 |
| Echogenicity | 98.4 ± 75.5 vs 46.1 ± 52.9 | 52.29 (40.31, 64.27) | <.001 | 0.69 |
| Solid lesions | 40.4 ± 49.1 vs 92.4 ± 75 | −51.96 (32.14, 71.78) | <.001 | 0.69 |
| Asymmetry | 74.4 ± 80.5 vs 96.5 ± 71.5 | −22.1 (−35.26, −8.93) | .001 | 0.31 |
Group comparisons were conducted as follows: BTV: ≥24 mL vs < 24 mL, TML: present (any grade) vs absent, Echotexture: homogenous vs inhomogeneous, Echogenicity: normoechoic vs hypoechoic, Solid lesions: present vs absent, Asymmetry: present vs absent.
Abbreviations: BTV, bitesticular volume; FSH, follicle‐stimulating hormone; LH, luteinizing hormone; TML, testicular microlithiasis; TTe, total testosterone; US, ultrasound.
Cohen's d.
Table 6.
Linear model of US predictors of seminal parameters and hormones
| β | b | P | |
|---|---|---|---|
| Semen analysis | |||
| Sperm concentration | |||
| BTV (mL) | 0.374 | 2.232 (1.853, 2.611) | <.001 |
| Inhomogeneous echotexture | −0.135 | −15.642 (−23.233, −8.050) | <.001 |
| Total sperm motility | |||
| BTV (mL) | 0.359 | 0.726 (0.592, 0.860) | <.001 |
| Inhomogeneous echotexture | −0.203 | −7.383 (−9.920, −4.847) | <.001 |
| Normal sperm morphology | |||
| BTV (mL) | 0.263 | 0.311 (0.229, 0.394) | <.001 |
| Inhomogeneous echotexture | −0.156 | −3.312 (−4.873, −1.752) | <.001 |
| Composite index | |||
| BTV (mL) | 0.337 | 1.016 (0.821, 1.212) | <.001 |
| Inhomogeneous echotexture | −0.124 | −7.275 (−11.191, −3.359) | <.001 |
| Hormonal data | |||
| FSH | |||
| BTV (mL) | −0.401 | −0.327 (−0.372, −0.282) | <.001 |
| Inhomogeneous echotexture | 0.131 | 2.149 (1.213, 3.085) | <.001 |
| Reduced echogenicity | 0.074 | 1.659 (0.366, 2.951) | .012 |
| TML | 0.076 | 2.837 (0.852, 4.822) | .005 |
| Solid lesions | 0.088 | 3.257 (1.260, 5.255) | .001 |
| LH | |||
| BTV (mL) | −0.302 | −0.104 (−0.124, −0.084) | <.001 |
| Reduced echogenicity | 0.107 | 1.000 (0.421, 1.578) | <.001 |
| TML | 0.058 | 0.927 (0.029, 1.825) | .043 |
| Solid lesions | 0.065 | 1.017 (0.122, 1.912) | .026 |
| Total testosterone | |||
| BTV (mL) | 0.102 | 0.068 (0.026, 0.110) | .002 |
| Inhomogeneous echotexture | −0.124 | −1.673 (−2.556, −0.790) | <.001 |
| TML | −0.088 | −2.818 (−4.777, −0.860) | .005 |
| Inhibin B | |||
| BTV (mL) | 0.469 | 3.415 (2.912, 3.919) | <.001 |
| Inhomogeneous echotexture | −0.175 | −26.274 (−36.922, −15.627) | <.001 |
Only the variables included in the models are reported in the table. 95% confidence intervals reported in parentheses.
Abbreviations: BTV, bitesticular volume; FSH, follicle‐stimulating hormone; LH, luteinizing hormone TML, testicular microlithiasis; US, ultrasound; VC, varicocoele.
Table 7.
Logistic regression analysis models for impaired spermatogenesis and hypogonadism
| Variables in the equation | β | P | OR | 95% CI Lower bound | 95% CI Upper bound | ||
|---|---|---|---|---|---|---|---|
| Normal composite index | BTV (mL) | 0.085 | <.001 | 1.089 | 1.068 | 1.110 | |
| Inhomogeneous echotexture | −0.789 | <.001 | 0.454 | 0.328 | 0.628 | ||
| Eugonadism | BTV (mL) | 0.059 | <.001 | 1.060 | 1.038 | 1.083 | |
| Inhomogeneous echotexture | −0.619 | .004 | 0.538 | 0.354 | 0.819 | ||
| Reduced echogenicity | −0.697 | .002 | 0.498 | 0.318 | 0.780 | ||
| TML | −0.857 | .013 | 0.424 | 0.216 | 0.834 |
Normal composite index: = .236, model χ2 172.927, P < .001. Eugonadism: = .191, model χ2 127,417, P < .001.
Abbreviations: BTV, bitesticular volume; TML, testicular microlithiasis.
Figure 3.
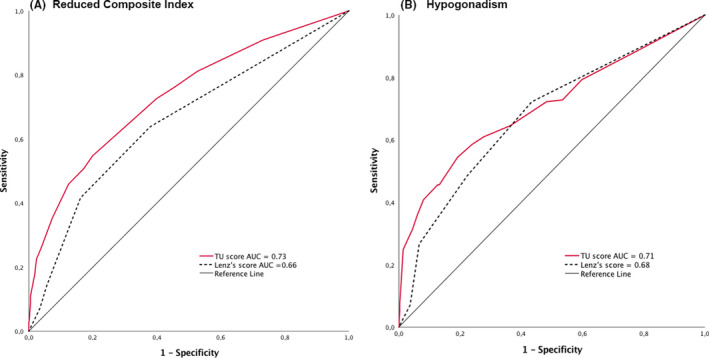
Left (a): ROC curve analysis of the TU score's ability to predict a reduced composite index; right (b): ROC curve analysis of the TU score's ability to predict hypogonadism, defined as TTe < 12 nmol/L
3.3. Relationship between ultrasound characteristics and hormonal values
FSH and LH values (mUI/mL) were significantly higher among patients with reduced BTV (P < .001 for both), presence of TML (P = .005 and P = .017), presence of inhomogenous echotexture (P < .001 for both), reduced echogenicity (P < .001 for both), presence of solid lesions (P < .001 and P = .002), and significant asymmetry (P = .001 and P = .005). TTe values (ng/dL) were significantly lower in patients with reduced BTV (P <.001), presence of TML (P = .001), presence of inhomogenous echotexture (P < .001), and reduced echogenicity (P < .001) (Table 5). On the contrary, cfTe (ng/dL) values were not found to significantly differ according to ultrasound parameters (not shown). The TTe/LH, a marker of Leydig cell function, was lower in patients with reduced BTV (5.48 ± 5.38 vs 7.52 ± 3.70; P < .001), significant asymmetry (6.30 ± 4.21 vs 6.93 ± 4.62; P = .036), inhomogeneous echotexture (5.91 ± 5.17 vs 7.53 ± 3.66; P < .001), reduced echogenicity (5.35 ± 7.25 vs 7.01 ± 3.75; P = .004), TML (5.18 ± 5.48 vs 6.83 ± 4.45; P = .043), and presence of solid lesions (4.52 ± 3.39 vs 6.88 ± 4.54; P < .001). The INHB/FSH, a marker of Sertoli cell function, was significantly lower in patients with reduced BTV (12.92 ± 19.18 vs 46.57 ± 44.44; P < .001), significant asymmetry (22.9 ± 26.67 vs 38.66 ± 44.21; P = .001), inhomogeneous echotexture (46.12 ± 43.78 vs 20.71 ± 31.63; P < .001), reduced echogenicity (15.52 ± 27.7 vs 37.53 ± 41.59; P < .001), TML (14.39 ± 18.6 vs 34.95 ± 41.01; P < .001), solid lesions (12.3 ± 20.42 vs 35.1 ± 40.9; P < .001). Among hypogonadal patients, 43.8% had a primary and 56.2% a secondary (or mixed) hypogonadism (based on TTe and LH values). A linear regression analysis was conducted to determine which US parameters were able to predict independently hormonal values; the significant results are summarized in Table 6. FSH values were predicted negatively by BTV (P < .001) and positively by inhomogeneous echotexture (P < .001), reduced echogenicity (P = .012), presence of TML (P = .005), and presence of solid lesions (P = .001). LH values were predicted negatively by BTV (P < .001) and positively by reduced echogenicity (P < .001), presence of TML (P = .043), and presence of solid lesions (P = .026). TTe values were predicted positively by BTV (P = .002) and negatively by presence of inhomogeneous echotexture (P < .001) and TML (P = .005). Inhibin B values were predicted positively by BTV (P < .001) and negatively by presence of inhomogeneous echotexture (P < .001). cfTe values were not predicted by any ultrasound characteristic alone (P = .847 for the model, not shown). The findings were maintained even when adjusted for the presence of VC.
A logistic regression analysis was also conducted to analyze which US parameters were able to predict independently eugonadism, as defined above. BTV was positively associated with normal TTe and LH values (OR 1.060 per mL; P < .001), while the following characteristics were associated with an increased risk of hypogonadism: presence of inhomogeneous echotexture (OR 0.538; P = .004), reduced echogenicity (OR 0.498; P = .002), and presence of TML (OR 0.424; P = .013) (Table 7). The model was found to be a good fit of the data, with an overall predicted correct percentage of 86.5%. The ROC curve analysis of the TU score's ability to predict hypogonadism showed an AUC of 0.71 (95% CI 0.69‐0.73, P < .001), significantly greater than the ROC obtained by applying Lenz's score (AUC = 0.68, 95% CI 0.65‐0.69, P = .008 for the comparison) with a cutoff ≥ 2 points associated with a sensitivity of 71% and a specificity of 53% (Figure 3B).
3.3.1. Odds ratios
We proceeded to determine the OR for normal gonadal function in our cohort of patients, to calculate the risk of a patient whose testicular US is available for reduced spermatogenesis and hypogonadism. As a reference, the prevalence of reduced composite index in our cohort of patients with a BTV of at least 24 mL and a homogenous echotexture was 18.3%, compared with an overall prevalence of 37.7%. As for hypogonadism, the prevalence among patients with a BTV of at least 24 mL, a homogenous echotexture, a normal echogenicity and absence of TML was 8.6%, compared with an overall prevalence of 17.6%.
3.3.2. ORs for normal composite index
The two parameters independently predicting a normal composite index were BTV and echotexture (Table 8). Assuming as reference an individual with a BTV of 24 mL and a homogenous echotexture, we can derive a baseline OR for a normal composite index of 26.14. By comparison, a patient presenting with a BTV of 16 mL and a homogenous echotexture will have a baseline OR of 17.42, while a patient with a BTV of 20 mL and a inhomogenous echotexture will have a baseline OR of 9.9. The first patient will present an OR for a normal composite index compared with our reference of 0.67 and as such a 33% higher risk of having a reduced semen quality, while the second patient will present an OR for a normal composite index of 0.38, with a 62% higher risk of presenting a reduced semen quality. Table 8 provides constant (α) values to be used. Constant (α) and Reference OR for BTV values are already set (respectively, 1.089 and 26.14), while echotexture α values should be chosen and inserted in the formula accordingly.
Table 8.
Constant (α) and Reference OR values to be used to calculate odds for normal composite index and eugonadism according to US parameters observed (see the text). Constant (α) and Reference OR for bitesticular volume values are already set (in bold), while echotexture, echogenicity, and microlithiasis α values should be chosen and inserted in the formula accordingly
| Reference OR (24 mL) | ||
|---|---|---|
| Normal composite index | ||
| Bitesticular volume | 1.089 (per mL) | 26.14 |
| Constant (α) | ||
| + homogeneous echotexture | 1.000 | |
| + inhomogeneous echotexture | 0.454 | |
| Eugonadism | ||
| Bitesticular volume | 1.060 (per mL) | 25.44 |
| Constant (α) | ||
| + homogeneous echotexture | 1.000 | |
| + normal echogenicity | 1.000 | |
| + absence of microlithiasis | 1.000 | |
| + inhomogeneous echotexture (IE) | 0.538 | |
| + reduced echogenicity (RE) | 0.498 | |
| + presence of microlithiasis (TML) | 0.424 | |
| + IE + RE | 0.268 | |
| + IE + TML | 0.228 | |
| + RE + TML | 0.211 | |
| + IE + RE +TML | 0.114 | |
Abbreviations: OR, odds ratio; US, ultrasound.
3.3.3. ORs for eugonadism
Four different ultrasound parameters are able to predict eugonadism: BTV, echotexture, echogenicity, and presence of TML (Table 8). Assuming our reference standard to have a BTV of 24 mL, a homogenous echotexture, normal echogenicity, and absence of TML, he will possess a baseline OR of 25.44. By comparison, a patient with a BTV of 30 mL, homogenous echotexture, normal echogenicity, and presence of TML will have a baseline OR of 13.48, while a patient with a BTV of 20 mL, inhomogenous echotexture, reduced echogenicity, and absence of TML will have a baseline OR of 5.68. The first patient will present an OR of 0.53 and as such a 47% higher risk of hypogonadism compared with our reference, while the second patients will have an OR of 0.22 and as such a 78% higher risk of hypogonadism. Table 8 provides constant (α) values to be used. Constant (α) and Reference OR for bitesticular volume values are already set (respectively, 1.060 and 25.44), while echotexture, echogenicity, and microlithiasis α values should be chosen and inserted in the formula accordingly.
4. DISCUSSION
To our knowledge, this is the first comprehensive study to incorporate and correlate the various testicular US, endocrine, and sperm parameters data from a large number of patients referred to a tertiary‐care andrological center, encompassing the entire spectrum of gonadal function (from normal to severely compromised). Aim of our work was to assess whether, and to what extent, testicular US is informative for normal sperm parameters (as a surrogate fertility marker) and eugonadal state, and which US parameters are most informative.
Knowledge of the many clinical information ultrasonography can provide in testicular disease workup is gaining momentum, as US scan is now becoming a readily available tool in the andrologist's office. The increasing number of surveillance programs assessing men's health also contributed to an expansion of testicular US scans. Thus, there is the need to simplify and standardize the diagnostic workup to avoid unnecessary laboratory requests. 45 Lenz et al 28 , 46 , 47 developed a US score as a possible screening method for testicular function based on testicular biopsies performed on patients affected by contralateral testicular tumors. Their score considered five possibilities, ranging from 1 (normal testis) to 5 (testis affected by a solid lesion). They found that a higher score was correlated with a lower sperm count, negatively correlated with US testicular volume and not correlated with tubular membrane thickness or the estimated number of Leydig cells. These findings supported the theory that irregular echotexture reflects testicular damage and that there is a correlation between testicular function, US volume and echotexture.
With this in mind, the TU score was developed on the assumption that testicular US can provide not only morphological but also functional information and that each feature observed during an US examination might offer a different piece of information and have its own weight in relation to testicular health and should therefore be considered separately. TML may be found in otherwise homogeneous and normally functioning testes; solid lesions can develop in testes with normal volume and function; reduction in gonadal function can be found in slightly inhomogeneous testes. The TU score is accurate and comprehensive, considers the most important features that should always be described in an US report, and has proved significantly more accurate than Lenz's score in predicting both impaired spermatogenesis and hypogonadism. The high statistical significance of linear and logistic regression models performed confirms the relevance of US‐assessed BTV, positively correlated with fertility status, testosterone levels, and TTe/LH ratio, 48 but also underscores the importance of echotexture and echogenicity, which should always be considered. Reduced BTV and inhomogeneous echotexture appear to be risk factors for reduced composite index, as a surrogate fertility marker, while reduced BTV, inhomogeneous echotexture, reduced echogenicity, and the presence of TML appear to be all risk factors for hypogonadism. This is consistent with the current awareness that inhomogeneous echotexture is indicative of testicular fibrosis, tubular sclerosis, and spermatogenic arrest, 11 while reduced echogenicity has also been associated with interstitial proliferation, 9 , 49 possibly involving Leydig cell function, although this finding needs to be further confirmed by future studies. The role of TML in predicting hypogonadism is less clear; hypogonadism could reflect other congenital or acquired risk factors, as it is associated with conditions such as cryptorchidism or testicular dysgenesis syndrome. 50
Taking into account our results, derived from a large cohort of subjects ranging from potentially “healthy” individuals—recruited during prevention campaigns—to testicular cancer patients, we calculated the individual TU score and recommend that patients scoring ≥ 2 should undergo further evaluation. Although the decision to request a semen analysis and/or hormonal evaluation in the clinical setting is based on clinical history, presenting complaint, and physical examination, the rationale of creating an US score is to provide the less experienced sonographer, or the non‐andrologists, with a more objective tool to triage patients toward the need for further investigations or follow‐up. Similarly, given the score's good ability to predict testicular function, it could be used, in the setting of large screening programs, to select those asymptomatic patients who can avoid further analyses, saving time and money. Finally, we derived a formula to calculate specific ORs for a normal composite index and eugonadism.
The strengths of this study are its high statistical power, deriving from a large cohort of patients, and the availability of hormonal data, semen analyses, and US examinations, all performed at a single tertiary center. It is limited by the lack of complete data on the various reasons why patients were originally referred to our center, and on the risk factors shown to be associated with reduced fertility. However, we provided US reasons of referral for approximately two thirds of our cohort and have excluded all patients with known genetic conditions associated with altered testicular function, patients who had undergone testicular or pituitary surgery and those on therapy with drugs active on the hypothalamic‐pituitary‐testicular axis. Another drawback was the need to consider total sperm motility instead of progressive motility, as patients were enrolled both before and after publication of the WHO 2010 semen analysis criteria. Moreover, we did not evaluate the predictive value of VC in terms of spermatogenesis or Leydig cell function: yet, the present study was not designed to assess its specific role, already defined, in determining testicular impairment, but rather aimed at describing the individual predictive contribution of each US parameter. Anyhow, our findings were maintained even when adjusted for the presence of VC. Further studies could explore the informative role of other testicular imaging methods, such as parenchymal Doppler and contrast‐enhanced US, elastosonography and magnetic resonance imaging, which has already proven valuable in characterizing small solid lesions, 51 , 52 and whether they possess different predictive values for different clinical conditions.
In conclusion, our study is the first to comprehensively describe the testicular US parameters independently associated with both impaired spermatogenesis and hypogonadism in a large cohort of patients. In addition, we propose the new TU score as a simple and informative screening tool, which could be applied during routine testicular US examination in order to identify patients who require further evaluation.
DISCLOSURES
The authors have nothing to disclose.
AUTHORS' CONTRIBUTIONS
AMI, CP, GK, DG guaranteed the integrity of entire study. CP, GK, DG, FC analyzed and interpreted the data. MT, MM, AL, FS, MGT contributed to literature research. AMI, CP, DG, AL, AA, DP, MT, RP, FS acquired data. FC performed statistical analysis. AMI, CP, GK, DG, FC, MT edited the manuscript. All authors were involved in study concept/study design or data acquisition, manuscript drafting or manuscript revision for important intellectual content, and manuscript final version approval.
ACKNOWLEDGEMENTS
We thank Marie‐Helene Hayles for revision of the English text.
Pozza C, Kanakis G, Carlomagno F, et al. Testicular ultrasound score: A new proposal for a scoring system to predict testicular function. Andrology. 2020;8:1051–1063. 10.1111/andr.12822
Pozza and Kanakis contributed equally to this work.
Funding information
The work was supported by Ministry of Research MIUR Grant PRIN 2017 2017TK7Z8L.
REFERENCES
- 1. Lotti F, Maggi M. Ultrasound of the male genital tract in relation to male reproductive health. Hum Reprod Update 2015;21(1):56‐83. [DOI] [PubMed] [Google Scholar]
- 2. Dogra VS, Gottlieb RH, Oka M, Rubens DJ. Sonography of the scrotum. Radiology. 2003;227(1):18‐36. [DOI] [PubMed] [Google Scholar]
- 3. Goede J, Hack WW, Sijstermans K, et al. Normative values for testicular volume measured by ultrasonography in a normal population from infancy to adolescence. Horm Res Paediatr. 2011;76(1):56‐64. [DOI] [PubMed] [Google Scholar]
- 4. Isidori A, Lenzi A. Ultrasound of the Testis for the Andrologist. Cham, Switzerland: Springer International Publishing; 2017. [Google Scholar]
- 5. Jorgensen N, Carlsen E, Nermoen I, et al. East‐West gradient in semen quality in the Nordic‐Baltic area: a study of men from the general population in Denmark, Norway, Estonia and Finland. Hum Reprod. 2002;17(8):2199‐2208. [DOI] [PubMed] [Google Scholar]
- 6. Fisher AD, Rastrelli G, Bandini E, et al. Metabolic and cardiovascular outcomes of fatherhood: results from a cohort of study in subjects with sexual dysfunction. J Sex Med. 2012;9(11):2785‐2794. [DOI] [PubMed] [Google Scholar]
- 7. Sakamoto H, Ogawa Y, Yoshida H. Relationship between testicular volume and testicular function: comparison of the Prader orchidometric and ultrasonographic measurements in patients with infertility. Asian J Androl. 2008;10(2):319‐324. [DOI] [PubMed] [Google Scholar]
- 8. Ventimiglia E, Ippolito S, Capogrosso P, et al. Primary, secondary and compensated hypogonadism: a novel risk stratification for infertile men. Andrology. 2017;5(3):505‐510. [DOI] [PubMed] [Google Scholar]
- 9. Loberant N, Bhatt S, McLennan GT, Dogra VS. Striated appearance of the testes. Ultrasound Q. 2010;26(1):37‐44. [DOI] [PubMed] [Google Scholar]
- 10. Einstein DM, Paushter DM, Singer AA, Thomas AJ, Levin HS. Fibrotic lesions of the testicle: sonographic patterns mimicking malignancy. Urol Radiol. 1992;14(3):205‐210. [DOI] [PubMed] [Google Scholar]
- 11. Harris RD, Chouteau C, Partrick M, Schned A. Prevalence and significance of heterogeneous testes revealed on sonography: ex vivo sonographic‐pathologic correlation. AJR Am J Roentgenol. 2000;175(2):347‐352. [DOI] [PubMed] [Google Scholar]
- 12. Spaggiari G, Granata ARM, Santi D. Testicular ultrasound inhomogeneity is an informative parameter for fertility evaluation. Asian J Androl. 2019;22:302‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Raza SA, Jhaveri KS. Imaging in male infertility. Radiol Clin North Am. 2012;50(6):1183‐1200. [DOI] [PubMed] [Google Scholar]
- 14. Richenberg J, Brejt N. Testicular microlithiasis: is there a need for surveillance in the absence of other risk factors? Eur Radiol. 2012;22(11):2540‐2546. [DOI] [PubMed] [Google Scholar]
- 15. Elzinga‐Tinke JE, Sirre ME, Looijenga LH, van Casteren N, Wildhagen MF, Dohle GR. The predictive value of testicular ultrasound abnormalities for carcinoma in situ of the testis in men at risk for testicular cancer. Int J Androl. 2010;33(4):597‐603. [DOI] [PubMed] [Google Scholar]
- 16. Barbonetti A, Martorella A, Minaldi E, et al. Testicular cancer in infertile men with and without testicular microlithiasis: a systematic review and meta‐analysis of case‐control studies. Front Endocrinol (Lausanne). 2019;10:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pedersen MR, Rafaelsen SR, Moller H, Vedsted P, Osther PJ. Testicular microlithiasis and testicular cancer: review of the literature. Int Urol Nephrol. 2016;48(7):1079‐1086. [DOI] [PubMed] [Google Scholar]
- 18. Richenberg J, Belfield J, Ramchandani P, et al. Testicular microlithiasis imaging and follow‐up: guidelines of the ESUR scrotal imaging subcommittee. Eur Radiol. 2015;25(2):323‐330. [DOI] [PubMed] [Google Scholar]
- 19. Yee WS, Kim YS, Kim SJ, Choi JB, Kim SI, Ahn HS. Testicular microlithiasis: prevalence and clinical significance in a population referred for scrotal ultrasonography. Korean J Urol. 2011;52(3):172‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tan MH, Eng C. Testicular microlithiasis: recent advances in understanding and management. Nat Rev Urol. 2011;8(3):153‐163. [DOI] [PubMed] [Google Scholar]
- 21. Hoei‐Hansen CE, Sommer P, Rajpert‐De Meyts E, Skakkebaek NE. A rare diagnosis: testicular dysgenesis with carcinoma in situ detected in a patient with ultrasonic microlithiasis. Asian J Androl. 2005;7(4):445‐447. [DOI] [PubMed] [Google Scholar]
- 22. Sansone A, Fegatelli DA, Pozza C, et al. Effects of percutaneous varicocele repair on testicular volume: results from a 12‐month follow‐up. Asian J Androl. 2018;21:408‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pozza C, Pofi R, Tenuta M, et al. Clinical presentation, management and follow‐up of 83 patients with Leydig cell tumors of the testis: a prospective case‐cohort study. Hum Reprod. 2019;34(8):1389‐1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Isidori AM, Pozza C, Gianfrilli D, et al. Differential diagnosis of nonpalpable testicular lesions: qualitative and quantitative contrast‐enhanced US of benign and malignant testicular tumors. Radiology. 2014;273(2):606‐618. [DOI] [PubMed] [Google Scholar]
- 25. Pozza C, Gianfrilli D, Fattorini G, et al. Diagnostic value of qualitative and strain ratio elastography in the differential diagnosis of non‐palpable testicular lesions. Andrology. 2016;4(6):1193‐1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Raheem OA. Surgical management of adolescent varicocele: Systematic review of the world literature. Urol Ann. 2013;5(3):133‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tanrikut C, McQuaid JW, Goldstein M. The impact of varicocele and varicocele repair on serum testosterone. Curr Opin Obstet Gynecol. 2011;23(4):227‐231. [DOI] [PubMed] [Google Scholar]
- 28. Lenz S, Giwercman A, Elsborg A, et al. Ultrasonic testicular texture and size in 444 men from the general population: correlation to semen quality. Eur Urol. 1993;24(2):231‐238. [DOI] [PubMed] [Google Scholar]
- 29. Beres J, Papp G, Pazonyi I, Czeizel E. Testicular volume variations from 0 to 28 years of age. Int Urol Nephrol. 1989;21(2):159‐167. [DOI] [PubMed] [Google Scholar]
- 30. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666‐3672. [DOI] [PubMed] [Google Scholar]
- 31. Jorgensen N, Joensen UN, Toppari J, et al. Compensated reduction in Leydig cell function is associated with lower semen quality variables: a study of 8182 European young men. Hum Reprod. 2016;31(5):947‐957. [DOI] [PubMed] [Google Scholar]
- 32. Grunewald S, Glander HJ, Paasch U, Kratzsch J. Age‐dependent inhibin B concentration in relation to FSH and semen sample qualities: a study in 2448 men. Reproduction. 2013;145(3):237‐244. [DOI] [PubMed] [Google Scholar]
- 33. WHO . WHO Laboratory Manual for the Examination of Human Semen and Sperm‐cervical Mucus Interaction, 4th edn Cambridge: Cambridge University Press; 1999. [Google Scholar]
- 34. WHO . WHO Laboratory Manual for the Examination and Processing of Human Semen. Geneva: World Health Organization, 2010. [Google Scholar]
- 35. Cooper TG, Noonan E, von Eckardstein S, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16(3):231‐245. [DOI] [PubMed] [Google Scholar]
- 36. Adamopoulos D, Kapolla N, Nicopoulou S, Pappa A, Koukkou E, Gregoriou A. Assessment of Sertoli cell functional reserve and its relationship to sperm parameters. Int J Androl. 2003;26(4):215‐225. [DOI] [PubMed] [Google Scholar]
- 37. Koukkou E, Billa E, Kapolla N, et al. An empiric treatment for idiopathic oligozoospermia revisited: a 20‐year investigative saga. Andrologia. 2012;44(5):337‐342. [DOI] [PubMed] [Google Scholar]
- 38. Adamopoulos DA, Pappa A, Billa E, Nicopoulou SC, Koukkou E, Venaki E. Seasonality in sperm parameters in normal men and dyspermic patients on medical intervention. Andrologia. 2009;41(2):118‐124. [DOI] [PubMed] [Google Scholar]
- 39. Oyen RH. Scrotal ultrasound. Eur Radiol. 2002;12(1):19‐34. [DOI] [PubMed] [Google Scholar]
- 40. Sanli O, Kadioglu A, Atar M, Acar O, Nane I, Kadioglu A. Grading of classical testicular microlithiasis has no effect on the prevalence of associated testicular tumors. Urol Int. 2008;80(3):310‐316. [DOI] [PubMed] [Google Scholar]
- 41. Niedzielski J, Pisarska K, Przewratil P. The usefulness of testicular atrophy index in the assessment of undescended testicle–preliminary report. Rocz Akad Med Bialymst. 2003;48:112‐114. [PubMed] [Google Scholar]
- 42. Sawilowsky SS. New effect size rules of thumb. J Modern Appl Stat Meth. 2009;8(2):597‐599. [Google Scholar]
- 43. Stevens JP. Applied Multivariate Statistics for the Social Sciences. New York, NY: Routledge; 2002. [Google Scholar]
- 44. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837‐845. [PubMed] [Google Scholar]
- 45. Gianfrilli D, Ferlin A, Isidori AM, et al. Risk behaviours and alcohol in adolescence are negatively associated with testicular volume: results from the Amico‐Andrologo survey. Andrology. 2019;7(6):769‐777. [DOI] [PubMed] [Google Scholar]
- 46. Lenz S, Skakkebaek NE, Hertel NT. Abnormal ultrasonic pattern in contralateral testes in patients with unilateral testicular cancer. World J Urol. 1996;14(Suppl 1):S55‐S58. [DOI] [PubMed] [Google Scholar]
- 47. Lenz S, Thomsen JK, Giwercman A, Hertel NT, Hertz J, Skakkebaek NE. Ultrasonic texture and volume of testicles in infertile men. Hum Reprod. 1994;9(5):878‐881. [DOI] [PubMed] [Google Scholar]
- 48. Ruiz‐Olvera SF, Rajmil O, Sanchez‐Curbelo JR, Vinay J, Rodriguez‐Espinosa J, Ruiz‐Castane E. Association of serum testosterone levels and testicular volume in adult patients. Andrologia. 2018;50(3):e12933. [DOI] [PubMed] [Google Scholar]
- 49. Whitworth PW 3rd, Dyer RB. The "striated" testis. Abdom Radiol. 2019;44(8):2943‐2944. [DOI] [PubMed] [Google Scholar]
- 50. Joensen UN, Jorgensen N, Rajpert‐De Meyts E, Skakkebaek NE. Testicular dysgenesis syndrome and Leydig cell function. Basic Clin Pharmacol Toxicol. 2008;102(2):155‐161. [DOI] [PubMed] [Google Scholar]
- 51. Manganaro L, Saldari M, Pozza C, et al. Dynamic contrast‐enhanced and diffusion‐weighted MR imaging in the characterisation of small, non‐palpable solid testicular tumours. Eur Radiol. 2018;28(2):554‐564. [DOI] [PubMed] [Google Scholar]
- 52. Manganaro L, Vinci V, Pozza C, et al. A prospective study on contrast‐enhanced magnetic resonance imaging of testicular lesions: distinctive features of Leydig cell tumours. Eur Radiol. 2015;25(12):3586‐3595. [DOI] [PubMed] [Google Scholar]


