Abstract
BACKGROUND
Urgency triage in the emergency department (ED) is important for early identification of potentially lethal conditions and extensive resource utilization. However, in older patients, urgency triage systems could be improved by taking geriatric vulnerability into account. We investigated the association of geriatric vulnerability screening in addition to triage urgency levels with 30‐day mortality in older ED patients.
DESIGN
Secondary analysis of the observational multicenter Acutely Presenting Older Patient (APOP) study.
SETTING
EDs within four Dutch hospitals.
PARTICIPANTS
Consecutive patients, aged 70 years or older, who were prospectively included.
MEASUREMENTS
Patients were triaged using the Manchester Triage System (MTS). In addition, the APOP screener was used as a geriatric screening tool. The primary outcome was 30‐day mortality. Comparison was made between mortality within the geriatric high‐ and low‐risk screened patients in every urgency triage category. We calculated the difference in explained variance of mortality by adding the geriatric screener (APOP) to triage urgency (MTS) by calculating Nagelkerke R2.
RESULTS
We included 2,608 patients with a median age of 79 (interquartile range = 74‐84) years, of whom 521 (20.0%) patients were categorized as high risk according to geriatric screening. Patients were triaged on urgency as standard (27.2%), urgent (58.5%), and very urgent (14.3%). In total, 132 (5.1%) patients were deceased within a period of 30 days. Within every urgency triage category, 30‐day mortality was threefold higher in geriatric high‐risk compared to low‐risk patients (overall = 11.7% vs 3.4%; P < .001). The explained variance of 30‐day mortality with triage urgency was 1.0% and increased to 6.3% by adding the geriatric screener.
CONCLUSION
Combining triage urgency with geriatric screening has the potential to improve triage, which may help clinicians to deliver early appropriate care to older ED patients. J Am Geriatr Soc 68:1755‐1762, 2020.
Keywords: emergency department, geriatric assessment, geriatric emergency medicine, risk stratification, triage
Emergency department (ED) urgency triage aims to prioritize patients based on their clinical urgency, rapidly diagnose potentially lethal illness, and reduce the negative impact of a delay in treatment on prognosis. Within the last 30 years, several triage tools have been developed and implemented within routine ED care to manage ED crowding.1 The Australasian Triage Scale,2 the Canadian Triage and Acuity Scale (CTAS),3 the Manchester Triage System (MTS),4 and the Emergency Severity Index5 are frequently used and have reasonable overall validity and reliability in allocating clinical priority.6, 7, 8 However, despite the increase in older patients visiting the ED, above‐mentioned commonly used triage tools seem to allocate urgency less effective within this population.9, 10, 11 Potentially, different reference values of vital signs, atypical disease presentations, or the presence of cognitive impairment could be contributing factors.12 Older patients are therefore at risk for “undertriage,” an assignment of an inappropriately low triage level, resulting in longer wait times and risk of adverse outcomes due to harm by delay in treatment.13, 14, 15, 16, 17
Although it is known that frail older patients have high risks of adverse outcomes and tend to have less functional organ capacity, making this population more vulnerable to adverse outcomes when ED treatment is delayed, this is not incorporated in urgency triage tools. However, several geriatric screening tools have been developed to identify vulnerable geriatric patients in the ED,18 like the Identification of Seniors at Risk (ISAR),19 Triage Risk Screening Tool (TRST),20 and the Acutely Presenting Older Patient (APOP) screener.21 Although there is still room for improvement in predictive performance,18 these geriatric vulnerability screening tools may still have added value as they enhance awareness and understanding of geriatric patients beyond the ED presenting complaint.22
Geriatric screening tools are prognostic tools on longer‐term adverse outcomes, while urgency triage tools are primarily designed as diagnostic tools to assign short‐term clinical priority and secondarily to predict short‐term mortality. Although geriatric screening tools and triage tools serve different purposes, it was hypothesized that the combination of these tools could improve triage and prediction of early mortality in older patients.23, 24, 25, 26 However, the added value of combining a geriatric screening tool and an urgency triage tool in the ED has not been studied before.
Therefore, the aim of this study was to explore the combination of geriatric screening with triage urgency by means of studying the association of geriatric screening in addition to triage urgency levels with 30‐day mortality in older ED patients. To explore this proof of principle, the APOP screener was used as a geriatric screening tool and the MTS was used as a triage tool.
METHODS
Study Design
This was a secondary analysis of the APOP study: a prospective multicenter cohort study that was performed in four Dutch hospitals. A detailed description has been published elsewhere.27 In short, patients visiting the ED at the Leiden University Medical Center (LUMC; September 2014‐November 2014), Alrijne Hospital (March 2015‐June 2015), Haaglanden Medical Center (HMC), location Bronovo (May 2016‐July 2016), and Erasmus University Medical Center (July 2016‐January 2017) were included. Inclusion occurred 24/7 within the LUMC, 7 days a week (from 10 am to 10 pm) within the Alrijne Hospital, 6 days a week (from 10 am to 10 pm) within the HMC Bronovo, and 4 days a week (from 10 am to 10 pm) within the Erasmus University Medical Center. Written informed consent was obtained from all patients. The study was approved by the Medical Ethics Committees of all four hospitals.
Setting
In all participating EDs, a triage nurse prioritized patients based on their disease severity by using the MTS as an urgency triage tool at patient arrival.4, 28 Triage nurses are trained to use the MTS by standardized approaches and protocols, which generally results in substantial interrater reliability.29 The MTS consists of 52 presenting complaint‐based flowcharts, and each of the flowcharts uses key discriminators to determine urgency in a five‐level scale: red (immediate assessment required; eg, respiratory failure, shock, coma); orange (very urgent, seen within 10 minutes; eg, chest pain); yellow (urgent, seen within 60 minutes; eg, pneumonia); green (standard, can wait 120 minutes; eg, ankle sprain); and blue (nonurgent, can wait 240 minutes; eg, abrasions). The 52 possible chief complaints were classified into seven main groups.21 As the nonurgent level is not used in routine care within the participating EDs and patients with the immediate urgency level were excluded, patients presenting with triage urgency levels standard, urgent, and very urgent were included in the present study.
Study Participants
In the APOP study, all consecutive patients, aged 70 years or older, visiting the ED were included. We excluded patients who were triaged “red” according to the MTS, because due to immediate required assessment geriatric screening would not be possible or beneficial for these patients.4 In addition, patients with an unstable medical condition, those with impaired mental status without a proxy to provide informed consent, those with a language barrier, and patients who refused to participate were excluded. For the present study, all older ED patients with an APOP screening result at baseline were included.
Outcomes
The primary outcome of the present study was 30‐day mortality. Secondary outcomes were hospital admission rate (after ED visit) and 7‐day mortality.
Data Collection
Patient Characteristics
At baseline in the ED, data on three domains were assessed: demographics, severity of disease indicators, and geriatric measurements. Demographics consisted of age, sex, and living arrangement. Severity of disease indicators consisted of arrival by ambulance, fall‐related ED visit, triage urgency, and chief complaint according to MTS. Geriatric measurements consisted of polypharmacy (≥5 different medications stated by the patient), use of a walking device, Katz activities of daily living questionnaire (functional status 2 weeks before the ED visit),30, 31 six‐item Cognitive Impairment Test (6‐CIT),32, 33, 34 and history of diagnosed dementia reported by the patient or a proxy.
Geriatric Screening
As a geriatric screening tool, the APOP screener was used. The APOP screener is a prognostic instrument that uses geriatric impairments on functional and cognitive domains to predict the individual risk of mortality and/or functional decline within 3 months in older patients presenting to the ED.27 The screener has been validated in one study in four Dutch hospitals and has been implemented in the electronic health record system (HiX, Chipsoft) of approximately half of all Dutch hospitals.35 The screener comprises seven predictors that are collected in less than 2 minutes after ED arrival: age, sex, arrival by ambulance, need of regular help, need for help with bathing and showering, hospitalization in the past 6 months, and impaired cognition (defined as having dementia, an incorrect answer on at least one of two 6‐CIT questions [“what year is it now?” and/or “say the months in reverse order”], or no data of cognition) (Supplementary Table S1). For the present study, the result of the APOP screener was retrospectively calculated. The APOP screener indicates patients with the highest 20% predicted risk on the composite outcome of mortality and/or functional decline within 3 months. The threshold for a “high‐risk” APOP screening result is a predicted risk of 45% or greater.27
Follow‐Up Data
Hospital admission rate was measured by using the discharge destination from the patientʼs electronic health record. Data on mortality were obtained from municipal records.
Data Analyses
Continuous data were presented as median (interquartile range [IQR]). Categorical data were presented as number (percentage). The χ2 test was used to compare differences in clinical outcomes within every MTS category between the APOP high‐risk and low‐risk screened patients. Relative risks (RRs) were calculated, and we presented outcomes with 95% confidence intervals (95% CIs).
The Nagelkerke R2 was used to calculate the proportion of the explained variance of clinical outcomes by MTS and APOP screening, separate and combined. For comparison with other studies, we additionally assessed the discrimination of the models with the area under the receiver operating characteristic curve (AUC [95% CI]) for the primary outcome, 30‐day mortality. To solely assess the effect of age on predicting mortality, we performed identical analyses with MTS and age younger or older than 80 years.
Finally, we developed a reclassification concept for 30‐day mortality, in which every patient with an APOP high‐risk screening result was upgraded one MTS category. Taking into consideration that up triage of patients to the highest urgency level requiring immediate assessment (MTS category red) would not be feasible in practice, very urgent patients with an APOP high‐risk result remained in the same very urgent category. We compared 30‐day mortality rates between the original MTS classification and the reclassification model. A P < .05 was determined as statistically significant. Statistical analyses were performed using IBM SPSS Statistics version 23.
RESULTS
Within the APOP study, 2,629 individual ED patients, aged 70 years or older, were included in four hospitals. We excluded 21 patients with an incomplete APOP screening, resulting in 2,608 patients included in the analyses (Supplementary Figure S1).
In the total study population, the median age was 79 (IQR = 74‐84) years, and 1,227 (47.0%) patients were male (Table 1). In total, 710 (27.2%) patients were assigned as standard, 1,525 (58.5%) patients were assigned as urgent, and 373 (14.3%) patients were assigned as very urgent. Half of all patients arrived by ambulance, with an increasing percentage with increasing urgency levels: standard (28.2%), urgent (55.7%), and very urgent (75.1%). The most common chief complaint was minor trauma in the standard category (46.3%), while in the very urgent category the most common complaint was chest pain (31.9%). The presence of polypharmacy increased with increasing urgency levels: standard (53.1%), urgent (59.0%), and very urgent (64.1%). In total, 521 (20.0%) patients were high risk according to the APOP screener, which showed an increase with increasing urgency levels: standard (13.7%), urgent (22.3%), and very urgent (22.5%).
Table 1.
Patient Characteristics Stratified by MTS Triage Urgency
| Characteristic | MTS Category | All (N = 2,608) | ||
|---|---|---|---|---|
| Standard (N = 710) | Urgent (N = 1,525) | Very Urgent (N = 373) | ||
| Demographics | ||||
| Age, median (IQR), y | 79 (74‐84) | 79 (74‐84) | 78 (74‐83) | 79 (74‐84) |
| Male, No. (%) | 315 (44.4) | 721 (47.3) | 191 (51.2) | 1,227 (47.0) |
| Living arrangement, No. (%) | ||||
| Independent alone or with others | 662 (93.2) | 1,390 (91.1) | 340 (91.2) | 2,392 (91.8) |
| Nursing home/residential care | 48 (6.8) | 134 (8.8) | 33 (8.8) | 215 (8.2) |
| Severity of disease indicators | ||||
| Arrival by ambulance, No. (%) | 200 (28.2) | 849 (55.7) | 280 (75.1) | 1,329 (51.0) |
| Fall‐related ED visit, No. (%) | 209 (29.4) | 396 (26.0) | 51 (13.7) | 656 (25.2) |
| Chief complaints, No. (%) | ||||
| Minor trauma | 239 (46.3) | 431 (28.3) | 47 (12.6) | 807 (30.9) |
| Malaise | 107 (15.1) | 300 (19.7) | 54 (14.5) | 461 (17.7) |
| Chest pain | 82 (11.5) | 192 (12.6) | 119 (31.9) | 393 (15.1) |
| Dyspnea | 63 (8.9) | 190 (12.5) | 64 (17.2) | 317 (12.2) |
| Loss of consciousness | 21 (3.0) | 96 (6.3) | 28 (7.5) | 145 (5.6) |
| Abdominal pain | 65 (9.2) | 179 (11.7) | 36 (9.7) | 280 (10.7) |
| Others | 43 (6.1) | 137 (9.0) | 25 (6.7) | 205 (7.9) |
| Geriatric measurements | ||||
| Polypharmacy, No. (%) | 377 (53.1) | 899 (59.0) | 239 (64.1) | 1,515 (58.1) |
| Use of walking device, No. (%) | 265 (37.4) | 684 (44.9) | 158 (42.4) | 1,107 (42.5) |
| Katz ADL score, median (IQR) | 0 (0‐1) | 0 (0‐1) | 0 (0‐1) | 0 (0‐1) |
| 6‐CIT score, median (IQR) | 4 (0‐8) | 4 (2‐10) | 4 (2‐8) | 4 (2‐8) |
| Diagnosis of dementia, No. (%) | 31 (4.4) | 89 (5.8) | 18 (4.8) | 138 (5.3) |
| APOP screening result | ||||
| Low risk | 613 (86.3) | 1,185 (77.7) | 289 (77.5) | 2,087 (80.0) |
| High risk | 97 (13.7) | 340 (22.3) | 84 (22.5) | 521 (20.0) |
Note: Missing data: 1 living arrangement, 5 use of walking device, 27 Katz ADL score, and 283 6‐CIT score.
Abbreviations: 6‐CIT, six‐item Cognitive Impairment Test; ADL, activities of daily living; APOP, Acutely Presenting Older Patient; ED, emergency department; IQR, interquartile range; MTS, Manchester Triage System.
In total, 132 (5.1%) patients died within 30 days after their ED visit: 23 (3.2%) standard patients, 83 (5.4%) urgent patients, and 26 (7.0%) very urgent patients (Figure 1). There was a higher mortality rate within 30 days in the APOP high‐risk patients compared to APOP low‐risk patients (11.7% vs 3.4%; P < .001).
Figure 1.
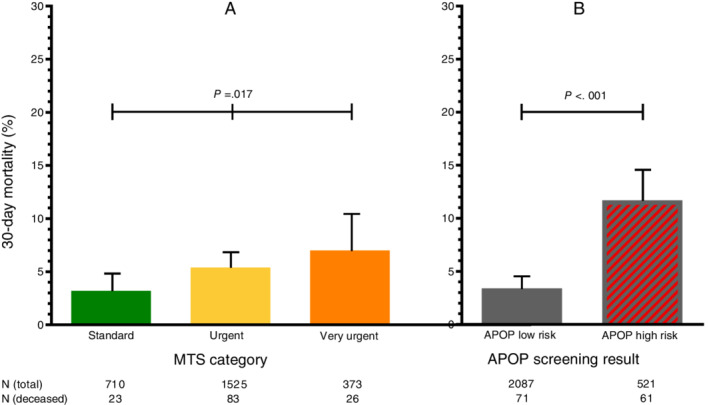
The 30‐day mortality by Manchester Triage System (MTS) category and Acutely Presenting Older Patient (APOP) screening result separately. A, The 30‐day mortality rate for patients stratified by MTS category standard, urgent, or very urgent. The χ2 test was used to compare differences in mortality between the MTS categories. B, The 30‐day mortality rate for patients stratified by APOP low‐risk or high‐risk screening result. The χ2 test was used to compare differences in mortality between the APOP low‐risk and high‐risk screened patients. The upper 95% confidence intervals for proportion are shown.
Figure 2 shows the percentages of deceased patients in the first 30 days stratified by MTS categories and the APOP screening result. Mortality increased with increasing urgency levels. The differences in mortality between APOP high‐ and low‐risk patients were statistically significant within the standard category (RR = 2.8; 95% CI = 1.2‐6.5; P = .021), the urgent category (RR = 3.4; 95% CI = 2.3‐5.1; P < .001), and the very urgent category (RR = 3.4; 95% CI = 1.7‐7.1; P = .001). APOP high‐risk patients triaged as standard had higher mortality rates (7.2%) than APOP low‐risk patients triaged as very urgent (4.5%). One percent of the variability in 30‐day mortality was explained by MTS category alone (Nagelkerke R2 = 1.0%), whereas 5.6% was explained by the APOP screener alone. The R2 increased to 6.3% when combining MTS with the APOP screener. The AUC was 0.57 (95% CI = 0.52‐0.61) for MTS alone, 0.64 (95% CI = 0.59‐0.69) for the APOP screener alone, and 0.66 (95% CI = 0.61‐0.72) for MTS and the APOP screener combined. To assess the effect of age alone on the variability of 30‐day mortality, we performed identical analyses with MTS and age younger or older than 80 years. In total, 2.5% of the variability in 30‐day mortality could be explained by high age alone, with an AUC of 0.60 (95% CI = 0.55‐0.65).
Figure 2.
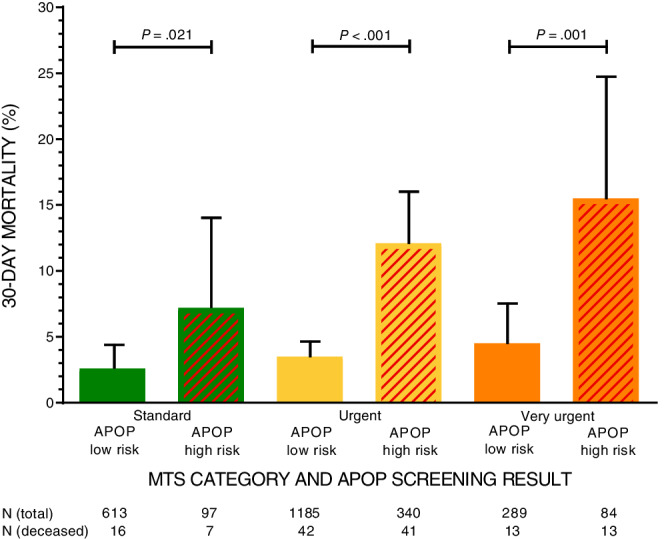
The 30‐day mortality by Manchester Triage System (MTS) category and Acutely Presenting Older Patient (APOP) screening result combined. The 30‐day mortality percentages for patients stratified by MTS category and APOP screening result combined. The upper 95% confidence intervals (CIs) for proportion are shown. Relative risks (RRs) were calculated to compare differences in mortality between APOP low‐risk and high‐risk screened patients within all three MTS categories, resulting in significant differences within the standard category (RR = 2.8; 95% CI = 1.2‐6.5; P = .021), the urgent category (RR = 3.4; 95% CI = 2.3‐5.1; P < .001), and the very urgent category (RR = 3.4; 95% CI = 1.7‐7.1; P = .001). Nagelkerke R2 was calculated for MTS alone (R2 = 0.010), APOP alone (R2 = 0.056), and MTS and APOP combined (R2 = 0.063).
The secondary outcomes hospital admission rate and 7‐day mortality are shown in Supplementary Figures S2 and S3. Similar trends were found as for the primary outcome. Overall, APOP high‐risk patients had a higher admission rate (high risk vs low risk = 61.4% vs 46.0%; P < .001) and higher 7‐day mortality rate (high risk vs low risk = 3.5% vs 1.5%; P = .003), compared to APOP low‐risk patients.
A reclassification concept for the primary outcome, 30‐day mortality, in which every patient with an APOP high‐risk screening result is upgraded one MTS category, is presented in Figure 3. This reclassification concept induces a decrease of 30‐day mortality in the standard category (reclassified vs original = 2.6% vs 3.2%) and the urgent category (reclassified vs original = 3.8% vs 5.4%), and an increase in the very urgent category (reclassified vs original = 9.4% vs 7.0%).
Figure 3.
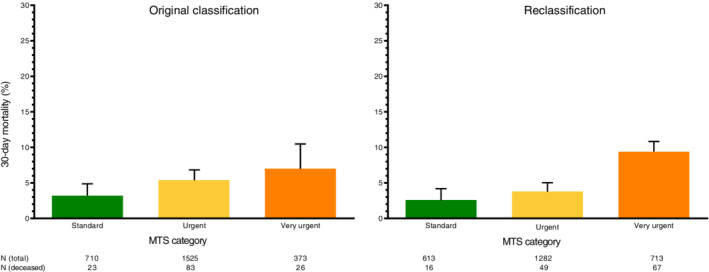
Reclassification concept: upgrade of one Manchester Triage System (MTS) category for Acutely Presenting Older Patient (APOP) high‐risk patients. A reclassification concept for the primary outcome, 30‐day mortality, in which every patient with an APOP high‐risk screening result is upgraded one MTS category. Very urgent patients with an APOP high‐risk result remained in the same very urgent category.
DISCUSSION
The main finding of this proof‐of‐principle study is that within every triage urgency category, older patients with a high‐risk geriatric screening result had a three times higher 30‐day mortality rate compared to patients who were identified as low risk during geriatric screening. Combining geriatric screening with triage urgency explained more of the variability of 30‐day mortality in older ED patients than triage urgency alone.
To proof the principle that addition of geriatric screening has the potential to improve routinely used urgency triage, we used the APOP screener as a geriatric screening tool and the MTS as an urgency triage system since these tools were already implemented in the study hospitals. Other commonly used triage or geriatric screening tools may have given the same results. We used reclassification and measures of predictive performance, like AUCs and correlation coefficients, to be able to compare the combination of geriatric screening and urgency triage in contrast with urgency triage alone, and to compare our results with literature, not to quantify predictive performance of the APOP screening or the MTS.
It was shown that the MTS alone had a low discriminative performance for 30‐day mortality in older ED patients with an AUC of 0.57, which is in line with literature.36, 37 We found that older patients who were identified by the APOP screener as high risk had a higher 30‐day mortality compared to APOP low‐risk patients. These results are in line with other studies demonstrating that frailty is associated with short‐term adverse outcomes, such as hospital admission or in‐hospital mortality.26, 38, 39 Previous studies of other geriatric screening tools, such as ISAR and TRST, did not evaluate short term (eg, 30‐day) mortality.18 In line with studies in which geriatric characteristics (impaired mobility40 or clinical frailty scale23) were combined with early warning scores, we also found that the combination of the MTS with the APOP screener improved the prediction of mortality. Recently, the CTAS guideline was revised with a “frailty modifier,” which allows triage nurses to manually increase triage urgency for nonurgent complaints based on geriatric impairments.3 To our best knowledge, this modification of CTAS has not been formally tested yet, but is supported by a recent study that investigated the relationship between triage acuity measured with CTAS and frailty.26 In comparison with the definition of the frailty modifier of the CTAS, our results within the MTS indicate that considering age older than 80 years during triage is already a good start to differentiate between older patients at risk for adverse outcomes. However, the explained variance for 30‐day mortality was higher when taking into account more geriatric characteristics than age only. The MTS is known for performing worse in allocating priority in both children and older adults.11, 41 Previously, the MTS has been modified for use in children41 additionally, the opportunity remains to improve the MTS for older adults as well.
Triage tools are diagnostic tools with the aim to determine urgency and early clinical need, while geriatric screening instruments are prognostic tools for adverse outcomes. Although triage tools and geriatric screening tools serve different purposes, they could be combined as predictors of “disease urgency” and “geriatric urgency” to improve prediction of early mortality in older patients. Combining triage urgency with geriatric impairment could be executed in two ways. First, current triage tools and existing geriatric screening tools can be used next to each other. Second, current triage tools can be adjusted, taking geriatric impairments into account. Adjusting triage by adding geriatric screening could improve risk stratification early at ED arrival and could in all probability reduce undertriage in older patients. Triage tools aim to prioritize patients who will benefit from early treatment (eg, patients with myocardial infarction [who benefit from early revascularization] or shock [who benefit from early fluid resuscitation]), thereby contributing to prevention of acute organ failure and thus mortality.42, 43 However, older patients are often undertriaged due to atypical disease presentations, nonspecific complaints (eg, generalized weakness), and inappropriate interpretation of vital signs.13, 14, 15, 16 Older patients with geriatric impairments will be generally more sensitive to delays in treatments (caused by undertriage) due to less physiological reserve related to chronic comorbidity. This may, at least partially, explain that the addition of the APOP screener to the MTS increases the explained variance and improves prediction of 30‐day mortality. Reclassification of APOP high‐risk patients to a higher triage urgency level will result in a higher number of older ED patients who are allocated to the very urgent urgency level (Figure 3), which would reduce time to treatment in the ED. Adjustment of triage by adding geriatric screening has the additional advantage that the atypical disease presentation and different interpretation of vital signs are automatically taken into account, potentially improving triage. Additionally, cognitive impairment can partially be explained by acute disturbance of brain perfusion and oxygenation, which might be improved with optimal resuscitation after early recognition with geriatric screening at triage.44 In other words, combining diagnostic triage tools with prognostic geriatric screening tools has the potential to provide a comprehensive understanding of the individual risk of poor outcomes using both disease severity and geriatric impairments, with the possibility to acquire more personalized care in acutely ill older patients as early as arrival in the ED. Future studies should investigate whether it is possible to replicate this proof of principle of combining urgency triage with geriatric screening by using other tools and whether implementation of a concept of reclassification would result in less undertriage and therefore less mortality in older patients, without unanticipated consequences like overtreatment.
This study has several limitations. First, patients with MTS category red were not included within the study due to immediately required care. However, given the severity of disease that required immediate action, these patients already belong to a vulnerable patient group who cannot be undertriaged by definition. Second, MTS might have had a better predictive performance in more short‐term outcomes, such as in‐hospital mortality, but, despite our large sample size, the numbers of the present study were too small to examine that outcome. Nonetheless, the same trend was found for 7‐day mortality as for our primary outcome, 30‐day mortality. Third, for the present study, the development and validation cohort of the APOP study was used, and the APOP screener was calculated retrospectively. However, we considered the degree of selection or information bias due to the retrospective design minimal because of the prospective follow‐up of the study and inclusion of all consecutive older ED patients. Finally, to explore the study aim, the APOP screener was used as a geriatric screening instrument that is developed and validated in The Netherlands, limiting generalizability. As this study explored a proof of principle, other geriatric screening instruments were not compared to the APOP screener with the purpose to investigate which geriatric screening tool has the best predictive performance. It would be interesting to study the concept of combining urgency triage with geriatric screening further by using other instruments in other countries.
Strengths of this study can be accounted to the broad and unselected inclusion of patients in four hospitals. In addition, there were no missing data within the outcome measures. Finally, the APOP screener can be performed in less than 2 minutes after ED arrival and is therefore feasible to use in clinical practice on a large scale. The fact that the APOP screener recently has been implemented in the electronic health record system (HiX, Chipsoft) used by approximately half of all Dutch hospitals and has been put into routine use by several EDs throughout The Netherlands is promising.35
In conclusion, combining triage urgency with geriatric screening has the potential to improve triage, which may help clinicians to deliver early appropriate care to older ED patients.
Supporting information
Supplementary Table S1: The Acutely Presenting Older Patient Screener.
Supplementary Figure S1: Flowchart of study population. The total study population of the APOP study was included, minus 21 patients with an incomplete APOP screening result.
Supplementary Figure S2: Hospital admission by MTS category and APOP screening result combined. Hospital admission rate for patients stratified by MTS category and APOP screening result combined. The upper 95% confidence intervals for proportion are shown. Nagelkerke R2 was calculated for MTS alone (R2 = 0.083), APOP alone (R2 = 0.020), and MTS and APOP combined (R2 = 0.096).
Supplementary Figure S3: The 7‐day mortality by MTS category and APOP screening result combined. The 7‐day mortality percentages for patients stratified by MTS category and APOP screening result combined. The upper 95% confidence intervals for proportion are shown. Nagelkerke R2 was calculated for MTS alone (R2 = 0.008), APOP alone (R2 = 0.017), and MTS and APOP combined (R2 = 0.019).
ACKNOWLEDGMENTS
The authors acknowledge the contribution of G.J. Blauw in the collaboration of the Haaglanden Medical Center (location Bronovo) as a participating center in the Acutely Presenting Older Patient study. The authors would like to thank L.C. Zweistra‐van de Lint for editing the English language of the manuscript.
Financial Disclosure
The Institute for Evidence‐Based Medicine in Old Age is supported by the Dutch Ministry of Health, Welfare, and Sport and supported by the Netherlands Organization for Health Research and Development (ZonMw project numbers 62700.3001 and 62700.4001).
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
L.C.B., B.d.G., and S.P.M.: designed the study. S.A., S.C.E.S., B.d.G., and S.P.M.: obtained funding. J.A.L. and J.d.G.: collected data. L.C.B. and C.S.: performed statistical analyses. E.W.S.: advised on statistical analyses. L.C.B.: drafted the article. J.G., B.d.G., and S.P.M.: advised during drafting process. C.S., J.A.L., J.d.G., S.A., S.C.E.S., E.W.S., J.G., B.d.G., and S.P.M.: revision for important intellectual content. All authors gave final approval of the current version of the article.
Sponsor's Role
The sponsor had no role in the design of the study, methods, or collection or analysis of the data, and had no role in the preparation of the manuscript.
Twitter handles for co‐authors: @__sint__; @ESteyerberg; @DrSimonPM
Meeting: Poster with short oral presentation at European Society for Emergency Medicine Congress (EUSEM), Prague, Czech Republic, October 14, 2019.
Contributor Information
Laura C. Blomaard, Email: l.c.blomaard@lumc.nl, @BlomaardLC.
Jacinta A. Lucke, @__sint__.
Ewout W. Steyerberg, @ESteyerberg.
Simon P. Mooijaart, @DrSimonPM.
REFERENCES
- 1. FitzGerald G, Jelinek GA, Scott D, Gerdtz MF. Emergency department triage revisited. Emerg Med J. 2010;27:86‐92. [DOI] [PubMed] [Google Scholar]
- 2. Australasian College for Emergency Medicine . Guidelines on the Implementation of the Australasian Triage Scale in Emergency Departments. https://acem.org.au/getmedia/51dc74f7-9ff0-42ce-872a-0437f3db640a/G24_04_Guidelines_on_Implementation_of_ATS_Jul-16.aspx. January 27, 2020.
- 3. Bullard MJ, Musgrave E, Warren D, et al. Revisions to the Canadian Emergency Department Triage and Acuity Scale (CTAS) guidelines 2016. CJEM. 2017;19:S18‐S27. [DOI] [PubMed] [Google Scholar]
- 4. Mackway‐Jones K, Marsden J, Windle J. Emergency Triage: Manchester Triage Group. Vol 18 3rd ed. London, England: BMJ; 2014. http://healthindisasters.com/images/Books/Emergency-Triage–Manchester-Triage-Group-Third-Edition.pdf [Google Scholar]
- 5. Gilboy N, Tanabe T, Travers D, Rosenau AM. Emergency Severity Index (ESI): A Triage Tool for Emergency Department Care, Version 4. Implementation Handbook 2012 Edition AHRQ Publication No.12‐0014 Rockville, MD: Agency for Healthcare Research and Quality, 2011. [Google Scholar]
- 6. Zachariasse JM, van der Hagen V, Seiger N, Mackway‐Jones K, van Veen M, Moll HA. Performance of triage systems in emergency care: a systematic review and meta‐analysis. BMJ Open. 2019;9:e026471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Christ M, Grossmann F, Winter D, Bingisser R, Platz E. Modern triage in the emergency department. Dtsch Arztebl Int. 2010;107:892‐898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Farrohknia N, Castren M, Ehrenberg A, et al. Emergency department triage scales and their components: a systematic review of the scientific evidence. Scand J Trauma Resusc Emerg Med. 2011;19:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Platts‐Mills TF, Travers D, Biese K, et al. Accuracy of the Emergency Severity Index triage instrument for identifying elder emergency department patients receiving an immediate life‐saving intervention. Acad Emerg Med. 2010;17:238‐243. [DOI] [PubMed] [Google Scholar]
- 10. Brouns SHA, Mignot‐Evers L, Derkx F, Lambooij SL, Dieleman JP, Haak HR. Performance of the Manchester triage system in older emergency department patients: a retrospective cohort study. BMC Emerg Med. 2019;19:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zachariasse JM, Seiger N, Rood PP, et al. Validity of the Manchester Triage System in emergency care: a prospective observational study. PLoS One. 2017;12:e0170811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Samaras N, Chevalley T, Samaras D, Gold G. Older patients in the emergency department: a review. Ann Emerg Med. 2010;56:261‐269. [DOI] [PubMed] [Google Scholar]
- 13. Grossmann FF, Zumbrunn T, Frauchiger A, Delport K, Bingisser R, Nickel CH. At risk of undertriage? testing the performance and accuracy of the emergency severity index in older emergency department patients. Ann Emerg Med. 2012;60:317‐325.e313. [DOI] [PubMed] [Google Scholar]
- 14. Graff I, Goldschmidt B, Glien P, Dolscheid‐Pommerich RC, Fimmers R, Grigutsch D. Validity of the Manchester Triage System in patients with sepsis presenting at the ED: a first assessment. Emerg Med J. 2017;34:212‐218. [DOI] [PubMed] [Google Scholar]
- 15. Rogers A, Rogers F, Bradburn E, et al. Old and undertriaged: a lethal combination. Am Surg. 2012;78:711‐715. [DOI] [PubMed] [Google Scholar]
- 16. Hendin A, Eagles D, Myers V, Stiell IG. Characteristics and outcomes of older emergency department patients assigned a low acuity triage score. CJEM. 2018;20:762‐769. [DOI] [PubMed] [Google Scholar]
- 17. Kodadek LM, Selvarajah S, Velopulos CG, Haut ER, Haider AH. Undertriage of older trauma patients: is this a national phenomenon? J Surg Res. 2015;199:220‐229. [DOI] [PubMed] [Google Scholar]
- 18. Carpenter CR, Shelton E, Fowler S, et al. Risk factors and screening instruments to predict adverse outcomes for undifferentiated older emergency department patients: a systematic review and meta‐analysis. Acad Emerg Med. 2015;22:1‐21. [DOI] [PubMed] [Google Scholar]
- 19. McCusker J, Bellavance F, Cardin S, Trepanier S, Verdon J, Ardman O. Detection of older people at increased risk of adverse health outcomes after an emergency visit: the ISAR screening tool. J Am Geriatr Soc. 1999;47:1229‐1237. [DOI] [PubMed] [Google Scholar]
- 20. Cousins G, Bennett Z, Dillon G, Smith SM, Galvin R. Adverse outcomes in older adults attending emergency department: systematic review and meta‐analysis of the triage risk stratification tool. Eur J Emerg Med. 2013;20:230‐239. [DOI] [PubMed] [Google Scholar]
- 21. de Gelder J, Lucke JA, de Groot B, et al. Predicting adverse health outcomes in older emergency department patients: the APOP study. Neth J Med. 2016;74:342‐352. [PubMed] [Google Scholar]
- 22. Hwang U, Carpenter C. Assessing geriatric vulnerability for post emergency department adverse outcomes: challenges abound while progress is slow. Emerg Med J. 2016;33:2‐3. [DOI] [PubMed] [Google Scholar]
- 23. Romero‐Ortuno R, Wallis S, Biram R, Keevil V. Clinical frailty adds to acute illness severity in predicting mortality in hospitalized older adults: an observational study. Eur J Intern Med. 2016;35:24‐34. [DOI] [PubMed] [Google Scholar]
- 24. Malinovska A, Pitasch L, Geigy N, Nickel CH, Bingisser R. Modification of the emergency severity index improves mortality prediction in older patients. West J Emerg Med. 2019;20:633‐640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brabrand M, Kellett J, Opio M, Cooksley T, Nickel CH. Should impaired mobility on presentation be a vital sign? Acta Anaesthesiol Scand. 2018;62:945‐952. [DOI] [PubMed] [Google Scholar]
- 26. Mowbray F, Brousseau AA, Mercier E, Melady D, Emond M, Costa AP. Examining the relationship between triage acuity and frailty to inform the care of older emergency department patients: findings from a large Canadian multisite cohort study. CJEM. 2020;22:74‐81. [DOI] [PubMed] [Google Scholar]
- 27. de Gelder J, Lucke JA, Blomaard LC, et al. Optimization of the APOP screener to predict functional decline or mortality in older emergency department patients: cross‐validation in four prospective cohorts. Exp Gerontol. 2018;110:253‐259. [DOI] [PubMed] [Google Scholar]
- 28. Parenti N, Reggiani ML, Iannone P, Percudani D, Dowding D. A systematic review on the validity and reliability of an emergency department triage scale, the Manchester Triage System. Int J Nurs Stud. 2014;51:1062‐1069. [DOI] [PubMed] [Google Scholar]
- 29. van der Wulp I, van Baar ME, Schrijvers AJ. Reliability and validity of the Manchester Triage System in a general emergency department patient population in the Netherlands: results of a simulation study. Emerg Med J. 2008;25:431‐434. [DOI] [PubMed] [Google Scholar]
- 30. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged: the index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914‐919. [DOI] [PubMed] [Google Scholar]
- 31. Rodriguez‐Molinero A, Lopez‐Dieguez M, Tabuenca AI, de la Cruz JJ, Banegas JR. Functional assessment of older patients in the emergency department: comparison between standard instruments, medical records and physiciansʼ perceptions. BMC Geriatr. 2006;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short orientation‐memory‐concentration test of cognitive impairment. Am J Psychiatry. 1983;140:734‐739. [DOI] [PubMed] [Google Scholar]
- 33. Carpenter CR, Banerjee J, Keyes D, et al. Accuracy of dementia screening instruments in emergency medicine: a diagnostic meta‐analysis. Acad Emerg Med. 2019;26:226‐245. [DOI] [PubMed] [Google Scholar]
- 34. OʼSullivan D, Brady N, Manning E, et al. Validation of the 6‐Item Cognitive Impairment Test and the 4AT test for combined delirium and dementia screening in older emergency department attendees. Age Ageing. 2017;0:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mooijaart SP, De Groot B, Blomaard LC et al. The APOP Screeningprogram: Handbook for Optimizing Care for the Acutely Presenting Older Patient in the Emergency Department. Leiden; 2018. http://www.apop.eu/UserFiles/File/apop_handboek_web.pdf. January 27, 2020.
- 36. Graff I, Goldschmidt B, Glien P, et al. The German version of the Manchester Triage System and its quality criteria–first assessment of validity and reliability. PLoS One. 2014;9:e88995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Steiner D, Renetseder F, Kutz A, et al. Performance of the Manchester triage system in adult medical emergency patients: a prospective cohort study. J Emerg Med. 2016;50:678‐689. [DOI] [PubMed] [Google Scholar]
- 38. Bagshaw SM, Stelfox HT, McDermid RC, et al. Association between frailty and short‐ and long‐term outcomes among critically ill patients: a multicentre prospective cohort study. CMAJ. 2014;186:E95‐E102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Basic D, Shanley C. Frailty in an older inpatient population: using the clinical frailty scale to predict patient outcomes. J Aging Health. 2015;27:670‐685. [DOI] [PubMed] [Google Scholar]
- 40. Nickel CH, Kellett J, Nieves Ortega R, Lyngholm L, Wasingya‐Kasereka L, Brabrand M. Mobility identifies acutely ill patients at low risk of in‐hospital mortality: a prospective multicenter study. Chest. 2019;156:316‐322. [DOI] [PubMed] [Google Scholar]
- 41. van Veen M, Steyerberg EW, Vanʼt Klooster M, et al. The Manchester triage system: improvements for paediatric emergency care. Emerg Med J. 2012;29:654‐659. [DOI] [PubMed] [Google Scholar]
- 42. Atzema CL, Austin PC, Tu JV, Schull MJ. Emergency department triage of acute myocardial infarction patients and the effect on outcomes. Ann Emerg Med. 2009;53:736‐745. [DOI] [PubMed] [Google Scholar]
- 43. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304‐377. [DOI] [PubMed] [Google Scholar]
- 44. Lucke JA, de Gelder J, Blomaard LC, et al. Vital signs and impaired cognition in older emergency department patients: the APOP study. PLoS One. 2019;14:e0218596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1: The Acutely Presenting Older Patient Screener.
Supplementary Figure S1: Flowchart of study population. The total study population of the APOP study was included, minus 21 patients with an incomplete APOP screening result.
Supplementary Figure S2: Hospital admission by MTS category and APOP screening result combined. Hospital admission rate for patients stratified by MTS category and APOP screening result combined. The upper 95% confidence intervals for proportion are shown. Nagelkerke R2 was calculated for MTS alone (R2 = 0.083), APOP alone (R2 = 0.020), and MTS and APOP combined (R2 = 0.096).
Supplementary Figure S3: The 7‐day mortality by MTS category and APOP screening result combined. The 7‐day mortality percentages for patients stratified by MTS category and APOP screening result combined. The upper 95% confidence intervals for proportion are shown. Nagelkerke R2 was calculated for MTS alone (R2 = 0.008), APOP alone (R2 = 0.017), and MTS and APOP combined (R2 = 0.019).


