Abstract
Objective
We analyzed the longitudinal profile of Alzheimer's disease (AD) cerebrospinal fluid (CSF) biomarkers in early Parkinson's disease (PD) compared with healthy controls (HCs) and tested baseline CSF biomarkers for prediction of clinical decline in PD.
Methods
Amyloid‐β 1 to 42 (Aβ42), total tau (t‐tau) and phosphorylated tau (p‐tau) at the threonine 181 position were measured using the high‐precision Roche Elecsys electrochemiluminescence immunoassay in all available CSF samples from longitudinally studied patients with PD (n = 416) and HCs (n = 192) followed for up to 3 years in the Parkinson's Progression Markers Initiative (PPMI). Longitudinal CSF and clinical data were analyzed with linear‐mixed effects models.
Results
We found patients with PD had lower CSF t‐tau (median = 157.7 pg/mL; range = 80.9–467.0); p‐tau (median = 13.4 pg/mL; range = 8.0–40.1), and Aβ42 (median = 846.2 pg/mL; range = 238.8–3,707.0) than HCs at baseline (CSF t‐tau median = 173.5 pg/mL; range = 82.0–580.8; p‐tau median = 15.4 pg/mL; range = 8.1–73.6; and Aβ42 median = 926.5 pg/mL; range = 239.1–3,297.0; p < 0.05–0.001) and a moderate‐to‐strong correlation among these biomarkers in both patients with PD and HCs (Rho = 0.50–0.97; p < 0.001). Of the patients with PD, 31.5% had pathologically low levels of CSF Aβ42 at baseline and these patients with PD had lower p‐tau levels (median = 10.8 pg/mL; range = 8.0–32.8) compared with 27.7% of HCs with pathologically low CSF Aβ42 (CSF p‐tau median = 12.8 pg/mL; range 8.2–73.6; p < 0.03). In longitudinal CSF analysis, we found patients with PD had greater decline in CSF Aβ42 (mean difference = −41.83 pg/mL; p = 0.03) and CSF p‐tau (mean difference = −0.38 pg/mL; p = 0.03) at year 3 compared with HCs. Baseline CSF Aβ42 values predicted small but measurable decline on cognitive, autonomic, and motor function in early PD.
Interpretation
Our data suggest baseline CSF AD biomarkers may have prognostic value in early PD and that the dynamic change of these markers, although modest over a 3‐year period, suggest biomarker profiles in PD may deviate from healthy aging. ANN NEUROL 2020;88:574–587
There is significant clinical and pathological heterogeneity of Parkinson's disease (PD), and whereas α‐synuclein (aSyn) Lewy pathology and the associated synapse and neuronal loss is the hallmark of this disease, there is varying severity of mixed Alzheimer's disease (AD) associated amyloid‐beta 1 to 42 (Aβ42) plaques and tau tangles found at autopsy in many patients with PD. Indeed, approximately 30% of autopsy confirmed PD have sufficient postmortem plaque and tangle pathology to meet neuropathologic criteria for a second diagnosis of AD, and these patients have a more rapid decline in cognition and overall survival than patients with PD with minimal AD co‐pathology. 1 , 2 Thus, identifying markers of AD pathology during life may have important prognostic indications in PD to guide clinical trials for homogeneous patient selection.
Cerebrospinal fluid (CSF) analysis provides a mechanism to detect and measure protein species related to the accumulation of these pathological proteins over time in living patients; cross‐sectional work finds CSF measures of AD pathology associate with cognitive performance 3 , 4 , 5 , 6 and postmortem severity of AD co‐pathology in PD. 7 Moreover, CSF tau and aSyn levels are highly correlated and, on average, lower in PD compared with controls 8 , 9 ; however, longitudinal AD CSF biomarker data in PD is rare 10 , 11 , 12 , 13 , 14 and detailed longitudinal modeling of progressive changes in values are lacking.
One obstacle to longitudinal CSF studies is inter‐ and intra‐assay variation, 15 which could reduce the sensitivity to detect changes between repeated measurements from an individual over time in longitudinal biomarker studies. The Roche Elecsys analytical platform is fully automated with high reliability for measurement of AD CSF biomarkers 16 , 17 , 18 and was previously validated with a reference measurement procedure approved by the Joint Committee for Traceability in Laboratory Medicine for CSF Aβ42. 19 Parkinson Progression Markers Initiative (PPMI 20 ) is a unique multicenter international observational study collecting long term annual detailed harmonized clinical measures and biomarkers in a large cohort of newly diagnosed drug‐naïve PD. We previously found CSF measurements of tau and Aβ42, as well as aSyn, related to cross‐sectional and longitudinal clinical features in this cohort with follow‐up up to 1 year. 8 , 9 , 12 , 21
Using the rich PPMI dataset with standardized longitudinal data for up to 3 years and the Elecsys high‐precision analytical platform, we evaluated the baseline and longitudinal progression of AD CSF biomarkers in PD and tested the relationship of these with clinical features.
Methods
Sample
The sample consisted of participants in 2 of the cohorts of the PPMI multicenter prospective longitudinal observational study: early PD, drug‐naïve at baseline, and healthy controls (HCs), 20 with diagnostic criteria for enrollment as described previously. 8 , 9 Those included for study (n = 608) had at least one CSF sample at any timepoint available as of May 7, 2018 (PD = 416, HC = 192). We did not include other PPMI cohort participants (symptomatic or asymptomatic individuals with PD‐related genetic mutations, prodromal PD, or participants with parkinsonism but without evidence of dopaminergic deficit syndrome). CSF and clinical data were obtained from PPMI database for baseline, 6 months and annual follow up visits at years 1, 2, and 3. A subset of these participants were previously reported in a cross‐sectional study of baseline CSF data (n = 601) 8 , 9 or longitudinal CSF with only 1‐year follow‐up (n = 285) 12 and using a different immunoassay platform (ie, Innogenetics AlzBio3 Luminex platform).
All procedures were performed with prior approval from ethical standards committees at each participating institution and with informed consent from all study participants. The study is registered in clinicaltrials.gov as NCT01141023.
CSF Analysis
CSF collection, shipment, and storage were performed using standard operating procedures at each institution, as described in detail previously (please see biologics manual ppmi.info.org). CSF samples were shipped from the PPMI Biorepository Core Laboratories to the University of Pennsylvania (Penn) Biomarker Research Laboratory for measurement of CSF Aβ42, total‐tau (t‐tau) and phosphorylated tau at threonine 181 position (p‐tau) using Elecsys electrochemiluminescence immunoassays on the cobas e 601 analysis platform (Roche Diagnostics) as described. 16 , 18 The analytical measurement range for the Aβ42 assay was 200 to 1,700 pg/mL, the t‐tau assay was 80 to 1,300 pg/mL, and the p‐tau 181 assay: 8 to 120 pg/mL. Roche extrapolated values above the upper technical limit from the calibration curve, 1,700 pg/mL, in 96 measurements of Aβ42. Performance of this platform has been previously reported with intra‐ and inter‐percent coefficient of variation (CV%) <5%. 17 , 18 , 19 CSF total aSyn data from baseline visits were obtained from PPMI database and measured by BioLegend (San Diego, CA) using a commercially available sandwich immunoassay, as previously described. 8 , 9 , 12
Clinical Data
Clinical data for each visit was obtained from the PPMI database, as above and described in detail previously. 22 Variables included for analysis were demographics (age at baseline, age at symptoms onset, disease duration at visit, years of education, and sex) and cognitive and motor testing scores. We chose continuous measures of cognitive functioning in several domains, including global functioning (Montreal Cognitive Assessment [MoCA]), episodic memory (Hopkins Verbal Learning Test [HVLT] discrimination recognition score), visuospatial functioning (Benton judgment of line orientation score [JOLO]), language (semantic fluency [SF]), and executive functioning (letter number sequencing [LNS]). For motor functioning, we used the Movement Disorders Society modified Unified Parkinson's disease rating scale (MDS‐UPDRS) part III total score and motor subscores for tremor and postural instability (PIGD), as previously defined, 9 as continuous variables. We also included the total score for the Scales for Outcomes in Parkinson's Disease‐Autonomic questionnaire (SCOPA‐AUT) to capture non‐motor/cognitive autonomic aspects of PD.
Genetic Data
Blood samples were analyzed for apolipoprotein E (APOE) genotype at the PPMI genetics core, as described, 8 and coded for analyses as the presence or absences of one or more ɛ4 alleles (ie, dominant model).
Statistical Analyses
Data used in this study were downloaded from PPMI database on May 7, 2018, and analyzed at the University of Iowa using SAS version 9.4 Software (SAS Institute, Cary, NC) or at Penn using SPSS version 24.0 (IBM, Chicago, IL). We used a significance threshold of p < 0.05 due to the hypothesis‐driven approach for CSF‐clinical correlations (please see Results section for specifics).
Continuous demographic, clinical, and biomarker data were compared between groups using Student's t‐test or Wilcoxon rank‐sum test, as appropriate, and nominal variables compared with a chi‐square or Fisher's exact test. For nonparametric comparisons, we calculated effect size r = z/√(N), where N is the total sample size. 23
To test for associations of needle type used in CSF collection, we used univariate comparisons for measures of each analyte using the Kruskal–Wallis test within PD subjects. The CSF needle was grouped by type coded in database: Quincke, Sprotte, or “other.”
Correlations between CSF biomarkers were computed using Spearman rank correlation and 95% confidence intervals (CIs) obtained based on Fisher's z transformation. To test biological associations of CSF biomarkers, we performed univariate subgroup analysis within patients with PD and HC groups comparing patients with one or more copies of APOE ε4 allele compared to those without.
To characterize the AD CSF profile of patients with PD and HCs we applied a cut off point for amyloid‐positivity established in AD. 16 To mitigate differences in pre‐analytical factors between PPMI and AD cohorts that influence CSF Aβ42 levels, 24 we used the transformation formula from Shaw et al 25 to convert Elecsys values to AlzBio3 equivalents [x = (CSF Aβ42 + 251.55)/3.74] and applied the established cut off point of <250 pg/mL of AlzBio3 equivalent values 16 to designate amyloid‐positivity. A chi‐square test was used to analyze proportional differences in amyloid‐positivity among patients with PD and HCs at baseline. Within patients with PD and HCs, we compared demographics and CSF biomarker values between amyloid‐positive and negative groups using univariate statistics.
For longitudinal analyses we focused on core AD CSF biomarkers (Aβ42, t‐tau, and p‐tau), rather than ratios of these analytes, to more directly test biomarker associations. To assess the difference in mean change from baseline for each AD CSF analyte between the patients with PD and control groups, we used rank‐based linear mixed models (LMMs) with adjustment for age, sex, and the baseline value of the CSF outcome. Akaike information criterion (AIC) was used in the determination to adjust for APOE and the model fit of including an interaction between time and group (ie, PD vs HC). We report the p value from the rank‐based LMM and mean estimates from a model based on the untransformed values for ease of interpretation. The model‐based mean estimates of the change in CSF within patients with PD and HCs and their differences adjust for group‐specific differences in the baseline covariates (age, sex, baseline CSF, and APOE, if applicable).
To test the associations between baseline CSF analyte levels and decline on clinical measures in patients with PD, we used LMM or rank‐based LMM with separate models for each baseline CSF measure as predictors for the dependent variable of change in each clinical measure (MoCA, HVLT, JOLO, SF, LNS, UPDRS III total, tremor UPDRS subscore, PIGD UPDRS subscore, and SCOPA‐AUT) from baseline in PD subjects. All models adjusted for baseline age, sex, disease duration, and the baseline value of the clinical measure. AIC was used in the determination to adjust for APOE in the final models and to compare the model fit of including an interaction between time and baseline CSF. If AIC indicated the interaction did not provide better fit, the interaction term was removed. Using this approach, we found the optimal model structure for MoCA, LNS, UPDRS III, and SCOPA‐AUT was a linear time model with a random intercept and slope and an unstructured covariance structure. The optimal model for SF was a linear time model with a random intercept and an unstructured covariance structure. A nonlinear time model had optimal fit for JOLO. The final models for the clinical outcomes in LNS adjusted for APOE along with the MoCA models for Aβ42 and aSyn. Rank‐based LMMs were fit for Tremor, PIGD, and HVLT. We report the p value from the rank‐based LMM and effect estimates from a model based on the untransformed values for ease of interpretation.
Results
Patient Demographics and Baseline Characteristics
PD and HC patient demographics are listed in Table 1. Similar to previous reports of this cohort at baseline, 8 , 9 PD and HC groups did not differ in age, sex, or APOE allele status.
TABLE 1.
Patient and Control Demographics and Baseline Characteristics
| Variables | PD N = 416 | HCs N = 192 | P | |
|---|---|---|---|---|
| DEMOGRAPHIC | Age, yr | 61.7 (9.6) | 60.8 (11.3) | 0.3 |
| Sex |
M = 272 (65.4%) F = 144 (34.6%) |
M = 123 (64.0%) F = 69 (35.9%) |
0.8 | |
| Education, yr | 15.5 (3.0) | 16.0 (2.9) | 0.06 | |
| APOE ε4 status |
0 alleles = 277 (73.3%) 1 allele = 92 (24.3%) 2 alleles = 9 (2.4%) Missing data = 38 |
0 alleles = 129 (73.7%) 1 allele = 42 (24.0%) 2 alleles = 4 (2.3%) Missing data = 17 |
>0.99 | |
| Age at onset, yr | 59.7 (9.9) | NA | — | |
| Disease duration, mo | 6.7 (6.5) | NA | — | |
| MOTOR | UPDRS III tremor |
0.5 (0.3) N = 415 Missing data = 1 |
0.03 (0.08) N = 191 Missing data = 1 |
<0.0001 |
| UPDRS III PIGD |
0.23 (0.22) N = 415 Missing data = 1 |
0.02 (0.09) N = 191 Missing data = 1 |
<0.0001 | |
| COGNITIVE | MoCA |
27.1 (2.3) N = 413 Missing data = 3 |
28.2 (1.1) N = 192 |
<0.0001 |
| HVLT |
10 (−4‐12) N = 414 Missing data = 2 |
11 (−4‐12) N = 192 |
<0.001 | |
| SFT |
48.8 (11.6) N = 415 Missing data = 1 |
51.9 (11.3) N = 192 |
<0.01 | |
| JOLO |
13 (5‐15) N = 415 Missing data = 1 |
14 (4‐15) N = 192 |
0.06 | |
| LNS |
10.6 (2.6) N = 415 Missing data = 1 |
10.9 (2.6) N = 192 |
0.1 | |
Data listed = mean (SD) for normally distributed variables or median (range) for non‐normally distributed variables and frequency (%) for categorical variables. Missing data noted in each cell where applicable. APOE = apolipoprotein E; HCs = healthy controls; HVLT = Hopkins Verbal Learning Test Discrimination Recognition Score; JOLO = Benton judgement of line orientation score; LNS = Letter‐number sequencing score; MoCA = Montreal Cognitive Assessment; PD = Parkinson's disease; PIGD = postural instability and gait disturbance subscore of UPDRS; SFT = semantic fluency total score; Tremor = Tremor subscore of UPDRS; UPDRS = Unified Parkinson's Disease Rating Scale.
Cross‐Sectional CSF Analysis
First, to test for pre‐analytical factors that could influence CSF measurements on this platform, we performed univariate comparisons of needle type used in CSF collection cross‐sectional data at each time point for CSF Aβ42, t‐tau, and p‐tau. We did not find any association of needle type with biomarker values (data not shown) and needle type did not have a significant effect on any of our subsequent CSF outcome models below.
Baseline levels of CSF Aβ42, t‐tau, p‐tau, aSyn, and the ratio of p‐tau/t‐tau were lower in patients with PD than HCs (effect size = 0.09–0.17; p < 0.03–0.0001), whereas the ratios of t‐tau/Aβ42, p‐tau/Aβ42, t‐tau/aSyn, p‐tau/aSyn, and Aβ42/aSyn were similar between groups (Fig 1). These group‐level differences were similar across timepoints (Table 2); however, despite group‐wise differences in these CSF biomarkers, there was individual patient overlap in values between groups (see Fig 1).
FIGURE 1.
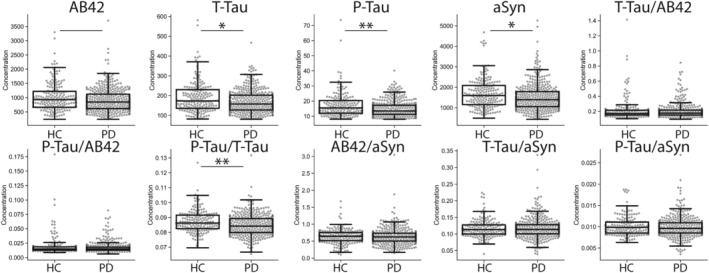
Group‐wise comparison of baseline CSF biomarkers in PD and HC. Solid line represents group‐wise difference between patients with PD and HCs p < 0.05, solid line plus single asterisk p ≤ 0.01, and solid line plus double‐asterisk p < 0.001.
TABLE 2.
Cross‐Sectional Cerebrospinal Fluid Biomarker Data
| Analyte | Visit | PD (N = 416) | HCs (N = 192) | Effect Size r | P |
|---|---|---|---|---|---|
| Aβ42 | Baseline | 846.15 (238.80–3707.00) Missing data = 6 | 926.45 (239.10–3297.00)Missing data = 4 | 0.09 | 0.02 |
| 6 mo | 849.70 (267.30–2888.00)Missing data = 77 | 938.90 (372.90–3272.00)Missing data = 35 | 0.14 | <0.002 | |
| Year 1 | 821.30 (249.50–2480.00)Missing data = 90 | 1019.50 (312.00 ‐2678.00)Missing data = 40 | 0.18 | <0.0001 | |
| Year 2 | 849.75 (260.30–3000.00)Missing data = 112 | 955.75 (248.60–3551.00)Missing data = 56 | 0.13 | <0.01 | |
| Year 3 | 855.25 (240.80–2396.00)Missing data = 194 | 954.30 (282.00–2842.00)Missing data = 79 | 0.12 | 0.03 | |
| t‐tau | Baseline | 157.70 (80.93–467.00)Missing data = 13 | 173.50 (81.96–580.80)Missing data = 5 | 0.12 | <0.01 |
| 6 mo | 153.60 (80.64–387.50)Missing data = 81 | 179.60 (82.64–551.50)Missing data = 37 | 0.19 | <0.0001 | |
| Year 1 | 155.60 (82.24–388.70)Missing data = 94 | 178.80 (82.36–600.10)Missing data = 40 | 0.18 | <0.0001 | |
| Year 2 | 156.35 (80.88–463.60)Missing data = 110 | 178.80 (85.92–619.70)Missing data = 60 | 0.18 | <0.001 | |
| Year 3 | 160.45 (80.98–444.50)Missing data = 190 | 173.60 (83.48–569.40)Missing data = 79 | 0.16 | <0.01 | |
| p‐tau | Baseline | 13.40 (8.01–40.13) Missing data = 37 | 15.44 (8.08–73.61) Missing data = 16 | 0.17 | 0.0001 |
| 6 mo | 13.34 (8.00–36.94) Missing data = 106 | 15.69 (8.53–69.10) Missing data = 42 | 0.20 | <0.0001 | |
| Year 1 | 13.41 (8.05–34.28) Missing data = 124 | 15.87 (8.30–80.08) Missing data = 48 | 0.21 | <0.0001 | |
| Year 2 | 13.39 (8.13–43.69) Missing data = 136 | 15.59 (8.00–80.54) Missing data = 66 | 0.21 | <0.0001 | |
| Year 3 | 13.31 (8.03–42.87) Missing data = 205 | 15.31 (8.05–78.34) Missing data = 86 | 0.22 | 0.0001 | |
| aSyn | Baseline | 1390.50 (432.40‐5256.90)Missing data = 2 | 1593.50 (488.60‐4683.10)Missing data = 2 | 0.13 | <0.002 |
| t‐tau/Aβ42 | Baseline | 0.18 (0.10–0.84) Missing data = 18 | 0.17 (0.10–1.41) Missing data = 7 | 0.02 | 0.5 |
| p‐tau/Aβ42 | Baseline | 0.01 (0.01–0.08) Missing data = 42 | 0.01 (0.01–0.18) Missing data = 18 | 0.01 | 0.8 |
| p‐tau/t‐tau | Baseline | 0.08 (0.07–0.13) Missing data = 37 | 0.09 (0.07–0.13) Missing data = 16 | 0.16 | <0.001 |
| Aβ42/aSyn | Baseline | 0.63 (0.15–3.04) Missing data = 7 | 0.65 (0.10–1.68) Missing data = 4 | 0.02 | 0.7 |
| t‐tau/aSyn | Baseline | 0.11 (0.04–0.34) Missing data = 14 | 0.11 (0.04–0.22) Missing data = 5 | 0.02 | 0.5 |
| p‐tau/aSyn | Baseline | 0.01 (0.00–0.03) Missing data = 38 | 0.01 (0.01–0.02) Missing data = 16 | 0.05 | 0.3 |
Data listed = median (range). Missing data noted in each cell where applicable. Aβ42 = cerebrospinal fluid amyloid‐beta 1 to 42; aSyn = cerebrospinal fluid total alpha‐synuclein; HCs = healthy controls; PD = Parkinson's disease; p‐tau = cerebrospinal fluid phosphorylated tau at threonine 181; t‐tau = cerebrospinal fluid total tau.
Next, to test the association of AD CSF biomarkers with a known genetic marker of AD pathology, 26 we compared both PD and HC individuals with one or more copies of APOE ε4 genotype to those with no copies of APOE ε4 at baseline and found lower CSF Aβ42 in APOE ε4 carriers for both patients with PD and HCs (effect size = 0.26–0.31; p < 0.0001), whereas there was no difference between APOE genotype groups within PD or HC for t‐tau or p‐tau (Table 3). Interestingly, there was also lower baseline CSF aSyn in PD APOE ε4 carriers than noncarriers (effect size = 0.13; p = 0.01), whereas CSF aSyn was similar between HC APOE groups (see Table 3).
TABLE 3.
Baseline CSF Data by APOE Genotype
| CSF analyte | PD | HCs | ||||||
|---|---|---|---|---|---|---|---|---|
| APOE 4 + N = 101 | APOE 4 – N = 277 | Effect size r | p | APOE 4 + N = 46 | APOE 4 – N = 129 | Effect Size r | p | |
| CSF Aβ42 | 697.1 (238.8–1795.0) Missing = 1 | 912.8 (249.0–3707.0) Missing = 4 | 0.26 | <0.0001 | 673.1 (239.1–1890.0) Missing = 1 | 994.8 (336.1–3297.0) Missing = 3 | 0.31 | <0.0001 |
| CSF t‐tau | 152.0 (85.0–349.8) Missing = 5 | 159.9 (80.9–467.0) Missing = 8 | 0.04 | 0.48 | 189.5 (93.3–554.5) Missing = 2 | 168.6 (82.0–580.8) Missing = 2 | 0.04 | 0.57 |
| CSF p‐tau | 13.3 (8.0–28.0) Missing = 12 | 13.6 (8.0–40.1) Missing = 22 | 0.03 | 0.56 | 17.0 (8.2–60.0) Missing = 4 | 15.3 (8.1–73.6) Missing = 10 | 0.05 | 0.52 |
| CSF aSyn | 1256.5 (432.4–3022.3) | 1432.7 (472.0–5256.9) Missing = 2 | 0.13 | 0.01 | 1522.0 (488.6–4683.1) Missing = 1 | 1662.6 (600.7–4271.3) Missing = 1 | 0.07 | 0.36 |
Data listed = median (range). Number of missing data points is noted in each cell. Aβ42 = cerebrospinal fluid amyloid‐beta 1 to 42; aSyn = cerebrospinal fluid total alpha‐synuclein; HCs = healthy controls; PD = Parkinson's disease; p‐tau = cerebrospinal fluid phosphorylated tau at threonine 181; t‐tau = cerebrospinal fluid total tau.
We found a moderate to strong correlation among AD CSF biomarkers (Aβ42 vs t‐tau Rho = 0.59; 95% CI = 0.53–0.64; p < 0.0001; n = 583; Aβ42 vs p‐tau Rho = 0.51; 95% CI = 0.45–0.57; p < 0.0001; n = 548; t‐tau vs p‐tau Rho = 0.97; 95% CI = 0.97–0.98; p < 0.0001; n = 555) and with AD CSF biomarkers and CSF aSyn (Aβ42 vs aSyn Rho = 0.60; 95% CI = 0.55–0.65; p < 0.0001; n = 597; t‐tau vs aSyn Rho = 0.80; 95% CI = 0.77–0.83; p < 0.0001; n = 589; p‐tau vs aSyn Rho = 0.80; 95% CI = 0.77–0.83; p < 0.0001; n = 554) in the total cohort at baseline (Fig 2).
FIGURE 2.
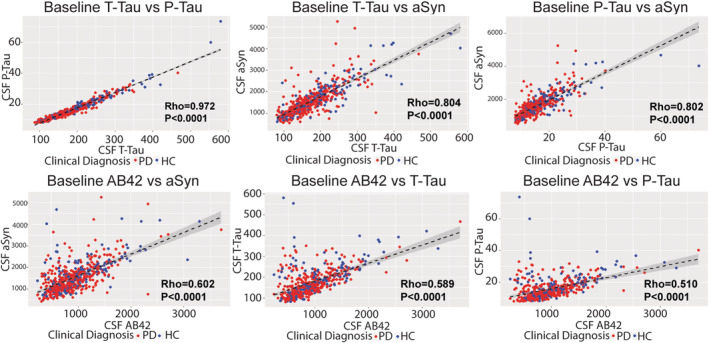
Baseline correlation of CSF biomarkers in patients with PD and HCs. Scatterplots depict individual patient datapoints for CSF values at baseline. Dashed‐line represents fitted line with 95% confidence interval. Red = patients with PD; and Blue = HCs.
Finally, we examined cross‐sectional profiles of patients with presumed amyloid‐positivity in patients with PD and HCs at baseline using an established cut off point in AD. 16 We found at baseline, relative equal frequencies of pathologically low CSF Aβ42 indicative of amyloidosis (+A) in patients with PD (31.5%) and HCs (27.7%; Table 4) with no differences in demographics between PD + A and PD with normal CSF Aβ42 (−A) or HC + A and HC – A; however, there were lower CSF t‐tau, p‐tau, and aSyn levels in PD + A vs PD – A (effect size = 0.29–0.45; p < 0.0001). In contrast, there was no difference in CSF p‐tau between HC + A and HC – A, but CSF p‐tau was lower in PD + A than HC + A (effect size = 0.19; p < 0.03), suggesting a divergent interaction between AD CSF biomarkers in PD compared with controls (see Table 4).
TABLE 4.
Baseline CSF AB Groups
| PD − A | PD + A | HC − A | HC + A | |
|---|---|---|---|---|
| N (% total) | 281 (68.5%) | 129 (31.5%) | 136 (72.3%) | 52 (27.7%) |
| Sex F (%F) | 99 (35.2%) | 41 (31.8%) | 50 (36.8%) | 18 (34.6%) |
| Age at CSF | 61.5 (9.6) | 62.2 (9.6) | 60.7 (10.8) | 61.0 (13.0) |
| Disease duration | 4.2 (0.4–34.8) | 4.6 (0.7–34.7) | NA | NA |
| CSF t‐tau | 169.50 (85.6–467.0) |
124.1 a (80.9–339.2) Missing = 12 |
183.0 (93.7–420.5) |
126.8b (81.96–580.8) Missing = 3 |
| CSF p‐tau |
14.04 (8.2–40.1) Missing = 3 |
Missing = 33 |
15.6 (8.1–39.1) Missing = 1 |
12.8 (8.2‐73.6) Missing = 13 |
| CSF aSyn |
1522.3 (606.1–5256.9) Missing = 1 |
1026.6 a (432.4–3638.3) | 1696.2 (733.8–4271.3) | 1131.9b (488.6–4683.1) |
Data listed = frequency (%) for categorical data, mean (standard deviation) for normally distributed data or median (range) for non‐normally distributed data. Number of missing data points is noted in each cell. aSyn = cerebrospinal fluid total alpha‐synuclein; HC − A = healthy controls with normal CSF Aβ42 levels; HC + A = HC with pathologically low CSF Aβ42 levels; PD − A = Parkinson's disease with normal CSF Aβ42 levels; PD + A = PD with pathologically low CSF Aβ42 levels; p‐tau = cerebrospinal fluid phosphorylated tau at threonine 181; t‐tau = cerebrospinal fluid total tau.
p < 0.0001 PD + A vs PD − A;
p < 0.0001 HC + A vs HC − A;
p < 0.03 PD + A vs HC + A.
Longitudinal Change in AD CSF Biomarkers
To further test the profile of AD CSF biomarkers longitudinally in patients with PD versus HCs, we performed LMM analysis to test the mean change from baseline at each timepoint between patients with PD and HCs. We did not find an interaction between group and time, suggesting the difference in change between patients with PD and HCs was largely constant over the 3‐year period (Fig 3). PD had greater decline in all 3 biomarkers over time; we found greater reduction in CSF Aβ42 (mean difference = −41.83 pg/mL; SE = 18.94; p = 0.03) and p‐tau (mean difference = −0.38 pg/mL; SE = 0.22; p = 0.03), in patients with PD compared to HCs with a trend for CSF t‐tau (mean difference = −3.7 pg/mL; SE = 2.7; p = 0.07; Table 5). Examination of estimates of mean change at each timepoint in our models finds an increasingly negative mean change in CSF Aβ42 in patients with PD compared to HCs, where there is mild decline only at year 3, and in patients with PD more modest mean increases in CSF t‐tau and p‐tau seen only at year 3 compared to more consistent increases over time in HCs (see Table 5).
FIGURE 3.
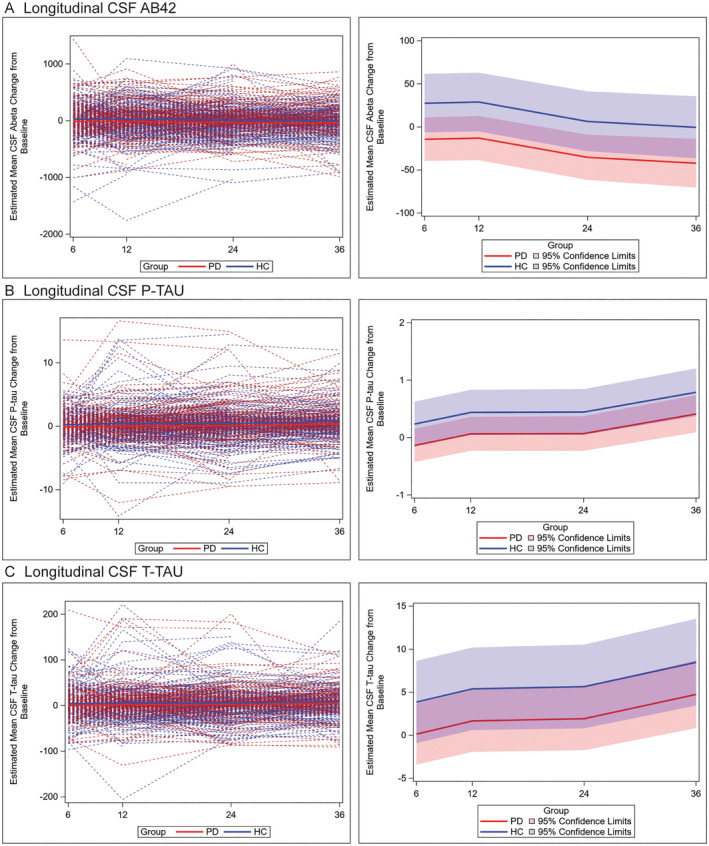
Individual patient data for median change in AD CSF biomarker measurements at each timepoint. Spaghetti plot depicts individual‐patient trajectories (left panels) and trend lines (right panels) depict mean values for PD (red) and HC (blue) and 95% CI for mean change in biomarker value at each time point using LMM adjusted for age, sex, and the baseline value of the CSF outcome for mean change in measurements for CSF Aβ42 (A) t‐tau (B) and p‐tau (C) at 6, 12, 24, and 36‐month timepoints. Across all timepoints we found a greater reduction in CSF Aβ42 (mean difference −41.83 pg/mL; SE = 18.94; p = 0.03) and p‐tau (mean difference = −0.38 pg/mL; SE = 0.22; p = 0.03), in patients with PD compared to HCs with a nonsignificant trend for CSF t‐tau (mean difference = −3.7 pg/mL; SE = 2.7; p = 0.07).
TABLE 5.
Mean Estimates of Change in AD CSF Biomarkers in PD and Healthy Controls
| Variable | PD | HCs | ||||||
|---|---|---|---|---|---|---|---|---|
| 6 mo | 1 yr | 2 yr | 3 yr | 6 mo | 1 yr | 2 yr | 3 yr | |
| Aβ42 | ||||||||
| Estimate (SE) | −14.29 (12.88) | −13.01 (13.08) | −35.39 (13.36) a | −42.27 (14.47) a | 27.53 (17.28) | 28.82 (17.37) | 6.44 (17.67) a | −0.44 (18.38) a |
| (95% CI) | (−39.56, 10.97) | (−38.67, 12.65) | (−61.60, −9.18) | (−70.65, −13.88) | (−6.38, 61.45) | (−5.26, 62.89) | (−28.23, 41.10) | (−36.50, 35.62) |
| p‐tau | ||||||||
| Estimate (SE) | −0.14 (0.15) | 0.06 (0.15) | 0.07 (0.15) a | 0.41 (0.16) a | 0.24 (0.20) | 0.44 (0.20) | 0.45 (0.20) a | 0.79 (0.21) a |
| (95% CI) | (−0.43, 0.15) | (−0.23, 0.36) | (−0.23, 0.37) | (0.09, 0.74) | (−0.15, 0.63) | (0.05, 0.83) | (0.05, 0.84) | (0.37, 1.20) |
| t‐tau | ||||||||
| Estimate (SE) | 0.13 (1.81) | 1.67 (1.83) | 1.93 (1.87) a | 4.75 (2.01) a | 3.87 (2.43) | 5.40 (2.44) | 5.66 (2.48) a | 8.49 (2.57) a |
| (95% CI) | (−3.41, 3.68) | (−1.93, 5.26) | (−1.73, 5.59) | (0.81, 8.69) | (−0.90, 8.63) | (0.61, 10.18) | (0.80, 10.53) | (3.45, 13.52) |
Estimates based on the raw values (not the ranks) from models adjusting for age, sex, and baseline CSF outcome value. AIC criteria determined APOE included in Aβ42 model.
Denotes p < 0.0001 for within‐group comparison of estimates between time point and 6‐mo reference category.
Prediction of Longitudinal Cognitive, Motor, and Autonomic Decline Using Baseline AD CSF Biomarkers
We performed exploratory analyses based on previous postmortem 27 , 28 , 29 , 30 and biomarker work 3 , 4 , 5 to test the predictive value of AD CSF biomarkers in patients with PD. We hypothesized that AD CSF biomarkers would relate to overall cognitive decline, and more specifically in temporal‐lobe mediated episodic memory and SF tasks. Moreover, we expected CSF aSyn would relate to decline on traditional‐reported cognitive deficits in early PD 22 , 31 , 32 : spatial and executive/attention/working memory tasks. Further, we hypothesized CSF aSyn would relate to progression of classic PD features of motor impairment and autonomic instability. Finally, based on recent postmortem work, 27 we expected greater increase in motor postural instability to associate with lower CSF AB42.
We found greater baseline p‐tau (β = −0.47 points per 10 pg/mL; 95% CI = −0.91 to −0.03; p < 0.05) and lower CSF Aβ42 (month 24 β = 0.06 points per 100 pg/mL; 95% CI = 0.01–0.12; p = 0.02; month 36 β = 0.09 points per 100 pg/mL; 95% CI = 0.03–0.15; p < 0.01) predicted greater decline in global cognition (ie, MoCA). We also found that both lower CSF baseline Aβ42 (β = 0.04 points per 100 pg/mL; 95% CI = 0.0003–0.09; p < 0.05) and aSyn (β = 0.03 points per 100 pg/mL; 95% CI = 0.003–0.06; p = 0.03) predicted greater decline in working memory (ie, LNS). There was a nonsignificant trend for greater baseline CSF t‐tau to be associated with longitudinal decline on SF (β = −0.57 points per 100 pg/mL; 95% CI = −1.17–0.03; p = 0.06).
We found both lower baseline CSF Aβ42 and aSyn were associated with increased postural instability subscores (aSyn β = −0.004 points per 100 pg/mL; 95% CI = −0.008 to −0.0007; p < 0.02; Aβ42 β = −0.007 points per 100 pg/mL; 95% CI = −0.01 to −0.001; p = 0.04) and total UPDRS III motor scores (aSyn β = −0.10 points per 100 pg/mL; 95% CI = −0.19 to −0.003; p = 0.04; Aβ42 β = −0.16 points per 100 pg/mL; 95% CI = −0.30 to −0.01; p = 0.03). Finally, lower baseline CSF Aβ42 was also associated with an increase in autonomic symptoms on SCOPA‐AUT (β = −0.12 points per 100 pg/mL; 95% CI = −0.21 to −0.02; p = 0.02). We did not find other associations with baseline CSF biomarkers and longitudinal clinical measures (data not shown).
Discussion
In this large‐scale longitudinal study of well‐characterized patients with PD over a 3‐year period using a precise analytical platform (the Roche Elecsys system) to measure AD CSF biomarker analytes, we have several important findings. First, we find lower overall AD CSF biomarker values in patients with PD versus HCS (see Fig 1, Table 2), with a moderate‐to‐strong correlation between markers in both patients with PD and HCs (see Fig 2). There were 31.5% of patients with PD who had pathologically low CSF Aβ42 at baseline with relatively low CSF p‐tau compared with HCs with pathological CSF Aβ42 (see Table 4). Moreover, we found modest but novel measurable group level changes in AD CSF biomarkers over time in patients with PD that were distinct from HCs, with greater overall decline in CSF Aβ42 and p‐tau in patients with PD (see Fig 3, Table 5). Finally, we find preliminary evidence for predictive value of CSF Aβ42 for global and domain‐specific cognitive decline, motor, and autonomic function in patients with PD. These data have important implications for the interpretation of these emerging CSF biomarkers in patients with PD.
Our group‐wise comparisons at baseline (see Fig 1, Table 2) using the high‐precision immunoassay replicated previous findings of lower CSF levels of t‐tau and p‐tau, on average, in patients with PD than HCs and a strong correlation with CSF aSyn (Rho = 0.8–0.9; see Fig 2). 8 , 9 , 10 Similar to another study of early PD, 5 we found lower CSF Aβ42 in patients with PD compared with HCs and moderate correlations of CSF Aβ42 with CSF t‐tau, p‐tau, and aSyn in both patients with PD and HCs (see Fig 2). Moreover, low baseline CSF Aβ42 in this PD cohort was, overall, associated with lower baseline levels of CSF t‐tau and p‐tau, rather than higher levels of CSF tau as in preclinical and clinical AD cohorts. 16 Indeed, in our unique analysis applying an established AD cut off point for CSF Aβ42, we found approximately one‐third of patients with PD had pathologically low CSF Aβ42 (PD positive [+] A). Moreover, these patients had, on average, lower p‐tau levels compared with HCs with pathologically low Aβ42 (HC + A; see Table 4), suggesting the profiles of CSF Aβ42 and p‐tau in PD may diverge from aging and AD. Interestingly, HC + A had lower CSF t‐tau and aSyn compared with HCs with normal CSF Aβ42m (HC negative [–] A; see Table 4), which is opposite than expected; however, there was heterogeneity in values with higher overall range in these analytes than seen in PD. Our observed frequency of 31% of early PD with positive AD CSF biomarker profile is similar to autopsy data in end‐stage PD, 1 but lower than a previous study using a CSF p‐tau/Aβ42 ratio to designate AD positive profile. 10 Our findings of low CSF p‐tau in patients with PD at baseline and follow‐up suggest that a CSF p‐tau cut off point established in AD cohorts may underestimate the frequency of AD co‐pathology in PD. This is important to consider as biomarker classification strategies are being used in AD and related neurodegenerative conditions. 33
To further clarify the biological context of our findings, we tested the association of CSF Aβ42 with APOE ε4 genotype, and similar to previous studies, 8 , 10 , 11 we found lower levels in APOE ε4 carriers versus non‐carriers for both PD and HC groups (see Table 3). These data suggest our measurements are related, at least in part, to amyloid‐beta pathophysiology in PD. Interestingly, we also found lower CSF aSyn in APOE ε4 carriers compared with non‐carriers for PD but not HC; previous autopsy work finds an association of APOE ε4 with pure aSyn neuropathology 34 suggesting shared genetic risk for amyloidosis and aSyn aggregation that may be reflected in our CSF findings here. Interestingly, our clinical correlations, although preliminary, found similar associations of both CSF Aβ42 and aSyn with core clinical features of PD (see below), further suggesting these biomarkers may, in part, reflect similar underlying pathophysiological processes in PD.
Longitudinal analysis of CSF biomarkers in PD are rare 10 , 12 , 14 with conflicting results. One study that included 30 sporadic patients with PD found lower CSF Aβ42, t‐tau, and p‐tau in patients with PD compared with controls at baseline and 24‐month follow‐up. 13 Whereas in 62 patients with PD of the BioFINDER study, on average, there was an increase in CSF t‐tau and p‐tau at 24 months that was most pronounced in patients with PD with longer disease duration. 14 In a large‐scale prospective PD cohort with follow‐up up to 8 years, there was lower CSF Aβ42 in patients with PD who developed cognitive impairment with more stable levels in PD without cognitive impairment, 11 but not this study, the similarly sized DATATOP study, 10 or other studies above modeled longitudinal change of CSF biomarkers over time.
Here, with the first automated high‐precision measurements in PD and statistical modeling to account for demographic factors in the longitudinal change in biomarkers, we find modest but measurable group‐wise changes in AD CSF biomarkers over a 3‐year period (see Table 5, Fig 3). Importantly, we find that the longitudinal profile in patients with PD diverges from HCs with greater overall decrease in CSF Aβ42 and lower overall increases in CSF t‐tau and p‐tau by year 3. We previously reported a slight increase in CSF Aβ42 and CSF p‐tau in the PPMI PD cohort at year 1 using the AlzBio3 assay and shorter follow‐up. 12 There are several possibilities for discrepancies in the previous literature, including the size and demographic makeup of the patient population (eg, stage/severity of disease), statistical approach, and increased precision of the automated analytical platform in this study. 19 Moreover, there was large individual patient variability in this study (Fig 3) and our statistical modeling helped account for demographic and APOE status, which could influence longitudinal measures of CSF analytes and obscure group‐wise differences using traditional cross‐sectional analyses used in previous work. Indeed, our observations in HCs here are congruent with previous longitudinal CSF data in cognitively normal aged patients with mild decreases in CSF Aβ42 and increases in CSF t‐tau and p‐tau. 35 , 36
It is interesting to hypothesize the mechanism for our observations of decline in CSF Aβ42 in patients with PD; as aforementioned, whereas low CSF Aβ42 has been linked to amyloid‐beta pathophysiology in PD, 7 , 37 low CSF Aβ42 may have independent associations with aSyn pathology 7 and perhaps in some patients with PD low CSF Aβ42 is reflective of mechanisms related to underlying aSyn pathology prior to, or in absence of, the accumulation of cerebral amyloidosis. We also found CSF t‐tau and p‐tau had divergent longitudinal profiles from HCs, with minimal change until years 2 to 3, where there was mild overall increase in levels compared to the greater mean increases seen in HCs (see Table 5). Thus, the longitudinal profile of increasing CSF t‐tau and p‐tau with age may be partially suppressed in the context of PD. Other longitudinal studies with more advanced PD suggest highly correlated levels CSF tau and aSyn levels may eventually increase over time in more advanced disease 14 and cross‐sectional work finds greater CSF t‐tau and p‐tau levels in PD with dementia compared to PD without dementia. 38 Moreover, both CSF aSyn and tau levels are elevated in patients with AD, 39 suggesting increasing neurodegeneration may lead to increased CSF tau and aSyn. Thus, future work with molecular imaging and autopsy data are needed to establish CSF cut off points to accurately detect AD co‐pathology in PD for prognosis and to elucidate the underlying pathophysiological changes contributing to patterns observed here.
Our longitudinal clinical correlation analyses provide further insight into the interpretation of these CSF markers in patients with PD. Although there are currently relative mild levels of overall cognitive impairment in the PPMI PD cohort even after 5 years, 21 , 40 we found evidence for lower baseline CSF Aβ42 to predict global cognitive decline (ie, change in MoCA score) in PD, similar to previous work. 3 , 5 , 11 , 13 , 21 , 41 , 42 , 43 , 44 , 45 Moreover, we also found more modest associations of greater baseline CSF p‐tau to predict decline in MoCA score in our PD cohort, similar to one study 44 but not others. 13 , 43 One possible interpretation is that, despite the overall trend of declining CSF p‐tau in the PD group, there is heterogeneity and some patients with PD at risk for cognitive impairment have an early increase in p‐tau levels. Future work with longer follow‐up can elucidate potential biomarker‐defined subgroups of patients with PD. Nonetheless, these data suggest that baseline AD CSF profiles may have prognostic value for overall incipient cognitive decline in PD.
Cognitive impairment in PD is heterogeneous and although attention, working memory, executive abilities, and visuospatial dysfunction are considered to be the core clinical features in the majority of initial PD cognitive deficits, 22 , 31 , 32 episodic memory loss and language dysfunction are not uncommon and previously linked to AD pathology. 28 , 29 , 30 Thus, we hypothesized domain‐specific associations of AD CSF biomarkers for episodic memory and SF but surprisingly did not find an association. Instead, we found both lower CSF Aβ42 and CSF aSyn had predictive value of cognitive decline in working memory (ie, a core cognitive feature of PD) and decline in motor UPDRS III total and PIGD subscores. Moreover, CSF Aβ42 alone predicted worsening of autonomic symptoms in patients with PD. One study of early PD similarly found lower CSF Aβ42 related to postural instability scores 46 and postmortem amyloid‐pathology has been linked to postural instability in PD 27 ; however, our data also conflict with some previous work that found associations of baseline AD CSF biomarkers with measures of memory impairment 5 and findings of greater baseline CSF aSyn associated with cognitive and motor decline in PD. 42 , 47 Moreover, another study of early PD did not find an association of CSF aSyn with cognitive or motor decline, 48 whereas p‐tau/t‐tau and p‐tau/Aβ42 ratios have been linked to motor decline in PD in one large‐scale study. 10 Thus, there is complex literature on baseline CSF biomarker prediction of progression in PD with varying methodologies and patient compositions, which could contribute to these discrepancies, necessitating replication with follow‐up capturing end‐stage disease to fully discern predictive values of CSF biomarkers in PD. Here, the effect sizes of these changes were relatively small and statistical associations marginal so these findings remain preliminary in this early stage of PD; however, the overall pattern of CSF Aβ42 clinical associations with core features of PD reinforce the possibility that this analyte may reflect biological processes integral to the pathophysiology of PD.
There are several limitations to acknowledge in this study. First, although this cohort represents a unique large‐scale international coordinated multicenter effort to collect standardized longitudinal assessments, findings in this dataset from a research setting require replication in independent population‐based cohorts to generalize findings. The Roche Elecsys platform has advantages of high precision (percent coefficient of variance [%CV] values <5%), linearity of dynamic range of measurements, 16 , 17 , 18 , 19 and standard operating procedures were used for harmonized methods of CSF collection across PPMI sites; we examined the effect of needle type used during the lumbar puncture (LP) procedure and found no significant association of needle type with any of our AD CSF biomarkers, similar to other recent work in AD, 49 providing further critical data to optimize large‐scale multicenter biomarker efforts needed to establish CSF biomarkers for use in clinical practice. Although our predictive models were robust, the magnitude of change in our clinical and biomarker values were relatively modest, likely due to the early stage of disease and relative short duration of follow‐up for longitudinal biomarker values that may take decades to show progression. 50 Finally, future work relating CSF biomarker profiles across the full natural history of PD to in vivo measures of pathology and autopsy data is needed to fully resolve the biological context of these analytes in PD. Nonetheless, our unique large‐scale longitudinal data suggest a distinct CSF AD biomarker profile in early PD with relatively greater decline in CSF Aβ42 and p‐tau. Moreover, we find preliminary evidence of early predictive value of subtle changes in CSF biomarkers for cognition, motor, and autonomic function in PD. Further follow‐up of the PPMI cohort and other ongoing longitudinal PD studies 5 , 11 , 14 , 45 will be needed to determine predictive value for clinically relevant changes.
Author Contributions
D.J.I., J.F., C.S.C., B.M., D.R.G., A.S., K.M., and L.M.S. were responsible for the conception and design of the study. D.J.I., J.F., C.S.C., C.C.G., J.H.K., T.S., T.F., A.W.T., C.M.T., K.K., L.M.C., A.R., S.H., D.W., B.M., D.R.G., A.S., K.M., J.Q.T., and L.M.S. acquisition and analysis of data. D.J.I., J.F., and L.M.S. drafting of the manuscript. The complete list of members of the PPMI group and their affiliations are contained in a Supplementary Online Table.
Potential Conflicts of Interest
Dr. Mollenhauer has received honoraria for consultancy from Roche, Biogen, UCB, and Sun Pharma Advanced Research Company. Dr. Kieburtz reports other from Clintrex Research Corp., other from Hoover Brown LLC, outside the submitted work; Dr. Galasko reports personal fees from Biogen, Inc., personal fees from vTv Pharmaceuticals, Inc., personal fees from Fujirebio, Inc., personal fees from Cognition Therapeutics, outside the submitted work. Dr. Simuni reports grants from Biogen, Roche, Neuroderm, Sanofi, Sun Pharma, Abbvie, IMPAX, and Prevail, and other from Acadia, Abbvie, Accorda, Adamas, Allergan, Amneal, Aptinyx, Denali, General Electric (GE), Kyowa, Neuroderm, Neurocrine, Sanofi, Sinopia, Sunovion, Roche, Takeda, Voyager, and US World Meds, during the conduct of the study. Dr. Tanner reports grants from Gateway LLC, grants from Roche/Genentech, grants and personal fees from Biogen Idec, personal fees from Acorda, personal fees from Adamas Therapeutics, personal fees from Amneal, personal fees from CNS Ratings, personal fees from Grey Matter LLC, personal fees from Northwestern University, personal fees from Partners, Harvard U, outside the submitted work. Dr. Marek reports consulting from Michael J Fox, GE Healthcare, Takeda, Lundbeck, Neuron23, Roche, Neuroderm, and Invicro, outside the submitted work. PPMI is supported in part by Roche who manufacture the Elecsys assays used in the study.
Supporting information
Appendix S1: Supporting Information
Acknowledgments
PPMI is sponsored by the Michael J. Fox Foundation for Parkinson's Research (MJFF) and is co‐funded by MJFF, Abbvie, Allergan, Avid Radiopharmaceuticals, Biogen, BioLegend, Bristol‐Myers Squibb, Celgene, Denali, Eli Lilly & Co., F. Hoffman‐La Roche, Ltd., GE Healthcare, Genentech, GlaxoSmithKline, Lundbeck, Merck, MesoScale, Piramal, Prevail Therapeutics, Pfizer, Roche, Sanofi Genzyme, Servier, Takeda, Teva, UCB, Berily, and Voyager Therapeutics. We are grateful to Roche for supplying all immunoassay reagents and supplies to the University of Pennsylvania Biomarker Research Laboratory to enable measurements of CSF biomarkers using the Elecsys β‐amyloid (1–42) CSF, the Elecsys phospho‐tau (181P) CSF and Elecsys total‐tau CSF on a cobas e 601 analyzer (software version 05.02). D.J.I. is supported by NIH NINDS (NS088341) and Penn Institute on Aging as well as NIA grant AG10124 (J.Q.T.). D.J.I. and J.F. were responsible for generation of figures and we thank Nicholas Cullen and Claire Peterson for their assistance.
Data used in the preparation of this article were obtained from the Parkinson Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). For up‐to‐date information on the study, visit www.ppmi-info.org.
References
- 1. Irwin DJ, White MT, Toledo JB, et al. Neuropathologic substrates of Parkinson disease dementia. Ann Neurol 2012;72:587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Irwin DJ, Grossman M, Weintraub D, et al. Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: a retrospective analysis. Lancet Neurol 2017;16:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siderowf A, Xie SX, Hurtig H, et al. CSF amyloid {beta} 1‐42 predicts cognitive decline in Parkinson disease. Neurology 2010;75:1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Montine TJ, Shi M, Quinn JF, et al. CSF Abeta(42) and tau in Parkinson's disease with cognitive impairment. Mov Disord 2010;25:2682–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alves G, Bronnick K, Aarsland D, et al. CSF amyloid‐beta and tau proteins, and cognitive performance, in early and untreated Parkinson's disease: the Norwegian ParkWest study. J Neurol Neurosurg Psychiatry 2010;81:1080–1086. [DOI] [PubMed] [Google Scholar]
- 6. Compta Y, Marti MJ, Ibarretxe‐Bilbao N, et al. Cerebrospinal tau, phospho‐tau, and beta‐amyloid and neuropsychological functions in Parkinson's disease. Mov Disord 2009;24:2203–2210. [DOI] [PubMed] [Google Scholar]
- 7. Irwin DJ, Xie SX, Coughlin D, et al. CSF tau and beta‐amyloid predict cerebral synucleinopathy in autopsied Lewy body disorders. Neurology 2018;90:e1038–e1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kang JH, Mollenhauer B, Coffey CS, et al. CSF biomarkers associated with disease heterogeneity in early Parkinson's disease: the Parkinson's progression markers initiative study. Acta Neuropathol 2016;131:935–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kang JH, Irwin DJ, Chen‐Plotkin A, et al. Association of cerebrospinal fluid Aβ1‐42, t‐tau, p‐tau181 and α‐synuclein levels with clinical features of early drug naïve Parkinson's disease patients. JAMA Neurol 2013;70:1277–1287. 10.1001/jamaneurol.2013.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang J, Mattison HA, Liu C, et al. Longitudinal assessment of tau and amyloid beta in cerebrospinal fluid of Parkinson disease. Acta Neuropathol 2013;126:671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lerche S, Wurster I, Roben B, et al. Parkinson's disease: evolution of cognitive impairment and CSF Abeta1‐42 profiles in a prospective longitudinal study. J Neurol Neurosurg Psychiatry 2019;90:165–170. [DOI] [PubMed] [Google Scholar]
- 12. Mollenhauer B, Caspell‐Garcia CJ, Coffey CS, et al. Longitudinal CSF biomarkers in patients with early Parkinson disease and healthy controls. Neurology 2017;89:1959–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brockmann K, Schulte C, Deuschle C, et al. Neurodegenerative CSF markers in genetic and sporadic PD: classification and prediction in a longitudinal study. Parkinsonism Relat Disord 2015;21:1427–1434. [DOI] [PubMed] [Google Scholar]
- 14. Hall S, Surova Y, Ohrfelt A, et al. Longitudinal measurements of cerebrospinal fluid biomarkers in Parkinson's disease. Mov Disord 2016;31:898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shaw LM, Vanderstichele H, Knapik‐Czajka M, et al. Qualification of the analytical and clinical performance of CSF biomarker analyses in ADNI. Acta Neuropathol 2011;121:597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shaw LM, Waligorska T, Fields L, et al. Derivation of cutoffs for the Elecsys((R)) amyloid beta (1‐42) assay in Alzheimer's disease. Alzheimers Dement 2018;10:698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rozga M, Bittner T, Hoglund K, Blennow K. Accuracy of cerebrospinal fluid Abeta1‐42 measurements: evaluation of pre‐analytical factors using a novel Elecsys immunosassay. Clin Chem Lab Med 2017;55:1545–1554. [DOI] [PubMed] [Google Scholar]
- 18. Hansson O, Seibyl J, Stomrud E, et al. CSF biomarkers of Alzheimer's disease concord with amyloid‐beta PET and predict clinical progression: a study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimers Dement 2018;14:1470–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bittner T, Zetterberg H, Teunissen CE, et al. Technical performance of a novel, fully automated electrochemiluminescence immunoassay for the quantitation of beta‐amyloid (1‐42) in human cerebrospinal fluid. Alzheimers Dement 2016;12:517–526. [DOI] [PubMed] [Google Scholar]
- 20. Marek K, Jennings D, Lasch S, et al. The Parkinson Progression Marker Initiative (PPMI). Prog Neurobiol 2011;95:629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Caspell‐Garcia C, Simuni T, Tosun‐Turgut D, et al. Multiple modality biomarker prediction of cognitive impairment in prospectively followed de novo Parkinson disease. PLoS One 2017;12:e0175674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weintraub D, Simuni T, Caspell‐Garcia C, et al. Cognitive performance and neuropsychiatric symptoms in early, untreated Parkinson's disease. Mov Disord 2015;30:919–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fritz CO, Morris PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen 2012;141:2–18. [DOI] [PubMed] [Google Scholar]
- 24. Stewart T, Shi M, Mehrotra A, et al. Impact of pre‐analytical differences on biomarkers in the ADNI and PPMI studies: implications in the era of classifying disease based on biomarkers. J Alzheimers Dis 2019;69:263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shaw LM, Hansson O, Manuilova E, et al. Method comparison study of the Elecsys(R) beta‐amyloid (1‐42) CSF assay versus comparator assays and LC‐MS/MS. Clin Biochem 2019;72:7–14. [DOI] [PubMed] [Google Scholar]
- 26. Wider C, Ross OA, Nishioka K, et al. An evaluation of the impact of MAPT, SNCA and APOE on the burden of Alzheimer's and Lewy body pathology. J Neurol Neurosurg Psychiatry 2012;83:424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Selikhova M, Williams DR, Kempster PA, et al. A clinico‐pathological study of subtypes in Parkinson's disease. Brain 2009;132:2947–2957. [DOI] [PubMed] [Google Scholar]
- 28. Coughlin D, Xie SX, Liang M, et al. Cognitive and pathological influences of tau pathology in Lewy body disorders. Ann Neurol 2019;85:259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kraybill ML, Larson EB, Tsuang DW, et al. Cognitive differences in dementia patients with autopsy‐verified AD, Lewy body pathology, or both. Neurology 2005;64:2069–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peavy GM, Edland SD, Toole BM, et al. Phenotypic differences based on staging of Alzheimer's neuropathology in autopsy‐confirmed dementia with Lewy bodies. Parkinsonism Relat Disord 2016;31:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord 2007;22:1689–1707. [DOI] [PubMed] [Google Scholar]
- 32. Litvan I, Goldman JG, Troster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society task force guidelines. Mov Disord 2012;27:349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jack CR Jr, Bennett DA, Blennow K, et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 2016;87:539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tsuang D, Leverenz JB, Lopez OL, et al. APOE epsilon4 increases risk for dementia in pure synucleinopathies. JAMA Neurol 2013;70:223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Toledo JB, Xie SX, Trojanowski JQ, Shaw LM. Longitudinal change in CSF tau and Abeta biomarkers for up to 48 months in ADNI. Acta Neuropathol 2013;126:659–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fagan AM, Mintun MA, Shah AR, et al. Cerebrospinal fluid tau and ptau(181) increase with cortical amyloid deposition in cognitively normal individuals: implications for future clinical trials of Alzheimer's disease. EMBO Mol Med 2009;1:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Buongiorno M, Antonelli F, Compta Y, et al. Cross‐sectional and longitudinal cognitive correlates of FDDNP PET and CSF amyloid‐beta and tau in Parkinson's Disease1. J Alzheimer's Dis 2017;55:1261–1272. [DOI] [PubMed] [Google Scholar]
- 38. Hall S, Ohrfelt A, Constantinescu R, et al. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch Neurol 2012;69:1445–1452. [DOI] [PubMed] [Google Scholar]
- 39. Shi M, Tang L, Toledo JB, et al. Cerebrospinal fluid alpha‐synuclein contributes to the differential diagnosis of Alzheimer's disease. Alzheimers Dement 2018;14:1052–1062. 10.1016/j.jalz.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Marek K, Chowdhury S, Siderowf A, et al. The Parkinson's progression markers initiative (PPMI) ‐ establishing a PD biomarker cohort. Ann Clin Transl Neurol 2018;5:1460–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schrag A, Siddiqui UF, Anastasiou Z, et al. Clinical variables and biomarkers in prediction of cognitive impairment in patients with newly diagnosed Parkinson's disease: a cohort study. Lancet Neurol 2017;16:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hall S, Surova Y, Ohrfelt A, et al. CSF biomarkers and clinical progression of Parkinson disease. Neurology 2015;84:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Terrelonge M Jr, Marder KS, Weintraub D, Alcalay RN. CSF beta‐amyloid 1‐42 predicts progression to cognitive impairment in newly diagnosed Parkinson disease. J Mol Neurosci 2016;58:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu C, Cholerton B, Shi M, et al. CSF tau and tau/Abeta42 predict cognitive decline in Parkinson's disease. Parkinsonism Relat Disord 2015;21:271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Alves G, Lange J, Blennow K, et al. CSF Abeta42 predicts early‐onset dementia in Parkinson disease. Neurology 2014;82:1784–1790. [DOI] [PubMed] [Google Scholar]
- 46. Alves G, Pedersen KF, Bloem BR, et al. Cerebrospinal fluid amyloid‐beta and phenotypic heterogeneity in de novo Parkinson's disease. J Neurol Neurosurg Psychiatry 2013;84:537–543. [DOI] [PubMed] [Google Scholar]
- 47. Stewart T, Liu C, Ginghina C, et al. Cerebrospinal fluid alpha‐synuclein predicts cognitive decline in Parkinson disease progression in the DATATOP cohort. Am J Pathol 2014;184:966–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Forland MG, Ohrfelt A, Dalen I, et al. Evolution of cerebrospinal fluid total alpha‐synuclein in Parkinson's disease. Parkinsonism Relat Disord 2018;49:4–8. [DOI] [PubMed] [Google Scholar]
- 49. Rembach A, Evered LA, Li QX, et al. Alzheimer's disease cerebrospinal fluid biomarkers are not influenced by gravity drip or aspiration extraction methodology. Alzheimer's Res Ther 2015;7:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jack CR Jr, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 2013;12:207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information


