Abstract
Introduction
In several studies, the chimeric antigen receptor T‐cell therapy tisagenlecleucel demonstrated encouraging rates of remission and lasting survival benefits in pediatric patients with relapsed/refractory (r/r) acute lymphoblastic leukemia (ALL). We assessed the cost‐effectiveness of tisagenlecleucel (list price: 320 000 EUR) among these patients when compared to clofarabine monotherapy (Clo‐M), clofarabine combination therapy (Clo‐C), and blinatumomab (Blina) from both a healthcare and a societal perspective. We also assessed future medical and future non‐medical consumption costs.
Methods
A three‐state partitioned survival model was used to simulate a cohort of pediatric patients (12 years of age) through different disease states until the end of life (lifetime horizon). Relevant outcomes were life years, quality‐adjusted life years (QALYs), healthcare costs, societal costs, and the incremental cost‐effectiveness ratio (ICER). Uncertainty was explored through deterministic and probabilistic sensitivity analyses as well as through several scenario analyzes.
Results
Total discounted costs for tisagenlecleucel were 552 679 EUR from a societal perspective, which was much higher than the total discounted costs from a healthcare perspective (ie, 409 563 EUR). Total discounted societal costs for the comparator regimens ranged between 160 803 EUR for Clo‐M and 267 259 EUR for Blina. Highest QALYs were estimated for tisagenlecleucel (11.26), followed by Blina (2.25), Clo‐C (1.70) and Clo‐M (0.74). Discounted societal ICERs of tisagenlecleucel ranged between 31 682 EUR/QALY for Blina and 37 531 EUR/QALY for Clo‐C and were considered cost‐effective with a willingness‐to‐pay (WTP) threshold of 80 000 EUR/QALY. None of the scenarios exceeded this threshold, and more than 98% of the iterations in the probabilistic sensitivity analysis were cost‐effective.
Discussion
At the current price and WTP threshold, tisagenlecleucel is cost‐effective from both a healthcare and a societal perspective. Nevertheless, long‐term effectiveness data are needed to validate the several assumptions that were necessary for this model.
Keywords: CAR‐T, cost‐effectiveness, pediatric ALL, tisagenlecleucel
Novelty statement.
This is the first European cost‐effectiveness analysis of tisagenlecleucel from a societal perspective.
Tisagenlecleucel is cost‐effective from both a healthcare and a societal perspective.
Our findings may be used to support decision‐making in both clinical application and reimbursement of tisagenlecleucel.
1. INTRODUCTION
With current first‐line treatment protocols, survival in pediatric B‐cell acute lymphoblastic leukemia (pALL) increased to 85%‐90% over the past years. Also in relapsed pALL, 40%‐60% of children can be cured with intensive chemotherapy regimens, often including allogeneic stem cell transplantation (alloSCT). 1 The prognosis for patients with a second relapse, with a relapse after alloSCT, or with refractory pALL remains however poor, ranging from 10% to 30% 2‐year overall survival (OS). 2 , 3 In this article, these patients are referred to as r/r pALL patients. Current regimens for r/r pALL include clofarabine monotherapy (Clo‐M), clofarabine combination therapy (Clo‐C), and blinatumomab (Blina), although no clearly defined standard of care yet exists. In countries such as the United States and the UK, salvage chemotherapy is also commonly used.
In several clinical trials, the chimeric antigen receptor (CAR) T‐cell therapy tisagenlecleucel showed high rates of remission 4 , 5 , 6 , 7 , 8 and lasting survival benefits with 12‐month event‐free survival (EFS) rates between 45% and 51%. 4 , 5 , 7 These promising results come at a high costs however. In the United States, tisagenlecleucel was made available at 475 000 USD (approx. 414 000 EUR) which included an outcome‐based commercial model. 9 The stated list price in the UK is 282 000 GBP (314 000 EUR; 360 000 USD), and after a confidential discount, it is currently available via the Cancer Drug Fund. 10 In the Netherlands, the list price is 320 000 EUR. Whether tisagenlecleucel is a cost‐effective alternative to existing treatments is a pressing question for policymakers, payers, clinicians and patients and can be explored by cost‐effectiveness modeling approaches. 11 Ideally, such a cost‐effectiveness analysis is not limited to a healthcare (or payer) perspective, including only direct healthcare costs. This is because treatment for r/r pALL also affects both personal and professional lives of the patients and their caretakers. When other aspects such as travel costs, informal care costs, and productivity losses or gains are incorporated, a cost‐effectiveness study is referred to as being conducted from a so‐called “societal perspective.” The Dutch EE guideline recommends such a perspective for all cost‐effectiveness analyses in the Netherlands. 12
To date, some economic evaluation studies have been performed estimating the cost‐effectiveness of tisagenlecleucel compared to Clo‐M, Clo‐C, or Blina over a lifetime horizon (ie, until all simulated patients died). 13 , 14 , 15 , 16 , 17 All of them found tisagenlecleucel cost‐effective in at least one scenario from a payer perspective 14 , 16 , 17 and a societal perspective. 13 , 15 To employ a societal perspective, Sarkar et al 13 included cost of caregivers, patient time, transportation, and parking, as well as meals. However, it remains unclear what specific cost items were considered with regard to caregivers and patient time. The initial manufacturer submission to the Canadian Agency for Drugs and Technologies in Health (CADTH) did not seem to include a societal perspective. Therefore, the CADTH considered travel and accommodation time for patients and caregivers, medical coinsurance amounts, copayment, and deductibles over a period of only 3 years for a scenario analysis from a societal perspective. 15 Assuming a lifetime horizon for the economic model, the considered total societal costs for tisagenlecleucel of approximately 16 500 CAD seem to be a drastic underestimation of the true societal costs that can be attributed to tisagenlecleucel in the lifetime of pediatric patients.
Our aim was to add to the existing evidence for tisagenlecleucel in r/r pALL patients by estimating the cost‐effectiveness of tisagenlecleucel for pediatric patients with r/r pALL from a broad societal perspective when compared to Clo‐C, Clo‐M, and Blina, respectively. We are the first study to consider both medical and non‐medical consumption costs in life years gained (ie, future medical costs). Furthermore, we considered productivity losses for patients’ caretakers rather than for the pediatric patients and explored the inclusion of potential productivity gains for children with long‐term EFS.
2. METHODS
The primary outcome of this analysis was the incremental cost‐effectiveness ratio (ICER) of tisagenlecleucel for each comparator from two perspectives over a lifetime horizon. 12 A healthcare perspective included costs and effects of pretreatment, treatment, adverse events, follow‐up period, subsequent HSCT, and future medical costs. A societal perspective included all costs and effects of the healthcare perspective in addition to costs for travel, the stay of caregivers at a charity hotel during treatment, productivity losses of patients’ caregivers, and informal care. Lastly, we also considered non‐medical consumption costs. 18 , 19 Results of all perspectives are reported separately. The base case is defined from a societal perspective, including future non‐medical consumption costs as this represents the most conservative estimates.
To estimate the clinical effectiveness outcomes such as life years (LYs) and quality‐adjusted life years (QALYs) of each treatment, we modeled a fictive cohort of pediatric patients (12 years of age) that receive tisagenlecleucel or either comparator treatment (ie, Clo‐M, Clo‐C, or Blina). At any time, the modeled patients could be in one of the three health states: (a) EFS, (b) progressive disease (PD), or (c) death (see Figure 1). The proportion of patients per health state was estimated from standard parametric survival functions (ie, exponential, Weibull, log‐logistic, log‐normal, Gompertz, and generalized Gamma) with the best statistical and clinical fit to the observed OS and EFS. 20 In addition, a set of flexible cubic spline models was considered to capture the potential curative nature of tisagenlecleucel. 21 Statistical fit was assessed with Akaike information criterion (AIC) and the Bayesian information criteria (BIC), while clinical plausibility was validated by a clinical expert (PMH). For tisagenlecleucel, survival (EFS and OS) was based on pooled data (N = 193) from the ELIANA (NCT02435849), ENSIGN (NCT02228096), and B2101J (NCT01626495) trials. 22 , 23 , 24 Overall survival for Clo‐M, 25 Clo‐C, 26 and Blina 3 was based on the literature. Since EFS data were not available for the comparator arms, EFS was considered proportional to OS, using a validated ratio from the literature. 27
Figure 1.
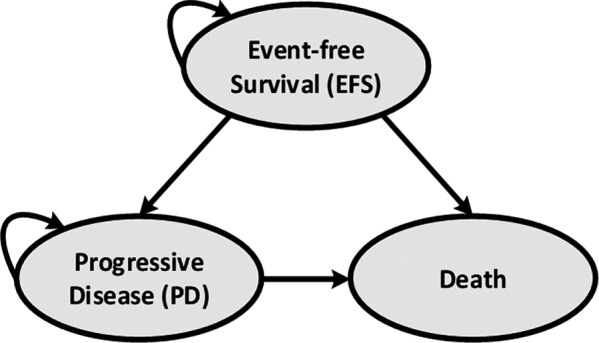
De novo model
Patients who remained in the EFS state after 5 years were assumed to be long‐term survivors of ALL (ie, considered cured). This assumption was based on the observed plateau phase and validated by expert opinion. OS of these patients was modeled by applying the standard mortality rate (SMR) of 15.2 for 5‐year ALL survivors. 28 The initial proportional relationship of EFS to OS was assumed for the first 5 years of the model. After the fifth year, the cumulative survival probabilities of EFS were assumed to flatten up until they reached OS. In the model, EFS could not exceed OS at any time point. Furthermore, we assumed that relapses and leukemia‐specific deaths only occurred within the first 5 years for all comparators.
The model cycle length was set to 1 month. To adhere to the Dutch guideline for economic evaluation research (Dutch EE guideline), costs and effects were discounted at a 4% and 1.5% rate, respectively. 12 , 29
Tisagenlecleucel was included as a one‐time infusion costing €320 000, and its dosing schedule was according to the ELIANA trial. 22 For the comparator treatments, dosing schedules were taken from the literature. 3 , 26 , 30 Adverse treatment events (AEs) were considered for all treatments and included cytokine release syndrome and B‐cell aplasia. After initial therapy, we assumed that a proportion of patients would receive HSCT (17%, 16%, 40%, and 34% for tisagenlecleucel, Clo‐M, Clo‐C, Blina, respectively). For patients staying alive (ie, in EFS or PD), we assumed follow‐up costs for outpatients visits and laboratory test and procedures with different resource use frequencies per model health state (see Appendix S1).
To calculate QALYs, health‐state utilities for EFS and PD were derived from the EQ‐5D‐3L data collected in the ELIANA trial and estimated with the Dutch tariff. 22 , 31 Additional disutilities (ie, for treatment and adverse events) and age‐related utility decrements were based on the literature. 32 , 33 , 34
Prices and costs for the societal perspective were based on the Dutch EE guideline and the literature (see Table 1 and Appendix S1). 12 , 35 Future costs (medical and non‐medical consumption) were based on the PAID tool (version 3.0). 36 Furthermore, we explored potential productivity gains due to the improved survival by assuming that 53% of the long‐term survivors aged 18 years or older would be employed. 37 These cost savings were explored in a scenario analysis to account for potential future productivity gains.
Table 1.
Model input parameters and values
| Variable | Value | Measurement of uncertainty in DSA and PSA | Distribution used in PSA | Source |
|---|---|---|---|---|
| Model settings | ||||
| Discount rate (costs) | 4.00% | NA | NA | Dutch EE guideline 12 |
| Discount rate (benefits) | 1.5% | NA | NA | |
| Time horizon | 88 y | NA | NA | NA |
| Patient characteristics | ||||
| Starting age (years) | 12 | 95% CI: 1; 25 | Normal | Pooled data |
| Percent female | 46.63% | SE: 0.04 | Beta | |
| Mean body surface area (BSA) | 1.3 | SE: 0.03 | Normal | |
| Mean weight (kg) | 41.7 | SE: 1.52 | Normal | |
| Efficacy | ||||
| OS distribution | Log normal | Different distributions selected in DSA | Bootstrapped | Assumption validated by clinical expert |
| EFS distribution | Gompertz | |||
| Duration of benefit in months | 60 | NA | NA | |
| EFS vs OS ratio for all comparators | 0.69 | SE: 25% of mean | Beta | Van den Berg et al, 2011 |
| Drug and procedure acquisition cost | ||||
| Pretreatment cost for lymphodepleting regimen | €521 | SE: 25% of mean | Gamma | ELIANA trial (resource use); Dutch Z‐index (unit cost) |
| Tisagenlecleucel | €320 000 | Dutch Z‐index public list price | ||
| Clofarabine monotherapy | €71 020 | Jeha et al 2006 (dosing schedule); Dutch Z‐index (unit cost) | ||
| Clofarabine combination therapy | €35 453 | Hijiya et al 2011 (dosing schedule); Dutch Z‐index (unit cost) | ||
| Blinatumomab | €117 934 | von Stackelberg 2016 (dosing schedule); Dutch Z‐index (unit cost) | ||
| Inpatient and outpatient administration cost | ||||
| Pretreatment cost for lymphodepleting regimen | €6301 | SE: 25% of mean | Gamma | ELIANA (resource use); Dutch EE guideline and Franken et al, (unit cost inpatient and daycare respectively) |
| Tisagenlecleucel | €15 932 | |||
| Clofarabine monotherapy | €2437 | Clinical expert opinion (resource use); Franken et al, 2018 (unit cost) | ||
| Clofarabine combination therapy | €4292 | Clinical expert opinion (resource use); Dutch EE guideline (unit cost) | ||
| Blinatumomab | €1997 | Clinical expert opinion (resource use); Franken et al, 2018 (unit cost); von Stackelberg et al 2016 (distribution of patients over treatment cycles) | ||
| Subsequent HSCT | ||||
| Rates for tisagenlecleucel | 16.58% | SE: 25% of mean | Beta | pooled data, (duration and percentage) |
| Rate for clofarabine monotherapy | 16.39% | SE: 0.07 | Evoltra product label (duration and percentage) | |
| Rate for clofarabine combination therapy | 40.00% | SE: 0.05 | Hijiya et al 2011 (duration and percentage) | |
| Rate for blinatumomab | 34.29% | SE: 0.10 | von Stackelberg et al 2016 (duration and percentage) | |
| Disutility (treatment) | ‐0.21 | SE: 25% of mean | Beta | Forsythe et al, 2018 |
| Disutility (6‐12 mo after treatment) | ‐0.02 | |||
| Costs: stem cell harvesting a | €66 581 | SE: 25% of mean | Gamma | Blommestein et al, 2012 |
| Costs: initial HSCT procedure a | €44 391 | |||
| Follow‐up costs after HSCT (up to 1 y) a | €106 618 | |||
| Health‐state utilities and disutilities | ||||
| Utility for EFS | 0.83 | SE: 0.03 | Beta | ELIANA trial |
| Utility for PD | 0.68 | SE 0.05 | ||
| Disutility for tisagenlecleucel (duration in days) | ‐0.20 (26) | SE: 25% of mean | Kwon et al 2018 (utility value) 33 ; Gaynon et al 2006 (duration Clo‐M) 47 ; Hijiya et al 2011 (duration (Clo‐C) 26 ; von Stackelberg et al 2016 (duration Blina) 3 | |
| Disutility for Clo‐M (duration in days) | ‐0.20 (66) | |||
| Disutility for Clo‐C therapy (duration in days) | ‐0.20 (47) | |||
| Disutility for Blina (duration in days) | ‐0.20 (61) | |||
| Age‐related utilities |
Age < 25:0.95 Age 25‐74:0.93 ‐ 0.89 Age 75+: 0.83 |
NA | Janssen et al 2014 34 | |
| Future costs | ||||
| Future medical costs | Various costs per treatment and age group | NA | NA | Van Baal et al, 2011 19 |
| Future non‐medical (consumption) costs | Various costs per treatment and age group | NA | NA | |
| Patient and family costs | ||||
| Distance to hospital | 79 kilometers | NA | NA | Own calculation |
| Travel costs b | €3.09 parking costs per visit, €0.19 per kilometer for travelling by car | NA | NA | Dutch EE guideline 12 |
| Average number of caregivers/ parents accompanying patient | 1.5 | NA | NA | Expert opinion |
| Parents stay at a charity hotel during treatment | €60 | NA | NA | Charity hotel website (Ronald McDonald) |
| Informal care c | € 14.52 per hour, 8 h per outpatient visit, daycare treatment, or inpatient hospital day | NA | NA | Dutch EE guideline 12 |
| Productivity losses | ||||
| Total productivity losses females | € 12 499 | SE: 25% of mean b | Gamma d | Dutch EE guideline 12 (wage per hour) |
| Total productivity losses males | € 8993 | |||
| Rate of females with productivity losses | 60% | Hovén et al, 2013 48 (proportion of parents going back to work) Statistics Netherlands (proportion of parents contributing to the labor force) | ||
| Rate of males with productivity losses | 85% | |||
Abbreviations: Allo‐SCT, allogeneic stem cell transplantation; CI, confidence interval; MUD, matched unrelated donor; NA, not applicable; SE, standard error; sib, sibling donor; UCB, umbilical cord blood.
Based on proportions for different HSCT type (see Appendix S1)
The amount of travel trips is dependent on the assumed treatment regimen and respective number of visits during treatment and follow‐up visits (see Appendix S1 for treatment schedules and follow‐up visit frequencies).
Informal care was assumed for patients aged < 18 y.
Only total costs of the productivity losses were varied in both DSA and PSA (ie, a combination of the rate and the costs).
Lastly, we conducted deterministic sensitivity analyses (DSA), probabilistic sensitivity analysis (PSA) to address uncertainty of the model input parameters and estimates (see Table 1). Several scenario analyses were performed to explore the influence of different assumptions on the ICER.
A list of key input parameters to the model including their source is presented in Table 1, and a more detailed description of the employed methodology can be found in the Appendix S1.
3. RESULTS
In the model base case, tisagenlecleucel yielded 14.01 discounted LYs and 11.26 discounted QALYs, which was much higher than any of the comparators. Undiscounted LYs and QALYs were 18.98 and 15.21, respectively. Figure 2 shows the observed EFS from the pooled data as well as the modeled EFS of all treatments. Figure 3 shows both observed and modeled OS of all treatments.
Figure 2.
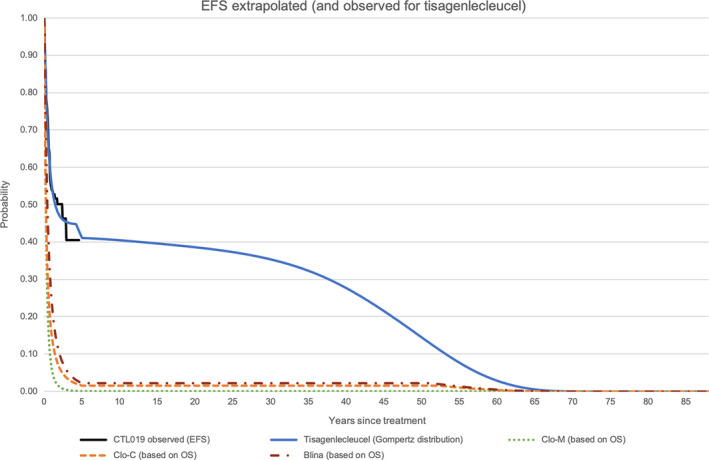
EFS extrapolated (and observed for tisagenlecleucel) [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 3.
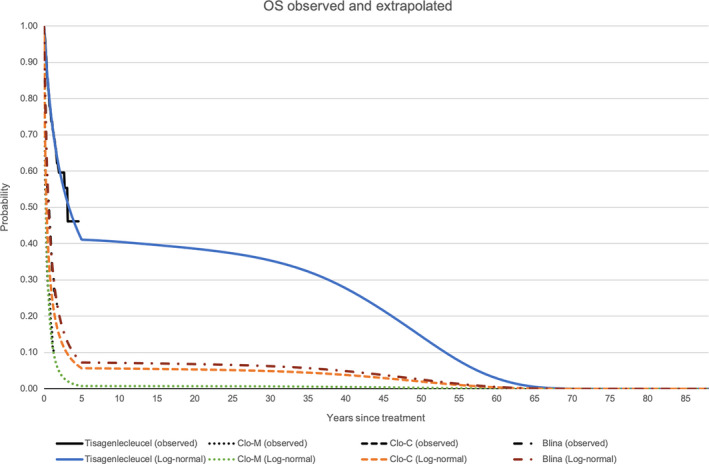
OS observed and extrapolated [Colour figure can be viewed at wileyonlinelibrary.com]
The total discounted treatment costs for tisagenlecleucel were 338 122 EUR and included costs for drug acquisition and administration as well as outpatient and inpatient days. These costs were the highest when compared to any comparator regimen (Clo‐M: 73 457 EUR, Clo‐C: 39 745 EUR, Blina: 119 931 EUR). The main cost driver were the much higher drug acquisition costs for tisagenlecleucel (320 000 EUR), when compared to all other drugs (See Table 1). Only for tisagenlecleucel was a pretreatment regimen (ie, lymphodepleting regimen) necessary. Total discounted costs for this pretreatment were 6821 EUR, with drug acquisition costs (ie, for fludarabine, cyclophosphamide, cytarabine, or etoposide) being the main cost driver. Considering both pretreatment and treatment costs of tisagenlecleucel together, the total treatment costs amounted to 344 943 EUR (discounted). Discounted costs for adverse events were highest for tisagenlecleucel (24 731 EUR), when compared to Clo‐M (4269 EUR), Clo‐C (8085 EUR), and Blina (4210 EUR). This was mainly due to the relatively high prevalence of B‐cell aplasia and the associated high costs of IVIG.
From a healthcare perspective, considering all discounted cost for treatment (including pretreatment for tisagenlecleucel), adverse events, follow‐up period, subsequent HSCT, and future medical costs of unrelated diseases, the total healthcare costs for tisagenlecleucel was 409 563 EUR. This was nearly four times as much when compared to Clo‐M (113 937 UER) or Clo‐C (136 069 EUR) and more than double the total healthcare costs of Blina (200 293 EUR).
For a societal perspective, we added costs of caretakers’ productivity losses, travel costs (for both caretakers and patients), informal care for patient below the age of 18 years, and caretakers’ stay at a charity hospital during the treatment period to the healthcare perspective. The total discounted costs from this perspective were 488 340 EUR for tisagenlecleucel, and 156 909 EUR, 182 496 EUR and 253 024 EUR for Clo‐M, Clo‐C, and Blina, respectively. Major cost drivers in all perspectives were the total costs of treatment for tisagenlecleucel, Clo‐M, and Blina. Only for Clo‐C, subsequent HSCT was more expensive than the treatment costs. When non‐medical consumption costs were added to the societal perspective, total costs increased for all treatment options. Total discounted costs for tisagenlecleucel, Clo‐M, Clo‐C, and Blina were 552 679 EUR, 160 803 EUR, 193 920 EUR, and 267 259 EUR, respectively.
When comparing total discounted costs of the healthcare perspective to the societal perspective, costs increased most for tisagenlecleucel (78 777 EUR), followed by Blina (42 972 EUR), Clo‐C (46 427 EUR), and Clo‐M (52 731 EUR). Considering future non‐medical consumption as part of the societal perspective, the additional costs when compared to the healthcare perspective were 143 116 EUR, 66 966 EUR, 57 851 EUR, and 46 866 EUR for tisagenlecleucel, Clo‐M, Clo‐C, and Blina, respectively.
The discounted ICERs per QALY gained, comparing tisagenlecleucel to Clo‐M, Clo‐C, and Blina from a healthcare perspective were 27 443 EUR, 28 611 EUR, and 23 229 EUR, respectively. When taking a societal perspective, the ICERs increased to 30 767 EUR/QALY, 31 996 EUR/QALY, and 26 120 EUR/QALY comparing tisagenlecleucel to Clo‐M, Clo‐C, and Blina, respectively. When future non‐medical consumption costs were added, ICERs of tisagenlecleucel compared to Clo‐M, Clo‐C, and Blina were 36 378 EUR/QALY, 37 531 EUR/QALY, and 31 682 EUR/QALY. Assuming a WTP threshold of 80 000 EUR/QALY gained, it can thus be concluded that tisagenlecleucel is a cost‐effective treatment when compared to any comparator examined in this study.
The estimation of potential lifetime productivity gains could be 202 563 EUR, 482 EUR, 8884 EUR, and 12 658 EUR per patient for tisagenlecleucel, Clo‐M, Clo‐C, and Blina. However, it needs to be noted that these estimates are subject to substantial uncertainty as explained in the discussion and are therefore not considered for any presented ICER.
All deterministic results of the cost‐effectiveness analysis are presented in Table 2.
Table 2.
Deterministic results of the model base case
| Item | Treatment | |||
|---|---|---|---|---|
| Tisagenlecleucel | Clofarabine monotherapy | Clofarabine combination therapy | Blinatumomab | |
| Costs | ||||
| Pretreatment | €6821 | ‐ | ‐ | ‐ |
| Treatment a | €338 122 | €73 457 | €39 745 | €119 931 |
| Adverse events | €24 731 | €4269 | €8085 | €4210 |
| Follow‐up | €3811 | €540 | €1204 | €1549 |
| Subsequent HSCT | €36 077 | €35 670 | €87 036 | €74 602 |
| Patient and family | €14 277 | €2627 | €2733 | €3319 |
| Productivity losses | €28 301 | €25 868 | €26 857 | €30 696 |
| Future medical costs (unrelated disease and consumption) | €100 538 | €18 371 | €28 262 | €32 952 |
| Total costs | €552 679 | €160 803 | €193 920 | €267 259 |
| Effects | ||||
| Life years | 14.01 | 0.74 | 2.46 | 3.17 |
| Quality‐adjusted life years | 11.26 | 0.49 | 1.70 | 2.25 |
| Increments (tisagenlecleucel vs each comparator) | ||||
| Costs | ‐ | €391 876 | €358 759 | €285 420 |
| Life years | ‐ | 13.27 | 11.55 | 10.84 |
| Quality‐adjusted life years | ‐ | 10.77 | 9.56 | 9.01 |
| ICERs | ||||
| Costs per life years gained | ‐ | €29 535 | €31 052 | €26 334 |
| Costs per quality‐adjusted life year gained | ‐ | €36 378 | €37 531 | €31 682 |
The treatment costs entail drug/procedure costs, and costs for the inpatient and outpatient visits.
Deterministic sensitivity analyses demonstrated that the variation of the starting age of the simulated cohort was the most influential factor for the ICER in all three comparators. Figure 4 depict the top 10 DSA results of ICERs per QALY for each comparator treatment in so‐called Tornado diagrams. Although the change in some parameters affected the ICER quite heavily, none of the calculation exceeded an ICER of 45 000 EUR per QALY gained. The impact of choosing different parametric survival models for OS and EFS and the impact of choosing different time horizons were tested in scenario analyses. Depending on the chosen parametric survival function for EFS, different proportions of cured patients were estimated. In this case, we refer to cured patients as those who stay in EFS 5 years after treatment until the end of life. The proportion of cured patients 5 years post‐treatment varied between 8% (exponential distribution for EFS) and 40% (log‐normal distribution). Choosing either parametric survival function (both for OS or EFS) did not cause the ICER to exceed the WTP threshold of 80 000 EUR per QALY gained.
Figure 4.

Tornado charts [Colour figure can be viewed at wileyonlinelibrary.com]
Results of the 5000 PSA iterations (societal perspective, including future non‐medical consumption) are depicted in the cost‐effectiveness (CE) plane in Figure 5. The average ICERs per QALY gained were in line with the deterministic results with 38 129 EUR, 42 289 EUR, and 34 564 EUR when compared to Clo‐M, Clo‐C, and Blina, respectively. At a WTP threshold of 80 000 EUR, the probability of all simulations being cost‐effective for Clo‐M, Clo‐C, and Blina was 100%, 98%, and 100%, respectively.
Figure 5.

CE‐plane [Colour figure can be viewed at wileyonlinelibrary.com]
Of the conducted scenario analyses, assuming a time horizon of 20 years had the highest impact on the ICER. In this scenario, the ICERs per QALY gained increased to 60 859 EUR, 63 341 EUR, and 53 698 EUR for Clo‐M, Clo‐C, and Blina, respectively. This implies that tisagenlecleucel is less cost‐effective with a shorter time horizon.
Assuming a plateau phase in EFS after 3 years (instead of 5 years) decreased the ICER per QALY gained to 31 798 EUR, 33 641 EUR, and 29 219 EUR for Clo‐M, Clo‐C, and Blina, respectively. This suggests that the sooner patients can be considered cured with tisagenlecleucel, the more cost‐effective the treatment is.
Our analyses also show that the prevalence of B‐cell aplasia substantially adds to the costs of the tisagenlecleucel treatment, mainly through the length of IVIG usage. Based on the literature, we assumed an average duration of B‐cell aplasia of 11.4 months. Testing this assumption in a scenario analysis and considering IVIG cost for the entire duration of EFS among those without subsequent HSCT increased the ICER to 49 969 EUR/QALY, 52 847 EUR/QALY, and 47 932 EUR/QALY gained for Clo‐M, Clo‐C, and Blina, respectively. Hence, a longer treatment duration with IVIG negatively affected the ICER.
4. DISCUSSION
Our results showed that assessing the cost‐effectiveness of tisagenlecleucel from a societal perspective as opposed to a healthcare perspective, increased all estimated total costs and ICERs. This was due to the relative higher increase in total costs for tisagenlecleucel when compared to Clo‐M, Clo‐C, or Blina. Nevertheless, we demonstrate that tisagenlecleucel is also cost‐effective for pediatric patients with relapsed/refractory B‐cell acute lymphoblastic leukemia from a societal perspective. Although all efficacy input parameters for the model stem from international clinical studies, background mortality and HRQoL data as well as all costs were analyzed from a Dutch perspective. Transferability to an international setting needs therefore to be considered with caution.
Nevertheless, considering all assumptions made, our model results can be regarded as robust: all explored scenarios rendered tisagenlecleucel cost‐effective with a WTP threshold of 80 000 EUR per QALY gained. In addition, all 5000 iterations of the PSA yielded a probability for tisagenlecleucel being cost‐effective of more than 98%. The deterministic sensitivity analysis showed that the cohort starting age together with the utility values for EFS and the assumed subsequent HSCT rates for the comparator treatment had the highest impact on the results. However, when any of these parameters were altered (ie, increased or decreased in their estimated value), none of them had the potential to bring the ICER above 48 000 EUR/QALY gained. Lastly, the conducted scenario analyses demonstrate an increase in the ICER with decreasing time horizons (ie, follow‐up time) and a considerable impact of the IVIG assumption (ie, how long IVIG is given) on the ICER.
The favorable results for tisagenlecleucel in our analysis can mainly be explained by the extensive survival gains when compared to other treatment options. With a total of 14.01 life years (discounted), tisagenlecleucel performed significantly better than any of the comparators. Since to date no randomized clinical trials for tisagenlecleucel in r/r ALL patients exist, the modeled effectiveness was based on single‐arm studies. Furthermore, no information about EFS was available in the publications of the comparative treatments. Based on a high correlation between EFS and OS, 38 we assumed that the missing EFS data could be estimated based on the available OS data. This may have influenced the EFS estimates of the comparative treatments.
According to the currently available evidence, tisagenlecleucel is a potential curative treatment thereby preventing young patients from premature death. Consequently, substantial life years and QALYs can be gained from a lifetime perspective. However, it needs to be noted that the long‐term effects of tisagenlecleucel are not yet captured by any study, registry, or clinical trial, because none of those have lifetime follow‐up data. We assumed no specific late side effects after tisagenlecleucel and regarded patients to be cured after 5 years. In our model, patients that remain in EFS for 5 years after treatment are considered being cured. This assumption helped to reduce some of the long‐term uncertainties arising from long‐term survival extrapolation data beyond the observed trial data. The 5‐year cut‐off was validated by clinical experts and our approach was similar to the one used for the National Institute for Health and Care Excellence (NICE) mock technology appraisal for chimeric antigen receptor T‐cell (CAR‐T) therapy as a treatment for r/r B‐cell pALL. 39 However, the exact time at which patients in long‐term EFS may be considered cured is uncertain. In a scenario analysis, we explored the impact of assuming three instead of 5 years as a cut‐off. Consequently, all ICERs decreased (ICER Clo‐M: 29 628 EUR/QALY, ICRE Clo‐C: 31 459 EUR/QALY, ICER Blina: 27 110 EUR/QALY gained), meaning that the sooner patients can be considered cured, the more cost‐effective tisagenlecleucel is. Irrespective of the time point at which patient may be considered cured, it is uncertain what fraction of patients can be considered disease free at that time. Up until the 5 years after treatment, the EFS in the model was based on parametric survival functions. Each of these functions estimated different probabilities for EFS 5 years after treatment start. These estimates ranged between 8% and 40% for EFS, but none of these scenarios exceeded an ICER of 45 000 EUR per QALY gained. Nonetheless, empirical long‐term follow‐up data are vital to reduce uncertainty in effectiveness outcomes. Data from patient registries such as the EBMT (European Society for Blood and Marrow Transplantation) registry may play a vital role in collecting the necessary information.
The estimated favorable survival outcomes (both in EFS and OS) indicate significant benefits for both patients and society. These can best be captured by extending the healthcare perspective to a societal perspective. Assuming a societal perspective made it possible to capture costs from a broad economic angle, including the impact of the treatment on patients and their families. To include these additional cost components, health economic researchers can choose from an abundance of validated methodological approaches in the literature or health economic guidelines. However, most of the available approaches only focus on adult patient populations and children as well as young adults remain understudied. 40 , 41 , 42 , 43 , 44 Costs of productivity losses (ie, the costs occurring when the productivity of individuals is affected by illness, treatment, disability, or premature death) for instance may be relevant to patients that already were (economically) productive before the onset of the disease. 45 In the case of most pediatric ALL patients, this is however not the case. Instead, patients’ parents or caregivers face these losses. Since Dutch‐specific data were unavailable concerning the productivity losses of parents and informal care, we made assumptions based upon available information in the literature.
For economic evaluations conducted from a US or Dutch perspective, it is recommended to consider future medical costs. 12 , 46 While the US guidelines recommend the inclusion future non‐medical (consumption) costs as well, the Dutch guideline does not mention its inclusion yet. 12 , 46 Our study is the first to fully include both components in an evaluation of CAR T‐cell therapy for pALL. Both aspects were added through the latest version of the iMTA PAID tool (version 3.0) which is available online (https://imta.shinyapps.io/PAID3/). The methodology of this tool is described elsewhere. 18 , 19 Due to the favorable survival of patients with tisagenlecleucel, the discounted future costs of this treatment were extensive (ie, 100 538 EUR), while these costs were significantly lower for Clo‐M, Clo‐C, or Blina (ie, 18 371 EUR, 28 262 EUR, and 32 952 EUR, respectively). Long‐term survivors of pALL may however not only induce costs in the future. Cured pediatric patients may be able to finish their school education and consequently join the workforce. We refer to these prospects as potential productivity gains and made an attempt to quantify them in our analysis.
However, little is known about both educational and employment prospects of long‐term survivors of childhood cancer. In addition, there is a lack of evidence and methodological guidance in how to integrate such gains in economic evaluations. Therefore, the inclusion of these cost savings in our model made use of rather simplistic assumptions and should be interpreted with caution. For instance, we assumed full and life‐long employment of the modeled patients as from the age of 18 years. Future fluctuations on the job market or employment rates could not be reliably modeled and were beyond the scope of this research. Besides, it yet needs to be determined whether patients in long‐term EFS can or will start on the job market once they attain majority. It is apparent that patients who can potentially be cured from ALL may be enabled to finish their education and join the workforce in the future. However, the here modeled patients were all relapsed or refractory to previous treatment lines and non‐attendance to school might have been significant during previous treatment lines. Research is needed to determine in how far the absence from school affects the job starting age and shapes future employment opportunities in this patient population. Furthermore, resulting from the uncertainty of the long‐term effectiveness, no future productivity losses for the modeled patients were assumed that might result from long‐term, disease‐related absenteeism. Nevertheless, our approach may be seen as an illustration of the magnitude of potential economic gains resulting from improved survival, especially in pediatric diseases. Further research could quantify the potential productivity gains by elucidating how this aspect can be captured and integrated into cost‐effectiveness analyses in a sound methodological manner.
Although this study is not the first to estimate the cost‐effectiveness of tisagenlecleucel, it is the first to adopt a full societal perspective. Following a “mock appraisal” commissioned by the UK National Institute for Health and Care Excellence (NICE) to assess whether changed to its methods and processes were needed, 39 several cost‐effectiveness analyses were published in the US and Canada. Two studies had considered societal aspects in a scenario analysis, but none of them had considered productivity losses of caregivers, travel and hotel costs for patients and caregivers, informal care costs, and future medical costs including consumption costs altogether. 13 , 15 In addition, input parameters and outcomes of the societal perspective were either not reported, 15 or not clearly defined and point to evidence of adult patients, while pediatric patients are studied (see patient time in Sarkar et al(2018)). 13
When comparing our results to the other cost‐effectiveness, studies we found some disparities. Differences in incremental costs were highest between our study and Sarkar et al 13 for Clo‐C, followed by costs for Clo‐M when compared to the NICE mock appraisal. 39 The reason for these discrepancies can be explained by major differences in several cost input parameters. For instance, costs for tisagenlecleucel are higher in the United States when compared to the Netherlands (475 000 USD [426 000 EUR] vs 320 000 EUR). Similarity, the NICE mock appraisal assumed even higher one‐off costs for tisagenlecleucel of 528 600 GBP (587 697 EUR) per patient. 39 In addition, estimated costs for HSCT in all US studies were significantly higher when compared to our study. Sarkar et al 13 assumed HSCT costs ranging between 299 987 USD (267 456 EUR) for successful HSCT and 459 682 USD (409 834 EUR) for failed HSCT. Lin et al 14 estimated the HSCT costs to be 555 000 USD (483 904 EUR), which was similar to the estimates of Whittington et al 16 (560 000 USD [488 264 EUR]). For every modeled patient that received subsequent HSCT, our model considered one‐time costs of 217 590 EUR per HSCT 31 and no distinction was made between successful or failed treatment.
With the exception for Whittington et al, 16 incremental effects in LYs could be regarded as similar between all studies. Incremental QALYs differed to a greater extend and were highest for the study of Lin et al 14 We hypothesize that this is mainly due to the use of different utility estimates. Lin et al 14 used a variety of utility estimates ranging between 0.56 and 0.92, depending on the health state or time. Our utility estimates were based on the ELIANA trial and ranged between 0.68 and 0.83, depending on the health states. Although we accounted for disutilities during any treatment, the stay at an intensive care unit, and graft‐vs‐host disease, our utilities were consistently higher when compared to Lin et al 14
Finally, the divergent ICERs per QALY between the studies are a result of the difference in both costs and outcomes as explained above. Despite the several assumptions made in this study, we conclude that our results are robust (as tested through several sensitivity and scenario analyses) and that the conclusion of tisagenlecleucel being cost‐effective is in line with all other cost‐effectiveness studies for pediatric patients with r/r ALL. Furthermore, total costs from a societal perspective were higher for each treatment option when compared to costs from a healthcare perspective. Although the increase in these costs was higher for tisagenlecleucel when compared to Clo‐M, Clo‐C, or Blina, none of the ICERs exceeded the WTP threshold of 80 000 EUR per QALY gained.
CONFLICT OF INTEREST
Frederick W. Thielen: This project was funded with an unrestricted grant by Novartis Pharma BV Annemieke van Dongen‐Leunis: This project was funded with an unrestricted grant by Novartis Pharma BV Alexander MM Arons is employed by Novartis Pharma BV Judith Ladestein is employed by Novartis Pharma BV Peter Hoogerbrugge: None. Carin Uyl‐de Groot: This project was funded with an unrestricted grant by Novartis Pharma BV.
AUTHOR CONTRIBUTIONS
Frederick W. Thielen and Annemieke van Dongen‐Leunis prepared and analyzed data and wrote the paper; Alexander MM Arons critically reviewed the paper and co‐developed methods; Judith Ladestein critically reviewed the paper; Peter Hoogerbrugge served as clinical expert and critically reviewed the paper; Carin Uyl‐de Groot critically reviewed the analysis and paper.
Supporting information
Appendix S1
Thielen FW, van Dongen‐Leunis A, Arons AMM, Ladestein JR, Hoogerbrugge PM, Uyl‐de Groot CA. Cost‐effectiveness of Anti‐CD19 chimeric antigen receptor T‐Cell therapy in pediatric relapsed/refractory B‐cell acute lymphoblastic leukemia. A societal view. Eur J Haematol. 2020;105:203–215. 10.1111/ejh.13427
REFERENCES
- 1. Parker C, Waters R, Leighton C, et al. Effect of mitoxantrone on outcome of children with first relapse of acute lymphoblastic leukaemia (ALL R3): an open‐label randomised trial. Lancet. 2010;376(9757):2009‐2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gore L, Locatelli F, Zugmaier G, et al. Survival after blinatumomab treatment in pediatric patients with relapsed/refractory B‐cell precursor acute lymphoblastic leukemia. Blood Cancer J. 2018;8(9):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. von Stackelberg A, Locatelli F, Zugmaier G, et al. Phase I/Phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016;34(36):4381‐4389. [DOI] [PubMed] [Google Scholar]
- 4. Maude SL, Teachey DT, Rheingold SR, et al. Sustained remissions with CD19‐specific chimeric antigen receptor (CAR)‐modified T cells in children with relapsed/ refractory ALL. J Clin Oncol. 2016;34(15_suppl):3011‐3011. [Google Scholar]
- 5. Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in Children and Young Adults with B‐Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):439‐448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507‐1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gardner RA, Finney O, Annesley C, et al. Intent‐to‐treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129(25):3322‐3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee DW, Kochenderfer JN, Stetler‐Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose‐escalation trial. Lancet. 2015;385(9967):517‐528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Forsberg MH, Das A, Saha K, Capitini CM. The potential of CAR T therapy for relapsed or refractory pediatric and young adult B‐cell ALL. Ther Clin Risk Manag. 2018;14:1573‐1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. National Institute for Health and Care Excellence . Tisagenlecleucel for Treating Relapsed or Refractory B‐Cell Acute Lymphoblastic Leukaemia in People Aged up to 25 Years. London:National Institute for Health and Care Excellence; https://www.nice.org.uk/guidance/ta554/chapter/2‐Information‐about‐tisagenlecleucel. Published 2018. Accessed March 11, 2020. [Google Scholar]
- 11. Whittington MD, McQueen RB, Campbell JD. Considerations for cost‐effectiveness analysis of curative pediatric therapies. JAMA Pediatr. 2018;172(5):409. [DOI] [PubMed] [Google Scholar]
- 12. Hakkaart‐van Roijen L, van der Linden N , Bouwamans C, Kanters T, Swan Tan S. Kostenhandleiding: Methodologie van Kostenonderzoek En Referentieprijzen Voor Economische Evaluaties in de Gezondheidszorg. Bijlage 1 [Costing Guideline: Methodology of Costing Studies and Reference Prices for Economic Evaluations in Health Care. Appendix 1]. 2016.
- 13. Sarkar R, Gloude NJ, Murphy JD. Cost‐effectiveness of chimeric antigen receptor T‐cell therapy in pediatric relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2018;36(15):719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin JK, Barnes J, Lerman BJ, et al. Cost Effectiveness of Chimeric Antigen Receptor T‐Cell Therapy in Relapsed or Refractory Pediatric B‐Cell Acute Lymphoblastic Leukemia Global Burden of Diseases View project NCD risk factors collaboration View project JOURNAL OF CLINICAL ONCOLOGY Cost Effectiveness of Chimeric Antigen Receptor T‐Cell Therapy in Relapsed or Refractory Pediatric B‐Cell Acute Lymphoblastic Leukemia. J Clin Oncol. 2018;36(32):3192–3202. [DOI] [PubMed] [Google Scholar]
- 15. CADTH . Tisagenlecleucel for Acute Lymphoblastic Leukemia: Economic Review Report. Ottawa:Canadian Agency for Drugs and Technologies in Health (CADTH); 2019:61. [Google Scholar]
- 16. Whittington MD, McQueen RB, Ollendorf DA, et al. Long‐term Survival and Value of Chimeric Antigen Receptor T‐Cell Therapy for Pediatric Patients with Relapsed or Refractory Leukemia. JAMA Pediatr. 2018;172(12):1161‐1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Walton M, Sharif S, Simmonds M, Claxton L, Hodgson R. Tisagenlecleucel for the treatment of relapsed or refractory B‐cell acute lymphoblastic leukaemia in people aged up to 25 years: an evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics. 2019;37:1209‐1217. [DOI] [PubMed] [Google Scholar]
- 18. Kellerborg K, Perry‐Duxbury M, de Vries L , van Baal P . Practical guidance for including future costs in economic evaluations. [Not yet published]. [DOI] [PubMed]
- 19. van Baal PHM, Wong A, Slobbe LCJ, Polder JJ, Brouwer WBF, de Wit GA. Standardizing the Inclusion of Indirect Medical Costs in Economic Evaluations. Pharmacoeconomics. 2011;29(3):175‐187. [DOI] [PubMed] [Google Scholar]
- 20. Latimer N. NICE DSU technical support document 14: survival analysis for economic evaluations alongside clinical trials‐extrapolation with patient‐level data. Sheffield: Decision Support Unit. 2013;(0). [PubMed]
- 21. Royston P, Parmar MKB. Flexible parametric proportional‐hazards and proportional‐odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med. 2002;21(15):2175‐2197. [DOI] [PubMed] [Google Scholar]
- 22. Determine Efficacy and Safety of CTL019 in Pediatric Patients With Relapsed and Refractory B‐cell ALL and High Risk B‐cell ALL at First Relapse. Determine Feasibility and Safety of CTL019 Therapy in Pediatric Patients With High Risk B‐cell ALL That Relapsed < 6 Months Post All‐HSCT. ‐ Full Text View ‐ ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02435849. Accessed March 16, 2020.
- 23. Study of Efficacy and Safety of CTL019 in Pediatric ALL Patients ‐ Full Text View ‐ ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02228096. Accessed March 16, 2020.
- 24. Phase I/IIA Study of CART19 Cells for Patients With Chemotherapy Resistant or Refractory CD19+ Leukemia and Lymphoma ‐ Full Text View ‐ ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT01626495. Accessed March 16, 2020.
- 25. Evoltra (Clofarabine, Sanofi) . Summary of product characeteristics. https://www.ema.europa.eu/en/documents/product‐information/evoltra‐epar‐product‐information_en.pdf. Accessed March 16, 2020.
- 26. Hijiya N, Thomson B, Isakoff MS, et al. Phase 2 trial of clofarabine in combination with etoposide and cyclophosphamide in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. Blood. 2011;118(23):6043‐6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van den Berg H, de Groot‐Kruseman HA, Damen‐Korbijn CM, de Bont ESJM, Meeteren AYNS, Hoogerbrugge PM. Outcome after first relapse in children with acute lymphoblastic leukemia: a report based on the Dutch Childhood Oncology Group (DCOG) relapse all 98 protocol. Pediatr Blood Cancer. 2011;57(2):210‐216. [DOI] [PubMed] [Google Scholar]
- 28. Armstrong GT, Chen Y, Yasui Y, et al. Reduction in late mortality among 5‐year survivors of childhood cancer. N Engl J Med. 2016;374(9):833‐842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Attema AE, Brouwer WBF, Claxton K. Discounting in economic evaluations. Pharmacoeconomics. 2018;36(7):745‐758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jeha S, Gaynon PS, Razzouk BI, et al. Phase II study of clofarabine in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. J Clin Oncol. 2006;24(12):1917‐1923. [DOI] [PubMed] [Google Scholar]
- 31. Lamers LM, McDonnell J, Stalmeier PFM, Krabbe PFM, Busschbach JJV. The Dutch tariff: results and arguments for an effective design for national EQ‐5D valuation studies. Health Econ. 2006;15(10):1121‐1132. [DOI] [PubMed] [Google Scholar]
- 32. Forsythe A, Brandt PS, Dolph M, Patel S, Rabe APJ, Tremblay G. Systematic review of health state utility values for acute myeloid leukemia. Clinicoecon Outcomes Res. 2018;10:83‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kwon J, Kim SW, Ungar WJ, Tsiplova K, Madan J, Petrou S. A systematic review and meta‐analysis of childhood health utilities. Med Decis Making. 2018;38(3):277‐305. [DOI] [PubMed] [Google Scholar]
- 34. Janssen B, Szende A. Population norms for the EQ‐5D In: Szende A, Janssen B, Cabases J. eds. Self‐Reported Population Health: An International Perspective Based on EQ‐5D. Dordrecht:Springer Netherlands; 2014:19–30. [PubMed] [Google Scholar]
- 35. Franken MG, Kanters TA, Coenen JL, et al. Potential cost savings owing to the route of administration of oncology drugs: a microcosting study of intravenous and subcutaneous administration of trastuzumab and rituximab in the Netherlands. Anticancer Drugs. 2018;29(8):791‐801. [DOI] [PubMed] [Google Scholar]
- 36. PAID [version 3.0] . iMTA. https://www.imta.nl/paid/. Accessed March 26, 2020.
- 37. Langeveld NE, Ubbink MC, Last BF, Grootenhuis MA, VoÛte PA, de Haan RJ. Educational achievement, employment and living situation in long‐term young adult survivors of childhood cancer in the Netherlands. Psycho Oncol. 2003;12(3):213‐225. [DOI] [PubMed] [Google Scholar]
- 38. Schlenk RF, Döhner H, Döhner K, et al. Event‐free survival is a surrogate for overall survival in patients treated for acute myeloid leukemia. Blood. 2015;126(23):3744‐3744. [Google Scholar]
- 39. Crabb N, Stevens A. Exploring the assessment and appraisal of regenerative medicines and cell therapy products. https://docplayer.net/18693201‐Exploring‐the‐assessment‐and‐appraisal‐of‐regenerative‐medicines‐and‐cell‐therapy‐products.html. Accessed March 24, 2020.
- 40. Keegan THM, Parsons HM. Adolescent angst: enrollment on clinical trials. Hematology Am Soc Hematol Educ Program. 2018;2018(1):154‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Weiss AR, Hayes‐Lattin B, Kutny MA, Stock W, Stegenga K, Freyer DR. Inclusion of adolescents and young adults in cancer clinical trials. Semin Oncol Nurs. 2015;31(3):197‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sullivan SM, Tsiplova K, Ungar WJ. A scoping review of pediatric economic evaluation 1980–2014: do trends over time reflect changing priorities in evaluation methods and childhood disease? Expert Rev Pharmacoecon Outcomes Res. 2016;16(5):599‐607. [DOI] [PubMed] [Google Scholar]
- 43. Roth ME, O’Mara AM, Seibel NL, et al. Low enrollment of adolescents and young adults onto cancer trials: insights from the community clinical oncology program. J Oncol Pract. 2016;12(4):e388‐e395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kromm SK, Bethell J, Kraglund F, et al. Characteristics and quality of pediatric cost‐utility analyses. Qual Life Res. 2012;21(8):1315‐1325. [DOI] [PubMed] [Google Scholar]
- 45. Krol M, Brouwer W, Rutten F. Productivity costs in economic evaluations: past, present. Future. PharmacoEconomics. 2013;31(7):537‐549. [DOI] [PubMed] [Google Scholar]
- 46. Carias C, Chesson HW, Grosse SD, et al. Recommendations of the second panel on cost effectiveness in health and medicine: a reference, not a rule book. Am J Prev Med. 2018;54(4):600‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gaynon PS, Harris RE, Altman AJ, et al. Bone marrow transplantation versus prolonged intensive chemotherapy for children with acute lymphoblastic leukemia and an initial bone marrow relapse within 12 months of the completion of primary therapy: children’s oncology group study CCG‐1941. J Clin Oncol. 2006;24(19):3150‐3156. [DOI] [PubMed] [Google Scholar]
- 48. Doorduijn JK, Buijt I, van der Holt B, van Agthoven M, Sonneveld P, Uyl‐de Groot CA. Economic evaluation of prophylactic granulocyte colony stimulating factor during chemotherapy in elderly patients with aggressive non‐Hodgkin’s lymphoma. Haematologica. 2004;89(9):1109‐1117. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


