Abstract
To evaluate the value of modified Cornell electrocardiographic criteria in the assessment of left ventricular hypertrophy (LVH) for patients with essential hypertension. A total of 381 patients with essential hypertension diagnosed in our hospital were selected. Using the left ventricle (LV) geometric patterns classified by the American Society of Echocardiography (ASE), we examined the distribution of the modified Cornell criteria of Ravl + SD (the deepest S wave in 12‐lead ECG) in different geometric patterns and analyzed the correlation of modified Cornell criteria with changes in the LV geometric patterns using multiple linear regression analysis. The distribution of modified Cornell criteria, Sokolow‐Lyon criteria (RV5/V6 + SV1), and Cornell criteria (Ravl + SV3) in gender‐specific hypertensive geometric patterns were significantly different (P ≤ .01 for all). The voltage of Ravl + SD in male patients showed an increase trend in the normal geometry (NG), concentric remodeling (CR), concentric hypertrophy (CH), and eccentric hypertrophy (EH) groups, and this increase trend was significantly in the unadjusted model and the adjusted model. The voltages of Ravl + SV3 and RV5/V6 + SV1 of male patients in CR, CH and RH groups showed a gradual increase trend, but the increase trend in CR group has no statistical significance compared to that in NG group (P ≥ .05). The voltages of Ravl + SD, RV5/V6 + SV1, and Ravl + SV3 in female patients in CR, CH and EH groups showed a trend of increase after decrease in the adjusted model. In conclusion, the modified Cornell criteria could dynamically reflect left ventricular hypertensive geometry of male patients.
Keywords: diagnostic criteria, electrocardiogram, essential hypertension, left ventricle geometry, left ventricular hypertrophy
1. INTRODUCTION
Over the past 20 years, the number of patients with hypertension has increased dramatically, with more than 1 billion people worldwide suffering from hypertension. With population aging and sedentary lifestyle suffusing, the number will increase to 1.5 billion by 2025. Hypertension is a major risk factor for premature death, heart failure, atrial fibrillation, chronic kidney disease (CKD), peripheral vascular disease, and cognitive decline. 1 Therefore, accurate diagnosis and evidence‐based treatment of hypertension is very important to reduce cardiovascular events. Left ventricular hypertrophy (LVH) occurs in approximately 18% of patients with hypertension and is a strong predictor of non‐fatal and fatal cardiovascular events. 2 , 3 In August 2018, European Society of Cardiology (ESC) and European Society of Hypertension (ESH) have jointly issued guidelines for the management of hypertension and emphasized the significance of damages of target organs including LVH. 4 Identification of abnormal LV geometry in asymptomatic individuals prior to the occurrence of cardiovascular adverse events is important for developing prevention strategies.
Changes in LV geometry could help us identify myocardial changes in patients with hypertension from the aspect of myocardial anatomy. 5 These changes differ in their cardiac morphological adaptations to chronic pressure overload. 6 LVH is a progressive process and constantly changes with the LV geometric patterns. Accurately and conveniently determining the LV geometry is extremely important for the risk stratification of patients with hypertension. Early identification of abnormal LV geometry and observation of changes in LV geometry after drug treatment can help improve the prognosis of patients with hypertension. 7 Electrocardiogram (ECG) is a cost‐effective and convenient tool for evaluating cardiac structures. Current guidelines recommend ECG as a first‐line routine examination for management of patients with hypertension. The high sensitivity and specificity has made echocardiography as an important standard tool for evaluating LVH and its geometric changes. 8 However, echocardiography is usually used to diagnose LVH of inpatients and is not suitable for community or outpatient screening. In addition, its widespread clinical application is also limited by the operator's technical level, cost and its temporospatial flexibility. The 12‐lead resting ECG is the most convenient, cost‐effective, non‐invasive method for detecting LVH in clinics and suitable for large‐scale screening.
At present, more than thirty criteria are used to evaluate LVH in clinical practice. Among them, Cornell criteria and Sokolow‐Lyon criteria are the most common ones. However, their low diagnostic sensitivity is an important factor affecting their application. 1 Peguero et al proposed that the deepest S wave (SD) in 12‐lead resting ECG is the most accurate joint single‐lead ECG for diagnosing LVH (AUC: 0.80; P < .001) because it can dynamically respond to changes in negative S waves. 9 In this study, we envisage using ECG as the reference to evaluate the value of the modified Cornell criteria (Ravl + SD) in determination of LV geometric patterns so as to improve cardiovascular disease risk stratification and treatment decisions.
2. METHODS
2.1. Cohort profile
A total of 381 patients with essential hypertension diagnosed in our hospital from September 2015 to September 2018 were selected. All patients were Chinese Han at age of 18‐75 years. Among them, 213 were males and 168 females. Essential hypertension was diagnosed based on the blood pressure criteria recommended by the World Health Organization (WHO), that is, adult systolic blood pressure(SBP) ≥140 mm Hg (1 mm Hg = 0.133 Kpa) and/or diastolic blood pressure(DBP) ≥90 mm Hg. Patients who met the criteria twice in three blood pressure measurements were considered to have hypertension. Patient information was collected including history of hypertension, family history, history of diabetes, peripheral vascular diseases, hypercholesterolemia, chronic kidney disease (CKD), blood pressure levels, history of hypertension medication use, regular exercise and sport, and so on. Diabetes mellitus (DM) was defined as fasting blood glucose > 126 mg/dL tested on multiple occasions, or self‐reported use of diabetes medications. 10 Hypercholesterolemia was defined as total serum cholesterol > 240 mg/dL, or self‐report of hypercholesterolemia with use of lipid‐lowering treatment. CKD refers to the presence of kidney damage or kidney function decline for more than 3 months. Exclusion criteria were patients with complete left or right bundle branch blocks, ventricular paced rhythm, secondary hypertension, arrhythmia, valvular heart diseases, cardiomyopathy, pulmonary diseases, cardiopulmonary infectious diseases, hepatorenal syndrome, immune diseases, mental diseases, acute/chronic pulmonary heart disease and hematopathy and patients who had undergone coronary stenting or coronary artery dilatation. This study was approved by the Medical Ethics Committee of our hospital and all patients signed the informed consents.
2.2. Electrocardiogramic measurement
A standard 12‐lead ECG was recorded at 25 mm/s paper speed and 10 mm/mV using a Fukuda CardiMax FX‐7402 electrocardiograph machine with and classified using the Minnesota Code. Prior to measurement, all patients were asked to rest calmly for 10 minutes to eliminate tension and lie down in the supine position. The electrodes were placed according to the standard protocol and the graphs of all 12‐leads were recorded synchronously. Each ECG should be clear, stable at baseline and free of interference. All ECG indexes were measured and calculated independently by two physicians in accordance with the “AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram.” 11 Each lead was analyzed by measuring the deepest S or QS waves of all the anteroposterior and limb leads with the PR segment as the baseline. Only the largest composite voltage was considered when different voltage appeared in the same lead. SD was considered as the deepest S or QS amplitude of the precordial and limb leads. Ravl + SD was used in the modified Cornell criteria, while Ravl + SV3 of the Cornell criteria and RV5 + SV1 of the Sokolow‐Lyon criteria recommended by the International ECG Guidelines were used as the control. A total of 4‐6 cardiac cycles were recorded and the indexes of three consecutive cycles with stable baseline were measured and averaged as the final result.
2.3. Echocardiographic measurement
Echocardiogramic measurement was performed on the same day as ECG using the Philips EPIQ 7C echocardiographer with X5‐1 probe at frequency of 50 MHz. Images were obtained at left lateral position under calm breath and the transthoracic images were used as a reference method for estimating LV mass. The limb lead ECG was simultaneously recorded. The interventricular septum thickness (IVSD), left ventricular end diastolic diameter (LVEDD) and left ventricular posterior wall thickness (PWD) were recorded at the parasternal long axis view. LVM was calculated based on the recommendation of American Society of Echocardiography (ASE) as LVM (g) = 0.8 × 1.04 × ([IVSD + LVEDD + PWD]3 − LVEDD3) + 0.6, and normalized by body surface area (BSA) as the left ventricular muscle mass index (LVMI). According to the new ASE standard, females with LVMI > 95 g/m2 and males with LVMI > 115 g/m2 were diagnosed to have LVH. 12 All data were independently interpreted by two attending doctors and averaged.
2.4. LV geometric patterns of patients with hypertension
Hypertensive geometric patterns were classified based on LVMI and relative wall thickness (RWT) as proposed by Ganau et al 6 RWT was calculated as 2 times of PWD divided by LVEDD. Patients with RWT > 0.42 was considered as abnormal. As recommended by ASE, based on LVMI and RWT, LV hypertensive geometry was further classified into four different categories: normal geometry (NG), concentric remodeling (CR), concentric hypertrophy (CH), and eccentric hypertrophy (EH). 12
2.5. Data analysis
Statistical analysis was performed using SPSS22.0 software. Count data were expressed as a composition ratio. The measurement data that conforms to the normal distribution were expressed as mean ± standard deviation ( ± s). The measurement data with skewed distribution was expressed as M (Q1‐Q3). Differences in numeric variables among multiple groups and between two groups were analyzed using analysis of variance (ANOVA) and Student‐Newman‐Keuls (SNK), respectively. Differences in ratio among multiple groups were analyzed using the Cochran‐Mantel‐Haenszel (CMH) method. The distribution of skewed data was compared according to the non‐parametric Friedman M test. Multivariate linear regression models were used to examine associations of multiple ECG indexes with changes of left ventricle in the categories. Three models were used to estimate the associations: Model 1 was unadjusted. Model 2 was adjusted for age and BMI, and Model 3 was additionally adjusted for heart rate, SBP, DBP, eject fraction (EF), time of hypertension, family history of hypertension, DM, regular exercise and sport, as well as use of antihypertensive medications. P < .05 in two‐sided test was considered statistically significant.
3. RESULTS
3.1. Basic clinical characteristics and echocardiographic data
Comparison of the basic clinical characteristics and echocardiographic data of patients in different hypertensive categories is given in Table 1. Statistic analyses found significant differences in BMI, DBP, IVSD, LVEDD, PWD, EF, LVMI, use of beta‐blockers, use of calcium channel blocker, and time of hypertension among different categories (all P < .05).
Table 1.
Comparison of basic clinical data and echocardiographic data among different hypertensive geometries (n = 381)
| Characteristics | NG (n = 111) | CR (n = 102) | CH (n = 109) | EH (n = 59) | P‐Value |
|---|---|---|---|---|---|
| Male gender(n, [%]) | 66 (59.5) | 58 (56.9) | 56 (51.4) | 33 (55.9) | .679 |
| Age (y) | 59.5 + 8.1 | 60.1 + 10.2 | 58.2 + 13.5 | 56.2 + 12.2 | .053 |
| BMI (kg/m2) | 20.7 ± 1.2 | 21.1 ± 1.5 | 21.7 ± 1.3 | 21.5 ± 1.6 | .001 |
| Time of hypertension (y) | 2.7 ± 1.5 | 2.9 ± 1.1 | 5.2 ± 2.7 | 5.6 ± 2.8 | .001 |
| Family history of hypertension (n, [%]) | 33 (29.7) | 30 (29.4) | 36 (33.0) | 25 (42.4) | .325 |
| Medications | |||||
| ACE‐I or ARB (n, [%]) | 65 (59.6) | 55 (53.9) | 65 (59.6) | 28 (47.5) | .492 |
| Beta blockers (n, [%]) | 87 (78.4) | 65 (63.7) | 76 (69.7) | 23 (39.0) | .001 |
| Calcium channel blocker (n, [%]) | 54 (48.6) | 65 (63.7) | 45 (41.3) | 30 (50.8) | .012 |
| Diuretics (n, [%]) | 20 (18.0) | 18 (17.6) | 16 (14.7) | 12 (20.3) | .933 |
| Complications | |||||
| Diabetes mellitus (n, [%]) | 13 (11.7) | 9 (8.8) | 12 (11.1) | 12 (20.3) | .176 |
| Peripheral vascular disease (n, [%]) | 5 (4.5) | 7 (6.9) | 8 (7.3) | 5 (8.5) | .743 |
| Dyslipidemia (n, [%]) | 12 (10.8) | 10 (9.8) | 8 (7.3) | 6 (10.2) | .835 |
| Chronic kidney disease (n, [%]) | 8 (7.2) | 5 (4.9) | 9 (8.3) | 7 (11.9) | .445 |
| Regular exercise and sport (n, [%]) | 45 (40.5) | 54 (52.9) | 50 (45.9) | 25 (42.4) | .307 |
| Heart rate (bpm) | 76.3 + 15.3 | 77.7 + 14.3 | 76.4 + 15.4 | 74.4 + 14.3 | .105 |
| SBP (mm Hg) | 133.7 ± 4.9 | 135.9 ± 5.8 | 141.4 ± 8.5 | 145.31 ± 5.6 | .064 |
| DBP (mm Hg) | 84.5 ± 6.8 | 90.6 ± 4.8 | 90.5 ± 3.3 | 91.7 ± 4.6 | .001 |
| EF (%) | 64.1 ± 3.1 | 61.8 ± 3.1 | 60.9 ± 3.5 | 59.8 ± 5.1 | .001 |
| LVEDD (mm) | 47.9 ± 3.4 | 43.3 ± 2.7 | 48.7 ± 3.9 | 55.8 ± 6.3 | .001 |
| IVSD (mm) | 8.5 ± 0.6 | 10.7 ± 1.2 | 12.8 ± 1.5 | 11.4 ± 2.0 | .001 |
| PWD (mm) | 8.5 ± 0.6 | 10.6 ± 1.0 | 12.3 ± 1.2 | 11.1 ± 1.7 | .001 |
| RWT | 0.36 ± 0.03 | 0.49 ± 0.04 | 0.52 ± 0.06 | 0.40 ± 0.04 | .001 |
| LVMI (male) (g/m2) | 79.4 ± 13.9 | 88.6 ± 15.4 | 141.2 ± 18.2 | 171.6 ± 41.9 | .001 |
| LVMI (female) (g/m2) | 77.4 ± 9.2 | 87.9 ± 6.9 | 121.4 ± 32.2 | 116.6 ± 34.0 | .001 |
Data are presented as the mean ± standard deviation, or as n (%).
Abbreviations: ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CH, concentric hypertrophy; CR, concentric remodeling; DBP, diastolic blood pressure; EH, eccentric hypertrophy; IVSD, Ventricular septal diameter; LVEDD, left ventricular end diastolic diameter; LVMI, left ventricular mass index; NG, normal geometry; PWD, posterior wall diameter; RWT, relative ventricular wall thickness; SBP, systolic blood pressure.
3.2. Distribution of gender‐specific LV ECG indexes
The distribution of modified Cornell criteria, Cornell criteria, and Sokolow‐Lyon criteria in the gender‐specific hypertensive categories is shown in Figure 1. ANOVA analysis indicated that their distribution was significantly different among the four hypertensive categories (P < .05). The voltage values of the three ECG indexes are gradually increasing in male patients, while there was no statistical difference between the NG group and the CR group in Sokolow‐Lyon criteria(P > .05). The maximum voltage of female patients was in CH group in three ECG indexes.
FIGURE 1.
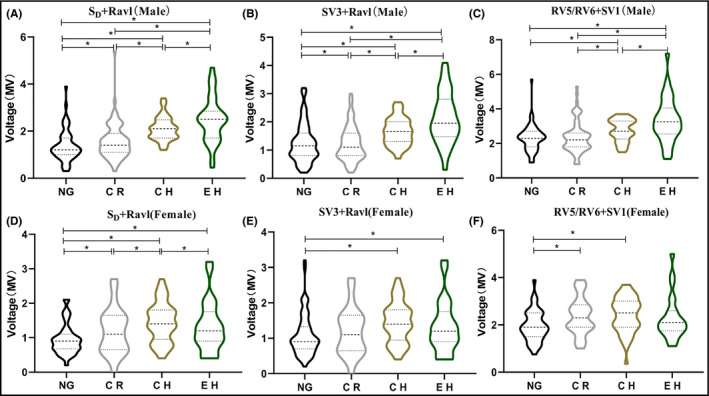
Distribution of ECG indexes in different hypertension geometries by gender. (A) Modified Cornell voltage in male, (B) Cornell voltage in male, (C) Sokolow–Lyon voltage in male, (D) Modified Cornell voltage in female, (E) Cornell voltage in female, and (F) Sokolow–Lyon voltage in female.*indicated P < .05. Data are shown as the mean median (interquartile range). NG, normal geometry; CR, concentric remodeling; CH concentric hypertrophy; EH, eccentric hypertrophy
3.3. Multivariable linear regression analysis
Multivariate linear regression analysis showed that compared with that in NG group, the voltage of Ravl + SD of male patients in CR, CH, and EH groups increased by 0.232, 0.745, and 1.119 mv, respectively, showing significant difference among these groups (Model 1, Table 2). After adjustments for potential mediators (Model 2 and Model 3), Ravl + SD still gradually increased with severity of hypertensive geometric abnormality increasing in male patients. Statistic analysis of the trend showed that the increasing trend of Ravl + SD of male patients with the severity of hypertensive geometric abnormality is statistically significant (P for trend <.001). The voltages of Ravl + SV3 and RV5/V6 + SV1 of male patients in CR, CH, and EH groups also showed a gradual increase trend, but the increase was not significantly different between CR group and NG group (P ≥ .05) (Table 2).
Table 2.
Multivariate regression model for male of the association between electrocardiogram indexes (per 1 mv) and LV hypertensive geometry
| SD + Ravl (mv) | RV5/V6 + SV1 (mv) | SV3 + RAVL (mv) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Β | 95% CI | P‐value | Β | 95% CI | P‐value | Β | 95% CI | P‐value | ||
| Model 1 (unadjusted) | ||||||||||
| NG | Reference | Reference | Reference | |||||||
| CR | 0.232 | 0.043, 0.421 | .017 | 0.006 | −0.208, 0.220 | .956 | .059 | −0.227, 0.345 | .686 | |
| EH | 0.745 | 0.493, 0.997 | <.001 | 0.433 | 0.201, 0.665 | .001 | .447 | 0.137, 0.757 | .005 | |
| CH | 1.119 | 0.826, 1.413 | <.001 | 0.910 | 0.640, 1.179 | <.001 | 1.041 | 0.681, 1.401 | <.001 | |
| P trend | <.001 | <.001 | <.001 | |||||||
| Model 2 (adjusted for BMI, age) | ||||||||||
| NG | Reference | Reference | Reference | |||||||
| CR | 0.395 | 0.154, 0.636 | .002 | 0.191 | −0.031, 0.412 | .093 | 0.261 | −0.038, 0.561 | .088 | |
| EH | 0.809 | 0.558, 0.061 | <.001 | 0.478 | 0.247, 0.709 | <.001 | 0.528 | 0.216, 0.840 | .001 | |
| CH | 1.134 | 0.838, 1.429 | <.001 | 0.909 | 0.637, 1.180 | <.001 | 1.076 | 0.709, 1.443 | <.001 | |
| P trend | <.001 | .001 | .001 | |||||||
| Model 3 (adjusted for BMI, age, heart rate, SBP, DBP, EF, hypertension time, family history of hypertension, history of diabetes, regular exercise and sport, and use of antihypertensive drugs) | ||||||||||
| NG | Reference | Reference | Reference | |||||||
| CR | 0.306 | 0.017, 0.595 | .039 | 0.159 | −0.107, 0.426 | .242 | 0.140 | −0.216, 0.497 | .441 | |
| EH | 0.743 | 0.480, 1.006 | <.001 | 0.456 | 0.214, 0.699 | <.001 | 0.475 | 0.150, 0.799 | .005 | |
| CH | 1.183 | 0.881, 1.484 | <.001 | 0.922 | 0.644, 1.200 | <.001 | 1.099 | 0.727, 1.470 | <.001 | |
| P trend | <.001 | <.001 | <.001 | |||||||
Abbreviations: BMI, body mass index; CH, concentric hypertrophy; CR, concentric remodeling; DBP, diastolic blood pressure; EF, eject fraction; EH, eccentric hypertrophy; NG, normal geometry; SBP, systolic blood pressure.
Multivariate linear regression analysis indicated that the voltage of Ravl + SD, RV5/V6 + SV1, and Ravl + SV3 in female patients showed a trend of gradual increase in CR, CH groups, and then followed by a decrease trend in EH groups (Model 1), but a trend of increase after decrease in Model 2 and Model 3. There was no relation among women between SV3 + Ravl in Model 2 (P for trend = .087) or in Model 3 (P for trend = .107) and LV geometry (Table 3).
Table 3.
Multivariate regression model for female of the association between electrocardiogram indexes (per 1 mv) and hypertensive geometry
| n = 168 | SD + Ravl (mv) | RV5/V6 + SV1 (mv) | SV3 + Ravl (mv) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Β | 95% CI | P‐value | Β | 95% CI | P‐value | Β | 95% CI | P‐value | |
| Model 1 (unadjusted) | |||||||||
| NG | Reference | Reference | Reference | ||||||
| CR | 0.383 | 0.067, 0.699 | .019 | 0.239 | −0.046, 0.524 | .102 | 0.417 | 0.047, 0.788 | .287 |
| EH | 0.696 | 0.439, 0.953 | <.001 | 0.495 | 0.263, 0.726 | <.001 | 0.474 | 0.173, 0.774 | .002 |
| CH | 0.385 | 0.069, 0.701 | .018 | 0.379 | 0.094, 0.664 | .010 | 0.331 | −0.039, 0.702 | .082 |
| P trend | <.001 | <.001 | .017 | ||||||
| Model 2 (adjusted for BMI, age) | |||||||||
| NG | Reference | Reference | Reference | ||||||
| CR | −0.036 | −0.401, 0.329 | .849 | −0.065 | −0.398, 0.269 | .705 | −0.145 | −0.570, 0.280 | .505 |
| EH | 0.347 | 0.054, 0.640 | .022 | 0.228 | −0.040, 0.496 | .097 | 0.039 | −0.302, 0.381 | .822 |
| CH | 0.354 | 0.054, 0.655 | .022 | 0.356 | 0.081, 0.631 | .012 | 0.303 | −0.047, 0.653 | .092 |
| P trend | .002 | .003 | .087 | ||||||
| Model 3 adjusted for BMI, age, heart rate, SBP, DBP, EF, hypertension time, family history of hypertension, history of diabetes, regular exercise and sport, and use of antihypertensive drugs) | |||||||||
| NG | Reference | Reference | Reference | ||||||
| CR | −0.196 | −0.624, 0.231 | .370 | −0.083 | −0.477, 0.311 | .679 | −0.333 | −0.831, 0.166 | .193 |
| EH | 0.308 | −0.000, 0.617 | .052 | 0.234 | −0.050, 0.518 | .109 | −0.013 | −0.372, 0.347 | .945 |
| CH | 0.332 | 0.019, 0.645 | .039 | 0.325 | 0.037, 0.613 | .029 | 0.286 | −0.079, 0.650 | .127 |
| P trend | .003 | .005 | .107 | ||||||
Abbreviations: BMI, body mass index; CH, concentric hypertrophy; CR, concentric remodeling; DBP, diastolic blood pressure; EF, eject fraction; EH, eccentric hypertrophy; NG, normal geometry; SBP, systolic blood pressure.
4. DISCUSSION
This study found that LV geometry progressed with prolonged time of hypertension and substandard blood pressure control. With the adjustment for other factors, the voltage of Ravl + SD in male patients with hypertension showed an increase trend with geometric changes and presented a clinical application value.
Left ventricular hypertrophy is an adaptation to the load after hypertension. In other words, hypertension induces the change in LV geometry. 8 With disease progressing, first, LV cavity is adaptively hypertrophic and gradually becomes eccentric hypertrophy. The mortality and morbidity of patients with different LV geometries were significantly different. 6 , 13 In addition, even during the concentric remodeling phase, which has distinctive geometric and hemodynamic characteristics, 6 LV geometry is also associated with increased cardiovascular morbidity and mortality. 14 Cardiac morphological characterization of hypertensive patients could provide clinicians with indirect information concerning the neurohormonal and hemodynamic profiles of these patients. 15 Most left ventricular hypertrophy ECG criteria are established based on the presumption that myocardium has more electrical activity and prolonged action potential duration (repolarization) is one of the main responses of cells to LVH. 16 Increased myocardial fibers and cardiomyocyte enlargement are the most important factors affecting the ECG voltage of patients with hypertension. The commonly used Cornell criteria and Sokolow‐Lyon criteria are not sensitive to early geometric changes in patients with hypertension. Aktoz et al studied 125 patients with essential hypertension and showed that the traditional ECG voltage criteria could not significantly distinguish ventricular geometric changes of patients with essential hypertension. 17 In addition, LV remodeling is not only accompanied with increased protein levels of certain cardiomyocytes, but also with changes in extracellular matrix such as increased fibroblasts, cardiac steatosis and vascular smooth muscle cell proliferation, 18 which are important influencing factors for the low sensitivity of ECG criteria. Meanwhile, increase in LV voltage is not only related to demographic factors (age, female, etc), ventricular surface area, intraluminal blood volume and the distance from ventricular surface to the chest wall, 19 but also affected by other diseases such as essential hypertension, diabetes, chronic obstructive pulmonary disease, abnormal chest anatomy, and obesity, all of which can significantly affect the diagnostic accuracy of certain ECG criteria. The traditional Cornell criteria comprehensively consider the forehead plus transverse leads. Although the longest axis of the transverse QRS vector ring is offset to the left and rear, most of them are perpendicular to the V3 lead axis. However, the maximum negative value of the S wave does not always occur in V3 lead. The simple Sokolow‐Lyon criteria only reflects left ventricle from the left‐rear direction at a cross‐sectional view, and cannot fully reflect the depolarization vectors of the walls of the entire hypertrophic ventricle.
In this study, we found that the distribution of modified Cornell criteria was statistically different in hypertensive patients with different geometric patterns. After grouping by gender and adjusting for factors such as age, BMI, heart rate, blood pressure, eject fraction and diabetes, the trend of gradual increasing in the distribution of modified Cornell criteria with the severity of geometric abnormality is still statistical significant in male patients. As LV geometry changing in hypertensive patients, the maximum QRS vector on the transverse plane shifts from the normal position first to the right and then gradually to the left and rear and the voltage shows a dynamic changing process. In this study, the modified Cornell criteria take the frontal and lateral leads into consideration and are more comprehensive. The deepest S wave response voltage in the 12‐lead ECG is selected for the horizontal vector and the largest QRS on the frontal plane points to the upper left. The combination of these two may be an important reason for increased sensitivity of the modified Cornell criteria in detecting changes in hypertensive LV geometry. Tomita et al showed that the combination of a high Sokolow‐Lyon voltage with a low RV6/V5 ratio for the CR group and a low Sokolow‐Lyon voltage for the NG group yielded modest sensitivity and specificity. The combination of a high Sokolow–Lyon voltage with a low Cornell voltage was moderately specific. 20 Aktoz et al divided LV geometry of selected 125 hypertensive patients according to their echocardiographic images. Although the current commonly used ECG criteria are very specific (range 97‐100%) and showed very high positive predictive values (range 94‐100%) for detecting abnormal geometry, they can not identify the distribution differences of different categories, 17 which may be related to the factors such as fewer research subjects, different calculation standards for LVM, and failure to classify by gender.
Data from several studies indicated that after adjustment for blood pressure and anthropometric parameters, LV volume and LVM are higher in men than in women. 21 How to diagnose LVH of different genders with unified ECG criteria is an urgent issue in ECG research. This study showed that the change in voltage with remodeling aggravation varied between men and women. The peak voltage appears earlier in females than in males. In the adjusted Model 2 and Model 3, the voltage change in females with geometric change showed a trend of decrease before increase. Previous studies have also revealed difference between females and males. Sokolow‐Lyon criteria tend to identify LVH in male, while Cornell criteria tend to identify LVH in female with a lower cutoff value for LVH diagnosis. 22 Therefore, it is necessary to establish gender‐specific criteria for LVH diagnosis.
The LV geometric change is an important form of hypertensive LV geometric changes. The evolution of left ventricle from normal geometry to concentric remodeling and concentric hypertrophy undoubtedly reflects the adjustment and adaptation process of cardiac structural function under the influence of pressure load. Among them, concentric remodeling is the starting point of hypertensive cardiac organic lesions. When it progresses to the eccentric hypertrophy stage, interstitial fibrosis, increased collagen deposition and changes in the proportion of collagen subtypes will appear, leading to anatomic uncoupling of adjacent muscle cells, which is also the electrophysiological basis of proarrhythmia of the hypertrophic myocardium. 23 The changes of LV geometry in patients with hypertension are also closely related to prognosis. 24 , 25 Therefore, it is important to identify abnormal geometric changes early. The modified Cornell criteria is superior to the existing criteria in increasing with change in hypertensive geometry and can identify hypertensive geometric changes as early as at the reversible stage so as to prevent disease development at early stage.
5. CONCLUSIONS
The improved Cornell criteria algorithm is relatively simple and could dynamically reflect the changes in myocardial voltage and well reflect the different stages of LV remodeling. This algorithm can be used in ECG built‐in software to detect LVH so that these indexes can be automatically provided to physicians and decision makers.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
MX, ZG, and JH contributed to design of work, analysis and interpretation of data, and drafting of manuscript. ZG and JH contributed to design of work and acquisition of data. JL, JY and XS contributed to design of work and acquisition of data and critical appraisal of manuscript. JL, JY and XS contributed to important intellectual content and critical appraisal of manuscript. JL, JY contributed to design of work, interpretation of data, and critical appraisal of manuscript.
Xu M, Ge Z, Huang J, et al. Modified Cornell electrocardiographic criteria in the assessment of left ventricular hypertrophy geometry of patients with essential hypertension. J Clin Hypertens. 2020;22:1239–1246. 10.1111/jch.13919
Funding information
This study was authorized and financially supported by Natural Science Foundation of China (Grant NO.81471690); Application basic Project of Changzhou City Science and Technology Bureau (Grant NO.CJ20190086). Application basic Project of Changzhou City Health Bureau (Grant NO.WZ201804).
Contributor Information
MRCP, Email: 13861058536@163.com, Email: yangjhsz1@163.com.
Junhua Yang, Email: yangjhsz1@163.com.
REFERENCES
- 1. Williams B, Mancia G, Agabiti Rosei E, et al. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC Task Force for the Management of Arterial Hypertension: Erratum. J Hypertens. 2019;37:456. [DOI] [PubMed] [Google Scholar]
- 2. Cuspidi C, Rescaldani M, Sala C, Negri F, Grassi G, Mancia G. Prevalence of electrocardiographic left ventricular hypertrophy in human hypertension: an updated review. J Hypertens. 2012;30:2066‐2073. [DOI] [PubMed] [Google Scholar]
- 3. Tadic M, Cuspidi C, Grassi G. The influence of sex on left ventricular remodeling in arterial hypertension. Heart Fail Rev. 2019;24:905‐914. [DOI] [PubMed] [Google Scholar]
- 4. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). G Ital Cardiol. 2006;2018(19):3‐73. [DOI] [PubMed] [Google Scholar]
- 5. Alp H, Karaarslan S, Eklioglu BS, Atabek ME, Baysal T. The effect of hypertension and obesity on left ventricular geometry and cardiac functions in children and adolescents. J Hypertens. 2014;32:1283‐1292. [DOI] [PubMed] [Google Scholar]
- 6. Ganau A, Devereux RB, Roman MJ, de Simone G, Pickering TG, Saba PS. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992;19:1550‐1558. [DOI] [PubMed] [Google Scholar]
- 7. Devereux RB, Palmieri V, Liu JE, et al. Progressive hypertrophy regression with sustained pressure reduction in hypertension: the losartan intervention for endpoint reduction study. J Hypertens. 2002;20:1445‐1450. [DOI] [PubMed] [Google Scholar]
- 8. Takasaki K, Miyata M, Imamura M, et al. Left ventricular dysfunction assessed by cardiac time interval analysis among different geometric patterns in untreated hypertension. Circ J. 2012;76:1409‐1414. [DOI] [PubMed] [Google Scholar]
- 9. Peguero JG, Lo Presti S, Perez J, et al. Electrocardiographic standard for the diagnosis of left ventricular hypertrophy. J Am Coll Cardiol. 2017;69:1694‐1703. [DOI] [PubMed] [Google Scholar]
- 10. Hope SV, Wienand‐Barnett S, Shepherd M, et al. Practical classification guidelines for diabetes in patients treated with insulin: a cross‐sectional study of the accuracy of diabetes diagnosis. Br J Gen Pract. 2016;66:e315‐e322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hancock EW, Deal BJ, Mirvis DM, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009;53:992‐1002. [DOI] [PubMed] [Google Scholar]
- 12. Marwick TH, Gillebert TC, Aurigemma G, et al. Recommendations on the use of echocardiography in adult hypertension: a report from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE). J Am Soc Echocardiogr. 2015;28:727‐754. [DOI] [PubMed] [Google Scholar]
- 13. Shenasa M, Shenasa H. Hypertension, left ventricular hypertrophy, and sudden cardiac death. Int J Cardiol. 2017;237:60‐63. [DOI] [PubMed] [Google Scholar]
- 14. Zabalgoitia M, Berning J, Koren MJ, et al. Impact of coronary artery disease on left ventricular systolic function and geometry in hypertensive patients with left ventricular hypertrophy (the LIFE study). Am J Cardiol. 2001;88:646‐650. [DOI] [PubMed] [Google Scholar]
- 15. Dávila DF, Donis JH, Odreman R, Gonzalez M, Landaeta A. Patterns of left ventricular hypertrophy in essential hypertension: should echocardiography guide the pharmacological treatment? Int J Cardiol. 2008;124:134‐138. [DOI] [PubMed] [Google Scholar]
- 16. Shenasa M, Shenasa H, El‐Sherif N. Left ventricular hypertrophy and arrhythmogenesis. Card Electrophysiol Clin. 2015;7:207‐220. [DOI] [PubMed] [Google Scholar]
- 17. Aktoz M, Erdogan O, Altun A. Electrocardiographic prediction of left ventricular geometric patterns in patients with essential hypertension. Int J Cardiol. 2007;120:344‐350. [DOI] [PubMed] [Google Scholar]
- 18. Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther. 2010;128:191‐227. [DOI] [PubMed] [Google Scholar]
- 19. Surawicz B. Electrocardiographic diagnosis of chamber enlargement. J Am Coll Cardiol. 1986;8:711‐724. [DOI] [PubMed] [Google Scholar]
- 20. Tomita S, Ueno H, Takata M, Yasumoto K, Tomoda F, Inoue H. Relationship between electrocardiographic voltage and geometric patterns of left ventricular hypertrophy in patients with essential hypertension. Hypertens Res. 1998;21:259‐266. [DOI] [PubMed] [Google Scholar]
- 21. Gjesdal O, Bluemke DA, Lima JA. Cardiac remodeling at the population level‐risk factors, screening, and outcomes. Nat Rev Cardiol. 2011;8:673‐685. [DOI] [PubMed] [Google Scholar]
- 22. Porthan K, Niiranen TJ, Varis J, et al. ECG left ventricular hypertrophy is a stronger risk factor for incident cardiovascular events in women than in men in the general population. J Hypertens. 2015;33:1284‐1290. [DOI] [PubMed] [Google Scholar]
- 23. Wolk R. Arrhythmogenic mechanisms in left ventricular hypertrophy. Europace. 2000;2:216‐223. [DOI] [PubMed] [Google Scholar]
- 24. Teh RO, M. Kerse N, M. Robinson E, A. Whalley G, J. Connolly M, N. Doughty R. Left ventricular geometry and all‐cause mortality in advanced age. Heart Lung Circ. 2015;24:32‐39. [DOI] [PubMed] [Google Scholar]
- 25. Lieb W, Gona P, Larson MG, et al. The natural history of left ventricular geometry in the community: clinical correlates and prognostic significance of change in LV geometric pattern. JACC Cardiovasc Imaging. 2014;7:870‐878. [DOI] [PMC free article] [PubMed] [Google Scholar]


