Abstract
Background
Serum markers currently used as indicators of iron status have clinical limitations. Hepcidin, a key regulator of iron homeostasis, is reduced in iron deficiency (ID) and increased in iron overload. We describe the first CLIA-validated immunoassay with excellent accuracy and precision to quantify human serum hepcidin. Its diagnostic utility for detecting ID in first-time blood donors was demonstrated.
Methods
A monoclonal competitive ELISA (C-ELISA) was developed for the quantitation of human hepcidin and validated according to CLIA guidelines. Sera from nonanemic first-time blood donors (n = 292) were analyzed for hepcidin, ferritin, transferrin, and serum iron. Logistic regression served to determine the utility of hepcidin as a predictor of ID.
Results
The C-ELISA was specific for human hepcidin and had a low limit of quantitation (4.0 ng/mL). The hepcidin concentration measured with the monoclonal C-ELISA was strongly correlated with a previously established, extensively tested polyclonal C-ELISA (Blood 2008;112:4292–7) (r = 0.95, P < 0.001). The area under the receiver operating characteristic curve for hepcidin as a predictor of ID, defined by 3 ferritin concentration thresholds, was >0.9. For predicting ID defined by ferritin <15 ng/mL, hepcidin <10 ng/mL yielded sensitivity of 93.1% and specificity of 85.5%, whereas the same hepcidin cutoff for ferritin <30 ng/mL yielded sensitivity of 67.6% and specificity of 91.7%.
Conclusion
The clinical measurement of serum hepcidin concentrations was shown to be a potentially useful tool for diagnosing ID.
Keywords: Hepcidin, ELISA, Iron Deficiency, Anemia
Impact Statement
Iron deficiency anemia is a chronic issue faced by populations around the world. Premenopausal women are at particular risk of iron deficiency with a higher prevalence of anemia compared to the general population. Hepcidin, the master regulator of iron homeostasis, has emerged as a reliable biomarker for iron availability and utilization. A clinically relevant blood test for hepcidin could aid clinicians in ruling out iron deficiency as a cause for patient anemia. We present a CLIA approved assay for hepcidin and demonstrate its utility to identify non-anemic, iron deficient first-time blood donors.
Introduction
Iron is essential for heme and hemoglobin synthesis, and restriction of iron delivery to erythrocyte precursors can limit erythropoiesis (1). Iron deficiency (ID) is a major contributor to the high global prevalence of anemia in both low-income and developed countries (2). Identifying patients with iron-restricted erythropoiesis before they progress to ID anemia (IDA) is a challenge for clinicians, especially when patients have comorbidities that give rise to anemia of chronic disease (ACD). Measurements of serum or plasma concentrations of hepcidin, the systemic iron regulatory hormone, can distinguish IDA from ACD in patients with mixed status in cases of tumor-related anemia (3), rheumatoid arthritis (4), inflammatory bowel disease (5), and critical illness (6) and in populations of African children (7).
Hepcidin-25 has been shown to be the principal regulator of systemic iron homeostasis (8). Hepcidin binds to the sole iron exporter ferroportin, causing its occlusion, internalization, and eventual degradation (9). This effectively shuts down the flow of iron into plasma from duodenal enterocytes that absorb dietary iron, macrophages that recycle senescent erythrocyte iron, and hepatocytes that store iron. Hepcidin is upregulated by iron loading (10) and inflammation (11) and downregulated by anemia and hypoxia (12). Measurement of serum hepcidin may allow the assessment of iron requirements and prove a valuable indicator of physiologic ID. The addition of hepcidin to the repertoire of iron indexes could aid clinicians in the initial exclusion of ID as the root cause of patient anemia or detection of ID progression before anemia develops.
We describe the Intrinsic Hepcidin IDx™ Test, validated using CLIA guidelines as a laboratory-developed test. This assay was tested in a first-time blood donor population.
Methods and Materials
Production of Antihepcidin Monoclonal Antibody
Monoclonal antibody was produced against a synthetic linear peptide containing 9 amino acids from the N-terminus of human hepcidin-25. Female BALB-C mice (8–10 weeks old) were immunized, and an IgG-secreting hybridoma was selected. Antibody was produced with a hollow-fiber bioreactor (Spectrum Laboratories) and purified via affinity chromatography (HiTrap Protein G) and buffer exchange (HiPrep 26/10; GE Healthcare). Aliquots were stored at −20 °C.
Competitive ELISA
ELISA plates (96-well) were coated with 100 µL of antibody, blocked, dried, and stored in foil pouches at 4 °C. The competitive ELISA (C-ELISA) was generally performed as described previously (13). Synthetic human hepcidin-25 and a patented biotin-labeled hepcidin-25 peptide competed for antibody binding sites. The C-ELISA was run on a Biomek FX Laboratory Automation Workstation (Beckman Coulter) using a 96-well head equipped with a selective tip loader and a Cytomat 2C15 temperature-controlled microplate incubator (Heraeus/Kendro Laboratory Products). A duplicate 8-point standard curve (0–400 ng/mL) was prepared in Tris-buffered saline containing biotinylated hepcidin-25 competitor peptide. Samples and controls diluted 1:5 in the biotinylated competitor peptide were added to their respective wells. Absorbance (450 nm) was recorded with a microplate reader (Molecular Devices DTX 880). All data were analyzed using a 4-parameter logistic regression software program (GraphPad Prism), and the hepcidin-25 concentration was calculated (ng/mL).
Validation of C-ELISA
Method validation consisted of characterization of synthetic hepcidin-25, including its bioactivity, assessments of monoclonal antibody selectivity, assay accuracy, intra- and interassay precision, sensitivity and analytical measurement range (AMR), interference by serum components, spike recovery, dilutional linearity, analyte stability, and determination of reference ranges. The reference test was performed as described previously (13). The validation experiments were performed according to the procedures developed by Intrinsic LifeSciences following CLIA guidelines (14).
Samples
Sera from first-time blood donors (self-identified with no history of previous donations) were obtained from the San Diego Blood Bank between June 2014 and December 2014 under institutional review board approval. All donors were prescreened for health status via questionnaire. Female and male donor inclusion criteria were as follows: at least 15 years of age (with parental consent), minimum weight of 114 pounds, good general health with no cold or flu-like symptoms, and a fingerstick hemoglobin result of at least 12.0 g/dL (HemoCue Hemoglobin system). A total of 292 serum samples were collected (149 female, 143 male) balanced across 4 general ethnic groups: black, Asian/Pacific Islander, white, and Hispanic/Latino.
Healthy iron status was defined by transferrin saturation (TSAT) between 15% and 45% and ferritin according to the age- and sex-specific brackets designated by ARUP Laboratories (Supplemental Table 1). Donors with missing data points were excluded from iron status classification.
Laboratory Measurements
Serum ferritin was measured with an Advia Centaur XP immunoassay system (Siemens), whereas serum iron, C-reactive protein, and transferrin were measured using a Cobas Integra 400 Plus clinical analyzer (Roche Diagnostics). TSAT was calculated as follows: TSAT % = (iron [µg/dL] / transferrin [mg/dL]) × 0.8 × 100.
Statistical Analysis
Given an estimated 18% prevalence of ID in the general nonanemic population, a minimum sample size of 280 would enable a sensitivity and specificity calculation of no less than 85% with a 95% CI of ±10% and ±5%, respectively.
ROC curves were calculated for hepcidin concentration as a test of ID compared with a surrogate gold standard: serum ferritin <30, 20, or 15 ng/mL (15). The sensitivity and specificity of hepcidin as an indicator of ID was determined for each possible hepcidin cutoff, and the area under the curve (AUC) for ROC curves (AUCROC) was generated. The curves were investigated to identify the hepcidin cutoffs that achieved the maximum percentage of correctly classified donors. Statistical significance was defined as P values <0.05.
Statistical analysis was performed using JMP12 (or greater) Pro software (SAS Institute). Log10 values for skewed measures, such as hepcidin, were used for statistical procedures with normality assumptions.
Results
Assay Characteristics
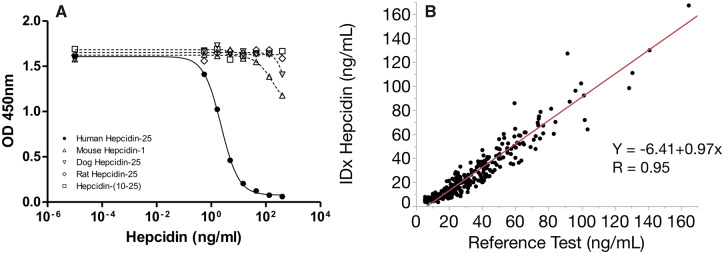
Complete assay performance characteristics are shown in Supplemental Table 2. The selectivity of our N-terminally directed monoclonal antibody to hepcidin-25 was assessed by performing dose response curves with structurally similar peptides (Fig. 1A). The removal of the N-terminus (1-9aa) from human hepcidin-25 (hepcidin-10 to -25) prevented the ability of the antibody to bind the antigen, demonstrating the requirement of the N-terminus for epitope recognition. We also tested dog, mouse, and rat hepcidin peptides, each with varying sequence similarity to the N-terminus of human hepcidin-25. We demonstrated 12%, 27%, and 0% reduction in overall signal, respectively, at the highest peptide concentration tested, despite the fact that rat hepcidin shares 80% identity with the N-terminus of human hepcidin-25.
Fig. 1.
Development and characterization of hepcidin C-ELISA. (A) Dose response curve starting at 400 ng/mL with serial dilutions. Structurally similar peptides were compared to assess selectivity. Hepcidin-10 to -25 is an N-terminally truncated hepcidin peptide. (B) Comparison of monoclonal hepcidin C-ELISA (n = 292) with a previously described polyclonal C-ELISA (1).
We also tested the selectivity of the C-ELISA to distinguish N-terminally truncated isoforms, representing <23% of the hepcidin species in circulation (16), from the full length hepcidin-25. The half maximal effective concentration of dose response curves generated from hepcidin-20 and hepcidin-22 was 55% and 59% of that for full length hepcidin-25, respectively (data not shown).
The AMR was defined as the lowest or highest measured value that achieves interassay precision (CV) and accuracy (relative error) within 25% and a total error <40%. Over 3 independent experiments, the AMR was found to be 4.0 to 200.0 ng/mL (1.4–71.6 pmol/L; lower AMR: CV = 10%, relative error = 12%, total error = 22%; upper AMR: CV = 11%, relative error = −8%, total error = 19%). The lower limit is consistent with the theoretical lower limit of quantitation, 5 SD from the zero calibrator, at 3.7 ng/mL (1.3 pmol/L). The lower limit of detection, 2 SD from the zero calibrator, was determined to be 1.5 ng/mL (0.5 pmol/L) (Supplemental Table 2).
Spike recovery was assessed using sera from 3 individual donors with low endogenous hepcidin (below the lower limit of detection). Each matrix spiked at concentrations of 200, 100, 50, 25, and 10 ng/mL yielded average percentage recoveries of 101%, 102%, 104%, 101%, and 97%, respectively. Spiking these low endogenous hepcidin samples above the AMR allowed the evaluation of dilutional linearity. Sera spiked at 1000, 500, and 250 ng/mL diluted 1:2, 1:4, and 1:8 yielded average percentage recoveries of 98%, 93%, and 90%, respectively.
We also examined the analytic interference from blood collection tube components by testing matching samples from 5 individual donors collected in serum separator tubes and Li-heparin. The average relative error of the serum separator tubes and Li-heparin samples compared with serum was 0% and −8%, respectively. All individual results for these matrices were within 15% of the serum values.
The intra- and interassay precision was evaluated by testing 3 serum samples representing high, medium, and low hepcidin concentrations. The average intra-assay CV (n = 5) over 3 independent experiments was 4% (98.6 ng/mL; 35.3 pmol/L), 5% (51.1 ng/mL; 18.3 pmol/L), or 7% (11.8 ng/mL; 4.2 pmol/L), respectively. The interassay precision studies were conducted with 28 measurements over 16 independent experiments and yielded CVs of 7% (100.0 ng/mL; 35.8 pmol/L), 8% (51.3 ng/mL; 18.4 pmol/L), or 9% (11.9 ng/mL; 4.3 pmol/L), respectively.
Hepcidin stability at room temperature for 4 hours or at 4 °C for 6 days was within 10% of those stored at −80 °C. Extending the room temperature stability to 24 hours leads to a further decline in measured hepcidin but still within 15%. Serum hepcidin was shown to be stable for up to 4 freeze-thaw cycles, with a consistent 88% to 106% recovery.
Although many assays and methods have been developed to measure serum hepcidin, the absolute measured value can vary significantly (17). The accuracy of our monoclonal C-ELISA was compared with a previously described polyclonal C-ELISA (13). The index test showed a high degree of accuracy (slope, 0.97; 95% CI, 0.94–1.01) and a strong correlation (R = 0.95) with the reference assay (Fig. 1B).
Blood Donor Evaluation
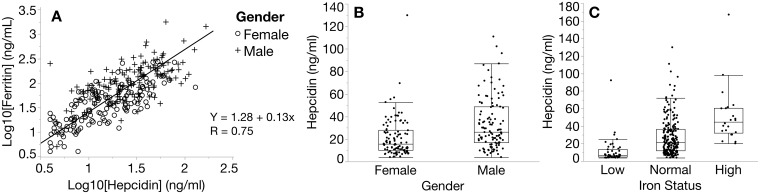
A reference population (n = 292) was obtained from first-time blood donors in good general health and screened for anemia (hemoglobin ≥12.0 g/dL). The median age for female and male donors was 29 years (interquartile range, 23–36) and 30 years (interquartile range, 25–39), respectively. Donors were equally distributed across gender and ethnicity. Donor hepcidin concentrations below the lower limit of quantitation (n = 4) could not be numerically reported so for the purpose of the reference range those values were set to the lower limit of quantitation (4.0 ng/mL). Hepcidin ranged from 4.0 to 167.2 ng/mL (1.4–59.9 pmol/L) with a median of 13.1 ng/mL (4.7 pmol/L) and 28.5 ng/mL (10.2 pmol/L) in female and male donors, respectively (Table 1). To assess the iron status of these individuals, additional iron markers were measured (Supplemental Tables 3 and 4). Only ferritin showed a strong correlation with hepcidin (Fig. 2A) (R = 0.75).
Table 1.
Hepcidin concentration in all blood donors and a subset with healthy iron status.a
| Type | Sex | n | Quantile (ng/mL) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 0% | 5% | 25% | Median | 75% | 95% | 100% | |||
| All Donors Mixed Iron Status | Female | 149 | 19.6 | 4.0 | 4.4 | 7.6 | 13.1 | 26.2 | 54.1 | 129.9 |
| Male | 143 | 35.4 | 4.0 | 6.1 | 17.1 | 28.5 | 48.4 | 91.2 | 167.2 | |
| Healthy Donors Normal Iron Status | Female | 104 | 21.4 | 4.0 | 6.2 | 10.4 | 15.9 | 27.9 | 51.5 | 129.9 |
| Male | 113 | 33.7 | 4.0 | 8.6 | 17.0 | 26.2 | 48.6 | 82.6 | 111.0 | |
Normal iron status was defined by age- and sex-specific ferritin cutoffs and TSAT 15%–45%. SI conversion: 1 ng/mL = 0.358 pmol/L.
Fig. 2.
Evaluation of first-time blood donors. (A) Donor comparison of matched serum hepcidin and ferritin concentrations (n = 292) (Demming regression). (B) Hepcidin by sex in donors with normal iron status (female, n = 104; male, n = 113; P < 0.0002). (C) Hepcidin in donors with low, normal, or high iron status classified using serum ferritin and TSAT (P < 0.0001, Steel-Dwass method).
Based on normal ferritin concentration ranges for sex and age (Supplemental Table 1) and normal TSAT from 15% to 45%, we observed that 22% (33/149) of female donors and 8% (12/143) of male donors were iron deficient. In addition, 6% (9/149) of female donors and 9% (13/143) of male donors had evidence of iron overload. Excluding donors with ID and iron overload and those with incomplete iron parameters, we defined a healthy, normal iron status reference range (n = 217) for hepcidin from 4.0 to 129.9 ng/mL (1.4–46.5 pmol/L) with a median of 15.9 ng/mL (5.7 pmol/L) and 26.2 ng/mL (9.4 pmol/L) in female and male donors, respectively (Table 1). Serum hepcidin in healthy female donors (n = 104) was significantly lower (p = 0.0002 by a median test) than in healthy male donors (n = 113) (Fig. 2B). Taken collectively, the median hepcidin for the groups with low, normal, and high iron status were 6.7 ng/mL (2.4 pmol/L, n = 45), 21.4 ng/mL (7.7 pmol/L, n = 217), and 44.9 ng/mL (16.1 pmol/L, n = 22), respectively (P < 0.0001, Steel-Dwass nonparametric multiple comparison test) (Fig. 2C).
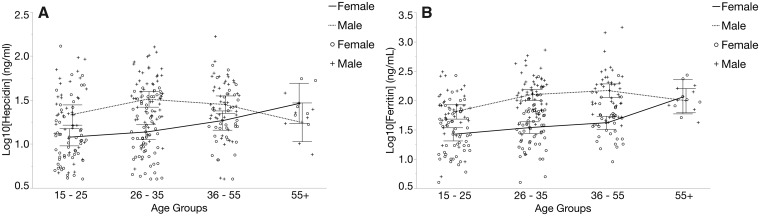
Based on the age distribution of our healthy donors, we grouped subjects into 4 age groups: 15–25, 26–35, 36–55, and >55 years. These definitions gave relatively equal distribution of subjects, with the exception of the oldest group, in which there were fewer subjects. For male donors, hepcidin (Fig. 3A) and ferritin (Fig. 3B) were consistent across all age groups, whereas for female donors, only the youngest and oldest age groups differed significantly (P < 0.04). Sex comparisons indicated that median hepcidin (Fig. 3A) and ferritin (Fig. 3B) levels were significantly different in the 2 youngest age groups (P < 0.016). No difference in hepcidin was observed for the 36- to 55-year age group; however, there was a significant difference (P < 0.0007) in the ferritin. Both hepcidin and ferritin were similar between female and male donors in the >55-year age group.
Fig. 3.
Age- and sex-dependent variation of hepcidin. Healthy donors were distributed into 4 bracketed age groups. Serum hepcidin (A) and ferritin (B) concentrations demonstrate similar distributions across age groups for both sexes.
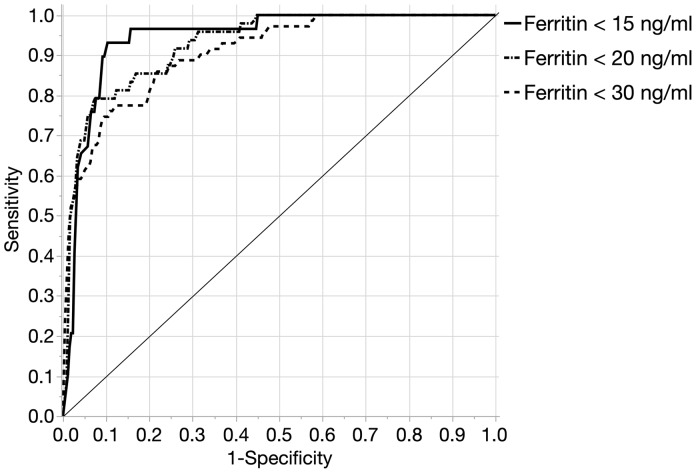
We then examined the utility of hepcidin as a diagnostic test for ID defined by a single ferritin cutoff. The appropriate ferritin cutoff to define ID is debated, but it is generally accepted to be <30 ng/mL (18–20). However, lower and more appropriate cutoffs have been tailored to specific study populations, as in premenopausal women (ferritin <15 ng/mL) (21) or children (ferritin <12 ng/mL) (7, 22). Using a range of common definitions of ID, ferritin <15, <20, or <30 ng/mL, the prevalence of ID in our study population was 10.2%, 16.5%, or 23.9%, respectively. These definitions were then used to evaluate the diagnostic potential of hepcidin for the detection of ID. In ROC curve analysis, the AUC for the defined ferritin cutoffs was 0.94 (95% CI, 0.89–0.97), 0.93 (95% CI, 0.88–0.95), and 0.91 (95% CI, 0.86–0.94), respectively (Fig. 4). When the potentially confounding effect of inflammation was removed by excluding donors with C-reactive protein >5.0 mg/L, the AUCs were essentially unchanged (n = 255; 0.94 (95% CI, 0.88–0.97), 0.93 (95% CI, 0.87–0.96), and 0.90 (95% CI, 0.85–0.94), respectively).
Fig. 4.
ROC curve for hepcidin as a predictor of ID. The AUCROC for hepcidin compared with ferritin cutoffs of <15, <20, or <30 ng/mL was 0.94, 0.93, and 0.90, respectively.
The diagnostic characteristics and their respective 95% CIs for representative hepcidin cutoffs to identify ID are depicted in Table 2. When hepcidin was <10 ng/mL (3.6 pmol/L), the maximum percentage correctly classified was achieved for all ferritin cutoffs of ID (ferritin <15 ng/mL, 86.3%; <20 ng/mL, 87.0%; < 30 ng/mL, 85.9%). The sensitivity and specificity of ferritin <15, 20, or 30 ng/mL were 93.1% and 85.5%, 78.7% and 88.6%, or 67.6% and 91.7%, respectively. Increasing the hepcidin cutoff to <15 ng/mL (5.4 pmol/L), the sensitivity and specificity for ferritin <15, 20, or 30 ng/mL were 96.6% and 69.8%, 91.5% and 73.8%, or 85.3% and 78.2%, respectively.
Table 2.
Properties of 2 hepcidin cutoffs as a diagnostic test of iron deficiency.
| ID ferritin cutoff, ng/mL | Hepcidin cutoff, ng/mL | Sensitivity, % (95% CI) | Specificity, % (95% CI) | Positive LR (95% CI) | Negative LR (95% CI) | Correctly classified, a % |
|---|---|---|---|---|---|---|
| <15 | <10 | 93.1 (78.0–98.1) | 85.5 (80.6–89.3) | 6.4 (4.0–9.2) | 0.08 (0.02–0.27) | 86.3 |
| <15 | 96.6 (82.8–99.4) | 69.8 (63.9–75.1) | 3.2 (2.3–4.0) | 0.05 (0.01–0.27) | 72.5 | |
| <20 | <10 | 78.7 (65.1–88.0) | 88.6 (83.9–92.1) | 6.9 (4.0–11.1) | 0.24 (0.13–0.42) | 87.0 |
| <15 | 91.5 (80.1–96.6) | 73.8 (67.9–79.0) | 3.5 (2.5–4.6) | 0.12 (0.04–0.29) | 76.7 | |
| <30 | <10 | 67.6 (55.8–77.6) | 91.7 (87.2–94.7) | 8.1 (4.4–14.6) | 0.35 (0.24–0.51) | 85.9 |
| <15 | 85.3 (75.0–91.8) | 78.2 (72.3–83.2) | 3.9 (2.7–5.5) | 0.19 (0.10–0.35) | 79.9 |
Specific to studied population.
Discussion
We have developed a CLIA-validated laboratory-developed test for the quantitative measurement of human hepcidin. This competitive ELISA, the Intrinsic Hepcidin IDx test, shows a high degree of correlation with a reference polyclonal assay with documented technical (13), physiologic (23), and diagnostic (21) validity but is based on a monoclonal antibody, a reagent that can be better chemically defined and standardized. The new assay has a wide AMR with a high degree of precision and spike recovery. The Intrinsic Hepcidin IDx test identified higher serum hepcidin in men compared with women and lower hepcidin associated with ID and demonstrated a direct positive correlation with serum ferritin. Our study of first-time blood donors demonstrated that hepcidin increases with age in women but remains relatively stable in men, a trend consistent with previously reported studies (18, 19).
Through an as yet unclear mechanism, loss of amino-terminal residues yields 2 smaller hepcidin isoforms: hepcidin-20 and -22 (24). Our assay detects all 3 isoforms; however, compared with hepcidin-25, hepcidin-20 and -22 had cross-reactivity of 55% and 59%, respectively. The truncated isoforms, which represent the minority of the hepcidin peptide in circulation, do not induce hypoferremia (25), whereas hepcidin-25 is the predominant and bioactive isoform. Whether the proportions of the 3 isoforms change depending on the underlying cause of ID remains to be determined. Likewise, the clinical relevance of distinguishing the various isoforms remains to be demonstrated.
Compared with first-time blood donors, those with 1 to 4 donations in the preceding 2 years had a 14-fold increase in the risk of ID (26). Our study demonstrated that in nonanemic first-time blood donors, hepcidin is an excellent diagnostic marker of ID. Compared with various ferritin definitions of ID, hepcidin had an AUCROC >0.9. Nevertheless, in the absence of point-of-care testing at blood center collection sites, additional measurement of hepcidin in the blood donor population would not be practical at this stage. However, our results indicated that prescreening of premenopausal women may be useful in preventing IDA in this population with high prevalence of ID. Furthermore, measurement of hepcidin may have broader diagnostic or prognostic utility in other physiologic or pathologic conditions associated with anemia.
Sensitivity and specificity are critical considerations when selecting a hepcidin cutoff for a particular population of clinical interest. Likelihood ratios (LRs), calculated from sensitivity and specificity, can be used to estimate and express diagnostic accuracy in clinical settings. LRs between 0 and 1 decrease the posttest probability of disease, and LRs >1 increase that probability, and these changes can be easily quantified (27). Our findings show that a hepcidin cutoff of <10 ng/mL increases the positive LR to ≥6.4 and correctly classifies >85% of subjects regardless of which ferritin level is used to define ID. With high sensitivity and low negative LRs, the Intrinsic Hepcidin IDx test could function as an effective way to initially rule out ID. However, this remains to be evaluated in appropriate clinical settings, and ongoing studies will be required to define the optimal cutoffs for different patient populations.
Variable time of blood sampling was a limitation of our study. Hepcidin can exhibit a diurnal circadian rhythm (28), but it appears to have no statistical effect on AUCROC for diagnosis of ID (21). When comparing sexes, our study showed a 2.75 times higher prevalence of ID in women than in men (22% vs 8%). This inclusion of female donors may overestimate the AUCROC for the clinical evaluation of male patients. However, a study of premenopausal women with the same 22% prevalence of ID generated a hepcidin AUCROC of 0.87 (21), whereas our study with the same ferritin cutoff, combining women and men, had an AUCROC of 0.94. This indicates the utility of hepcidin as a general diagnostic tool to detect ID in a diverse population.
Apart from diagnosing ID in general populations, hepcidin has been postulated to have clinical utility to predict nonresponders to oral iron in IDA (29) and responsiveness to intravenous iron in chemotherapy-related anemia (30). The Intrinsic Hepcidin IDx test was used recently to develop an index, serum iron/log10(hepcidin), to screen patients with chronic ID for iron-refractory ID anemia cause by TMPRSS6 mutations (31). This approach may simplify diagnosis of patients with ID iron-refractory ID anemia using a simple immunoassay without the cost of more expensive gene sequencing.
Identifying patients with iron-restricted erythropoiesis before it progresses to IDA is a challenge for clinicians when considering comorbidities that can give rise to ACD. Several diagnostic strategies have been proposed to distinguish IDA from ACD, but to date they seem to be of limited utility (32). The iron storage protein ferritin can be an indicator of iron stores but becomes upregulated under conditions of inflammation, causing iron sequestration and eventual hypoferremia (33). Soluble transferrin receptor has been reported to be a sensitive indicator of ID because it is released by erythropoietic precursors in proportion to their expanding population and is not influenced by inflammation. However, it is not specific for ID in all cases because it is increased in conditions that have increased red cell production, such as hemolytic anemias (34). The ferritin index combines these markers into a single formula, soluble transferrin receptor/log10(ferritin), and has been incorporated into a diagnostic plot in combination with reticulocyte hemoglobin (35). However, hepcidin has been proposed as a replacement for the ferritin index in part because of the very rapid hepcidin response within hours of hematologic changes, whereas the ferritin index takes several days to reflect those same changes (36). In addition, in patients with IDA, nonresponsiveness to oral iron can be predicted from patients’ baseline hepcidin levels, which have better positive predictive value than TSAT or ferritin concentrations (29).
Critical illness is another area of medicine that may benefit from routine hepcidin testing. ID defined by hepcidin <20 or <10 ng/mL was an independent predictor of mortality or poor quality of life, respectively, in intensive care unit patients at 1 year after discharge, and the odds ratios were superior to ID defined by ferritin <100 ng/mL (37). Both ferritin and hepcidin are upregulated during inflammation, but in critical illness, ID is often missed by ferritin alone, whereas hepcidin, suppressed by functional ID, can capture more of those false-negative patients. In the intensive care unit study, 6% of discharged patients were considered ID by ferritin <100 ng/mL, whereas 37% were considered ID by hepcidin <20 ng/mL, a result more in accordance with expectations, given the prevalence of ID in the general population and the high prevalence of bleeding complications and iatrogenic blood loss in the intensive care unit population (37).
As evidence accumulates of the opposing signals that concurrently regulate hepcidin, the diagnostic potential of hepcidin in various iron-related disorders becomes more apparent (38). Further clinical trials utilizing this CLIA-validated hepcidin assay will be essential for implementing improved diagnostic protocols for detecting ID.
Supplementary Material
Nonstandard abbreviations
- ID
iron deficiency
- IDA
iron deficiency anemia
- ACD
anemia of chronic disease
- C-ELISA
competitive ELISA
- AMR
analytical measurement range
- TSAT
transferrin saturation
- AUC
area under the curve
- LR
likelihood ratio
Previous presentation: Portions of this study were presented in poster form at the annual meeting of the American Society of Hematology, San Diego, CA, on December 4, 2016.
Nonstandard Abbreviations: ID, iron deficiency; IDA, iron deficiency anemia; ACD, anemia of chronic disease; C-ELISA, competitive ELISA; AMR, analytical measurement range; TSAT, transferrin saturation; AUC, area under the curve; LR, likelihood ratio.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 4 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; (c) final approval of the published article; and (d) agreement to be accountable for all aspects of the article thus ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest: Employment or Leadership: P. Gutschow, Intrinsic LifeSciences; H. Han, Intrinsic LifeSciences; G. Olbina, Intrinsic LifeSciences; K. Westerman, Intrinsic LifeSciences; V. Ostland, Intrinsic LifeSciences. Consultant or Advisory Role: E. Nemeth, Intrinsic LifeSciences, Silarus Pharma, Protagonist, Vifor, Ionis; T. Ganz, Intrinsic LifeSciences, Silarus Pharma, Ionis Pharma, Ambys Pharma, Sierra Oncology, Protagonist, Akebia, Keryx Pharma; K. Copeland, Intrinsic LifeSciences. Stock Ownership: P. Gutschow, Intrinsic LifeSciences; H. Han, Intrinsic LifeSciences; G. Olbina, Intrinsic LifeSciences; K. Westerman, Intrinsic LifeSciences; E. Nemeth, Intrinsic LifeSciences, Silarus Pharma; T. Ganz, Intrinsic LifeSciences, Silarus Pharma; V. Ostland, Intrinsic LifeSciences. Honoraria: None declared. Research Funding: Funding from the NIH (R44DK083843) awarded to Intrinsic LifeSciences. Expert Testimony: None declared. Patents: P. Gutschow, 9657098; H. Han, 9657098; G. Olbina, 9657098; K. Westerman, 9657098; M. Westerman, 9657098; V. Ostland, 9657098.
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, preparation of the manuscript, or final approval of the manuscript.
Acknowledgments: This project was the vision of Dr. Mark Westerman, who died August 30, 2017.
REFERENCES
- 1. Hentze MW, Muckenthaler MU, Galy B, Camaschella C.. Two to tango: regulation of mammalian iron metabolism. Cell 2010;142:24–38. [DOI] [PubMed] [Google Scholar]
- 2. McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B.. Worldwide prevalence of anaemia, who vitamin and mineral nutrition information system, 1993-2005. Public Health Nutr 2009;12:444–54. [DOI] [PubMed] [Google Scholar]
- 3. Shu T, Jing C, Lv Z, Xie Y, Xu J, Wu J.. Hepcidin in tumor-related iron deficiency anemia and tumor-related anemia of chronic disease: pathogenic mechanisms and diagnosis. Eur J Haematol 2015;94:67–73. [DOI] [PubMed] [Google Scholar]
- 4. van Santen S, van Dongen-Lases EC, de Vegt F, Laarakkers CMM, van Riel P, van Ede AE, Swinkels DW.. Hepcidin and hemoglobin content parameters in the diagnosis of iron deficiency in rheumatoid arthritis patients with anemia. Arthritis Rheum 2011;63:3672–80. [DOI] [PubMed] [Google Scholar]
- 5. Bergamaschi G, Di Sabatino A, Albertini R, Costanzo F, Guerci M, Masotti M, et al. Serum hepcidin in inflammatory bowel diseases: biological and clinical significance. Inflamm Bowel Dis 2013;19:2166–72. [DOI] [PubMed] [Google Scholar]
- 6. Lasocki S, Baron G, Driss F, Westerman M, Puy H, Boutron I, et al. Diagnostic accuracy of serum hepcidin for iron deficiency in critically ill patients with anemia. Intensive Care Med 2010;36:1044–8. [DOI] [PubMed] [Google Scholar]
- 7. Pasricha SR, Atkinson SH, Armitage AE, Khandwala S, Veenemans J, Cox SE, et al. Expression of the iron hormone hepcidin distinguishes different types of anemia in African children. Sci Transl Med 2014;6:235re3. [DOI] [PubMed] [Google Scholar]
- 8. Ganz T. Hepcidin and iron regulation, 10 years later. Blood 2011;117:4425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004;306:2090–3. [DOI] [PubMed] [Google Scholar]
- 10. Pigeon C, Ilyin G, Courselaud B, Leroyer P, Turlin B, Brissot P, Loréal O.. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem 2001;276:7811–9. [DOI] [PubMed] [Google Scholar]
- 11. Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T.. Il-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest 2004;113:1271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest 2002;110:1037–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M.. Immunoassay for human serum hepcidin. Blood 2008;112:4292–7. [DOI] [PubMed] [Google Scholar]
- 14. Westgard J. Basic method validation. 3rd ed Madison, WI: Westgard QC Inc; 2008:320 p. [Google Scholar]
- 15.World Health Organization. Iron Deficiency Anaemia: Assessment, Prevention, and Control.A Guide for Programme Managers. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- 16. Addo L, Ikuta K, Tanaka H, Toki Y, Hatayama M, Yamamoto M, et al. The three isoforms of hepcidin in human serum and their processing determined by liquid chromatography-tandem mass spectrometry (LC-tandem MS). Int J Hematol 2016;103:34–43. [DOI] [PubMed] [Google Scholar]
- 17. van der Vorm LN, Hendriks JC, Laarakkers CM, Klaver S, Armitage AE, Bamberg A, et al. Toward worldwide hepcidin assay harmonization: Identification of a commutable secondary reference material. Clin Chem 2016;62:993–1001. [DOI] [PubMed] [Google Scholar]
- 18. Traglia M, Girelli D, Biino G, Campostrini N, Corbella M, Sala C, et al. Association of HFE and TMPRSS6 genetic variants with iron and erythrocyte parameters is only in part dependent on serum hepcidin concentrations. J Med Genet 2011;48:629–34. [DOI] [PubMed] [Google Scholar]
- 19. Galesloot TE, Vermeulen SH, Geurts-Moespot AJ, Klaver SM, Kroot JJ, van Tienoven D, et al. Serum hepcidin: reference ranges and biochemical correlates in the general population. Blood 2011;117:e218–e225.e25. [DOI] [PubMed] [Google Scholar]
- 20. Camaschella C. Iron-deficiency anemia. N Engl J Med 2015;372:1832–43. [DOI] [PubMed] [Google Scholar]
- 21. Pasricha SR, McQuilten Z, Westerman M, Keller A, Nemeth E, Ganz T, Wood E.. Serum hepcidin as a diagnostic test of iron deficiency in premenopausal female blood donors. Haematologica 2011;96:1099–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weiler HA, Jean-Philippe S, Cohen TR, Vanstone CA, Agellon S.. Depleted iron stores and iron deficiency anemia associated with reduced ferritin and hepcidin and elevated soluble transferrin receptors in a multiethnic group of preschool-age children. Appl Physiol Nutr Metab 2015;40:887–94. [DOI] [PubMed] [Google Scholar]
- 23. Girelli D, Trombini P, Busti F, Campostrini N, Sandri M, Pelucchi S, et al. A time course of hepcidin response to iron challenge in patients with hfe and tfr2 hemochromatosis. Haematologica 2011;96:500–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schranz M, Bakry R, Creus M, Bonn G, Vogel W, Zoller H.. Activation and inactivation of the iron hormone hepcidin: Biochemical characterization of prohepcidin cleavage and sequential degradation to n-terminally truncated hepcidin isoforms. Blood Cells Mol Dis 2009;43: 169–79. [DOI] [PubMed] [Google Scholar]
- 25. Rivera S, Nemeth E, Gabayan V, Lopez MA, Farshidi D, Ganz T.. Synthetic hepcidin causes rapid dose-dependent hypoferremia and is concentrated in ferroportin-containing organs. Blood 2005;106:2196–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cable RG, Glynn SA, Kiss JE, Mast AE, Steele WR, Murphy EL, et al. Iron deficiency in blood donors: analysis of enrollment data from the reds-ii donor iron status evaluation (rise) study. Transfusion 2011;51:511–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McGee S. Simplifying likelihood ratios. J Gen Intern Med 2002;17:647–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schaap CC, Hendriks JC, Kortman GA, Klaver SM, Kroot JJ, Laarakkers CM, et al. Diurnal rhythm rather than dietary iron mediates daily hepcidin variations. Clin Chem 2013;59:527–35. [DOI] [PubMed] [Google Scholar]
- 29. Bregman DB, Morris D, Koch TA, He A, Goodnough LT.. Hepcidin levels predict non-responsiveness to oral iron therapy in patients with iron deficiency anemia. Am J Hematol 2013;88:97–101. [DOI] [PubMed] [Google Scholar]
- 30. Steensma DP, Sasu BJ, Sloan JA, Tomita DK, Loprinzi CL.. Serum hepcidin levels predict response to intravenous iron and darbepoetin in chemotherapy-associated anemia. Blood 2015;125:3669–71. [DOI] [PubMed] [Google Scholar]
- 31. Heeney MM, Guo D, De Falco L, Campagna DR, Olbina G, Kao PP, et al. Normalizing hepcidin predicts TMPRSS6 mutation status in patients with chronic iron deficiency. Blood 2018;132:448–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goodnough LT, Nemeth E, Ganz T.. Detection, evaluation, and management of iron-restricted erythropoiesis. Blood 2010;116:4754–61. [DOI] [PubMed] [Google Scholar]
- 33. Ganz T, Nemeth E.. Iron sequestration and anemia of inflammation. Semin Hematol 2009;46:387–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brugnara C, Adamson J, Auerbach M, Kane R, Macdougall I, Mast A.. Iron deficiency: what are the future trends in diagnostics and therapeutics? Clin Chem 2013;59:740–5. [DOI] [PubMed] [Google Scholar]
- 35. Thomas C, Kirschbaum A, Boehm D, Thomas L.. The diagnostic plot: a concept for identifying different states of iron deficiency and monitoring the response to epoetin therapy. Med Oncol 2006;23:23–36. [DOI] [PubMed] [Google Scholar]
- 36. Thomas C, Kobold U, Balan S, Roeddiger R, Thomas L.. Serum hepcidin-25 may replace the ferritin index in the Thomas plot in assessing iron status in anemic patients. Int J Lab Hematol 2011;33:187–93. [DOI] [PubMed] [Google Scholar]
- 37. Lasocki S, Lefebvre T, Mayeur C, Puy H, Mebazaa A, Gayat E; on behalf of the FROG-ICU study group. Iron deficiency diagnosed using hepcidin on critical care discharge is an independent risk factor for death and poor quality of life at one year: an observational prospective study on 1161 patients. Crit Care 2018;22:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Armitage AE, Drakesmith H.. The diagnostic potential of the iron-regulatory hormone hepcidin. HemaSphere 2019;3:100–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.